Seasonal Changes and Age-Related Effects on the Intestinal Microbiota of Captive Chinese Monals (Lophophorus lhuysii)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. DNA Extraction and 16S rRNA Sequencing Processing
2.3. Biological Information Analysis
2.4. Analysis of Potential Pathogen
2.5. Data Processing and Analysis
3. Results
3.1. Effective Data Statistics of Samples
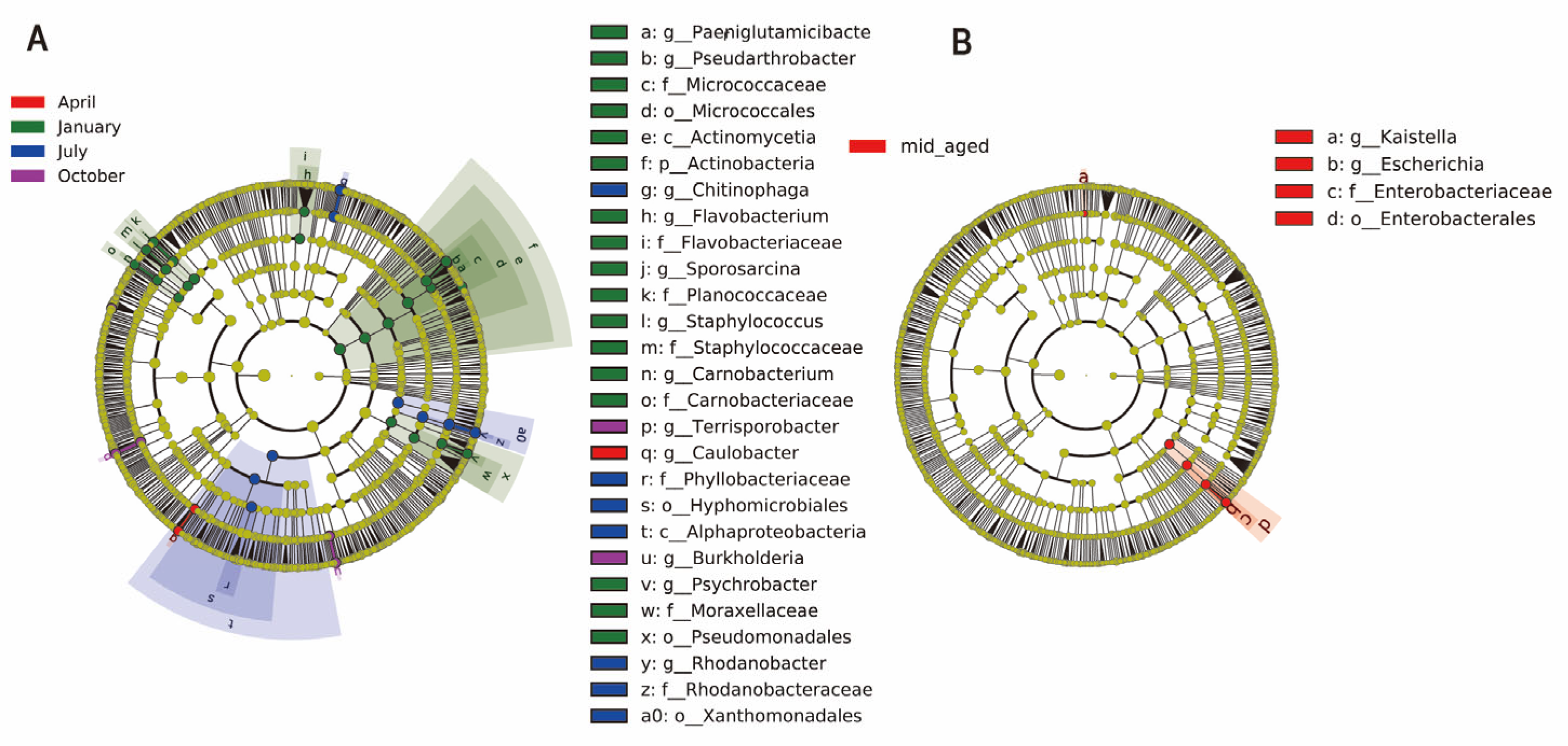
3.2. Structure and Composition of Gut Microbiota in Chinese Monal of Different Seasons and Ages
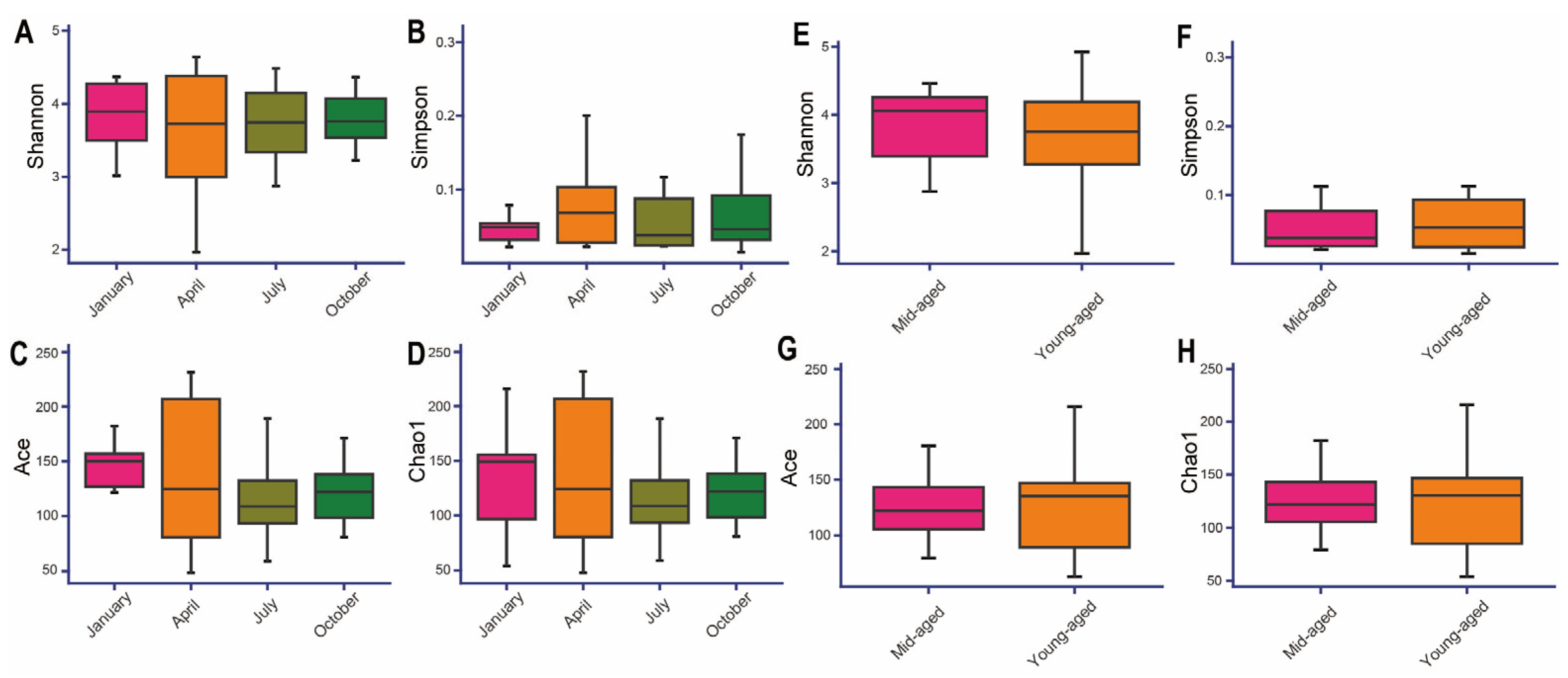
3.3. Alpha and Beta Diversity of Captive Chinese Monals Across Seasons and Ages
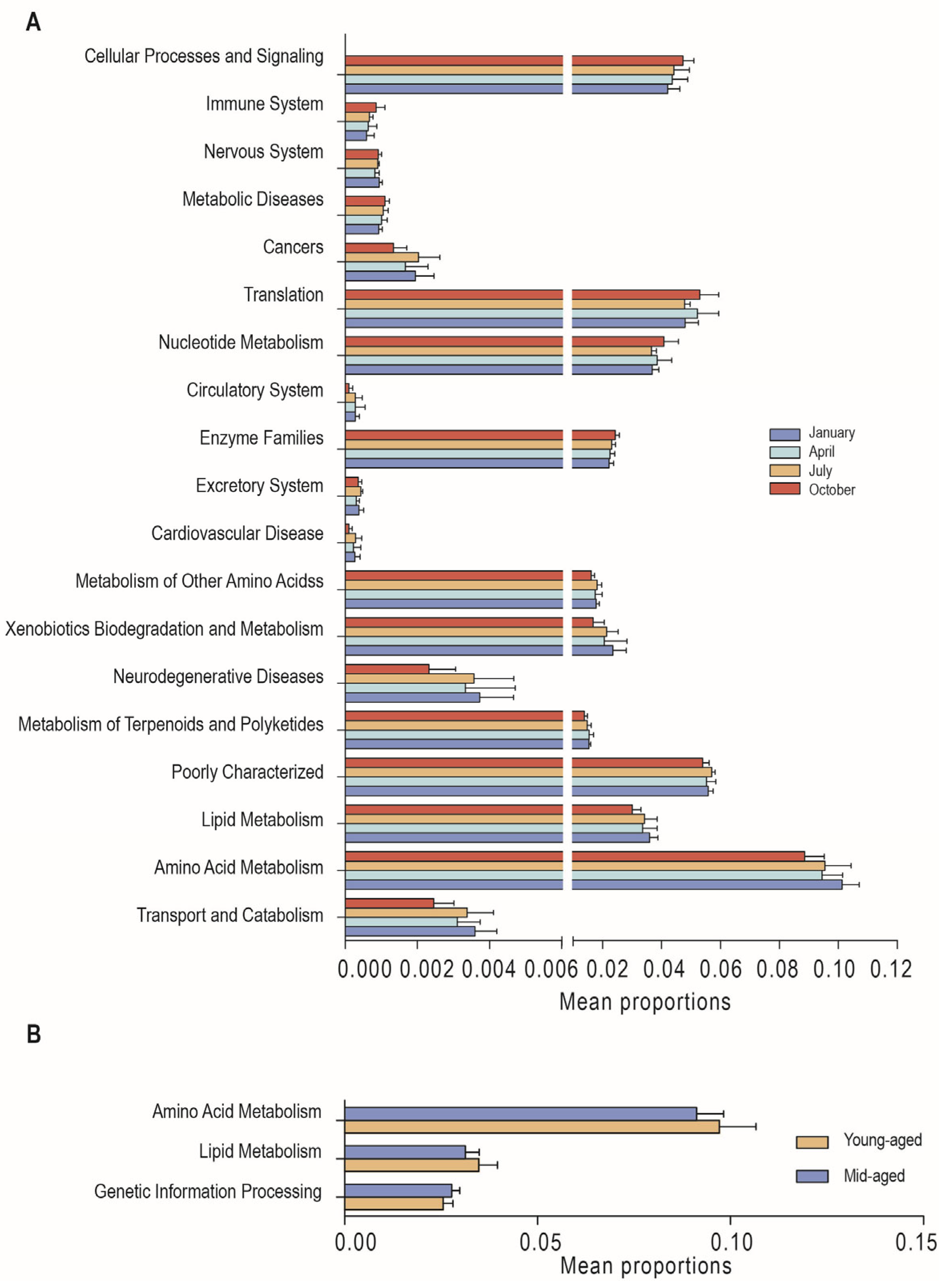
3.4. Functional Predictions
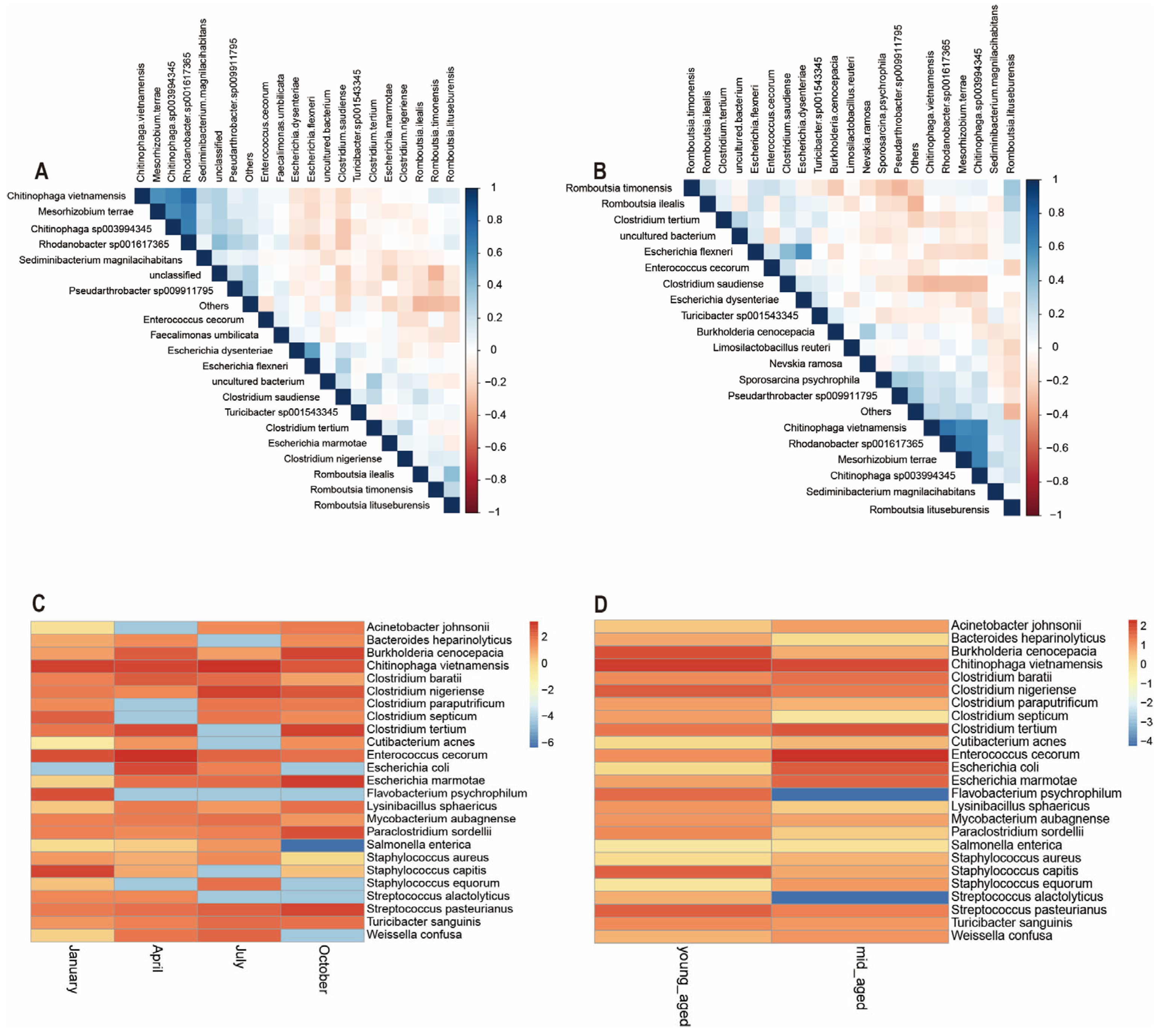
3.5. The Relationship Among Microbial Communities
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chinese Monal (Lophophorus lhuysii)-BirdLife Species Factsheet. Available online: https://datazone.birdlife.org/species/factsheet/chinese-monal-lophophorus-lhuysii (accessed on 10 November 2024).
- Wang, B.; Xu, Y.; Ran, J. Predicting Suitable Habitat of the Chinese Monal (Lophophorus lhuysii) Using Ecological Niche Modeling in the Qionglai Mountains, China. PeerJ 2017, 5, e3477. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wang, B.; Zhou, C.; Yang, H.; Zhong, X.; Li, W.; Chen, L.; Jian, Y.; Zhang, L. Characterization of Highly Polymorphic Microsatellite Markers for the Chinese Monal (Lophophorus lhuysii, Galliformes) Using Illumina MiSeq Sequencing. Mol. Biol. Rep. 2023, 50, 3903–3908. [Google Scholar] [CrossRef] [PubMed]
- IUCN Red List of Threatened Species. Available online: https://www.iucnredlist.org/species/22679192/219003994 (accessed on 7 June 2024).
- CITES. Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES) Appendices I, II and III, (2023-5-21). Available online: https://cites.org/eng/app/appendices.php (accessed on 7 June 2024).
- Keulartz, J. Captivity for Conservation? Zoos at a Crossroads. J. Agric. Environ. Ethics 2015, 28, 335–351. [Google Scholar] [CrossRef]
- Andreychev, A.; Kuznetsov, V.; Lapshin, A. Distribution and Population Density of the Russian Desman (Desmana moschata L., Talpidae, Insectivora) in the Middle Volga of Russia. For. Stud. 2019, 71, 48–68. [Google Scholar] [CrossRef]
- National Forestry and Grassland Administration. “14th Five-Year Plan” for the Protection and Development of Forestry and Grasslands. Available online: https://www.gov.cn/xinwen/2021-08/19/content_5632156.htm (accessed on 7 June 2024).
- Jandhyala, S.M.; Talukdar, R.; Subramanyam, C.; Vuyyuru, H.; Sasikala, M.; Nageshwar Reddy, D. Role of the Normal Gut Microbiota. World J. Gastroenterol. 2015, 21, 8787–8803. [Google Scholar] [CrossRef]
- Takiishi, T.; Fenero, C.I.M.; Câmara, N.O.S. Intestinal Barrier and Gut Microbiota: Shaping Our Immune Responses throughout Life. Tissue Barriers 2017, 5, e1373208. [Google Scholar] [CrossRef]
- Zhou, B.; Yuan, Y.; Zhang, S.; Guo, C.; Li, X.; Li, G.; Xiong, W.; Zeng, Z. Intestinal Flora and Disease Mutually Shape the Regional Immune System in the Intestinal Tract. Front. Immunol. 2020, 11, 575. [Google Scholar] [CrossRef]
- Ley, R.E.; Hamady, M.; Lozupone, C.; Turnbaugh, P.J.; Ramey, R.R.; Bircher, J.S.; Schlegel, M.L.; Tucker, T.A.; Schrenzel, M.D.; Knight, R.; et al. Evolution of Mammals and Their Gut Microbes. Science 2008, 320, 1647–1651. [Google Scholar] [CrossRef]
- Tidjani Alou, M.; Lagier, J.-C.; Raoult, D. Diet Influence on the Gut Microbiota and Dysbiosis Related to Nutritional Disorders. Hum. Microbiome J. 2016, 1, 3–11. [Google Scholar] [CrossRef]
- Wang, S.; Wang, L.; Fan, X.; Yu, C.; Feng, L.; Yi, L. An Insight into Diversity and Functionalities of Gut Microbiota in Insects. Curr. Microbiol. 2020, 77, 1976–1986. [Google Scholar] [CrossRef]
- Salgado-Flores, A.; Tveit, A.T.; Wright, A.-D.; Pope, P.B.; Sundset, M.A. Characterization of the Cecum Microbiome from Wild and Captive Rock Ptarmigans Indigenous to Arctic Norway. PLoS ONE 2019, 14, e0213503. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Hao, Y.; Zhang, Q.; Liu, X.; Wang, L.; Li, J.; Li, M.; Li, D. Coping with Extremes: Alternations in Diet, Gut Microbiota, and Hepatic Metabolic Functions in a Highland Passerine. Sci. Total Environ. 2023, 905, 167079. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Wang, X.; Li, L. Human Gut Microbiome: The Second Genome of Human Body. Protein Cell 2010, 1, 718–725. [Google Scholar] [CrossRef]
- Espírito Santo, C.; Caseiro, C.; Martins, M.J.; Monteiro, R.; Brandão, I. Gut Microbiota, in the Halfway between Nutrition and Lung Function. Nutrients 2021, 13, 1716. [Google Scholar] [CrossRef]
- Dallas, J.W.; Warne, R.W. Captivity and Animal Microbiomes: Potential Roles of Microbiota for Influencing Animal Conservation. Microb. Ecol. 2023, 85, 820–838. [Google Scholar] [CrossRef]
- Kers, J.G.; Velkers, F.C.; Fischer, E.A.J.; Hermes, G.D.A.; Stegeman, J.A.; Smidt, H. Host and Environmental Factors Affecting the Intestinal Microbiota in Chickens. Front. Microbiol. 2018, 9, 235. [Google Scholar] [CrossRef]
- Grond, K.; Sandercock, B.K.; Jumpponen, A.; Zeglin, L.H. The Avian Gut Microbiota: Community, Physiology and Function in Wild Birds. J. Avian Biol. 2018, 49, e01788. [Google Scholar] [CrossRef]
- Yang, G.; Xiang, Y.; Wang, S.; Tao, Y.; Xie, L.; Bao, L.; Shen, K.; Li, J.; Hu, B.; Wen, C.; et al. Response of Intestinal Microbiota to the Variation in Diets in Grass Carp (Ctenopharyngodon idella). Metabolites 2022, 12, 1115. [Google Scholar] [CrossRef]
- Xia, T.; Yao, Y.; Wang, C.; Dong, M.; Wu, Y.; Li, D.; Xie, M.; Ni, Q.; Zhang, M.; Xu, H. Seasonal Dynamics of Gut Microbiota in a Cohort of Wild Tibetan Macaques (Macaca thibetana) in Western China. Glob. Ecol. Conserv. 2021, 25, e01409. [Google Scholar] [CrossRef]
- Jin, L.; Huang, Y.; Yang, S.; Wu, D.; Li, C.; Deng, W.; Zhao, K.; He, Y.; Li, B.; Zhang, G.; et al. Diet, Habitat Environment and Lifestyle Conversion Affect the Gut Microbiomes of Giant Pandas. Sci. Total Environ. 2021, 770, 145316. [Google Scholar] [CrossRef]
- Xu, W.; Xu, N.; Zhang, Q.; Tang, K.; Zhu, Y.; Chen, R.; Zhao, X.; Ye, W.; Lu, C.; Liu, H. Association between Diet and the Gut Microbiome of Young Captive Red-Crowned Cranes (Grus japonensis). BMC Vet. Res. 2023, 19, 80. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Wang, L.; Li, J.; Hou, R.; Wang, M.; Wang, Z.; Qu, Q.; Zhou, W.; Nie, Y.; Hu, Y.; et al. Seasonal Shift of the Gut Microbiome Synchronizes Host Peripheral Circadian Rhythm for Physiological Adaptation to a Low-Fat Diet in the Giant Panda. Cell Rep. 2022, 38, 110203. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, W.; Hou, R.; Zhang, L.; Schmitz-Esser, S.; Sun, H.; Xie, J.; Zhang, Y.; Wang, C.; Li, L.; et al. Age-Associated Microbiome Shows the Giant Panda Lives on Hemicelluloses, Not on Cellulose. ISME J. 2018, 12, 1319–1328. [Google Scholar] [CrossRef]
- Liu, Y.; Yan, T.; Ren, Z.; Yang, X. Age-Associated Changes in Caecal Microbiome and Their Apparent Correlations with Growth Performances of Layer Pullets. Anim. Nutr. 2021, 7, 841–848. [Google Scholar] [CrossRef]
- Ran, J.; Wan, Q.-H.; Fang, S.-G. Gut Microbiota of Endangered Crested Ibis: Establishment, Diversity, and Association with Reproductive Output. PLoS ONE 2021, 16, e0250075. [Google Scholar] [CrossRef]
- Kohl, K.D.; Brun, A.; Caviedes-Vidal, E.; Karasov, W.H. Age-Related Changes in the Gut Microbiota of Wild House Sparrow Nestlings. Ibis 2019, 161, 184–191. [Google Scholar] [CrossRef]
- Tang, K.; Tao, L.; Wang, Y.; Wang, Q.; Fu, C.; Chen, B.; Zhang, Z.; Fu, Y. Temporal Variations in the Gut Microbiota of the Globally Endangered Sichuan Partridge (Arborophila rufipectus): Implications for Adaptation to Seasonal Dietary Change and Conservation. Appl. Environ. Microbiol. 2023, 89, e0074723. [Google Scholar] [CrossRef]
- Dietz, M.W.; Matson, K.D.; Versteegh, M.A.; van der Velde, M.; Parmentier, H.K.; Arts, J.A.J.; Salles, J.F.; Tieleman, B.I. Gut Microbiota of Homing Pigeons Shows Summer-Winter Variation under Constant Diet Indicating a Substantial Effect of Temperature. Anim. Microbiome 2022, 4, 64. [Google Scholar] [CrossRef]
- Sakda, P.; Xiang, X.; Song, Z.; Wu, Y.; Zhou, L. Impact of Season on Intestinal Bacterial Communities and Pathogenic Diversity in Two Captive Duck Species. Animals 2023, 13, 3879. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, X.; Gong, X.; Sui, J. Gut Microbiome Differences in Rescued Common Kestrels (Falco tinnunculus) Before and After Captivity. Front. Microbiol. 2022, 13, 858592. [Google Scholar] [CrossRef]
- Skeen, H.R.; Willard, D.E.; Jones, A.W.; Winger, B.M.; Gyllenhaal, E.F.; Tsuru, B.R.; Hackett, S.J.; Novembre, J. Intestinal Microbiota of Nearctic-Neotropical Migratory Birds Vary More over Seasons and Years than between Host Species. Mol. Ecol. 2023, 32, 3290–3307. [Google Scholar] [CrossRef] [PubMed]
- Grond, K.; Santo Domingo, J.W.; Lanctot, R.B.; Jumpponen, A.; Bentzen, R.L.; Boldenow, M.L.; Brown, S.C.; Casler, B.; Cunningham, J.A.; Doll, A.C.; et al. Composition and Drivers of Gut Microbial Communities in Arctic-Breeding Shorebirds. Front. Microbiol. 2019, 10, 2258. [Google Scholar] [CrossRef] [PubMed]
- Myhrvold, N.P.; Baldridge, E.; Chan, B.; Sivam, D.; Freeman, D.L.; Ernest, S.K.M. An Amniote Life-History Database to Perform Comparative Analyses with Birds, Mammals, and Reptiles. Ecology 2015, 96, 3109. [Google Scholar] [CrossRef]
- Griesser, M.; Drobniak, S.M.; Nakagawa, S.; Botero, C.A. Family Living Sets the Stage for Cooperative Breeding and Ecological Resilience in Birds. PLoS Biol. 2017, 15, e2000483. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science Using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahé, F. VSEARCH: A Versatile Open Source Tool for Metagenomics. PeerJ 2016, 4, e2584. [Google Scholar] [CrossRef]
- Xiao, Y.; Kong, F.; Xiang, Y.; Zhou, W.; Wang, J.; Yang, H.; Zhang, G.; Zhao, J. Comparative Biogeography of the Gut Microbiome between Jinhua and Landrace Pigs. Sci. Rep. 2018, 8, 5985. [Google Scholar] [CrossRef]
- Langille, M.G.I.; Zaneveld, J.; Caporaso, J.G.; McDonald, D.; Knights, D.; Reyes, J.A.; Clemente, J.C.; Burkepile, D.E.; Vega Thurber, R.L.; Knight, R.; et al. Predictive Functional Profiling of Microbial Communities Using 16S rRNA Marker Gene Sequences. Nat. Biotechnol. 2013, 31, 814–821. [Google Scholar] [CrossRef]
- Sun, F.; Chen, J.; Liu, K.; Tang, M.; Yang, Y. The Avian Gut Microbiota: Diversity, Influencing Factors, and Future Directions. Front. Microbiol. 2022, 13, 934272. [Google Scholar] [CrossRef]
- Hird, S.M.; Sánchez, C.; Carstens, B.C.; Brumfield, R.T. Comparative Gut Microbiota of 59 Neotropical Bird Species. Front. Microbiol. 2015, 6, 1403. [Google Scholar] [CrossRef]
- Patel, V.; Patel, A.K.; Parmar, N.R.; Patel, A.B.; Reddy, B.; Joshi, C.G. Characterization of the Rumen Microbiome of Indian Kankrej Cattle (Bos indicus) Adapted to Different Forage Diet. Appl. Microbiol. Biotechnol. 2014, 98, 9749–9761. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, G.; Xu, H.; Xin, H.; Zhang, Y. Metagenomic Analyses of Microbial and Carbohydrate-Active Enzymes in the Rumen of Holstein Cows Fed Different Forage-to-Concentrate Ratios. Front. Microbiol. 2019, 10, 649. [Google Scholar] [CrossRef] [PubMed]
- Becker, A.A.M.J.; Harrison, S.W.R.; Whitehouse-Tedd, G.; Budd, J.A.; Whitehouse-Tedd, K.M. Integrating Gut Bacterial Diversity and Captive Husbandry to Optimize Vulture Conservation. Front. Microbiol. 2020, 11, 1025. [Google Scholar] [CrossRef]
- Liu, G.; Guo, Z.; Liu, D.; Meng, H.; Zheng, Y.; Zhao, X.; Gu, L.; Chen, Z.; Chen, X.; Li, M.; et al. Does Gut Microbiota Regulate Brooding in Geese? Anim. Biol. 2021, 71, 361–373. [Google Scholar] [CrossRef]
- Du, X.; Li, F.; Kong, F.; Cui, Z.; Li, D.; Wang, Y.; Zhu, Q.; Shu, G.; Tian, Y.; Zhang, Y.; et al. Altitude-Adaption of Gut Microbiota in Tibetan Chicken. Poult. Sci. 2022, 101, 101998. [Google Scholar] [CrossRef]
- Sepulveda, J.; Moeller, A.H. The Effects of Temperature on Animal Gut Microbiomes. Front. Microbiol. 2020, 11, 384. [Google Scholar] [CrossRef]
- Zi, M.; Zhang, Y.; Hu, C.; Zhang, S.; Chen, J.; Yuan, L.; Cheng, X. A Literature Review on the Potential Clinical Implications of Streptococci in Gastric Cancer. Front. Microbiol. 2022, 13, 1010465. [Google Scholar] [CrossRef]
- Yu, L.C.-H.; Wang, J.-T.; Wei, S.-C.; Ni, Y.-H. Host-Microbial Interactions and Regulation of Intestinal Epithelial Barrier Function: From Physiology to Pathology. World J. Gastrointest. Pathophysiol. 2012, 3, 27–43. [Google Scholar] [CrossRef]
- Zhu, L.; Wu, Q.; Dai, J.; Zhang, S.; Wei, F. Evidence of Cellulose Metabolism by the Giant Panda Gut Microbiome. Proc. Natl. Acad. Sci. USA 2011, 108, 17714–17719. [Google Scholar] [CrossRef]
- Coyte, K.Z.; Schluter, J.; Foster, K.R. The Ecology of the Microbiome: Networks, Competition, and Stability. Science 2015, 350, 663–666. [Google Scholar] [CrossRef]
- Chevalier, C.; Stojanović, O.; Colin, D.J.; Suarez-Zamorano, N.; Tarallo, V.; Veyrat-Durebex, C.; Rigo, D.; Fabbiano, S.; Stevanović, A.; Hagemann, S.; et al. Gut Microbiota Orchestrates Energy Homeostasis during Cold. Cell 2015, 163, 1360–1374. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Yao, Y.; Dong, M.; Xiao, H.; Xiong, Y.; Yang, S.; Li, D.; Xie, M.; Ni, Q.; Zhang, M.; et al. Diet and High Altitude Strongly Drive Convergent Adaptation of Gut Microbiota in Wild Macaques, Humans, and Dogs to High Altitude Environments. Front. Microbiol. 2023, 14, 1067240. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Liu, L.; Du, C.; Hou, Q. Comparative Analysis of Fecal Bacterial Microbiota of Six Bird Species. Front. Vet. Sci. 2021, 8, 791287. [Google Scholar] [CrossRef] [PubMed]
- Schaefers, M.M. Regulation of Virulence by Two-Component Systems in Pathogenic Burkholderia. Infect. Immun. 2020, 88, e00927-19. [Google Scholar] [CrossRef]
- Depoorter, E.; Bull, M.J.; Peeters, C.; Coenye, T.; Vandamme, P.; Mahenthiralingam, E. Burkholderia: An Update on Taxonomy and Biotechnological Potential as Antibiotic Producers. Appl. Microbiol. Biotechnol. 2016, 100, 5215–5229. [Google Scholar] [CrossRef]
- Anandan, R.; Dharumadurai, D.; Manogaran, G.P.; Anandan, R.; Dharumadurai, D.; Manogaran, G.P. An Introduction to Actinobacteria. In Actinobacteria-Basics and Biotechnological Applications; IntechOpen: London, UK, 2016; ISBN 978-953-51-2248-7. [Google Scholar]
- Takahashi, Y.; Nakashima, T. Actinomycetes, an Inexhaustible Source of Naturally Occurring Antibiotics. Antibiotics 2018, 7, 45. [Google Scholar] [CrossRef]
- Barka, E.A.; Vatsa, P.; Sanchez, L.; Gaveau-Vaillant, N.; Jacquard, C.; Meier-Kolthoff, J.P.; Klenk, H.-P.; Clément, C.; Ouhdouch, Y.; van Wezel, G.P. Taxonomy, Physiology, and Natural Products of Actinobacteria. Microbiol. Mol. Biol. Rev. 2016, 80, 1–43. [Google Scholar] [CrossRef]
- Jiang, F.; Gao, H.; Qin, W.; Song, P.; Wang, H.; Zhang, J.; Liu, D.; Wang, D.; Zhang, T. Marked Seasonal Variation in Structure and Function of Gut Microbiota in Forest and Alpine Musk Deer. Front. Microbiol. 2021, 12, 699797. [Google Scholar] [CrossRef]
- Ayeni, E.A.; Aldossary, A.M.; Ayejoto, D.A.; Gbadegesin, L.A.; Alshehri, A.A.; Alfassam, H.A.; Afewerky, H.K.; Almughem, F.A.; Bello, S.M.; Tawfik, E.A. Neurodegenerative Diseases: Implications of Environmental and Climatic Influences on Neurotransmitters and Neuronal Hormones Activities. Int. J. Environ. Res. Public Health 2022, 19, 12495. [Google Scholar] [CrossRef]
- Scott, S.L.; Christopherson, R.J.; Thompson, J.R.; Baracos, V.E. The Effect of a Cold Environment on Protein and Energy Metabolism in Calves. Br. J. Nutr. 1993, 69, 127–139. [Google Scholar] [CrossRef]
- Liang, F.; Yan, L.; Li, Y.; Jin, Y.; Zhang, J.; Che, H.; Diao, J.; Gao, Y.; He, Z.; Sun, R.; et al. Effect of Season on Slaughter Performance, Meat Quality, Muscle Amino Acid and Fatty Acid Composition, and Metabolism of Pheasants (Phasianus colchicus). Anim. Sci. J. 2022, 93, e13735. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Dong, H.; Zhang, Y.; Li, Y. Discovery of Potential Genes Contributing to the Biosynthesis of Short-Chain Fatty Acids and Lactate in Gut Microbiota from Systematic Investigation in E. coli. NPJ Biofilms Microbiomes 2019, 5, 19. [Google Scholar] [CrossRef] [PubMed]
- Conway, T.; Krogfelt, K.A.; Cohen, P.S. The Life of Commensal Escherichia coli in the Mammalian Intestine. EcoSal Plus 2004, 1, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.L.; Preta, G. Lipids in the Cell: Organisation Regulates Function. Cell. Mol. Life Sci. 2018, 75, 1909–1927. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, Y.; Liu, S.; Huang, J.; Zhai, Z.; He, C.; Ding, J.; Wang, J.; Wang, H.; Fan, W.; et al. The Dynamic Distribution of Porcine Microbiota across Different Ages and Gastrointestinal Tract Segments. PLoS ONE 2015, 10, e0117441. [Google Scholar] [CrossRef]
- Joat, N.; Van, T.T.H.; Stanley, D.; Moore, R.J.; Chousalkar, K. Temporal Dynamics of Gut Microbiota in Caged Laying Hens: A Field Observation from Hatching to End of Lay. Appl. Microbiol. Biotechnol. 2021, 105, 4719–4730. [Google Scholar] [CrossRef]
- Dupont, A.; Sommer, F.; Zhang, K.; Repnik, U.; Basic, M.; Bleich, A.; Kühnel, M.; Bäckhed, F.; Litvak, Y.; Fulde, M.; et al. Age-Dependent Susceptibility to Enteropathogenic Escherichia coli (EPEC) Infection in Mice. PLoS Pathog. 2016, 12, e1005616. [Google Scholar] [CrossRef]
- Martinson, J.N.V.; Walk, S.T. Escherichia coli Residency in the Gut of Healthy Human Adults. EcoSal Plus 2020, 9, 1–19. [Google Scholar] [CrossRef]
- Ramakrishna, B.S. Role of the Gut Microbiota in Human Nutrition and Metabolism. J. Gastroenterol. Hepatol. 2013, 28, 9–17. [Google Scholar] [CrossRef]
- Hasan, N.; Yang, H. Factors Affecting the Composition of the Gut Microbiota, and Its Modulation. PeerJ 2019, 7, e7502. [Google Scholar] [CrossRef]
- Irina. Study on the Differences in the Gut Microbiota of Wild Sichuan Golden Monkeys in Shennongjia across Different Seasons, Ages, and Genders. Master’s Thesis, University of Chinese Academy of Sciences, Beijing, China, 2019. [Google Scholar]
- Dillon, R.J.; Webster, G.; Weightman, A.J.; Keith Charnley, A. Diversity of Gut Microbiota Increases with Aging and Starvation in the Desert Locust. Antonie Van Leeuwenhoek 2010, 97, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Yao, C.; Shen, X.; Jin, H.; Guo, Z.; Zhai, Q.; Yu-Kwok, L.; Zhang, H.; Sun, Z. The Diversity and Composition of the Human Gut Lactic Acid Bacteria and Bifidobacterial Microbiota Vary Depending on Age. Appl. Microbiol. Biotechnol. 2021, 105, 8427–8440. [Google Scholar] [CrossRef] [PubMed]
- Tong, Q.; Liu, X.-N.; Hu, Z.-F.; Ding, J.-F.; Bie, J.; Wang, H.-B.; Zhang, J.-T. Effects of Captivity and Season on the Gut Microbiota of the Brown Frog (Rana dybowskii). Front. Microbiol. 2019, 10, 1912. [Google Scholar] [CrossRef]
- Clark, R.I.; Salazar, A.; Yamada, R.; Fitz-Gibbon, S.; Morselli, M.; Alcaraz, J.; Rana, A.; Rera, M.; Pellegrini, M.; Ja, W.W.; et al. Distinct Shifts in Microbiota Composition during Drosophila Aging Impair Intestinal Function and Drive Mortality. Cell Rep. 2015, 12, 1656–1667. [Google Scholar] [CrossRef]
- Claesson, M.J.; Cusack, S.; O’Sullivan, O.; Greene-Diniz, R.; de Weerd, H.; Flannery, E.; Marchesi, J.R.; Falush, D.; Dinan, T.; Fitzgerald, G.; et al. Composition, Variability, and Temporal Stability of the Intestinal Microbiota of the Elderly. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. S1), 4586–4591. [Google Scholar] [CrossRef]
- Shin, N.-R.; Whon, T.W.; Bae, J.-W. Proteobacteria: Microbial Signature of Dysbiosis in Gut Microbiota. Trends Biotechnol. 2015, 33, 496–503. [Google Scholar] [CrossRef]
- Badal, V.D.; Vaccariello, E.D.; Murray, E.R.; Yu, K.E.; Knight, R.; Jeste, D.V.; Nguyen, T.T. The Gut Microbiome, Aging, and Longevity: A Systematic Review. Nutrients 2020, 12, 3759. [Google Scholar] [CrossRef]
- Chen, J.; Potlapalli, R.; Quan, H.; Chen, L.; Xie, Y.; Pouriyeh, S.; Sakib, N.; Liu, L.; Xie, Y. Exploring DNA Damage and Repair Mechanisms: A Review with Computational Insights. BioTech 2024, 13, 3. [Google Scholar] [CrossRef]
- Neumann, E.J.; Hall, W.F. Disease Control, Prevention, and Elimination. In Diseases of Swine; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2019; pp. 123–157. ISBN 978-1-119-35092-7. [Google Scholar]
- Mollentze, N.; Streicker, D.G. Viral Zoonotic Risk Is Homogenous among Taxonomic Orders of Mammalian and Avian Reservoir Hosts. Proc. Natl. Acad. Sci. USA 2020, 117, 9423–9430. [Google Scholar] [CrossRef]
- Hilker, F.M.; Sun, T.A.; Allen, L.J.S.; Hamelin, F.M. Separate Seasons of Infection and Reproduction Can Lead to Multi-Year Population Cycles. J. Theor. Biol. 2020, 489, 110158. [Google Scholar] [CrossRef]
- Hou, D.; Hong, M.; Wang, Y.; Dong, P.; Cheng, H.; Yan, H.; Yao, Z.; Li, D.; Wang, K.; Zhang, D. Assessing the Risks of Potential Bacterial Pathogens Attaching to Different Microplastics during the Summer-Autumn Period in a Mariculture Cage. Microorganisms 2021, 9, 1909. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhu, Q.; Chen, J.; Khattak, R.H.; Li, Z.; Teng, L.; Liu, Z. Insights into the Composition of Gut Microbiota in Response to Environmental Temperature: The Case of the Mongolia Racerunner (Eremias argus). Glob. Ecol. Conserv. 2022, 36, e02125. [Google Scholar] [CrossRef]
- Gavriilidou, A.; Gutleben, J.; Versluis, D.; Forgiarini, F.; van Passel, M.W.J.; Ingham, C.J.; Smidt, H.; Sipkema, D. Comparative Genomic Analysis of Flavobacteriaceae: Insights into Carbohydrate Metabolism, Gliding Motility and Secondary Metabolite Biosynthesis. BMC Genom. 2020, 21, 569. [Google Scholar] [CrossRef]
- Aljahdali, N.H.; Sanad, Y.M.; Han, J.; Foley, S.L. Current Knowledge and Perspectives of Potential Impacts of Salmonella Enterica on the Profile of the Gut Microbiota. BMC Microbiol. 2020, 20, 353. [Google Scholar] [CrossRef]
- Brodin, A. Theoretical Models of Adaptive Energy Management in Small Wintering Birds. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2007, 362, 1857–1871. [Google Scholar] [CrossRef]
- Ma, L.; Lyu, W.; Song, Y.; Chen, K.; Lv, L.; Yang, H.; Wang, W.; Xiao, Y. Anti-Inflammatory Effect of Clostridium Butyricum-Derived Extracellular Vesicles in Ulcerative Colitis: Impact on Host microRNAs Expressions and Gut Microbiome Profiles. Mol. Nutr. Food Res. 2023, 67, 2200884. [Google Scholar] [CrossRef]
- Xiao, Y.; Zou, H.; Li, J.; Song, T.; Lv, W.; Wang, W.; Wang, Z.; Tao, S. Impact of Quorum Sensing Signaling Molecules in Gram-Negative Bacteria on Host Cells: Current Understanding and Future Perspectives. Gut Microbes 2022, 14, 2039048. [Google Scholar] [CrossRef]
- Clostridium Butyricum and Its Derived Extracellular Vesicles Modulate Gut Homeostasis and Ameliorate Acute Experimental Colitis|Microbiology Spectrum. Available online: https://journals.asm.org/doi/full/10.1128/spectrum.01368-22 (accessed on 21 June 2024).
- Yang, H.; Lyu, W.; Lu, L.; Shi, X.; Li, N.; Wang, W.; Xiao, Y. Biogeography of Microbiome and Short-Chain Fatty Acids in the Gastrointestinal Tract of Duck. Poult. Sci. 2020, 99, 4016–4027. [Google Scholar] [CrossRef]
- Kim, H.; Seo, H.; Park, S.; Chung, H.; Sung, H.; Kim, M.-N.; Bae, S.; Jung, J.; Kim, M.J.; Kim, S.-H.; et al. Clinical Significance and Outcomes of Clostridium Tertium Bacteremia: Analysis of 62 Cases in Neutropenic and Non-Neutropenic Patients. Eur. J. Clin. Microbiol. Infect. Dis. 2023, 42, 183–191. [Google Scholar] [CrossRef]
- Drevinek, P.; Mahenthiralingam, E. Burkholderia Cenocepacia in Cystic Fibrosis: Epidemiology and Molecular Mechanisms of Virulence. Clin. Microbiol. Infect. 2010, 16, 821–830. [Google Scholar] [CrossRef]
- Toepfner, N.; Shetty, S.; Kunze, M.; Orlowska-Volk, M.; Krüger, M.; Berner, R.; Hentschel, R. Fulminant Neonatal Sepsis Due to Streptococcus Alactolyticus—A Case Report and Review. Apmis 2014, 122, 654–656. [Google Scholar] [CrossRef] [PubMed]
- Aronoff, D.M.; Marrazzo, J.M. Infections Caused by Clostridium Perfringens and Paeniclostridium Sordellii after Unsafe Abortion. Lancet Infect. Dis. 2023, 23, e48–e55. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, L.; Zheng, Y.; Feng, S.; Wu, B.; Chen, L.; Xu, X.; Wang, B.; Li, W.; Zhou, C.; Zhang, L. Seasonal Changes and Age-Related Effects on the Intestinal Microbiota of Captive Chinese Monals (Lophophorus lhuysii). Animals 2024, 14, 3418. https://doi.org/10.3390/ani14233418
Huang L, Zheng Y, Feng S, Wu B, Chen L, Xu X, Wang B, Li W, Zhou C, Zhang L. Seasonal Changes and Age-Related Effects on the Intestinal Microbiota of Captive Chinese Monals (Lophophorus lhuysii). Animals. 2024; 14(23):3418. https://doi.org/10.3390/ani14233418
Chicago/Turabian StyleHuang, Lijing, Yanchu Zheng, Shaohua Feng, Bangyuan Wu, Li Chen, Xiaoqin Xu, Bin Wang, Wanhong Li, Caiquan Zhou, and Long Zhang. 2024. "Seasonal Changes and Age-Related Effects on the Intestinal Microbiota of Captive Chinese Monals (Lophophorus lhuysii)" Animals 14, no. 23: 3418. https://doi.org/10.3390/ani14233418
APA StyleHuang, L., Zheng, Y., Feng, S., Wu, B., Chen, L., Xu, X., Wang, B., Li, W., Zhou, C., & Zhang, L. (2024). Seasonal Changes and Age-Related Effects on the Intestinal Microbiota of Captive Chinese Monals (Lophophorus lhuysii). Animals, 14(23), 3418. https://doi.org/10.3390/ani14233418





