Dynamic Shifts in Antibiotic Residues and Gut Microbiome Following Tilmicosin Administration to Silkie Chickens
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
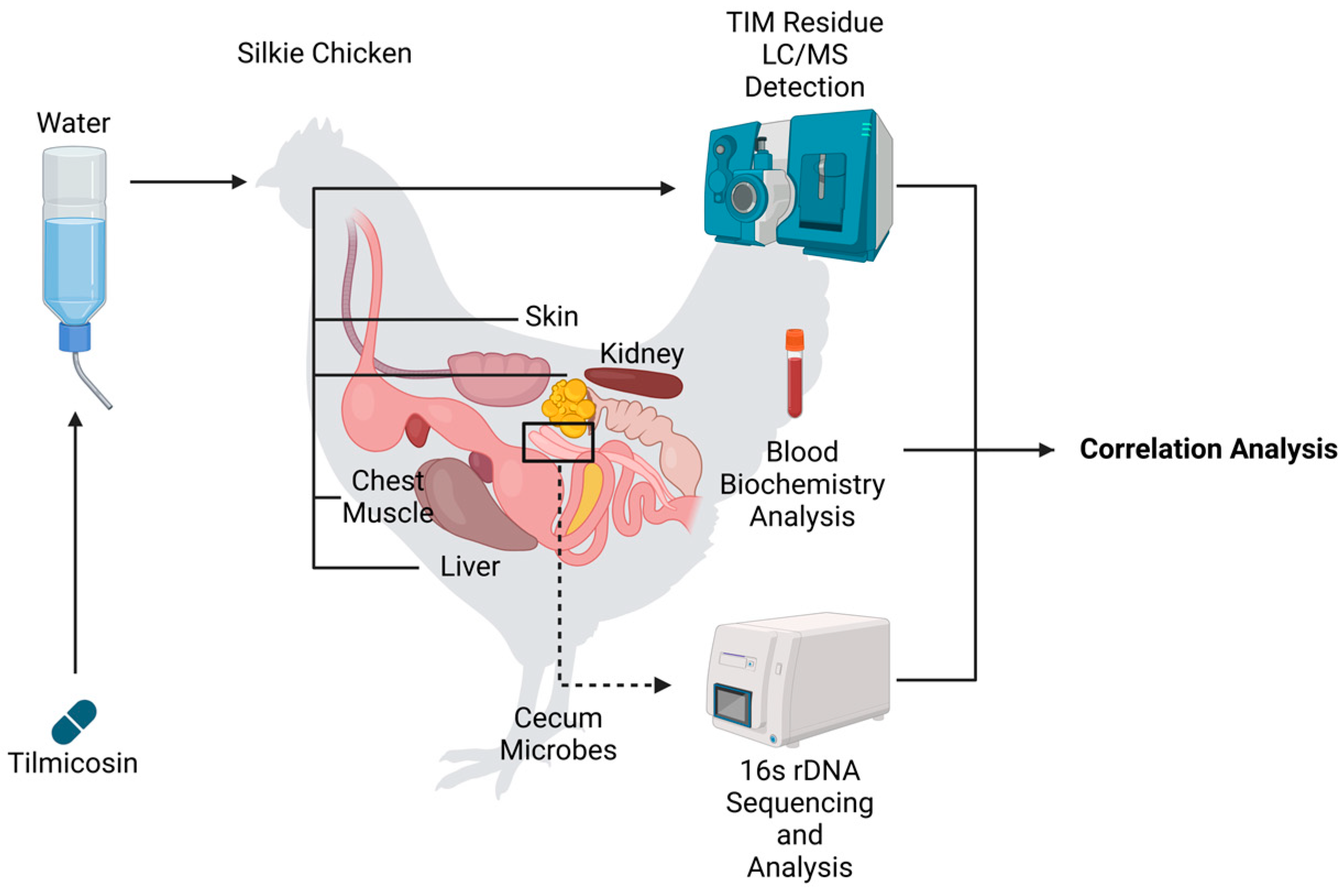
2.1. Silkie Chickens and Study Design
2.2. Determination of TIM Concentration
2.3. Biochemical Analysis
2.4. Microbial Genomic DNA Extraction
2.5. Microbial 16S rRNA Gene Sequencing
2.6. Sequencing Data Analysis
2.7. Statistical Analysis
3. Results
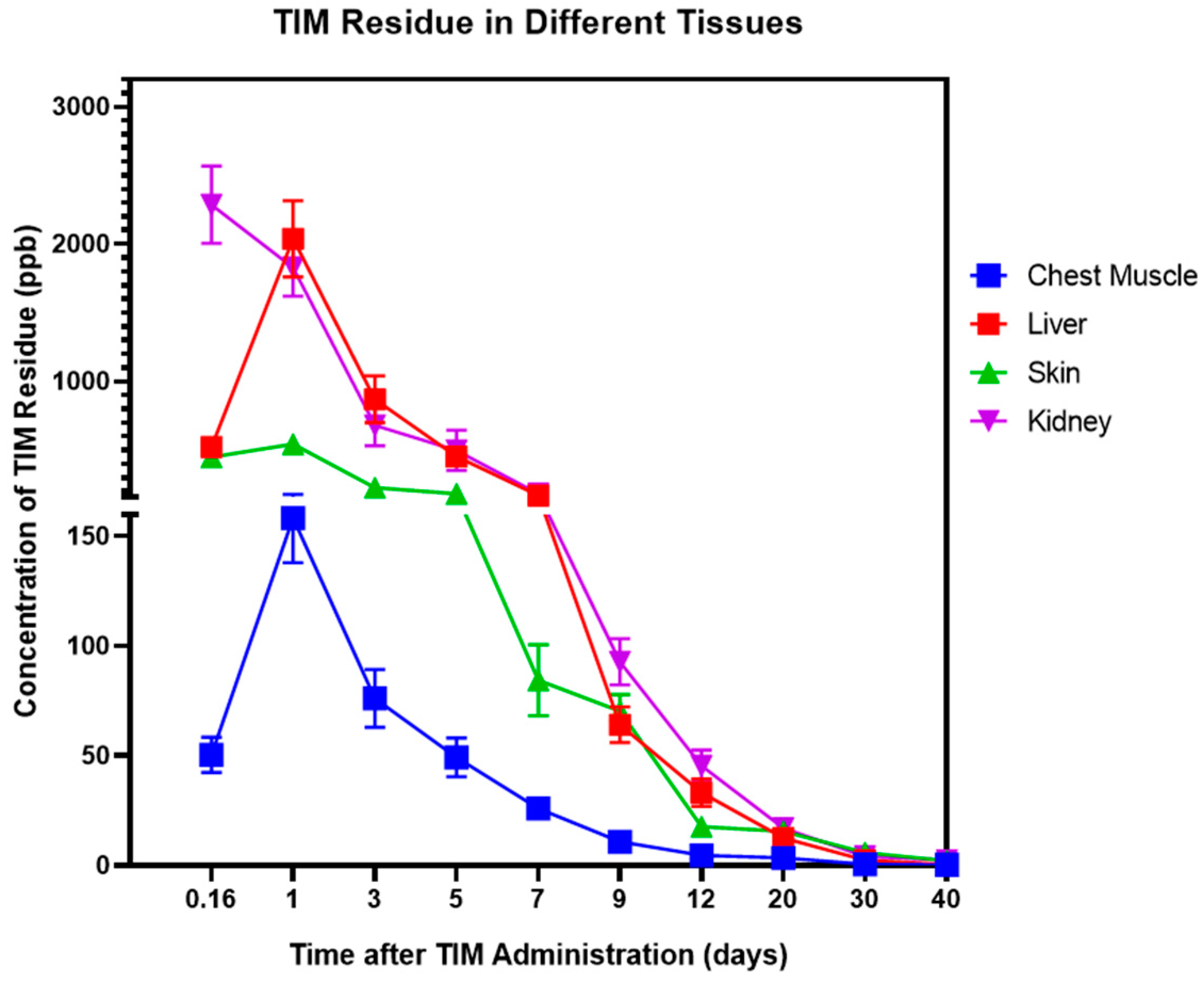
3.1. Patterns of TIM Residue Elimination Across Different Tissues
3.2. Effect of TIM Treatment on Blood Biochemical Parameters
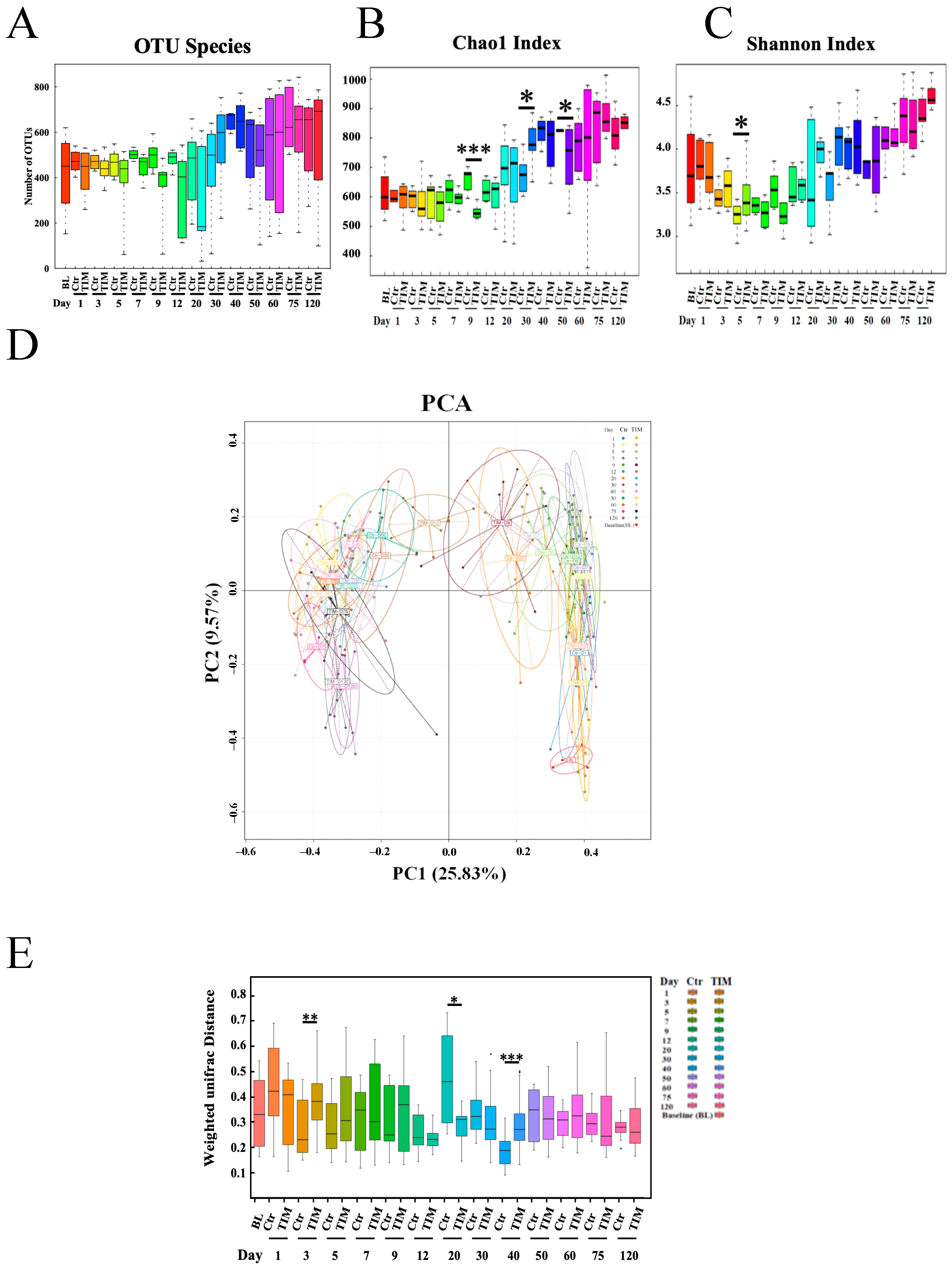
3.3. The Affect of TIM on Microbiota Structure
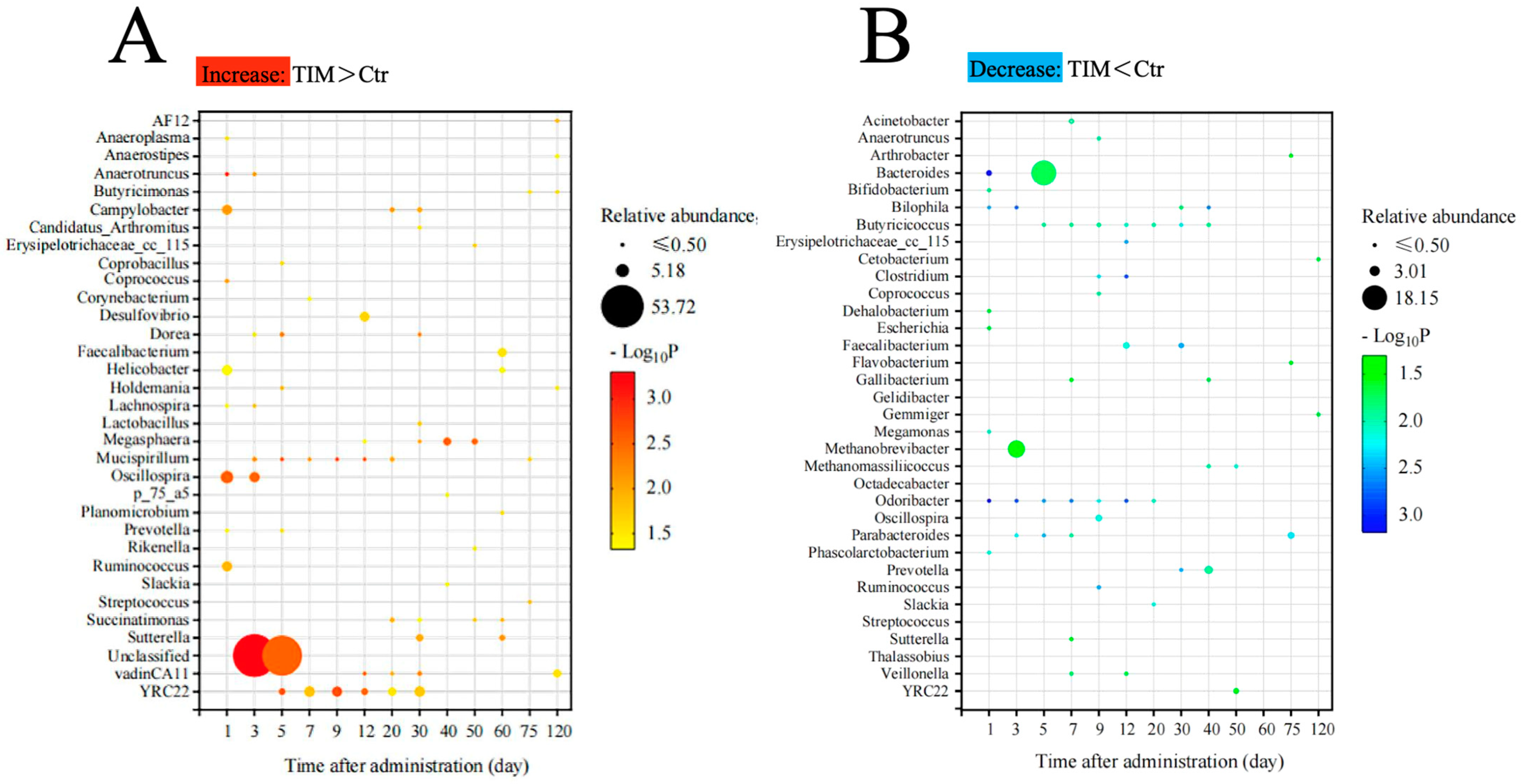
3.4. Shifts in Microbial Composition After TIM Treatment
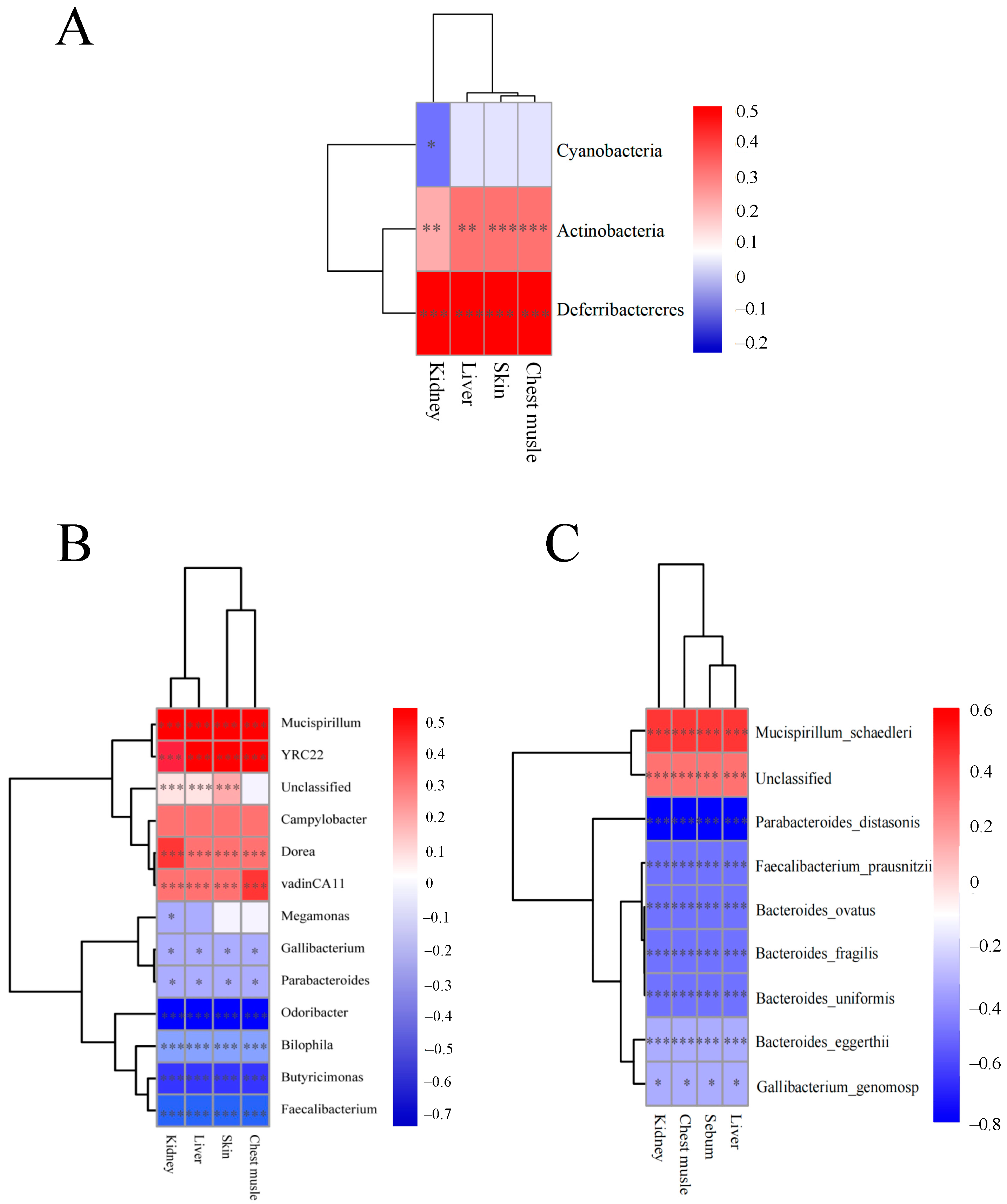
3.5. Correlation Analysis Between Microbiota and TIM Residue Levels in Four Tissues
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arsic, B.; Barber, J.; Čikoš, A.; Mladenovic, M.; Stankovic, N.; Novak, P. 16-Membered Macrolide Antibiotics: A Review. Int. J. Antimicrob. Agents 2018, 51, 283–298. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Zhu, Q.; Zhao, Y.; Yang, S.; Cao, J.; Qiu, Y. Tilmicosin Enteric Granules and Premix to Pigs: Antimicrobial Susceptibility Testing and Comparative Pharmacokinetics. Vet. Pharm. Ther. 2019, 42, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Hu, Z.; Zheng, H.; Xia, X.; Gu, X.; Shen, X.; Yang, H.; Ding, H. The PK/PD Integration and Resistance of Tilmicosin against Mycoplasma Hyopneumoniae. Pathogens 2020, 9, 487. [Google Scholar] [CrossRef]
- Zhang, X.; Akhtar, M.; Chen, Y.; Ma, Z.; Liang, Y.; Shi, D.; Cheng, R.; Cui, L.; Hu, Y.; Nafady, A.A.; et al. Chicken Jejunal Microbiota Improves Growth Performance by Mitigating Intestinal Inflammation. Microbiome 2022, 10, 107. [Google Scholar] [CrossRef]
- Bacanlı, M.; Başaran, N. Importance of Antibiotic Residues in Animal Food. Food Chem. Toxicol. 2019, 125, 462–466. [Google Scholar] [CrossRef]
- Van Boeckel, T.P.; Brower, C.; Gilbert, M.; Grenfell, B.T.; Levin, S.A.; Robinson, T.P.; Teillant, A.; Laxminarayan, R. Global Trends in Antimicrobial Use in Food Animals. Proc. Natl. Acad. Sci. USA 2015, 112, 5649–5654. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ying, G.-G.; Deng, W.-J. Antibiotic Residues in Food: Extraction, Analysis, and Human Health Concerns. J. Agric. Food Chem. 2019, 67, 7569–7586. [Google Scholar] [CrossRef]
- Bombaywala, S.; Mandpe, A.; Paliya, S.; Kumar, S. Antibiotic Resistance in the Environment: A Critical Insight on Its Occurrence, Fate, and Eco-Toxicity. Environ. Sci. Pollut. Res. 2021, 28, 24889–24916. [Google Scholar] [CrossRef]
- Su, X.; Gao, Y.; Yang, R. Gut Microbiota-Derived Tryptophan Metabolites Maintain Gut and Systemic Homeostasis. Cells 2022, 11, 2296. [Google Scholar] [CrossRef]
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut Microbiota Functions: Metabolism of Nutrients and Other Food Components. Eur. J. Nutr. 2018, 57, 1–24. [Google Scholar] [CrossRef]
- Khan, S.; Moore, R.J.; Stanley, D.; Chousalkar, K.K. The Gut Microbiota of Laying Hens and Its Manipulation with Prebiotics and Probiotics To Enhance Gut Health and Food Safety. Appl. Environ. Microbiol. 2020, 86, e00600-20. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Lee, H.; Jeong, H. Gut Microbiota in Reductive Drug Metabolism. In Progress in Molecular Biology and Translational Science; Elsevier: Amsterdam, The Netherlands, 2020; Volume 171, pp. 61–93. ISBN 978-0-12-820000-1. [Google Scholar]
- Zimmermann, M.; Zimmermann-Kogadeeva, M.; Wegmann, R.; Goodman, A.L. Mapping Human Microbiome Drug Metabolism by Gut Bacteria and Their Genes. Nature 2019, 570, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Charleston, B.; Gate, J.J.; Aitken, I.A.; Reeve-Johnson, L. Assessment of the Efficacy of Tilmicosin as a Treatment for Mycoplasma Gallisepticum Infections in Chickens. Avian Pathol. 1998, 27, 190–195. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zou, J.; Xu, M.; Zou, Y.; Yang, B. Chemical Compositions and Sensory Characteristics of Pork Rib and Silkie Chicken Soups Prepared by Various Cooking Techniques. Food Chem. 2021, 345, 128755. [Google Scholar] [CrossRef]
- Horwitz, W. Official Methods of Analysis of AOAC International, 18th ed.; AOAC International: Gaithersburg, MD, USA, 2006; ISBN 978-0-935584-77-6. [Google Scholar]
- Tiseo, K.; Huber, L.; Gilbert, M.; Robinson, T.P.; Van Boeckel, T.P. Global Trends in Antimicrobial Use in Food Animals from 2017 to 2030. Antibiotics 2020, 9, 918. [Google Scholar] [CrossRef]
- Zhang, N.; Zhou, M.; Yan, X.; Liu, J.; Yuan, S.; Yang, H.; Ding, H.; Zhang, D.; Bai, Y. Pharmacokinetic and Pharmacodynamic Integration of Tilmicosin against Mycoplasma Gallisepticum in the Target Infection Site in Chickens. Front. Vet. Sci. 2022, 9, 952599. [Google Scholar] [CrossRef]
- Zhang, N.; Liu, Z.; Wei, Y.; Zhang, C.; Mao, C.; Cai, Q.; Shen, X.; Ding, H. Comparison of the Pharmacokinetics of Tilmicosin in Plasma and Lung Tissue in Healthy Chickens and Chickens Experimentally Infected with Mycoplasma Gallisepticum. Vet. Pharm. Ther. 2020, 43, 347–354. [Google Scholar] [CrossRef]
- Zhang, N.; Ye, X.; Wu, Y.; Huang, Z.; Gu, X.; Cai, Q.; Shen, X.; Jiang, H.; Ding, H. Determination of the Mutant Selection Window and Evaluation of the Killing of Mycoplasma Gallisepticum by Danofloxacin, Doxycycline, Tilmicosin, Tylvalosin and Valnemulin. PLoS ONE 2017, 12, e0169134. [Google Scholar] [CrossRef]
- Wongsuvan, G.; Wuthiekanun, V.; Hinjoy, S.; Day, N.P.; Limmathurotsakul, D. Antibiotic Use in Poultry: A Survey of Eight Farms in Thailand. Bull. World Health Organ. 2018, 96, 94–100. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, J.-Y.; He, Y.; Li, L.; Huang, J.-X.; Tian, Y.-X.; Wang, H.; Xu, Z.-L.; Shen, Y.-D. Development of Time-Resolved Fluorescence Immunochromatographic Assays for Simultaneously Detecting Tylosin and Tilmicosin in Milk in Group-Screening Manner. Foods 2021, 10, 1838. [Google Scholar] [CrossRef]
- Baietto, L.; Corcione, S.; Pacini, G.; Perri, G.; D’Avolio, A.; De Rosa, F. A 30-Years Review on Pharmacokinetics of Antibiotics: Is the Right Time for Pharmacogenetics? CDM 2014, 15, 581–598. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, M.; Elkomy, A.; Aboubakr, M.; Morad, M. Tissue Residues, Hematological and Biochemical Effects of Tilmicosin in Broiler Chicken. Vet. Med. Int. 2014, 2014, 502872. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Qian, K.; Li, G.; Duan, M.; Li, Z.; Dai, Y.; Chen, J.; Yang, F. Depletion of Tilmicosin Residue in Gushi Chickens Following Oral Administration via Drinking Water. Vet. Pharm. Ther. 2024, 47, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Bourdo, K.; Fadel, C.; Giorgi, M.; Šitovs, A.; Poapolathep, A.; Łebkowska-Wieruszewska, B. Disposition Kinetics and Tissue Residues of Tilmicosin Following Intravenous, Subcutaneous, Single and Multiple Oral Dosing in Geese (Anser Anser Domesticus). Vet. Pharm. Ther. 2024, 47, 416–426. [Google Scholar] [CrossRef]
- Hioki, A.; Ohtomo, H. Significance of Blood Urea Nitrogen as an Index of Renal Function in Mice Infected with Plasmodium Berghei. Parasitol. Res. 1989, 76, 127–130. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, J.; Liu, S.; Fan, W.; Ding, C.; Gao, Z.; Tang, Z.; Luo, Y.; Shi, X.; Tan, L.; et al. Bromoacetic Acid Causes Oxidative Stress and Uric Acid Metabolism Dysfunction via Disturbing Mitochondrial Function and Nrf2 Pathway in Chicken Kidney. Environ. Toxicol. 2022, 37, 2910–2923. [Google Scholar] [CrossRef]
- Creeden, J.F.; Gordon, D.M.; Stec, D.E.; Hinds, T.D. Bilirubin as a Metabolic Hormone: The Physiological Relevance of Low Levels. Am. J. Physiol.-Endocrinol. Metab. 2021, 320, E191–E207. [Google Scholar] [CrossRef]
- Wu, H.; Wang, Y.; Yao, Q.; Fan, L.; Meng, L.; Zheng, N.; Li, H.; Wang, J. Alkaline Phosphatase Attenuates LPS-Induced Liver Injury by Regulating the miR-146a-Related Inflammatory Pathway. Int. Immunopharmacol. 2021, 101, 108149. [Google Scholar] [CrossRef]
- Tamber, S.S.; Bansal, P.; Sharma, S.; Singh, R.B.; Sharma, R. Biomarkers of Liver Diseases. Mol. Biol. Rep. 2023, 50, 7815–7823. [Google Scholar] [CrossRef]
- Wexler, H.M. Bacteroides: The Good, the Bad, and the Nitty-Gritty. Clin. Microbiol. Rev. 2007, 20, 593–621. [Google Scholar] [CrossRef]
- Hooper, L.V.; Stappenbeck, T.S.; Hong, C.V.; Gordon, J.I. Angiogenins: A New Class of Microbicidal Proteins Involved in Innate Immunity. Nat. Immunol. 2003, 4, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, J.; He, T.; Becker, S.; Zhang, G.; Li, D.; Ma, X. Butyrate: A Double-Edged Sword for Health? Adv. Nutr. 2018, 9, 21–29. [Google Scholar] [CrossRef]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The Firmicutes/Bacteroidetes Ratio: A Relevant Marker of Gut Dysbiosis in Obese Patients? Nutrients 2020, 12, 1474. [Google Scholar] [CrossRef]
- Weiland-Bräuer, N. Symbiotic Interactions of Archaea in Animal and Human Microbiomes. Curr. Clin. Microbiol. Rep. 2023, 10, 161–173. [Google Scholar] [CrossRef]
- Rizzatti, G.; Lopetuso, L.R.; Gibiino, G.; Binda, C.; Gasbarrini, A. Proteobacteria: A Common Factor in Human Diseases. BioMed Res. Int. 2017, 2017, 9351507. [Google Scholar] [CrossRef]
- Bornet, E.; Westermann, A.J. The Ambivalent Role of Bacteroides in Enteric Infections. Trends Microbiol. 2022, 30, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Ju, T.; Bhardwaj, T.; Korver, D.R.; Willing, B.P. Week-Old Chicks with High Bacteroides Abundance Have Increased Short-Chain Fatty Acids and Reduced Markers of Gut Inflammation. Microbiol. Spectr. 2023, 11, e03616-22. [Google Scholar] [CrossRef] [PubMed]
- Bernatchez, E.; Gold, M.J.; Langlois, A.; Blais-Lecours, P.; Boucher, M.; Duchaine, C.; Marsolais, D.; McNagny, K.M.; Blanchet, M.-R. Methanosphaera Stadtmanae Induces a Type IV Hypersensitivity Response in a Mouse Model of Airway Inflammation. Physiol. Rep. 2017, 5, e13163. [Google Scholar] [CrossRef]
- Mohammadzadeh, R.; Mahnert, A.; Duller, S.; Moissl-Eichinger, C. Archaeal Key-Residents within the Human Microbiome: Characteristics, Interactions and Involvement in Health and Disease. Curr. Opin. Microbiol. 2022, 67, 102146. [Google Scholar] [CrossRef]
- Yang, X. Transcriptome Analysis Identifies Candidate Genes and Signaling Pathways Associated with Feed Efficiency in Xiayan Chicken. Front. Genet. 2021, 12, 607719. [Google Scholar]
- Ho, Y.B.; Zakaria, M.P.; Latif, P.A.; Saari, N. Simultaneous Determination of Veterinary Antibiotics and Hormone in Broiler Manure, Soil and Manure Compost by Liquid Chromatography–Tandem Mass Spectrometry. J. Chromatogr. A 2012, 1262, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Baker, M.; Zhang, X.; Maciel-Guerra, A.; Dong, Y.; Wang, W.; Hu, Y.; Renney, D.; Hu, Y.; Liu, L.; Li, H.; et al. Machine Learning and Metagenomics Reveal Shared Antimicrobial Resistance Profiles across Multiple Chicken Farms and Abattoirs in China. Nat. Food 2023, 4, 707–720. [Google Scholar] [CrossRef] [PubMed]
- Noormohamed, A.; Fakhr, M.K. Incidence and Antimicrobial Resistance Profiling of Campylobacter in Retail Chicken Livers and Gizzards. Foodborne Pathog. Dis. 2012, 9, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Dai, M.-Y.; Huang, R.-Y.; Duan, J.-Y.; Zhang, T.; Bao, W.-M.; Zhang, J.-Y.; Gui, S.-Q.; Xia, S.-M.; Dai, C.-T.; et al. Parabacteroides Distasonis Ameliorates Hepatic Fibrosis Potentially via Modulating Intestinal Bile Acid Metabolism and Hepatocyte Pyroptosis in Male Mice. Nat. Commun. 2023, 14, 1829. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Wong, C.C.; Jia, Z.; Liu, W.; Liu, C.; Ji, F.; Pan, Y.; Wang, F.; Wang, G.; Zhao, L.; et al. Parabacteroides Distasonis Uses Dietary Inulin to Suppress NASH via Its Metabolite Pentadecanoic Acid. Nat. Microbiol. 2023, 8, 1534–1548. [Google Scholar] [CrossRef]
- Dai, W.; Zhang, J.; Chen, L.; Yu, J.; Zhang, J.; Yin, H.; Shang, Q.; Yu, G. Discovery of Bacteroides Uniformis F18-22 as a Safe and Novel Probiotic Bacterium for the Treatment of Ulcerative Colitis from the Healthy Human Colon. Int. J. Mol. Sci. 2023, 24, 14669. [Google Scholar] [CrossRef]
- Yan, Y. Bacteroides Uniformis-Induced Perturbations in Colonic Microbiota and Bile Acid Levels Inhibit TH17 Differentiation and Ameliorate Colitis Developments. NPJ Biofilms Microbiomes 2023, 9, 56. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, Q.; Xie, C.; Gebreselase, H.B.; Yuan, Y.; He, J.; Xie, L.; Luo, C.; Ji, J. Dynamic Shifts in Antibiotic Residues and Gut Microbiome Following Tilmicosin Administration to Silkie Chickens. Animals 2024, 14, 3428. https://doi.org/10.3390/ani14233428
Liang Q, Xie C, Gebreselase HB, Yuan Y, He J, Xie L, Luo C, Ji J. Dynamic Shifts in Antibiotic Residues and Gut Microbiome Following Tilmicosin Administration to Silkie Chickens. Animals. 2024; 14(23):3428. https://doi.org/10.3390/ani14233428
Chicago/Turabian StyleLiang, Qiying, Chunlin Xie, Haile Berihulay Gebreselase, Yushan Yuan, Jingyi He, Lu Xie, Chenglong Luo, and Jian Ji. 2024. "Dynamic Shifts in Antibiotic Residues and Gut Microbiome Following Tilmicosin Administration to Silkie Chickens" Animals 14, no. 23: 3428. https://doi.org/10.3390/ani14233428
APA StyleLiang, Q., Xie, C., Gebreselase, H. B., Yuan, Y., He, J., Xie, L., Luo, C., & Ji, J. (2024). Dynamic Shifts in Antibiotic Residues and Gut Microbiome Following Tilmicosin Administration to Silkie Chickens. Animals, 14(23), 3428. https://doi.org/10.3390/ani14233428




