Prognostic Significance of Microvessel Density and Hypoxic Markers in Canine Osteosarcoma: Insights into Angiogenesis and Tumor Aggressiveness
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Clinical Data
2.2. Histopathology
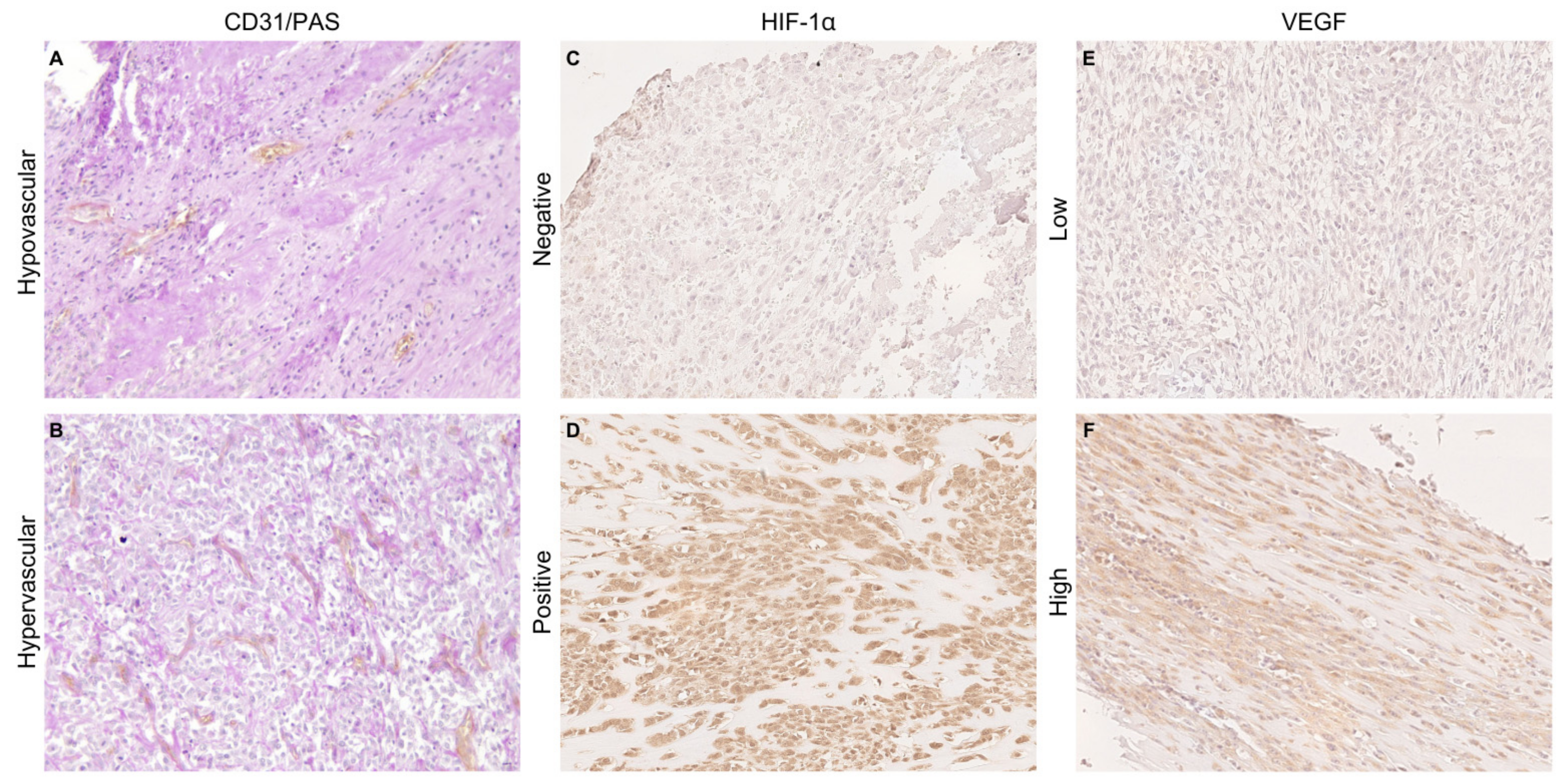
2.3. Hypoxic Markers Immunohistochemistry
2.4. Microvessel Density Measurement
2.5. Statistical Analyses
3. Results
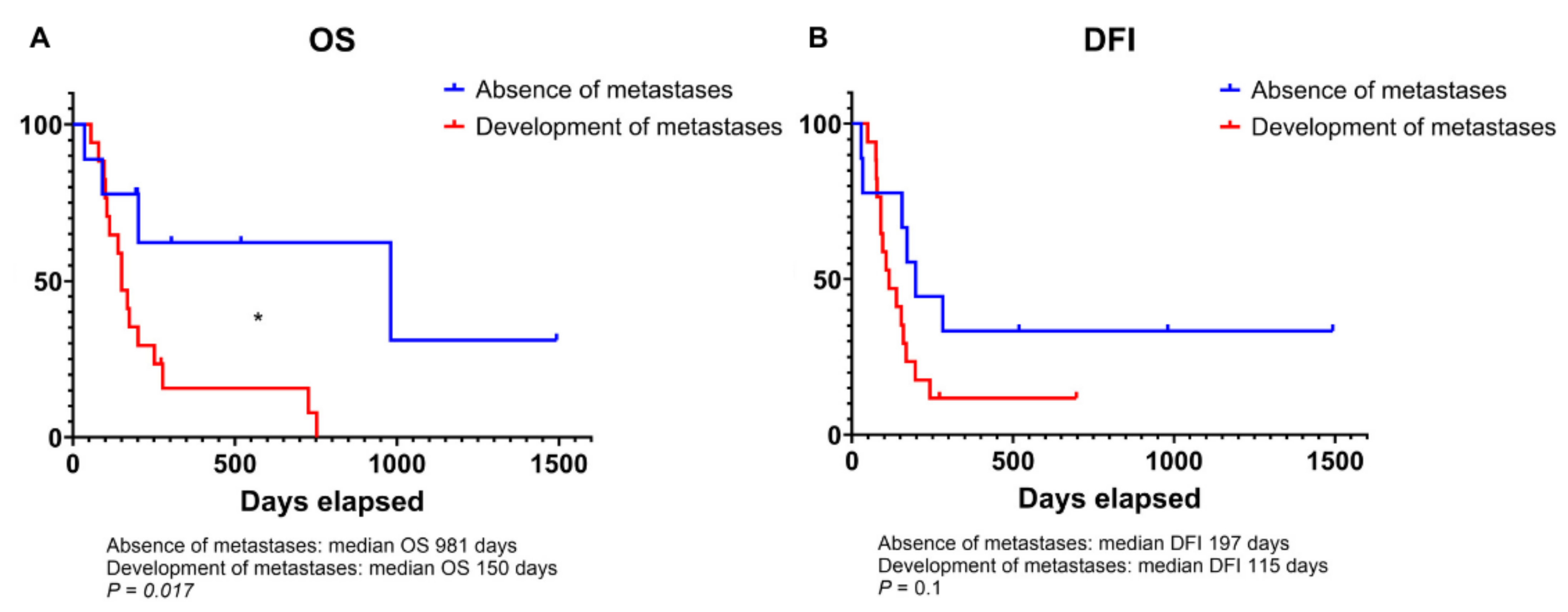
3.1. Clinicopathological Characteristics, and Survival Outcomes in Dogs with Osteosarcoma
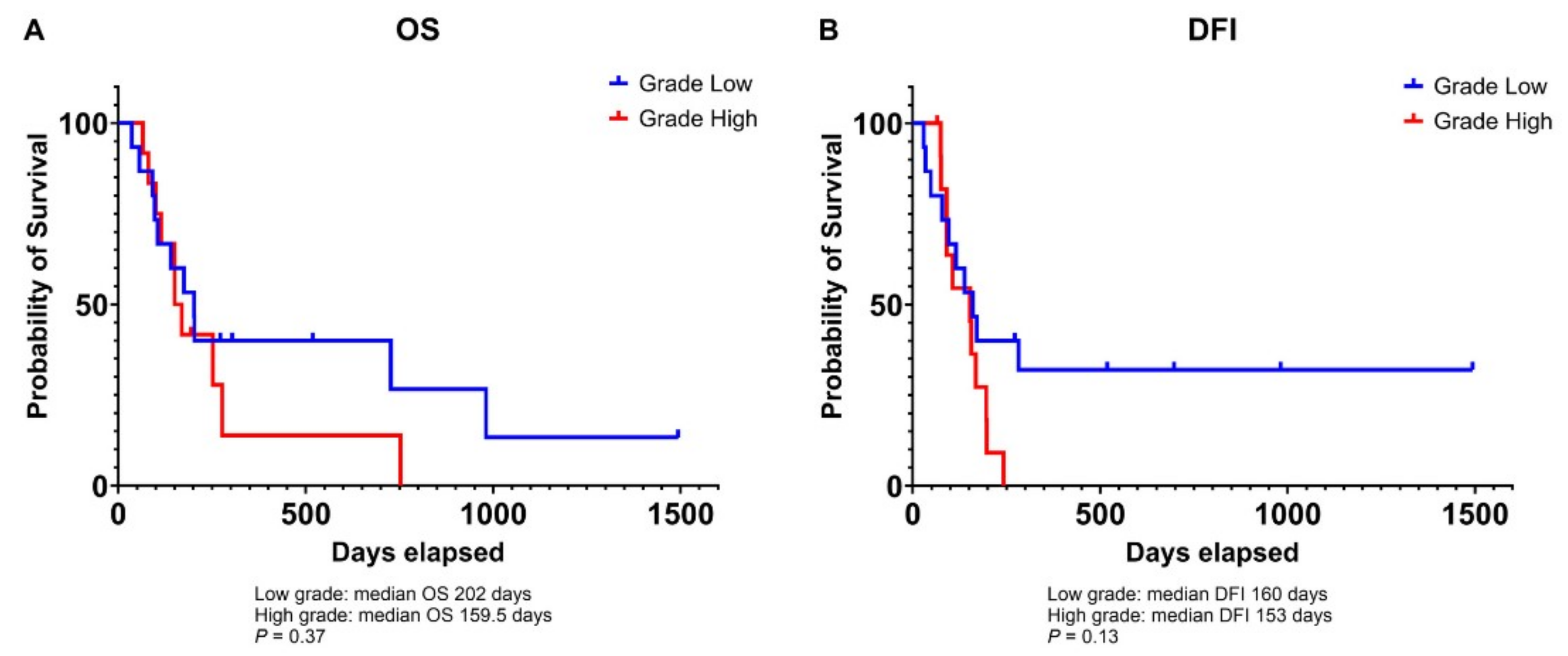
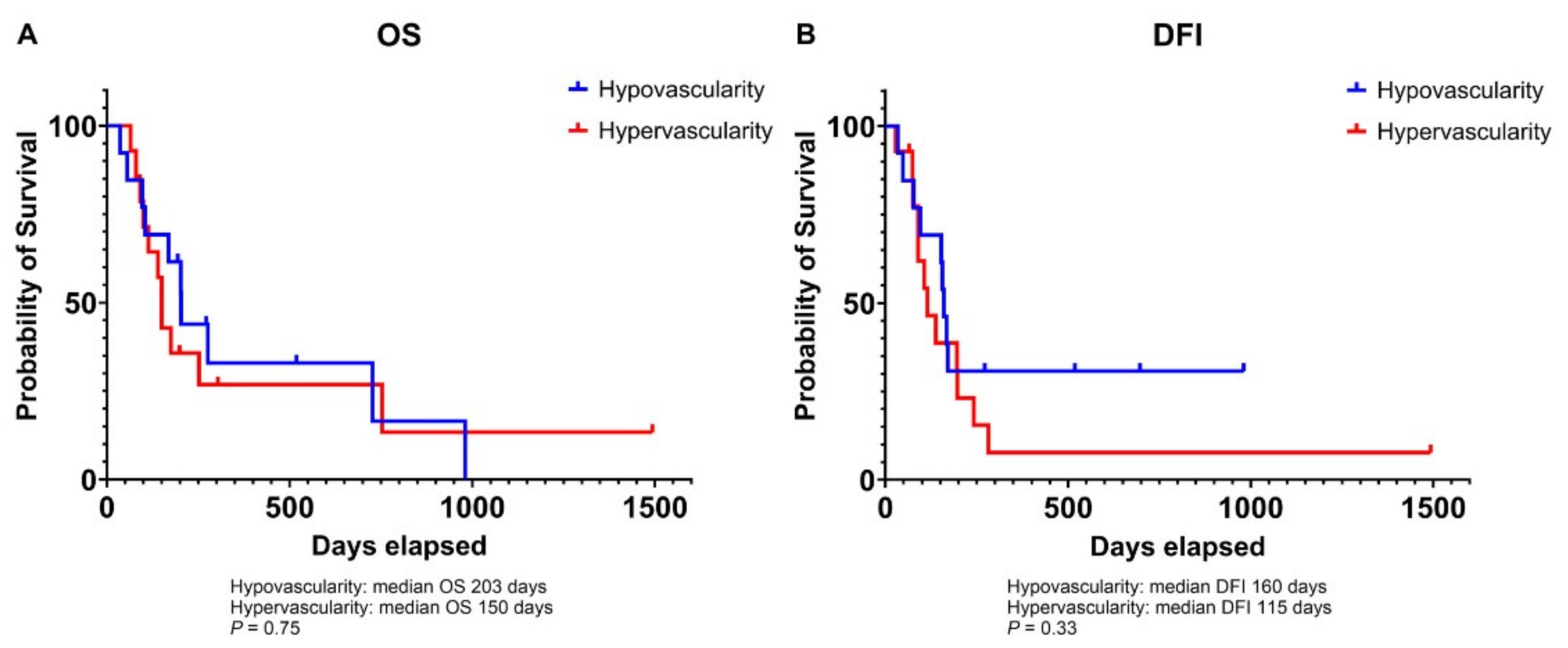
3.2. Association of Microvessel Density with Tumor Grade in Canine Osteosarcoma
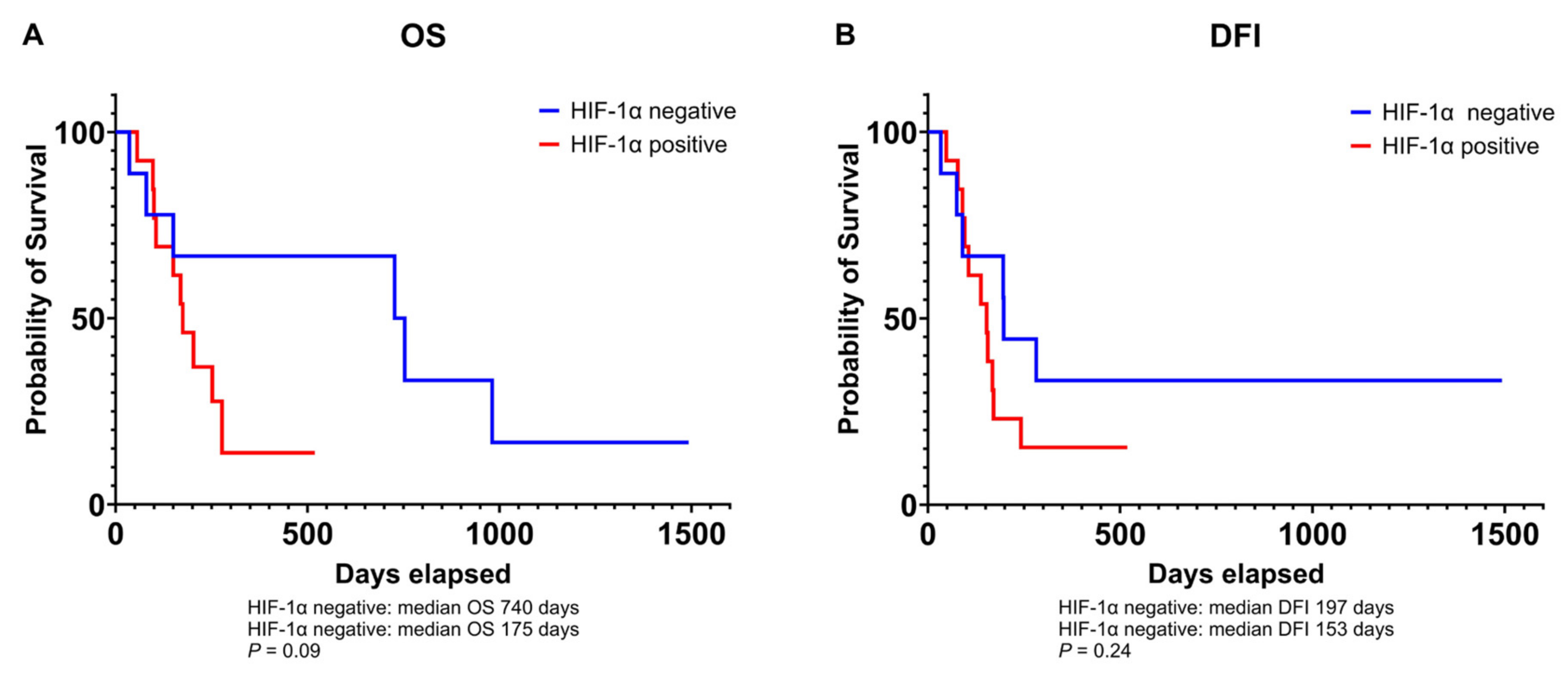
3.3. Relationship Between VEGF and HIF-1α Expression and Prognosis in Canine Osteosarcoma
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Antibody | Staining | ||
|---|---|---|---|
| HIF-1α | Negative = only cytoplasmatic staining Positive = cytoplasmatic and nuclear staining | ||
| Antibody | % of positive cells | Intensity | Total score [% × Int] |
| VEGF-A | 0 = 0–10% 1 = 10–30% 2 = 31–50% 3 = 51–75% 4 = >75% | 0 = negative 1 = yellow 2 = light brown 3 = dark brown | Low (1) = 0–8 High (2) = 9–12 |
References
- Mizobuchi, H.; García-Castellano, J.M.; Philip, S.; Healey, J.H.; Gorlick, R. Hypoxia markers in human osteosarcoma: An exploratory study. Clin. Orthop. Relat. Res. 2008, 466, 2052–2059. [Google Scholar] [CrossRef] [PubMed]
- Schito, L.; Semenza, G.L. Hypoxia-Inducible Factors: Master Regulators of Cancer Progression. Trends Cancer 2016, 2, 758–770. [Google Scholar] [CrossRef] [PubMed]
- Wicks, E.E.; Semenza, G.L. Hypoxia-inducible factors: Cancer progression and clinical translation. J. Clin. Investig. 2022, 132, e159839. [Google Scholar] [CrossRef] [PubMed]
- Ozel, I.; Duerig, I.; Domnich, M.; Lang, S.; Pylaeva, E.; Jablonska, J. The Good, the Bad, and the Ugly: Neutrophils, Angiogenesis, and Cancer. Cancers 2022, 14, 536. [Google Scholar] [CrossRef]
- Liu, Z.L.; Chen, H.H.; Zheng, L.L.; Sun, L.P.; Shi, L. Angiogenic signaling pathways and anti-angiogenic therapy for cancer. Signal Transduct. Target. Ther. 2023, 8, 198. [Google Scholar] [CrossRef] [PubMed]
- Bui, B.P.; Nguyen, P.L.; Lee, K.; Cho, J. Hypoxia-Inducible Factor-1: A Novel Therapeutic Target for the Management of Cancer, Drug Resistance, and Cancer-Related Pain. Cancers 2022, 14, 6054. [Google Scholar] [CrossRef]
- Kreuter, M.; Bieker, R.; Bielack, S.S.; Auras, T.; Buerger, H.; Gosheger, G.; Jurgens, H.; Berdel, W.E.; Mesters, R.M. Prognostic relevance of increased angiogenesis in osteosarcoma. Clin. Cancer Res. 2004, 10, 8531–8537. [Google Scholar] [CrossRef]
- Maiborodin, I.; Mansurova, A.; Chernyavskiy, A.; Romanov, A.; Voitcitctkii, V.; Kedrova, A.; Tarkhov, A.; Chernyshova, A.; Krasil’nikov, S. Cancer Angiogenesis and Opportunity of Influence on Tumor by Changing Vascularization. J. Pers. Med. 2022, 12, 327. [Google Scholar] [CrossRef]
- Nishida, N.; Yano, H.; Nishida, T.; Kamura, T.; Kojiro, M. Angiogenesis in cancer. Vasc. Health Risk Manag. 2006, 2, 213–219. [Google Scholar] [CrossRef]
- Hoff, P.M.; Machado, K.K. Role of angiogenesis in the pathogenesis of cancer. Cancer Treat. Rev. 2012, 38, 825–833. [Google Scholar] [CrossRef]
- Hasan, J.; Byers, R.; Jayson, G.C. Intra-tumoural microvessel density in human solid tumours. Br. J. Cancer 2002, 86, 1566–1577. [Google Scholar] [CrossRef] [PubMed]
- Luong, R.H.; Baer, K.E.; Craft, D.M.; Ettinger, S.N.; Scase, T.J.; Bergman, P.J. Prognostic Significance of Intratumoral Microvessel Density in Canine Soft-Tissue Sarcomas. Vet. Pathol. 2006, 43, 622–631. [Google Scholar] [CrossRef] [PubMed]
- Preziosi, R.; Sarli, G.; Paltrinieri, M. Prognostic value of intratumoral vessel density in cutaneous mast cell tumors of the dog. J. Comp. Pathol. 2004, 130, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Sakalauskaitė, S.; Riškevičienė, V.; Šengaut, J.; Juodžiukynienė, N. Association of mast cell density, microvascular density and endothelial area with clinicopathological parameters and prognosis in canine mammary gland carcinomas. Acta Vet. Scand. 2022, 64, 14. [Google Scholar] [CrossRef] [PubMed]
- Kubo, T.; Shimose, S.; Fujimori, J.; Arihiro, K.; Ochi, M. Diversity of angiogenesis among malignant bone tumors. Mol. Clin. Oncol. 2013, 1, 131–136. [Google Scholar] [CrossRef][Green Version]
- Perivoliotis, K.; Ntellas, P.; Dadouli, K.; Samara, A.A.; Sotiriou, S.; Ioannou, M.; Tepetes, K. Microvessel Density (MVD) in Patients with Osteosarcoma: A Systematic Review and Meta-Analysis. Cancer Investig. 2024, 42, 104–114. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, L.; Xiong, C.; Zhao, R.; Liang, H.; Luo, X. The role of vascular endothelial growth factor as a prognostic and clinicopathological marker in osteosarcoma: A systematic review and meta-analysis. J. Orthop. Surg. Res. 2021, 16, 738. [Google Scholar] [CrossRef] [PubMed]
- Coomber, B.L.; Denton, J.; Sylvestre, A.; Kruth, S. Blood vessel density in canine osteosarcoma. Can. J. Vet. Res. 1998, 62, 199–204. [Google Scholar]
- Gola, C.; Iussich, S.; Noury, S.; Martano, M.; Gattino, F.; Morello, E.; Martignani, E.; Maniscalco, L.; Accornero, P.; Buracco, P.; et al. Clinical significance and in vitro cellular regulation of hypoxia mimicry on HIF-1α and downstream genes in canine appendicular osteosarcoma. Vet. J. 2020, 264, 105538. [Google Scholar] [CrossRef]
- Simpson, S.; Dunning, M.D.; de Brot, S.; Grau-Roma, L.; Mongan, N.P.; Rutland, C.S. Comparative review of human and canine osteosarcoma: Morphology, epidemiology, prognosis, treatment and genetics. Acta Vet. Scand. 2017, 59, 71. [Google Scholar] [CrossRef]
- Makielski, K.M.; Mills, L.J.; Sarver, A.L.; Henson, M.S.; Spector, L.G.; Naik, S.; Modiano, J.F. Risk Factors for Development of Canine and Human Osteosarcoma: A Comparative Review. Vet. Sci. 2019, 6, 48. [Google Scholar] [CrossRef] [PubMed]
- Romanucci, M.; De Maria, R.; Morello, E.M.; Della Salda, L. Editorial: Canine osteosarcoma as a model in comparative oncology: Advances and perspective. Front. Vet. Sci. 2023, 10, 1141666. [Google Scholar] [CrossRef] [PubMed]
- Slayter, M.V.; Boosinger, T.R.; Pool, R.R.; Dammrich, K.; Misdorp, W.; Larsen, S. Histological Classification of Bone and Joint Tumors of Domestic Animals; WHO International Classification of Tumors of Domestic Animals; Armed Forces Institute of Pathology: Washington, DC, USA, 1994. [Google Scholar]
- Loukopoulos, P.; Robinson, W.F. Clinicopathological relevance of tumour grading in canine osteosarcoma. J. Comp. Pathol. 2007, 136, 65–73. [Google Scholar] [CrossRef]
- Schott, C.R.; Tatiersky, L.J.; Foster, R.A.; Wood, G.A. Histologic Grade Does Not Predict Outcome in Dogs with Appendicular Osteosarcoma Receiving the Standard of Care. Vet. Pathol. 2018, 55, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Weidner, N.; Semple, J.P.; Welch, W.R.; Folkman, J. Tumor angiogenesis and metastasis--correlation in invasive breast carcinoma. N. Engl. J. Med. 1991, 324, 1–8. [Google Scholar] [CrossRef]
- Vermeulen, P.B.; Gasparini, G.; Fox, S.B.; Toi, M.; Martin, L.; McCulloch, P.; Pezzella, F.; Viale, G.; Weidner, N.; Harris, A.L.; et al. Quantification of angiogenesis in solid human tumours: An international consensus on the methodology and criteria of evaluation. Eur. J. Cancer 1996, 32, 2474–2484. [Google Scholar] [CrossRef]
- Spiliopoulos, K.; Peschos, D.; Batistatou, A.; Ntountas, I.; Papoudou-Bai, A.; Zioga, A.; Agnantis, N.; Kitsos, G. Immunohistochemical Study of Vasculogenic Mimicry and Angiogenesis in Melanocytic Tumors of the Eye and the Periocular Area. Anticancer Res. 2017, 37, 1113–1120. [Google Scholar]
- Chen, Z.; Han, F.; Du, Y.; Shi, H.; Zhou, W. Hypoxic microenvironment in cancer: Molecular mechanisms and therapeutic interventions. Signal Transduct. Target. Ther. 2023, 8, 70. [Google Scholar] [CrossRef]
- Avallone, G.; Rasotto, R.; Chambers, J.K.; Miller, A.D.; Behling-Kelly, E.; Monti, P.; Berlato, D.; Valenti, P.; Roccabianca, P. Review of Histological Grading Systems in Veterinary Medicine. Vet. Pathol. 2021, 58, 809–828. [Google Scholar] [CrossRef]
- Broadhead, M.L.; Clark, J.C.; Myers, D.E.; Dass, C.R.; Choong, P.F. The molecular pathogenesis of osteosarcoma: A review. Sarcoma 2011, 2011, 959248. [Google Scholar] [CrossRef]
- Magnussen, A.L.; Mills, I.G. Vascular normalisation as the stepping stone into tumour microenvironment transformation. Br. J. Cancer 2021, 125, 324–336. [Google Scholar] [CrossRef] [PubMed]
- Ek, E.T.; Ojaimi, J.; Kitagawa, Y.; Choong, P.F. Does the degree of intratumoural microvessel density and VEGF expression have prognostic significance in osteosarcoma? Oncol. Rep. 2006, 16, 17–23. [Google Scholar] [CrossRef] [PubMed]
- de Queiroz, G.F.; Dagli, M.L.; Fukumasu, H.; Zavala, A.A.; Matera, J.M. Vascular endothelial growth factor expression and microvascular density in soft tissue sarcomas in dogs. J. Vet. Diagn Investig. 2010, 22, 105–108. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, N.; Liao, S.; Rothzerg, E.; Yao, F.; Li, Y.; Wood, D.; Xu, J. Current research progress in targeted anti-angiogenesis therapy for osteosarcoma. Cell Prolif. 2021, 54, e13102. [Google Scholar] [CrossRef]
- Kolb, A.D.; Bussard, K.M. The Bone Extracellular Matrix as an Ideal Milieu for Cancer Cell Metastases. Cancers 2019, 11, 1020. [Google Scholar] [CrossRef] [PubMed]
- Oda, Y.; Yamamoto, H.; Tamiya, S.; Matsuda, S.; Tanaka, K.; Yokoyama, R.; Iwamoto, Y.; Tsuneyoshi, M. CXCR4 and VEGF expression in the primary site and the metastatic site of human osteosarcoma: Analysis within a group of patients, all of whom developed lung metastasis. Mod. Pathol. 2006, 19, 738–745. [Google Scholar] [CrossRef]
- Chang, W.H.; Lai, A.G. The hypoxic tumour microenvironment: A safe haven for immunosuppressive cells and a therapeutic barrier to overcome. Cancer Lett. 2020, 487, 34–44. [Google Scholar] [CrossRef]
- Zhao, H.; Wu, Y.; Chen, Y.; Liu, H. Clinical significance of hypoxia-inducible factor 1 and VEGF-A in osteosarcoma. Int. J. Clin. Oncol. 2015, 20, 1233–1243. [Google Scholar] [CrossRef]
- Al-Ostoot, F.H.; Salah, S.; Khamees, H.A.; Khanum, S.A. Tumor angiogenesis: Current challenges and therapeutic opportunities. Cancer Treat. Res. Commun. 2021, 28, 100422. [Google Scholar] [CrossRef]
- Wilk, S.S.; Zabielska-Koczywąs, K.A. Molecular Mechanisms of Canine Osteosarcoma Metastasis. Int. J. Mol. Sci. 2021, 22, 3639. [Google Scholar] [CrossRef]

| Age (years) | Mean | 8.4 |
| Median | 9 | |
| Range | 2–13 | |
| Gender, n (%) | Female | 13 (46.4) |
| Male | 15 (53.6) | |
| Breed, n (%) | Crossbreed | 10 (35.71) |
| Boxer | 3 (10.72) | |
| Rottweiler | 3 (10.72) | |
| Labrador Retriever | 2 (7.14) | |
| German Shepherd | 2 (7.14) | |
| Czechoslovakian Wolfdog | 2 (7.14) | |
| Other breeds | 6 (21.43) | |
| Weight (kg) 1 | Mean | 34.3 |
| Median | 34 | |
| Range | 7.5–55 | |
| Localization, n (%) 2 | Forelimb | 16 (59.26) |
| Hindimb | 11 (40.74) | |
| Development of metastases, n (%) 1 | No | 9 (34.62) |
| Yes | 17 (65.38) | |
| Follow-up (days) 3 | Mean | 249 |
| DFI | Median | 153 |
| Range | 29–1493 | |
| Mean | 293 | |
| OS | Median | 175 |
| Range | 36–1493 | |
| Histological type, n (%) | Osteoblastic OSA | 18 (64.29) |
| Chondroblastic OSA | 4 (14.28) | |
| Poorly differentiated OSA | 3 (10.72) | |
| Giant-cell OSA | 1 (3.57) | |
| Fibroblastic OSA | 1 (3.57) | |
| Telangiectatic OSA | 1 (3.57) | |
| Grading, n (%) | Low-grade | 15 (53.57) |
| High-grade | 13 (46.43) |
| Variable | Hypovascular MVD ≤ 16 n (%) | Hypervascular MVD > 16 n (%) | Fischer’s Exact Test p-Value | MVD Median (n) | Mann–Whitney Test p-Value |
|---|---|---|---|---|---|
| Sex | 0.15 | 0.06 | |||
| Male | 4 (14.29) | 9 (32.14) | 23.33 (13) | ||
| Female | 9 (32.14) | 6 (21.43) | 16 (15) | ||
| Tumor grade | 0.029 | 0.026 | |||
| Low | 10 (35.71) | 5 (17.86) | 15 (15) | ||
| High | 3 (10.72) | 10 (35.71) | 19 (13) | ||
| Development of metastases 1 | 1 | 0.48 | |||
| No | 5 (19.23) | 4 (15.38) | 16 (9) | ||
| Yes | 8 (30.77) | 9 (34.62) | 17 (17) | ||
| HIF-1α | 0.25 | 0.66 | |||
| Negative | 3 (10.72) | 7 (25) | 18 (10) | ||
| Positive | 10 (35.71) | 8 (28.57) | 16.17 (18) | ||
| VEGF expression | 1 | 0.81 | |||
| Low | 7 (25) | 9 (32.14) | 17 (16) | ||
| High | 6 (21.43) | 6 (21.43) | 15.67 (12) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gola, C.; Massimini, M.; Morello, E.; Maniscalco, L.; Conti, L.C.; Romanucci, M.; Olimpo, M.; Della Salda, L.; De Maria, R. Prognostic Significance of Microvessel Density and Hypoxic Markers in Canine Osteosarcoma: Insights into Angiogenesis and Tumor Aggressiveness. Animals 2024, 14, 3181. https://doi.org/10.3390/ani14223181
Gola C, Massimini M, Morello E, Maniscalco L, Conti LC, Romanucci M, Olimpo M, Della Salda L, De Maria R. Prognostic Significance of Microvessel Density and Hypoxic Markers in Canine Osteosarcoma: Insights into Angiogenesis and Tumor Aggressiveness. Animals. 2024; 14(22):3181. https://doi.org/10.3390/ani14223181
Chicago/Turabian StyleGola, Cecilia, Marcella Massimini, Emanuela Morello, Lorella Maniscalco, Luiza Cesar Conti, Mariarita Romanucci, Matteo Olimpo, Leonardo Della Salda, and Raffaella De Maria. 2024. "Prognostic Significance of Microvessel Density and Hypoxic Markers in Canine Osteosarcoma: Insights into Angiogenesis and Tumor Aggressiveness" Animals 14, no. 22: 3181. https://doi.org/10.3390/ani14223181
APA StyleGola, C., Massimini, M., Morello, E., Maniscalco, L., Conti, L. C., Romanucci, M., Olimpo, M., Della Salda, L., & De Maria, R. (2024). Prognostic Significance of Microvessel Density and Hypoxic Markers in Canine Osteosarcoma: Insights into Angiogenesis and Tumor Aggressiveness. Animals, 14(22), 3181. https://doi.org/10.3390/ani14223181





