The Anti-Inflammatory Properties of Polysaccharides Extracted from Moringa oleifera Leaves on IEC6 Cells Stimulated with Lipopolysaccharide In Vitro
Simple Summary
Abstract
1. Introduction
2. Materials and Method
2.1. Plant Source and Extraction of Polysaccharide from M. oleifera
2.2. Structural Analysis of MOLP
2.3. IEC-6 Cell Culture
2.4. Cell Viability
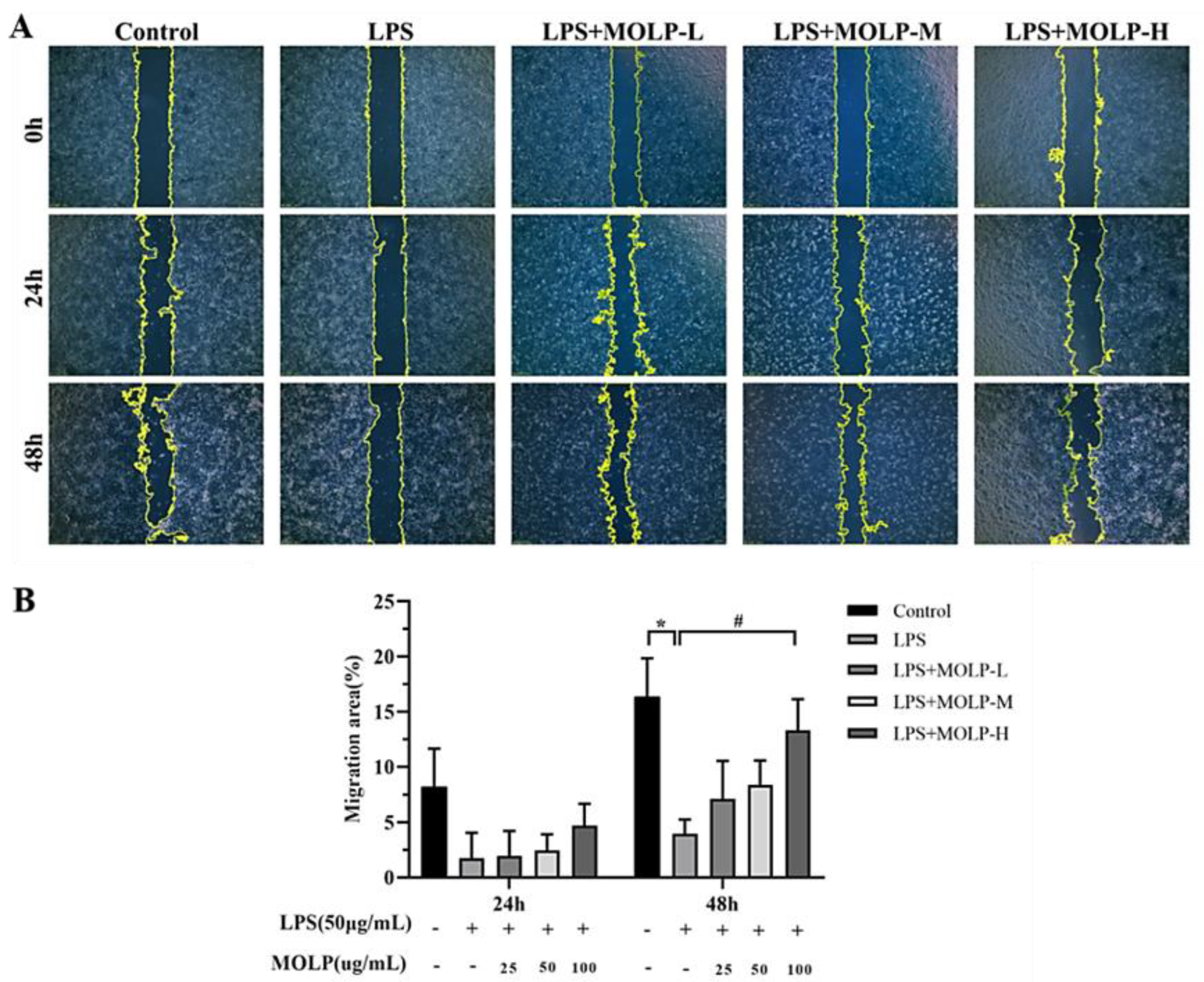
2.5. Cell Migration
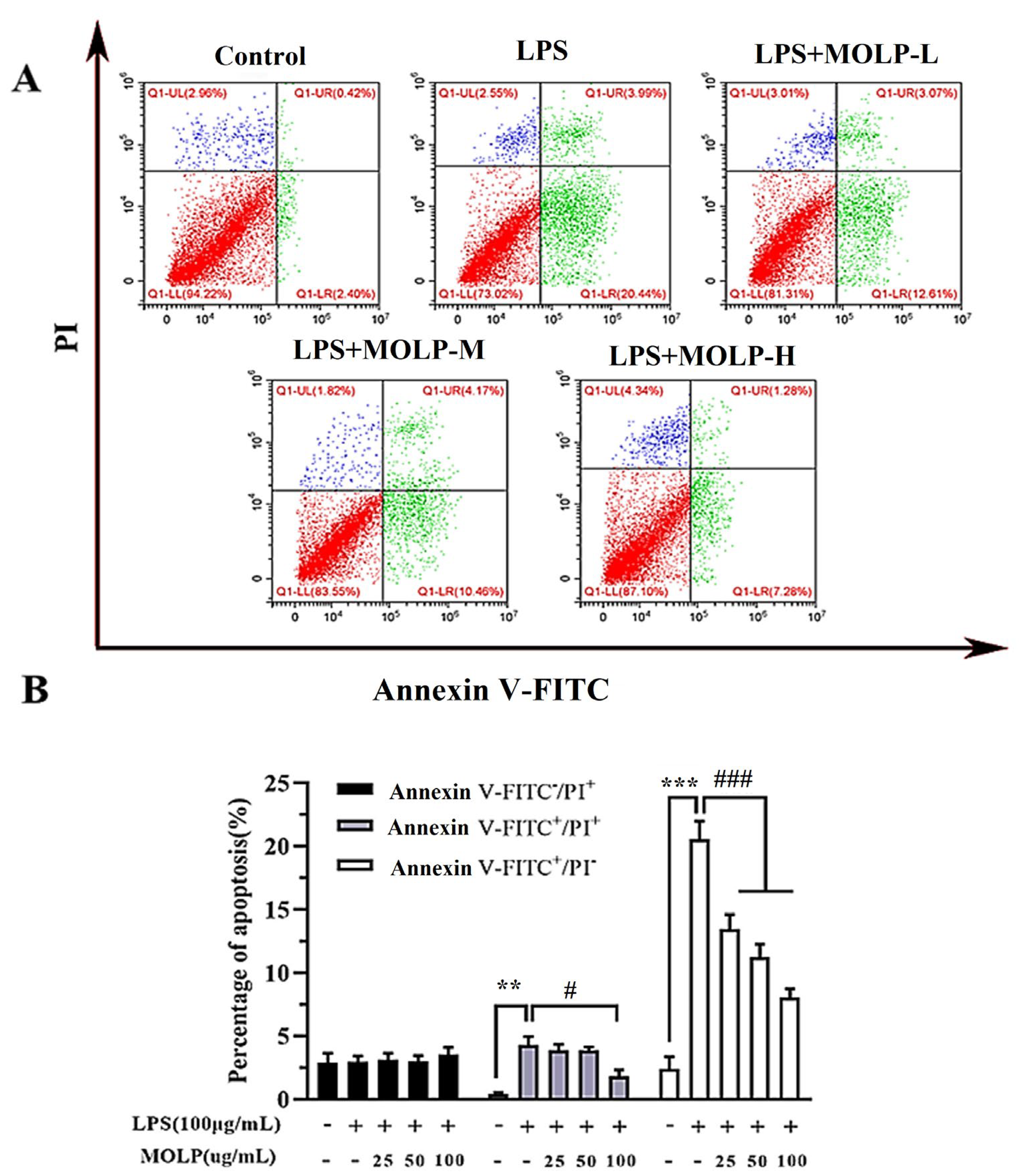
2.6. Cell Apoptosis
2.7. RT-qPCR Analysis
2.8. Western Blot Approach
2.9. Immunofluorescence
2.10. Statistical Analysis
3. Results and Discussion
3.1. Impact of MOLP on Cell Viability
3.2. Impact of MOLP on IEC-6 Cell Migration
3.3. MOLP Decreases Apoptosis in LPS-Activated IEC6 Cells
3.4. MOLP Suppresses Pro-Inflammatory Cytokine Production in LPS-Stimulated IEC6 Cells
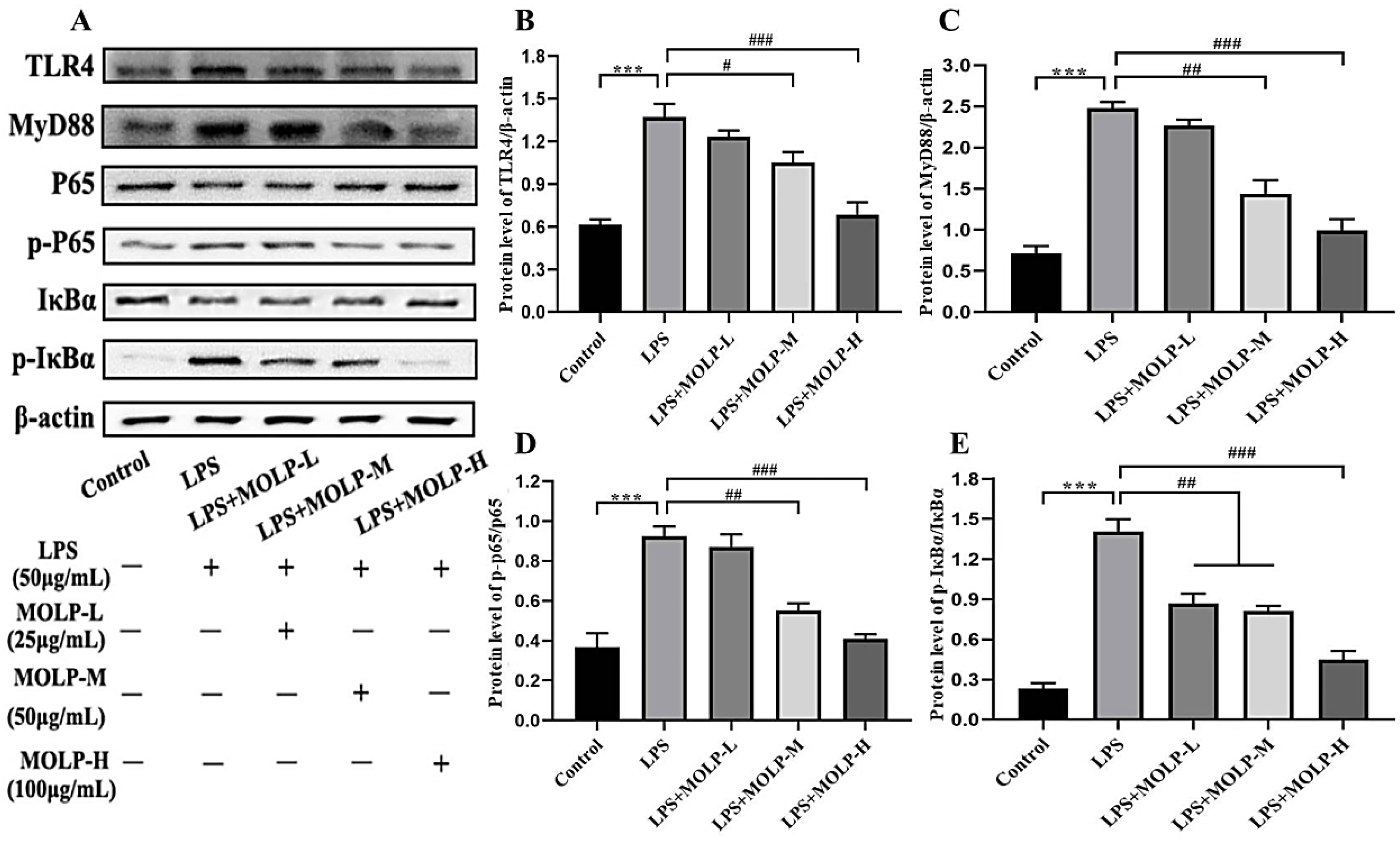
3.5. MOLP Suppresses the TLR4/MyD88/NF-κB Signalling Pathway in LPS-Activated IEC6 Cells
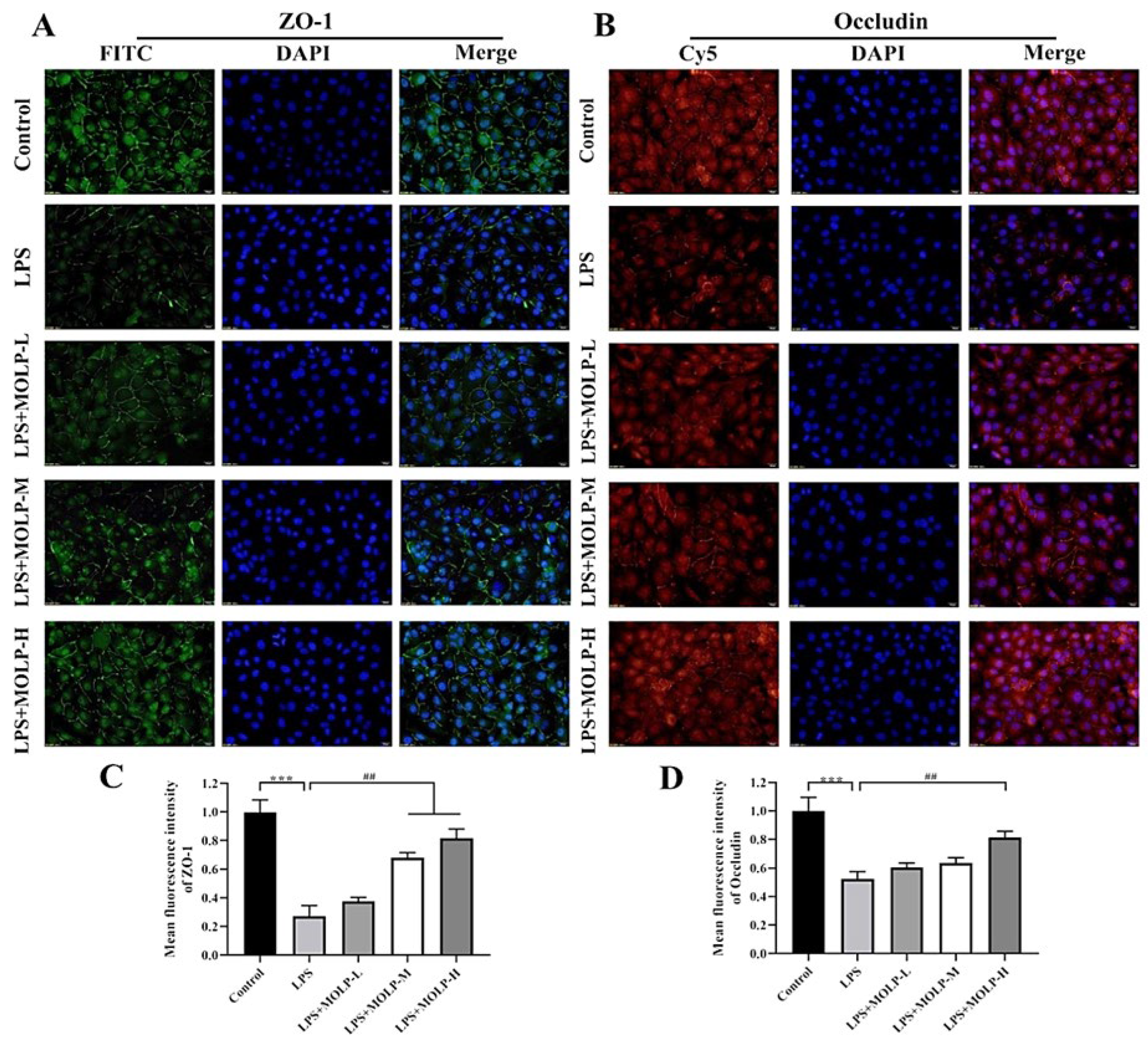
3.6. MOLP Protects Tight Junction Proteins in LPS-Stimulated IEC6 Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Santa Barbara, P.; van den Brink, G.R.; Roberts, D.J. Development and Differentiation of the Intestinal Epithelium. Cell. Mol. Life Sci. 2003, 60, 1322–1332. [Google Scholar] [CrossRef]
- Turner, J.R. Molecular Basis of Epithelial Barrier Regulation: From Basic Mechanisms to Clinical Application. Am. J. Pathol. 2006, 169, 1901–1909. [Google Scholar] [CrossRef] [PubMed]
- Howarth, G.S.; Shoubridge, C.A. Enhancement of Intestinal Growth and Repair by Growth Factors. Curr. Opin. Pharmacol. 2001, 1, 568–574. [Google Scholar] [CrossRef]
- Iizuka, M.; Konno, S. Wound Healing of Intestinal Epithelial Cells. World J. Gastroenterol. 2011, 17, 2161–2171. [Google Scholar] [CrossRef] [PubMed]
- Coskun, M. Intestinal Epithelium in Inflammatory Bowel Disease. Front. Med. 2014, 1, 24. [Google Scholar] [CrossRef]
- Okumura, R.; Takeda, K. Roles of Intestinal Epithelial Cells in the Maintenance of Gut Homeostasis. Exp. Mol. Med. 2017, 49, e338. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, R.; Watanabe, M. Role of Epithelial Cells in the Pathogenesis and Treatment of Inflammatory Bowel Disease. J. Gastroenterol. 2016, 51, 11–21. [Google Scholar] [CrossRef]
- Stohs, S.J.; Hartman, M.J. Review of the Safety and Efficacy of Moringa oleifera. Phytother. Res. 2015, 29, 796–804. [Google Scholar] [CrossRef]
- Wang, L.; Zou, Q.; Wang, J.; Zhang, J.; Liu, Z.; Chen, X. Proteomic Profiles Reveal the Function of Different Vegetative Tissues of Moringa oleifera. Protein J. 2016, 35, 440–447. [Google Scholar] [CrossRef]
- Anwar, F.; Bhanger, M.I. Analytical Characterization of Moringa oleifera Seed Oil Grown in Temperate Regions of Pakistan. J. Agric. Food Chem. 2003, 51, 6558–6563. [Google Scholar] [CrossRef]
- Olson, M.E.; Fahey, J.W. Moringa oleifera: Un Árbol Multiusos para las Zonas Tropicales Secas. Rev. Mex. Biodiv. 2011, 82, 1071–1082. [Google Scholar] [CrossRef]
- Leone, A.; Spada, A.; Battezzati, A.; Schiraldi, A.; Aristil, J.; Bertoli, S. Cultivation, Genetic, Ethnopharmacology, Phytochemistry and Pharmacology of Moringa oleifera Leaves: An Overview. Int. J. Mol. Sci. 2015, 16, 12791–12835. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, S.G.; Mali, R.G.; Mehta, A.A. Protective Effect of Ethanolic Extract of Seeds of Moringa oleifera Lam. Against Inflammation Associated with Development of Arthritis in Rats. J. Immunotoxicol. 2007, 4, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Mbikay, M. Therapeutic Potential of Moringa oleifera Leaves in Chronic Hyperglycemia and Dyslipidemia: A Review. Front. Pharmacol. 2012, 3, 24. [Google Scholar] [CrossRef] [PubMed]
- Thurber, M.D.; Fahey, J.W. Adoption of Moringa oleifera to Combat Under-Nutrition Viewed Through the Lens of the “Diffusion of Innovations” Theory. Ecol. Food Nutr. 2009, 48, 212–225. [Google Scholar] [CrossRef]
- Oliveira, J.T.A.; Silveira, S.B.; Vasconcelos, I.M.; Cavada, B.S.; Moreira, R.A. Compositional and Nutritional Attributes of Seeds from the Multiple Purpose Tree Moringa oleifera Lamarck. J. Sci. Food Agric. 1999, 79, 815–820. [Google Scholar] [CrossRef]
- Amaglo, N.K.; Bennett, R.N.; Lo Curto, R.B.; Rosa, E.A.S.; Turco, V.L.; Giuffrida, A.; Lo Curto, A.; Crea, F.; Timpo, G.M. Profiling Selected Phytochemicals and Nutrients in Different Tissues of the Multipurpose Tree Moringa oleifera L., Grown in Ghana. Food Chem. 2010, 122, 1047–1054. [Google Scholar] [CrossRef]
- Atawodi, S.E.; Atawodi, J.C.; Idakwo, G.A.; Pfundstein, B.; Haubner, R.; Wurtele, G.; Bartsch, H.; Owen, R.W. Evaluation of the Polyphenol Content and Antioxidant Properties of Methanol Extracts of the Leaves, Stem, and Root Barks of Moringa oleifera Lam. J. Med. Food 2010, 13, 710–716. [Google Scholar] [CrossRef]
- Ndhlala, A.R.; Mulaudzi, R.; Ncube, B.; Abdelgadir, H.A.; du Plooy, C.P.; Van Staden, J. Antioxidant, Antimicrobial and Phytochemical Variations in Thirteen Moringa oleifera Lam. Molecules 2014, 19, 10480–10494. [Google Scholar] [CrossRef]
- Gupta, A.; Gautam, M.K.; Singh, R.K.; Kumar, M.V.; Rao, C.h.V.; Goel, R.K.; Anupurba, S. Immunomodulatory Effect of Moringa oleifera Lam. Extract on Cyclophosphamide Induced Toxicity in Mice. Indian J. Exp. Biol. 2010, 48, 1157–1160. [Google Scholar]
- Waterman, C.; Cheng, D.M.; Rojas-Silva, P.; Poulev, A.; Dreifus, J.; Lila, M.A.; Raskin, I. Stable, Water Extractable Isothiocyanates from Moringa oleifera Leaves Attenuate Inflammation in Vitro. Phytochemistry 2014, 103, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. Proinflammatory Cytokines. Chest 2000, 118, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.S.; Xiang, X.W.; Jin, H.X.; Guo, X.Y.; Liu, L.J.; Huang, Y.N.; OuYang, X.K.; Qu, Y.L. Composition and Anti-Inflammatory Effect of Polysaccharides from Sargassum horneri in RAW264.7 Macrophages. Int. J. Biol. Macromol. 2016, 88, 403–413. [Google Scholar] [CrossRef]
- Wang, L.; Yu, X.; Yang, X.; Li, Y.; Yao, Y.; Lui, E.M.; Ren, G. Structural and Anti-Inflammatory Characterization of a Novel Neutral Polysaccharide from North American Ginseng (Panax quinquefolius). Int. J. Biol. Macromol. 2015, 74, 12–17. [Google Scholar] [CrossRef]
- Palapattu, G.S.; Sutcliffe, S.; Bastian, P.J.; Platz, E.A.; De Marzo, A.M.; Isaacs, W.B.; Nelson, W.G. Prostate Carcinogenesis and Inflammation: Emerging Insights. Carcinogenesis 2005, 26, 1170–1181. [Google Scholar] [CrossRef]
- Liu, C.J.; Lin, J.Y. Anti-Inflammatory and Anti-Apoptotic Effects of Strawberry and Mulberry Fruit Polysaccharides on Lipopolysaccharide-Stimulated Macrophages Through Modulating Pro-/Anti-Inflammatory Cytokines Secretion and Bcl-2/Bak Protein Ratio. Food Chem. Toxicol. 2012, 50, 3032–3039. [Google Scholar] [CrossRef]
- Dore, C.M.; das C Faustino Alves, M.G.; Will, L.S.; Costa, T.G.; Sabry, D.A.; de Souza Rêgo, L.A.; Accardo, C.M.; Rocha, H.A.; Filgueira, L.G.; Leite, E.L. A Sulfated Polysaccharide, Fucans, Isolated from Brown Algae Sargassum vulgare with Anticoagulant, Antithrombotic, Antioxidant and Anti-Inflammatory Effects. Carbohydr. Polym. 2013, 91, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Ding, C.; Liu, X.; Zhao, Y.; Ding, Q.; Sun, S.; Zhang, J.; Yang, J.; Liu, W.; Li, W. Research Progress on Extraction, Isolation, Structural Analysis and Biological Activity of Polysaccharides from Panax Genus. Molecules 2023, 28, 3733. [Google Scholar] [CrossRef]
- Medeiros, V.P.; Queiroz, K.C.; Cardoso, M.L.; Monteiro, G.R.; Oliveira, F.W.; Chavante, S.F.; Guimaraes, L.A.; Rocha, H.A.; Leite, E.L. Sulfated galactofucan from Lobophora variegata: Anticoagulant and anti-inflammatory properties. Biochemistry 2008, 73, 1018–1024. [Google Scholar] [CrossRef]
- Ji, X.L.; Chun, Y.H.; Yong, G.G.; Yu, Q.X.; Yi, Z.Y.; Xu, D.G. Metagenomic Analysis of Gut Microbiota Modulatory Effects of Jujube (Ziziphus jujuba Mill.) Polysaccharides in a Colorectal Cancer Mouse Model. Food Funct. 2020, 11, 163–173. [Google Scholar] [CrossRef]
- Mohamed, H.H.; Peng, W.; Su, H.; Zhou, R.; Tao, Y.; Huang, J.; Liu, M.; Bo, R.; Li, J. Moringa oleifera Leaf Polysaccharide Alleviates Experimental Colitis by Inhibiting Inflammation and Maintaining Intestinal Barrier. Front. Nutr. 2022, 9, 1055791. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.L.; Guo, J.H.; Tian, J.Y.; Ma, K.; Liu, Y. Research Progress on Degradation Methods and Product Properties of Plant Polysaccharides. J. Light Indust. 2023, 38, 55–62. [Google Scholar]
- Liu, X.; Hong, H.; Wang, J.; Huang, J.; Li, J.; Tao, Y.; Liu, M.; Pang, H.; Li, J.; Bo, R. Mucosal Immune Responses and Protective Efficacy Elicited by Oral Administration of AMP-ZnONPs-Adjuvanted Inactivated H9N2 Virus in Chickens. Poult. Sci. 2024, 103, 103496. [Google Scholar] [CrossRef] [PubMed]
- Bo, R.; Liu, X.; Wang, J.; Wei, S.; Wu, X.; Tao, Y.; Zhu, Y.; Li, F.; Zhang, L.; Chen, J. Polysaccharide from Atractylodes macrocephala Koidz Binding with Zinc Oxide Nanoparticles: Characterization, Immunological Effect and Mechanism. Front. Nutr. 2022, 9, 992502. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.J.; Choi, J.W.; Lee, M.K.; Choi, Y.H.; Nam, T.J. Spirulina Crude Protein Promotes the Migration and Proliferation in IEC-6 Cells by Activating EGFR/MAPK Signaling Pathway. Mar. Drugs 2019, 17, 205. [Google Scholar] [CrossRef]
- Li, L.; Wan, G.; Han, B.; Zhang, Z. Echinacoside Alleviated LPS-Induced Cell Apoptosis and Inflammation in Rat Intestine Epithelial Cells by Inhibiting the mTOR/STAT3 Pathway. Biomed. Pharmacother. 2018, 104, 622–628. [Google Scholar] [CrossRef]
- McCormack, S.A.; Viar, M.J.; Johnson, L.R. Migration of IEC-6 Cells: A Model for Mucosal Healing. Am. J. Physiol. 1992, 263, G426–G435. [Google Scholar] [CrossRef]
- Yeomans, N.D.; Saint John, D.J.; de Boer, W.G. Regeneration of Gastric Mucosa After Aspirin-Induced Injury in the Rat. Am. J. Dig. Dis. 1973, 18, 773–780. [Google Scholar] [CrossRef]
- Yu, Y.; Yang, W.; Bilotta, A.J.; Zhao, X.; Cong, Y.; Li, Y. L-Lactate Promotes Intestinal Epithelial Cell Migration to Inhibit Colitis. FASEB J. 2021, 35, e21554. [Google Scholar] [CrossRef]
- Liu, L.; Han, L.; Wong, D.Y.; Yue, P.Y.; Ha, W.Y.; Hu, Y.H.; Wang, P.X.; Wong, R.N. Effects of Si-Jun-Zi Decoction Polysaccharides on Cell Migration and Gene Expression in Wounded Rat Intestinal Epithelial Cells. Br. J. Nutr. 2005, 93, 21–29. [Google Scholar] [CrossRef]
- Islam, M.D.; Hori, M.; Ozaki, H. Interleukin-33, an Interleukin-1 Like Cytokine Accelerates Cell Migration in IEC-6 Cells. Asian J. Med. Biol. Res. 2017, 2, 577. [Google Scholar] [CrossRef]
- Wang, Y.; Chang, X.; Zheng, B.; Chen, Y.; Xie, J.; Shan, J.; Hu, X.; Ding, X.; Hu, X.; Yu, Q. Protective Effect of Ganoderma atrum Polysaccharide on Acrolein-Induced Apoptosis and Autophagic Flux in IEC-6 Cells. Foods 2022, 11, 240. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Chen, S.; Wang, X.; Yuan, G.; Jiang, F.; Chen, X.; Wang, L. Characterization of Moringa oleifera Roots Polysaccharide MRP-1 with Anti-Inflammatory Effect. Int. J. Biol. Macromol. 2019, 132, 844–851. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Ahmad, A.A.; Yang, Y.; Liang, Z.; Shen, W.; Feng, M.; Shen, J.; Lan, X.; Ding, X. Lactobacillus rhamnosus CY12 Enhances Intestinal Barrier Function by Regulating Tight Junction Protein Expression, Oxidative Stress, and Inflammation Response in Lipopolysaccharide-Induced Caco-2 Cells. Int. J. Mol. Sci. 2022, 23, 11162. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Chung, K.S.; Jin, B.R.; Cheon, S.Y.; Nugroho, A.; Roh, S.S.; An, H.J. Anti-Inflammatory Effects of an Ethanol Extract of Aster glehni via Inhibition of NF-κB Activation in Mice with DSS-Induced Colitis. Food Funct. 2017, 8, 2611–2620. [Google Scholar] [CrossRef]
- Xu, J.; Zhou, L.; Ji, L.; Chen, F.; Fortmann, K.; Zhang, K.; Liu, Q.; Li, K.; Wang, W.; Wang, H.; et al. The REGγ-Proteasome Forms a Regulatory Circuit with IκBɛ and NFκB in Experimental Colitis. Nat. Commun. 2016, 7, 10761. [Google Scholar] [CrossRef]
- Yu, Q.H.; Yang, Q. Diversity of Tight Junctions (TJs) Between Gastrointestinal Epithelial Cells and Their Function in Maintaining the Mucosal Barrier. Cell Biol. Int. 2009, 33, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Chandrashekharappa, S.; Bodduluri, S.R.; Baby, B.V.; Hegde, B.; Kotla, N.G.; Hiwale, A.A.; Saiyed, T.; Patel, P.; Vijay-Kumar, M.; et al. Enhancement of the Gut Barrier Integrity by a Microbial Metabolite Through the Nrf2 Pathway. Nat. Commun. 2019, 10, 89. [Google Scholar] [CrossRef]
- Alizadeh, A.; Akbari, P.; Garssen, J.; Fink-Gremmels, J.; Braber, S. Epithelial Integrity, Junctional Complexes, and Biomarkers Associated with Intestinal Functions. Tissue Barriers 2022, 10, 1996830. [Google Scholar] [CrossRef]
- Wang, S.; Hu, Q.; Chang, Z.; Liu, Y.; Gao, Y.; Luo, X.; Zhou, L.; Chen, Y.; Cui, Y.; Wang, Z.; et al. Moringa oleifera Leaf Polysaccharides Exert Anti-Lung Cancer Effects upon Targeting TLR4 to Reverse the Tumor-Associated Macrophage Phenotype and Promote T-Cell Infiltration. Food Funct. 2023, 14, 4607–4620. [Google Scholar] [CrossRef]
- Husien, H.M.; Rehman, S.U.; Duan, Z.; Wang, M. Effect of Moringa oleifera Leaf Polysaccharide on the Composition of Intestinal Microbiota in Mice with Dextran Sulfate Sodium-Induced Ulcerative Colitis. Front. Nutr. 2024, 11, 1409026. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, E.; Blundell, R. A Comprehensive Review of the Phytochemicals, Health Benefits, Pharmacological Safety and Medicinal Prospects of Moringa oleifera. Heliyon 2024, 10, e27807. [Google Scholar] [CrossRef]
- Arora, S.; Arora, S. Nutritional Significance and Therapeutic Potential of Moringa oleifera: The Wonder Plant. J. Food Biochem. 2021, 45, e13933. [Google Scholar] [CrossRef]
- Srivastava, S.; Pandey, V.K.; Dash, K.K.; Dayal, D.; Wal, P.; Debnath, B.; Singh, R.; Dar, A.H. Dynamic Bioactive Properties of Nutritional Superfood Moringa oleifera: A Comprehensive Review. J. Agric. Food Res. 2023, 14, 100860. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; Alqhtani, A.H.; Swelum, A.A.; El-Saadony, M.T.; Salem, H.M.; Babalghith, A.O.; Taha, A.E.; Ahmed, O.; Abdo, M.; El-Tarabily, K.A. Pharmacological, Nutritional and Antimicrobial Uses of Moringa oleifera Lam. Leaves in Poultry Nutrition: An Updated Knowledge. Poult. Sci. 2022, 101, 102031. [Google Scholar] [CrossRef]
- Shen, M.; Cai, R.; Li, Z.; Chen, X.; Xie, J. The Molecular Mechanism of Yam Polysaccharide Protected H2O2-Induced Oxidative Damage in IEC-6 Cells. Foods 2023, 12, 262. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xi, C.; Li, W.; Su, H.; Yang, H.; Bai, Z.; Tian, Y.; Song, S. Moringa oleifera Leaves Protein Enhances Intestinal Permeability by Activating TLR4 Upstream Signaling and Disrupting Tight Junctions. Int. J. Mol. Sci. 2023, 24, 16425. [Google Scholar] [CrossRef] [PubMed]
- Gu, F.; Tao, L.; Chen, R.; Zhang, J.; Wu, X.; Yang, M.; Sheng, J.; Tian, Y. Ultrasonic-Cellulase Synergistic Extraction of Crude Polysaccharides from Moringa oleifera Leaves and Alleviation of Insulin Resistance in HepG2 Cells. Int. J. Mol. Sci. 2022, 23, 12405. [Google Scholar] [CrossRef]
- Paradowska, M.; Dunislawska, A.; Siwek, M.; Slawinska, A. Avian Cell Culture Models to Study Immunomodulatory Properties of Bioactive Products. Animals 2022, 12, 670. [Google Scholar] [CrossRef]
- Zvinorova, P.I.; Lekhanya, L.; Erlwanger, K.; Chivandi, E. Dietary Effects of Moringa oleifera Leaf Powder on Growth, Gastrointestinal Morphometry and Blood and Liver Metabolites in Sprague Dawley Rats. J. Anim. Physiol. Anim. Nutr. 2015, 99, 21–28. [Google Scholar] [CrossRef]
- Khalid, A.R.; Yasoob, T.B.; Zhang, Z.; Zhu, X.; Hang, S. Dietary Moringa oleifera Leaf Powder Improves Jejunal Permeability and Digestive Function by Modulating the Microbiota Composition and Mucosal Immunity in Heat Stressed Rabbits. Environ. Sci. Pollut. Res. Int. 2022, 29, 80952–80967. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Zaneb, H.; Masood, S.; Yousaf, M.S.; Rehman, H.F.; Rehman, H. Effect of Moringa oleifera Leaf Powder Supplementation on Growth Performance and Intestinal Morphology in Broiler Chickens. J. Anim. Physiol. Anim. Nutr. 2017, 101 (Suppl. S1), 114–121. [Google Scholar] [CrossRef] [PubMed]


| Gene | Sequence (5′-----3′) | |
|---|---|---|
| TNFα | F1- | TGAAGCAGCAGCCAGCAA |
| R1- | GCAGCCTGTCTCCTTCTATGA | |
| IL1β | F2- | CCAGCAGGTTATCATCACATCC |
| R2- | ATCTCGCAGCAGCACATCA | |
| IL6 | F3- | AATTAAGCCTCCGACTTGTGAA |
| R3- | TTCCATCCAGTTGCCTTCTTG | |
| IL10 | F4- | GGCAGCCTTGTCCCTTG |
| R4- | AACATACTGCTAACCGACTCCTT | |
| GAPDH | F5- | CACCATCTTCCAGGAGCGAG |
| R5- | GGGGCCATCCACAGTCTTC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Husien, H.M.; Peng, W.; Essa, M.O.A.; Adam, S.Y.; Ur Rehman, S.; Ali, R.; Saleh, A.A.; Wang, M.; Li, J. The Anti-Inflammatory Properties of Polysaccharides Extracted from Moringa oleifera Leaves on IEC6 Cells Stimulated with Lipopolysaccharide In Vitro. Animals 2024, 14, 3508. https://doi.org/10.3390/ani14233508
Husien HM, Peng W, Essa MOA, Adam SY, Ur Rehman S, Ali R, Saleh AA, Wang M, Li J. The Anti-Inflammatory Properties of Polysaccharides Extracted from Moringa oleifera Leaves on IEC6 Cells Stimulated with Lipopolysaccharide In Vitro. Animals. 2024; 14(23):3508. https://doi.org/10.3390/ani14233508
Chicago/Turabian StyleHusien, Hosameldeen Mohamed, Weilong Peng, Mohamed Osman Abdalrahem Essa, Saber Y. Adam, Shahab Ur Rehman, Rahmat Ali, Ahmed A. Saleh, Mengzhi Wang, and Jingui Li. 2024. "The Anti-Inflammatory Properties of Polysaccharides Extracted from Moringa oleifera Leaves on IEC6 Cells Stimulated with Lipopolysaccharide In Vitro" Animals 14, no. 23: 3508. https://doi.org/10.3390/ani14233508
APA StyleHusien, H. M., Peng, W., Essa, M. O. A., Adam, S. Y., Ur Rehman, S., Ali, R., Saleh, A. A., Wang, M., & Li, J. (2024). The Anti-Inflammatory Properties of Polysaccharides Extracted from Moringa oleifera Leaves on IEC6 Cells Stimulated with Lipopolysaccharide In Vitro. Animals, 14(23), 3508. https://doi.org/10.3390/ani14233508









