Ensiling as a Conservation Technique for Opuntia ficus indica (L.) By-Products: Peel and Pastazzo
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ensiling Process
2.2. Physicochemical Analyses
2.3. Water-Soluble Carbohydrates
2.4. Microbiological Analyses
2.5. Organic Acids
2.6. Total Phenolic Content and Antioxidant Capacity
2.7. Minerals
2.8. Statistical Analysis
3. Results
3.1. Physicochemical Analyses
3.2. Microbiological Analysis
3.3. Organic Acids
3.4. Total Phenolic Content and Antioxidant Capacity
3.5. Minerals
4. Discussion
4.1. Chemical Composition
4.2. Fermentation Process and Microbial Loads
4.3. Organic Acids
4.4. Total Phenolic Content (TPC) and Antioxidant Capacity (AOC)
4.5. Minerals
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- García-Rodríguez, J.; Ranilla, M.J.; France, J.; Alaiz-Moretón, H.; Carro, M.D.; López, S. Chemical Composition, In Vitro Digestibility and Rumen Fermentation Kinetics of Agro-Industrial By-Products. Animals 2019, 9, 861. [Google Scholar] [CrossRef] [PubMed]
- Correddu, F.; Lunesu, M.F.; Buffa, G.; Atzori, A.S.; Nudda, A.; Battacone, G.; Pulina, G. Can Agro-Industrial By-Products Rich in Polyphenols Be Advantageously Used in the Feeding and Nutrition of Dairy Small Ruminants? Animals 2020, 10, 131. [Google Scholar] [CrossRef] [PubMed]
- Vastolo, A.; Calabrò, S.; Cutrignelli, M.I. A review on the use of agro-industrial CO-products in animals’ diets. Ital. J. Anim. Sci. 2022, 21, 577–594. [Google Scholar] [CrossRef]
- Jalal, H.; Giammarco, M.; Lanzoni, L.; Akram, M.Z.; Mammi, L.M.; Vignola, G.; Chincarini, M.; Formigoni, A.; Fusaro, I. Potential of fruits and vegetable by-products as an alternative feed source for sustainable ruminant nutrition and production: A review. Agriculture 2023, 13, 286. [Google Scholar] [CrossRef]
- Todaro, M.; Alabiso, M.; Di Grigoli, A.; Scatassa, M.L.; Cardamone, C.; Mancuso, I.; Mazza, F.; Bonanno, A. Prickly Pear By-Product in the Feeding of Livestock Ruminants: Preliminary Investigation. Animals 2020, 10, 949. [Google Scholar] [CrossRef]
- Vastolo, A.; Calabrò, S.; Cutrignelli, M.I.; Raso, G.; Todaro, M. Silage of prickly pears (Opuntia spp.) juice by-products. Animals 2020, 10, 1716. [Google Scholar] [CrossRef]
- Gannuscio, R.; Vastolo, A.; Maniaci, G.; Lucia, C.; Calabrò, S.; Todaro, M.; Cutrignelli, M.I. Improve nutritive value of silage based on prickly pear peel by-products. Ital. J. Anim. Sci. 2024, 23, 492–503. [Google Scholar] [CrossRef]
- Maniaci, G.; Ponte, M.; Giosuè, C.; Gannuscio, R.; Pipi, M.; Gaglio, R.; Busetta, G.; Di Grigoli, A.; Bonanno, A.; Alabiso, M. Cladodes of Opuntia ficus-indica (L.) as a source of bioactive compounds in dairy products. J. Dairy Sci. 2024, 107, 1887–1902. [Google Scholar] [CrossRef]
- ISTAT. Istituto Nazionale di Statistica. Available online: http://dati.istat.it/Index.aspx?DataSetCode=DCSP_COLTIVAZIONI (accessed on 13 July 2024).
- Dillard, C.; German, B. Phytochemicals: Nutraceuticals and human health. J. Sci. Food Agric. 2000, 80, 1744–1756. [Google Scholar] [CrossRef]
- Melgar, B.; Dias, M.I.; Ciric, A.; Sokovic, M.; Garcia-Castello, E.M.; Rodriguez-Lopez, A.D.; Barros, L.; Ferreira, I. By-product recovery of Opuntia spp. peels: Betalainic and phenolic profiles and bioactive properties. Ind. Crops Prod. 2017, 107, 353–359. [Google Scholar] [CrossRef]
- Tegegne, F.; Kijora, C.; Peters, K. Study on the optimal level of cactus pear (Opuntia ficus-indica) supplementation to sheep and its contribution as source of water. Small Rumin. Res. 2007, 72, 157–164. [Google Scholar] [CrossRef]
- Amaya-Cruz, D.M.; Pérez-Ramírez, I.F.; Delgado-García, J.; Mondragón-Jacobo, C.; Dector-Espinoza, A.; Reynoso-Camacho, R. An integral profile of bioactive compounds and functional properties of prickly pear (Opuntia ficus indica L.) peel with different tonalities. Food Chem. 2019, 278, 568–578. [Google Scholar] [CrossRef] [PubMed]
- Pastorelli, G.; Serra, V.; Vannuccini, C.; Attard, E. Opuntia spp. as alternative fodder for sustainable livestock production. Animals 2022, 12, 1597. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Mahato, N.; Cho, M.H.; Lee, Y.R. Converting Citrus Wastes into Value-Added Products: Economic and Environmently Friendly Approaches. Nutrition 2017, 34, 29–46. [Google Scholar] [CrossRef]
- AOAC International. Official Methods of Analysis of AOAC International, 18th ed.; AOAC International: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fibre, neutral detergent fibre, and no starch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Martin, P.C.B.; Schlienz, M.; Greger, M. Production of bio-hydrogen and methane during semi-continuous digestion of maize silage in a two-stage system. Int. J. Hydrogen Energy 2017, 42, 5768–5779. [Google Scholar] [CrossRef]
- ISO 4833-1; Microbiology of the Food Chain—Horizontal Method for the Enumeration of Microorganisms Part 1: Colony Count at 30 °C by the Pour Plate Technique. International Organization for Standardization: Geneva, Switzerland, 2013.
- ISO 21528-2; Microbiology of the Food Chain—Horizontal Method for the Detection and Enumeration of Enterobacteriaceae. Part 2: Colony-Count Technique. International Organization for Standardization: Geneva, Switzerland, 2017.
- UNI ISO 16649-2; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Beta-Glucuronidase-Positive Escherichia coli. Part 2: Colony-Count Technique at 44 Degrees C Using 5-bromo-4-chloro-3-indolyl beta-D-glucuronide. International Organization for Standardization: Geneva, Switzerland, 2010.
- UNI ISO 6888-2; Microbiology of the Food Chain—Horizontal Method for the Enumeration of Coagulase-Positive Staphylococci (Staphylococcus aureus and Other Species)—Part 2: Method Using Rabbit Plasma Fibrinogen Agar Medium. International Organization for Standardization: Geneva, Switzerland, 2023.
- ISO 15213-1; Microbiology of the Food Chain—Horizontal Method for the Detection and Enumeration of Clostridium spp. Part 1: Enumeration of Sulfite-Reducing Clostridium spp. by Colony-Count Technique. International Organization for Standardization: Geneva, Switzerland, 2023.
- ISO 21527-1; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Yeasts and Molds. Part 1: Colony Count Technique in Products with Water Activity Greater than 0,95. International Organization for Standardization: Geneva, Switzerland, 2008.
- Martillotti, F.; Puppo, P. Liquid chromatographic determination of organic acids in silages and rumen fluids. Ann. Ist. Sper. Zootec. 1985, 18, 1–10. [Google Scholar]
- Viola, E.; Buzzanca, C.; Tinebra, I.; Settanni, L.; Farina, V.; Gaglio, R.; Di Stefano, V. A Functional End-Use of Avocado (Cv. Hass) Waste through Traditional Semolina Sourdough Bread Production. Foods 2023, 12, 3743. [Google Scholar] [CrossRef]
- Di Stefano, V.; Buzzanca, C.; Ruvutuso, F.; Scuderi, D.; Palazzolo, E.; Gugliuzza, G.; Tinebra, I.; Farina, V. Chemical Composition and Anti-Radical Properties of Coffee Cherry Cultivated in Mediterranean Climate. Food Biosci. 2023, 56, 103349. [Google Scholar] [CrossRef]
- El Hajji, L.; Azzouzi, H.; Achchoub, M.; Elfazazi, K.; Salmaoui, S. Ensiling characteristics of prickly pear (Opuntia-ficus indica) rejects with and without molasses for animal feed. Int. J. Recycl. Org. Waste Agric. 2022, 11, 541–552. [Google Scholar]
- Underwood, E.J.; Suttle, N.F. The Mineral Nutrition of Livestock, 3rd ed.; CABI Publishing: Oxon, UK, 1999. [Google Scholar]
- Collins, M.; Moore, K.J.; Nelson, C.J.; Barnes, R.F. Preservation of forage as hay and silage. Forages 2017, 1, 321. [Google Scholar]
- Kuti, J.O.; Galloway, C.M. Sugar composition and lnvertase activity in prickly pear fruit. J. Food Sci. 1994, 59, 387–388. [Google Scholar] [CrossRef]
- Muck, R.E. Factors influencing silage quality and their implications for man-agement. J. Dairy Sci. 1988, 71, 2992–3002. [Google Scholar] [CrossRef]
- McDonald, P.; Henderson, A.R.; Whittenbury, R. The effect of temperature on ensilage. J. Sci. Food Agric. 1966, 17, 476–480. [Google Scholar] [CrossRef] [PubMed]
- Ávila, C.L.S.; Carvalho, B.F. Silage fermentation-updates focusing on the performance of micro-organisms. J. Appl. Microbiol. 2020, 128, 966–984. [Google Scholar] [CrossRef]
- Weinberg, Z.G.; Muck, R.E. New trends and opportunities in the development and use of inoculants for silage. FEMS Microbiol. Rev. 1996, 19, 53–68. [Google Scholar] [CrossRef]
- Soundharrajan, I.; Park, H.S.; Rengasamy, S.; Sivanesan, R.; Choi, K.C. Application and future prospective of lactic acid bacteria as natural additives for silage production—A review. Appl. Sci. 2021, 11, 8127. [Google Scholar] [CrossRef]
- Xiang, Q.; Zhang, J.; Huang, X.; Ma, M.; Zhao, K.; Yu, X.; Chen, Q.; Zhang, X.; Penttinen, P.; Gu, Y. Changes in the taxonomic and functional structures of microbial communities during vegetable waste mixed silage fermentation. Can. J. Microbiol. 2022, 68, 281–293. [Google Scholar] [CrossRef]
- Carvalho, B.F.; Ávila, C.L.S.; Pinto, J.C.; Neri, J.; Schwan, R.F. Microbiological and chemical profile of sugar cane silage fermentation inoculated with wild strains of lactic acid bacteria. Anim. Feed Sci. Technol. 2014, 195, 1–13. [Google Scholar] [CrossRef]
- Vohra, A.; Syal, P.; Madan, A. Probiotic yeast in livestock sector. Anim. Feed Sci. Technol. 2016, 219, 31–47. [Google Scholar] [CrossRef]
- Weinberg, Z.G.; Szakacs, G.; Ashbell, G.; Hen, Y. The effect of temperature on the ensiling process of corn and wheat. J. Appl. Microbiol. 2001, 90, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Kung, L.; Shaver, R. Interpretation and use of silage fermentation analysis reports. Focus Forage 2001, 3, 1–5. [Google Scholar]
- Jasaitis, D.K.; Wohlt, J.E.; Evans, J.L. Influence of feed ion content on buffering capacity of ruminant feedstuffs in vitro. J. Dairy Sci. 1987, 70, 1391–1403. [Google Scholar] [CrossRef]
- Rodrigues, P.H.M.; Gomes, R.D.C.; Meyer, P.M.; Borgatti, L.M.O.; Franco, F.M.J.; Godoy, G.L.A.D. Effects of microbial inoculants and amino acid production by-product on fermentation and chemical composition of sugarcane silages. Rev. Bras. Zootec. 2012, 41, 1394–1400. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Ahmed, A.R.; Mohamed, H.I.; Al-Otaibi, H.H.; Ramadan, K.M.; Elkatry, H.O. Utilization of prickly pear peels flour as a natural source of minerals, dietary fiber and antioxidants: Effect on cakes production. Agronomy 2023, 13, 439. [Google Scholar] [CrossRef]
- Yeddes, N.; Chérif, J.K.; Guyot, S.; Sotin, H.; Ayadi, M.T. Comparative study of antioxidant power, polyphenols, flavonoids and betacyanins of the peel and pulp of three Tunisian Opuntia forms. Antioxidants 2013, 2, 37–51. [Google Scholar] [CrossRef]
- Jiménez-Aguilar, D.M.; López-Martínez, J.M.; Hernández-Brenes, C.; Gutiérrez-Uribe, J.A.; Welti-Chanes, J. Dietary fiber, phytochemical composition and antioxidant activity of Mexican commercial varieties of cactus pear. J. Food Compos. Anal. 2015, 41, 66–73. [Google Scholar] [CrossRef]
- García-Cayuela, T.; Gómez-Maqueo, A.; Guajardo-Flores, D.; Welti-Chanes, J.; Cano, M.P. Characterization and quantification of individual betalain and phenolic compounds in Mexican and Spanish prickly pear (Opuntia ficus-indica L. Mill) tissues: A comparative study. J. Food Compos. Anal. 2019, 76, 1–13. [Google Scholar] [CrossRef]
- Hassan Amer, F.; Mobaraz, S.; Basyony, M.; Mahrose, K.; El-Medany, S. Effect of using prickly pear and its by-products as alternative feed resources on performance of growing rabbit. Egypt. J. Rabbit. Sci. 2019, 29, 99–124. [Google Scholar] [CrossRef][Green Version]
- Łozicki, A.; Koziorzębska, A.; Halik, G.; Dymnicka, M.; Arkuszewska, E.; Niemiec, T.; Bogdan, J. Effect of ensiling pumpkin (Cucurbita maxima D.) with dried sugar beet pulp on the content of bioactive compounds in silage and its antioxidant potential. Anim. Feed Sci. Technol. 2015, 206, 108–113. [Google Scholar] [CrossRef]
- Acin-Albiac, M.; Filannino, P.; Arora, K.; Da Ros, A.; Gobbetti, M.; Di Cagno, R. Role of lactic acid bacteria phospho-β-glucosidases during the fermentation of cereal by-products. Foods 2021, 10, 97. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, T.S.; Vale, R.C.; Almeida, R.R.; Ferreira, T.P.S.; Guimarães, L.G.L. Antioxidant potential and its correlation with the contents of phenolic compounds and flavonoids of methanolic extracts from different medicinal plants. Rev. Virtual Química 2017, 9, 1546–1559. [Google Scholar] [CrossRef]
- Georgievskii, V.I.; Annenkov, B.N.; Samokhin, V.T. Mineral Nutrition of Animals: Studies in the Agricultural and Food Sciences; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Arafa, S.G.; Badawy, W.Z.; El-Bana, M.A.; Mohamed, A.S. Utilization of prickly pear by-products to improve the nutritional value of balady bread. J. Food Dairy Sci. 2023, 14, 99–107. [Google Scholar] [CrossRef]
- El Kossori, R.L.; Villaume, C.; El Boustani, E.; Sauvaire, Y.; Méjean, L. Composition of pulp, skin and seeds of prickly pears fruit (Opuntia ficus indica sp.). Plant Foods Hum. Nutr. 1998, 52, 263–270. [Google Scholar] [CrossRef]
- Pulina, G. Alimentazione Degli Ovini da Latte; Avenue Media: Bologna, Italy, 2001. [Google Scholar]
- El-Beltagi, H.S.; Mohamed, H.I.; Elmelegy, A.A.; Eldesoky, S.E.; Safwat, G. Phytochemical screening, antimicrobial, antioxidant, anticancer activities and nutritional values of cactus (Opuntia ficus indica) pulp and peel. Fresenius Environ. Bull. 2019, 28, 1534–1551. [Google Scholar]

| Items | Raw Materials | Silages | SEM | p-Value | |||
|---|---|---|---|---|---|---|---|
| Bran | PPP | PPS | PPP | PPS | |||
| Dry matter | 89.17 A | 14.97 E | 38.47 C | 20.03 D | 41.37 B | 0.516 | 0.001 |
| Crude protein | 17.86 A | 5.16 E | 6.52 D | 12.02 B | 9.55 C | 0.250 | 0.001 |
| Ether extract | 5.07 ABb | 1.49 CDd | 3.10 BDc | 6.15 Aa | 3.78 Bb | 0.520 | 0.001 |
| aNDFom | 28.96 C | 20.45 D | 71.89 A | 25.31 C | 66.66 B | 0.734 | 0.001 |
| ADFom | 9.34 D | 10.10 D | 57.68 A | 11.38 C | 50.03 B | 0.315 | 0.001 |
| ADL | 3.16 C | 1.63 C | 36.12 A | 2.18 C | 26.77 B | 0.164 | 0.001 |
| NFC | 43.02 B | 60.69 A | 9.90 C | 45.01 B | 11.92 C | 0.820 | 0.001 |
| WSC | 4.16 B | 18.60 A | 1.50 B | 3.36 B | 1.10 B | 1.051 | 0.001 |
| Ash | 5.54 C | 12.43 A | 8.70 B | 11.52 A | 8.08 B | 0.354 | 0.001 |
| pH | 5.07 B | 5.71 A | 4.15 C | 3.75 C | 4.04 C | 0.144 | 0.001 |
| Activity water | 0.66 C | 0.97 A | 0.97 A | 0.96 AB | 0.95 B | 0.005 | 0.001 |
| Items | Raw Materials | Silages | SEM 4 | p-Value | |||
|---|---|---|---|---|---|---|---|
| Bran | PPP | PPS | PPP | PPS | |||
| Plate Count Agar (PCA) | 3.94 B | 6.72 A | 6.70 A | 7.41 A | 7.40 A | 0.16 | 0.002 |
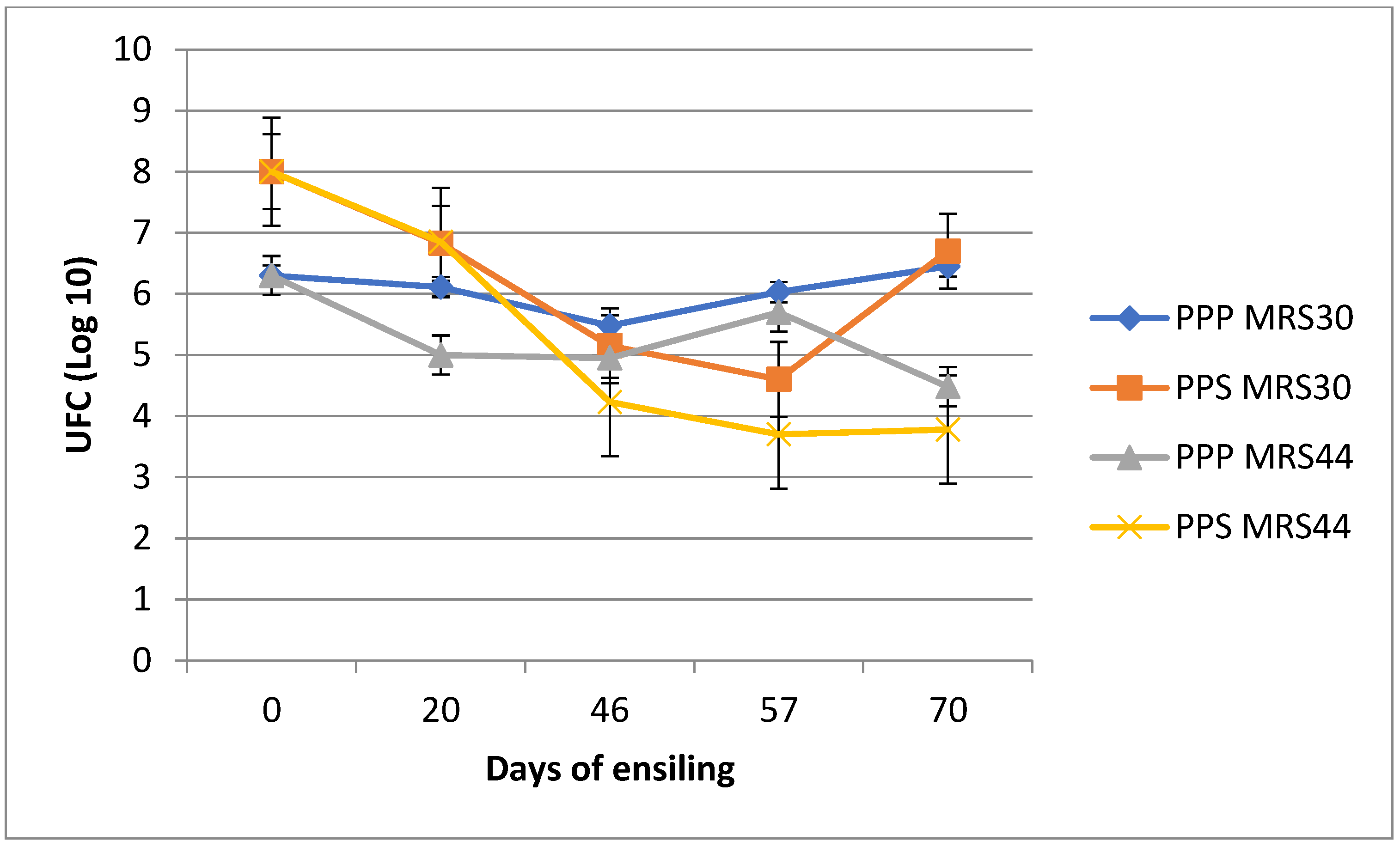
| MRS 30 °C 1 | 3.34 | 8.00 | 8.00 | 4.83 | 5.67 | 1.09 | 0.405 |
| MRS 44 °C 1 | 3.23 | 8.00 | 8.00 | 4.10 | 3.16 | 0.85 | 0.117 |
| M17 44 °C 2 | <1 b | <1 b | <1 b | 2.43 a | 3.38 a | 0.38 | 0.040 |
| VRBGA 3 | 3.38 | 6.41 | <1 | <1 | <1 | - | - |
| Mold | 2.95 A | 3.08 A | 2.08 B | 1.10 B | <1 B | 0.07 | 0.002 |
| Yeasts | <1 B | 5.48 Ab | 5.00 Ab | 6.30 Aa | <1 B | 0.12 | 0.001 |
| PPP Silage | PPS Silage | SEM | p-Value | |
|---|---|---|---|---|
| Lactate (g/kg DM) | 20.02 A | 1.26 B | 1.167 | 0.001 |
| Acetate (g/kg DM) | 1.96 | 1.92 | 0.145 | 0.846 |
| Propionate (g/kg DM) | 0.482 | 0.236 | 0.171 | 0.334 |
| Butyrate (g/kg DM) | 0.699 A | 0.020 B | 0.054 | 0.001 |
| Lactate/acetate | 10.54 A | 0.68 B | 0.723 | 0.001 |
| N-NH3/N (g/100 g) | 1.277 | 1.525 | 0.204 | 0.411 |
| Buffering capacity (meq NaOH/100 g DM) | 114 A | 78 B | 4.25 | 0.001 |
| Items | Raw Materials | Silages | SEM 4 | p-Value | |||
|---|---|---|---|---|---|---|---|
| Bran | PPP | PPS | PPP | PPS | |||
| TPC 1 (mg GAE/g DM) | 8.69 E | 28.96 B | 20.79 D | 30.24 A | 24.22 C | 0.11 | 0.001 |
| DPPH 2 (mmol TEAC/100 g DM) | 4.02 E | 11.57 B | 7.85 D | 15.75 A | 10.06 C | 0.13 | 0.001 |
| ABTS 3 (mmol TEAC/100 g DM) | 12.62 E | 25.55 B | 17.69 D | 26.46 A | 21.58 C | 0.14 | 0.003 |
| Items | Raw Materials | Silages | SEM 1 | p-Value | |||
|---|---|---|---|---|---|---|---|
| Bran | PPP | PPS | PPP | PPS | |||
| Ca (g kg−1 DM) | 2.72 Bc | 20.78 Ab | 31.51 Aa | 23.50 Aab | 22.48 Ab | 2.00 | 0.001 |
| K (g kg−1 DM) | 13.40 Bc | 40.51 Aa | 23.98 Bb | 43.43 Aa | 20.40 Bbc | 3.30 | 0.001 |
| Mg (g kg−1 DM) | 3.37 C | 11.21 A | 5.54 B | 10.01 A | 5.39 B | 0.63 | 0.001 |
| Na (g kg−1 DM) | 0.41 | 0.51 | 0.46 | 0.54 | 0.41 | 0.17 | 0.977 |
| Zn (mg kg−1 DM) | 120.5 A | 11.21 E | 21.16 D | 55.51 B | 45.81 C | 2.39 | 0.001 |
| Cd (mg kg−1 DM) | nd | nd | nd | nd | nd | - | - |
| Fe (mg kg−1 DM) | 229.1 Bbc | 33.7 C | 174.5 Bc | 266.6 ABb | 354.7 Aa | 23.26 | 0.001 |
| Cu (mg kg−1 DM) | 16.26 A | 6.68 CDd | 5.24 D | 12.27 B | 8.84 Cc | 0.60 | 0.001 |
| Ni (mg kg−1 DM) | 0.75 D | 0.32 E | 1.41 B | 1.07 C | 2.21 A | 0.07 | 0.001 |
| Pb (mg kg−1 DM) | 0.60 | nd | nd | nd | nd | - | - |
| Mn (mg kg−1 DM) | 81.14 Bbc | 67.26 Bc | 75.09 Bbc | 86.42 Bb | 113.74 Aa | 6.21 | 0.004 |
| Cr (mg kg−1 DM) | 3.45 A | 3.34 A | 2.97 A | 1.75 B | 1.35 B | 0.27 | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gannuscio, R.; Cardamone, C.; Vastolo, A.; Lucia, C.; D’Amico, A.; Maniaci, G.; Todaro, M. Ensiling as a Conservation Technique for Opuntia ficus indica (L.) By-Products: Peel and Pastazzo. Animals 2024, 14, 3196. https://doi.org/10.3390/ani14223196
Gannuscio R, Cardamone C, Vastolo A, Lucia C, D’Amico A, Maniaci G, Todaro M. Ensiling as a Conservation Technique for Opuntia ficus indica (L.) By-Products: Peel and Pastazzo. Animals. 2024; 14(22):3196. https://doi.org/10.3390/ani14223196
Chicago/Turabian StyleGannuscio, Riccardo, Cinzia Cardamone, Alessandro Vastolo, Caterina Lucia, Angela D’Amico, Giuseppe Maniaci, and Massimo Todaro. 2024. "Ensiling as a Conservation Technique for Opuntia ficus indica (L.) By-Products: Peel and Pastazzo" Animals 14, no. 22: 3196. https://doi.org/10.3390/ani14223196
APA StyleGannuscio, R., Cardamone, C., Vastolo, A., Lucia, C., D’Amico, A., Maniaci, G., & Todaro, M. (2024). Ensiling as a Conservation Technique for Opuntia ficus indica (L.) By-Products: Peel and Pastazzo. Animals, 14(22), 3196. https://doi.org/10.3390/ani14223196








