Simple Summary
Partial traumatic brachial plexus injury due to road traffic accidents is one of the most common and challenging disorders requiring neurorehabilitation in cats. The implementation of early intensive neurorehabilitation, including electrical stimulation with specific parameters and physical activity, may contribute to faster sensory-motor recovery and resumption of ambulation, possibly avoiding amputation of the affected limb.
Abstract
This prospective observational cohort pilot study included 22 cats diagnosed with partial traumatic brachial plexus injury (PTBPI), aiming to explore responses to an early intensive neurorehabilitation protocol in a clinical setting. This protocol included functional electrical stimulation (FES), locomotor treadmill training and kinesiotherapy exercises, starting at the time with highest probability of nerve repair. The synergetic benefits of this multimodal approach were based on the potential structural and protective role of proteins and the release of neurotrophic factors. Furthermore, FES was parametrized according to the presence or absence of deep pain. Following treatment, 72.6% of the cats achieved ambulation: 9 cats within 15 days, 2 cats within 30 days and 5 cats within 60 days. During the four-year follow-up, there was evidence of improvement in both muscle mass and muscle weakness, in addition to the disappearance of neuropathic pain. Notably, after the 60 days of neurorehabilitation, 3 cats showed improved ambulation after arthrodesis of the carpus. Thus, early rehabilitation, with FES applied in the first weeks after injury and accurate parametrization according to the presence or absence of deep pain, may help in functional recovery and ambulation, reducing the probability of amputation.
1. Introduction
The brachial plexus is a complex anatomic structure that is formed from a network of ventral roots of the spinal cord segments C6 to T2; in cats, it is located between the intervertebral foramen and the thoracic limb [1].
Partial traumatic brachial plexus injury (PTBPI) is one of the most common peripheric nervous system lesions that affects the brachial plexus in cats [2,3,4], most commonly due to road traffic accidents [5]. This type of lesion may occur due to excessive traction of the thoracic limb or severe abduction of the scapula [5,6,7].
The diagnosis of PTBPI is usually based on history, clinical signs and findings of both clinical and neurological examinations [2,6]. However, electrodiagnostic assessment can be useful as a precise tool for identifying damaged nerves through the resulting compound muscle action potentials (CMAPs), which are a reflection of muscle force in normal and re-innervated muscles [8]. These tests have already been used in cats and may be sufficient to localize injury of the brachial plexus, but they are still not sufficient to pinpoint the exact location of specific injured nerves [9].
Previous studies based on nerve conduction and electromyograms (EMG), which are not invasive, showed that these tools may have a delayed diagnostic role but are not best used immediately after injury, being more effective, for example, six weeks later, when fibrillations in the de-innervated muscles occur [10,11,12]. In addition, magnetic resonance imaging has been described as a possible complementary exam to identify brachial plexus masses [9].
Seddon (1943) [13] was the first to implement a classification system for nerve injuries, with three categories based on the presence of demyelination and the extent of damage to the axons and/or the connective tissues around the nerve [14,15]. Thus, since 1947, nerve injuries have been classified as follows: neuropraxia, which involves focal demyelination without damage to the axons and/or connective tissues and is mostly due to mild compression or nerve traction, leading to a transitory interruption or decrease in velocity conduction and muscle weakness, with recovery possible in three to six weeks; axonotmesis (crush injury), which involves direct damage to the axons and focal demyelination, while maintaining the continuity of nervous connective tissue, with recovery possible in 12–18 months; and neurotmesis, which involves a full transection of the axons and connective tissue [10,13,14,15,16,17].
In human medicine, a more aggressive approach, with acute repair, leads to better functional recovery; the preference is to avoid the “wait and see” approach, which can be much more costly [10,18]. Additionally, early nerve repair may result in improved functional outcomes, with less muscle fibrosis and secondary atrophy, as atrophy begins soon after denervation. However, the success rate may depend on various factors, such as the time between injury and surgery, the distance from the injury site to the target muscle, location of the injury (i.e., nerve root avulsion) and the regeneration rate. Thus, the time needed for re-innervation of sensory receptors is much longer than that needed for motor nerves, but better outcomes can be achieved with early repair [10].
PTBPI is mostly seen in cats, particularly as a result of road traffic collisions, when an impact force promotes excessive medial or caudal movement of the limb. Lesions between C8–T1 are the most common; however, C6–C7 can also be involved [5].
Clinical signs include a gait alteration compatible with proximal radial nerve paralysis, inability to support weight and inability to extend the carpus/digits, resulting in pain and dragging of the limb on the floor [3,19]. In addition, the elbow can be ventrally positioned, resulting in a dropped elbow posture. This change in positioning is due to shoulder plexus paralysis, specifically, paralysis of the latissimus dorsi and triceps long head, which are innervated by the caudal roots of the plexus [1,19,20].
Avulsion of the C8 rootlets affects the cutaneous trunci reflex on that side, regardless of the intensity of sensory stimulation. If the cranial roots of the plexus are preserved (C6-C7), with the musculocutaneous nerve intact, the elbow stays in a flexed position, resulting in a lameness score of five [1,3,19,20].
Cases of total brachial plexus avulsion may result in an absence of superficial pain distal to the elbow and the lateral surface of the limb. However, if the cranial portion of the brachial plexus is preserved, this pain can be absent only distal to the elbow, with sensation on the medial side of the thoracic limb intact [1,3,20].
Electrical stimulation (ES) is a treatment modality for peripheral nerve injury that can allow reinnervation of affected muscles and enhance functional outcomes. This treatment modality is well accepted in cats [21], and outcomes can be improved by daily stimulation. The underlying mechanism involves upregulation of intramuscular neurotrophic factors, which may be essential for long-term changes in the functional muscle. An understanding of this mechanism has already changed the paradigm in rats [22,23,24].
A therapeutic approach based on ES aims to improve post-traumatic neuromuscular recovery and can be applied either to the damaged nerve [25,26,27] or the denervated muscles [28,29,30], as was already tried in rat models of axonotmesis [25,31,32,33]. The degree of tissue healing is highly variable [20,34,35], but early neurorehabilitation protocols may help to improve the outcome.
In human medicine, neurorehabilitation protocols for treatment of denervated muscles based on single sessions of ES in combination with physical exercise, muscle stretching and passive movements lead to enhanced contraction without muscle fatigue [25,29].
Research in animal models (i.e., rats), also revealed that applying low-intensity ES directly to the nerve through implanted electrodes increased functional and morphological regeneration of the nerve, possibly via a delay in axonal degeneration, stimulation of nerve sprouting and regeneration of the myelin sheath [23,36]. Thus, low-frequency stimulation can increase the number of myelinated fibers and the axon density and result in a higher ratio of blood vessels to total nerve area compared to the values seen in non-stimulated and injured nerves. Furthermore, other studies have shown that ES is a strong delayer of degeneration in denervated muscle [25,37] and regulates molecular alterations in skeletal muscles [24,29].
The main effects of ES are still under investigation, and there is a need to elucidate how different frequencies may affect the regeneration of nerve fibers.
Treatment of PTBPI can be conservative, surgical or mixed. Conservative management can be based on functional neurorehabilitation and prevention of the self-mutilation and secondary wounds that can be associated with carpal arthrodesis, with the aim of preventing contracture and loss of carpus functionality [7,38,39,40].
The first aim of this study was to describe recovery from PTBPI in cats after implementation of a mixed multidisciplinary protocol based on neurorehabilitation, including functional electrical stimulation (FES), in a clinical rehabilitation center. The second aim was to assess various parameters of FES according to the presence or absence of deep pain perception (DPP). Finally, another aim was to evaluate recovery under the intensive neurorehabilitation protocol (INRP) over time and define the limitations of this protocol.
2. Material and Methods
This prospective observational cohort pilot study in cats with PTBPI was performed at the Arrábida Animal Rehabilitation Center (CRAA, Portugal) between 2016 and 2023 after approval by the Lusófona Veterinary Medicine Faculty Ethics committee, with signed consent from the cats’ owners.
2.1. Participants
All of the test subjects were domestic cats (n = 22) with previous traumatic injury (only from road traffic accidents) with acute non-progressive presentation that had been diagnosed with PTBPI. All had to present the following inclusion criteria: monoplegia with inability to support weight and fifth-degree lameness; shoulder extension and inability to extend the carpus and digits; presence of absence of ipsilateral hemiplegia (Figure 1); absent peripheral reflexes; and presence of absence of the cutaneous trunci reflex.
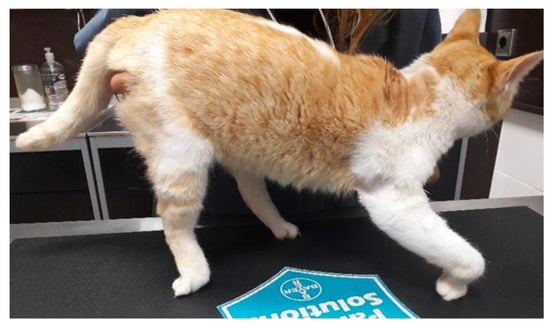
Figure 1.
Cat with ipsilateral monoplegia and proprioceptive deficits, showing knuckling posture.
In addition, subjects were required to have an absence of superficial pain distal to the elbow and lateral surface of the thoracic limb, while the medial side of the paw could have either absent or decreased pain perception. Deep pain could be decreased, present or absent. Lastly, all subjects had to show decreased triceps brachialis muscle tonus and could also present with Horner’s syndrome.
Additionally, to be selected, all cats had to have computed tomography or resonance imaging exams through which the brachial plexus injury was localized to a caudal lesion (C8, T1, T2). No conduction studies, EMG, functional tests (i.e., force plates) or nerve biopsies were conducted to evaluate the progress of the lesion.
Exclusion criteria encompassed all other diseases that can affect the brachial plexus, such as inflammatory, infectious or neoplastic disease and other trauma lesions with different clinical presentations.
2.2. Study Design
All 22 cats with PTBPI were referred to the rehabilitation center three to seven days after trauma and were enrolled in this prospective observational cohort study following hemodynamic stabilization.
All had received a previous emergency-setting consultation in different hospitals, where they underwent stabilization involving restrictive resuscitation fluid therapy, blood work and point-of-care ultrasound. Upon admission to the rehabilitation center, all cats presented with a normal mental state, heart rate, respiratory rate, blood pressure and temperature. The neurorehabilitation examination involved the assessment of the following: passive standing posture; spinal reflexes of the thoracic limb (withdrawal reflex and extensor carpi radialis reflex); and palpation of all muscles that are innervated by the caudal brachial plexus nerves (Table 1), with specific palpation of the medial region of the thoracic limb, as described in Figure 2.

Table 1.
Muscles innervated by the caudal brachial plexus nerves.
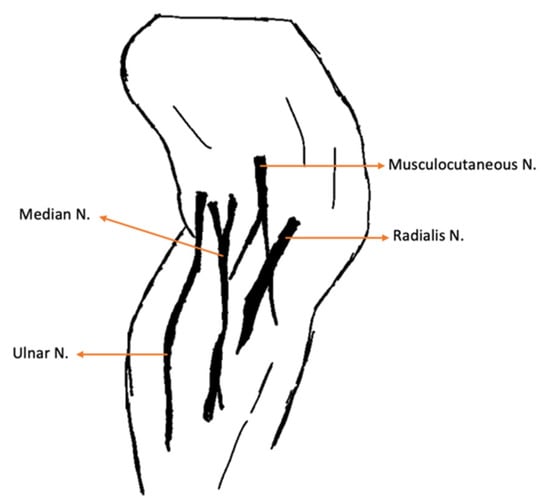
Figure 2.
Medial aspect of the thoracic limb. N.: Nerve.
Key points of the neurorehabilitation exam used to assess C8-T2 injury are described in Table 2 [1,20].

Table 2.
Key points of the brachial plexus neurorehabilitation examination [1,20].
The study design necessitated a specific examination of the brachial plexus region, given that EMG was not performed as an outcome measure. Therefore, it was critical to assess superficial pain and map the thoracic limb dermatomes to evaluate the presence of deep pain in the first and fifth digits and to monitor for paresthesia by tracking behavior changes, such as biting, licking or self-mutilation. The evaluation of superficial and deep pain was performed with a 12 cm Halsted mosquito forceps, and an ink marker was used to draw the cut-off point of the dermatomes map for further evaluation (Figure 3).
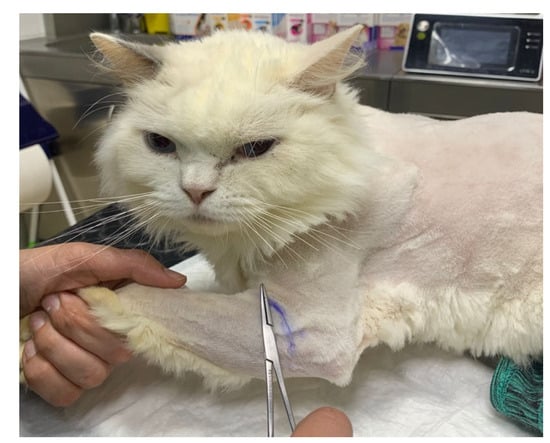
Figure 3.
Cut-off point of the dermatomes map on the thoracic limb, marked with ink for further evaluation.
For these reasons, neurorehabilitation examinations were repeated each week to assess neurological changes. Also, possible iatrogenic outcomes of the protocol, such as burns, pruritus, hyperemia and signs of worsening paresthesia, were evaluated and recorded.
2.3. Procedures
The neurorehabilitation protocol for PTBPI had multiple aims: (a) to maintain/restore the joint’s range of motion and neuromuscular function, preventing pain, trauma and self-mutilation [41]; (b) to prevent muscle contractures [39,42] and joint contractures, maintaining the elbow and carpus flexion/extension end-feel [43]; (c) to minimize neurogenic atrophy, promoting muscle strengthening and muscle flexibility; (d) to reduce the possibility of paresthesia and promote recovery of sensation [44]; (e) to achieve ambulation and improve quality of life.
All cats (n = 22) were subjected to the same early INRP. Procedures took place six times per day in a controlled and quiet environment in the cat´s rehabilitation room, with different modalities, such as laser therapy and ultrasounds, followed by a 30 min window in which to perform the electrotherapy protocols (functional electrostimulation of the radial nerve) in association with locomotor training, according to each patient´s cardiorespiratory condition and phase of neurological recovery.
All animals were hospitalized and were clinically discharged when they achieved ambulation, although the maximum time given to this study´s protocol was two months. Furthermore, all cats had their nails cut and wore Elizabethan collars to prevent self-mutilation, and wore harnesses during the administration of the protocol. Positive reinforcement was given in the form of treats, toys or play with other friendly cats. Modalities and exercises were executed by three veterinarians, a certified canine rehabilitation practitioner (CCRP) and two CCRP students. All evaluations were performed by a veterinarian diplomate of the European College of Veterinary Sports Medicine and Rehabilitation with a PhD in neurorehabilitation.
2.3.1. Intensive Neurorehabilitation Protocol (INRP)
1st–3rd Week of INRP
In the first three weeks the protocol was similar (Table 3). It was essential to avoid active movements with limb stretching, and the main focus was to increase depolarization of the radial, median and ulnar nerves. This aim was achieved using class IV laser therapy (LiteCure Companion Therapy Laser®, New Castle, DE, USA), which was selected for its regenerative properties, applied to the anatomic pathway of the nerves. In addition, a 4-point technique using the same laser was applied to the carpal, elbow, and shoulder joints, promoting analgesia, anti-inflammatory effects, and edema reduction [45].
Additionally, the ultrasound modality (BTL-4820 Smart®, Hertfordshire, UK) was prescribed to promote neural depolarization [46], beyond its primary role in muscle relaxation and ligament flexibility. This treatment aimed to reduce secondary muscle contractures [47] and allow the use of passive range-of-motion exercises for the shoulder, elbow and carpal joints (Figure 4). These exercises could be repeated 4–6 times/day (10–30 sets per session), finishing with manipulation of all digits, individually or jointly [48].
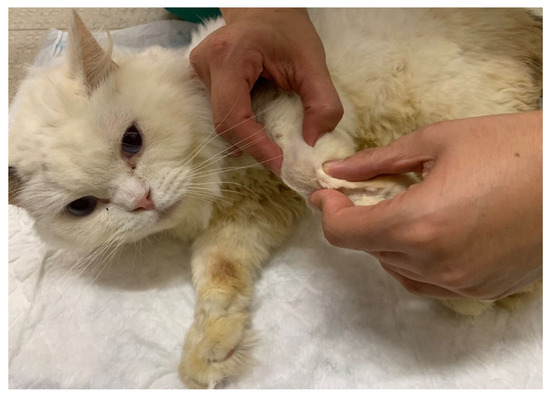
Figure 4.
Passive range-of-motion exercises on the carpal joint of a cat.
FES (BTL-4820 Smart®, Hertfordshire, UK) was implemented based on the anatomic pathway of the radial nerve, with one electrode placed to stimulate the C6, C7, C8, T1 and T2 nerve roots and another placed in the motor point of the triceps brachialis muscle (Figure 5). Electrical parameters were selected according to DPP guidelines (Table 3).
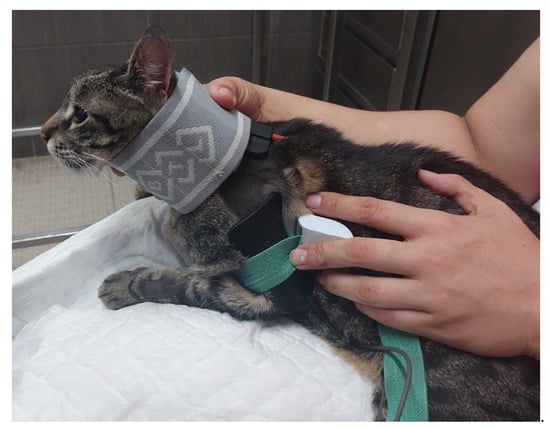
Figure 5.
Functional electrical stimulation of the radial nerve (one electrode placed to stimulate the C6, C7, C8, T1 and T2 nerve roots and another placed in the motor point of the triceps brachialis muscle).
All rehabilitation modalities and exercises were immediately followed by the application of a corrective carpal splint (Buster Leg Splint, Kruuse, Denmark) with vet-wrap tape (Vet-Flex, Kruuse, Denmark), which was placed for 2 h in the morning and afternoon (Figure 6) to prevent joint contracture.

Figure 6.
Corrective carpal splint. (A) Material for the splint. (B) Splint applied to the carpus of a cat.

Table 3.
Intensive neurorehabilitation protocol in the 1st, 2nd and 3rd weeks.
Table 3.
Intensive neurorehabilitation protocol in the 1st, 2nd and 3rd weeks.
| Rehabilitation Modality/Exercise | Parameters | Implementation |
|---|---|---|
| Laser therapy (A) | 18–22 J/cm2 Class IV laser Radial nerve pathway; SID | Regenerative |
| 5–10 J/cm2 Class IV laser 4-point joint technique; SID (Shoulder, Elbow, Carpus) | Analgesia, Anti-inflammatory effects | |
| FES of the radial nerve (B) | 30–40 Hz; 6–16 mA; 200 µs [49] Trapezoidal modulation 1:4 duty cycle 2–4 s ramp up; 8 s plateau; 1–2 s ramp down; 10 min; TID | Deep pain |
| 30–40 Hz; 6–24 mA; 200 µs [49] Trapezoidal modulation 1:4 or 1:5 duty cycle 2–4 s ramp up; 8 s plateau; 1–2 s ramp down; 10 min; TID | No deep pain | |
| Range-of-motion exercises (C) | 10–30 sets 4–6 times/day | All joints: shoulder, elbow, carpal and digits |
| Postural standing position (D) | 2–3 min 4–6 times/day | |
| Ultrasound (E) | 1 MHz; 1.5 w/cm2; 10 min Pulsed mode; Duty cycle of 20%; Pulse ratio 1:4; 5 cm transducer head | Muscles: triceps brachialis; biceps brachialis; extensor carpi radialis; lateral and common digital extensor |
FES: functional electrical stimulation; J: Joules; Hz: hertz; mA: milliamperes; s: seconds; MHz: megahertz.
Regarding frequency, the sequence B + C+ D (Table 3) was repeated three times daily, whereas the other modalities were performed only once a day.
ES on the pelvic limb was also performed to promote distal depolarization if the cat showed signs of proprioceptive deficits in the ipsilateral pelvic limb. This therapy included the sciatic nerve FES, with one electrode placed to stimulate the L7-S1 nerves and the other on the motor point of the flexor muscle group (50 Hz; 6–24 mA for cats without deep pain; 6–14 mA for cats with deep pain), as well as stimulation of the peroneal nerve branch (one electrode placed to stimulate nerves L7-S1 and the other on the dorsal region of the paw, with the same parameters) (Figure 7).
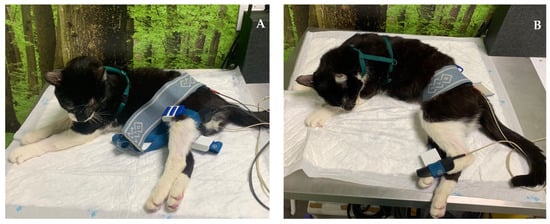
Figure 7.
Functional electrical stimulation of the hindlimb. (A) Sciatic nerve stimulation (one electrode placed to stimulate L7-S1 and the other on the motor point of the flexor muscle group); (B) stimulation of the peroneal nerve branch (one electrode placed on L7-S1 and the other on the dorsal region of the paw).
Between the second and third week, if a cat recovered joint motion of the elbow, stimulation of the distal radial nerve was performed, aiming to improve mobility of the carpal joint and reduce the possibility of contracture. Thus, radial nerve FES was performed for carpal extension, with one electrode at C7, C8, T1 and T2 and the other one on the dorsal region of the paw (Figure 8).
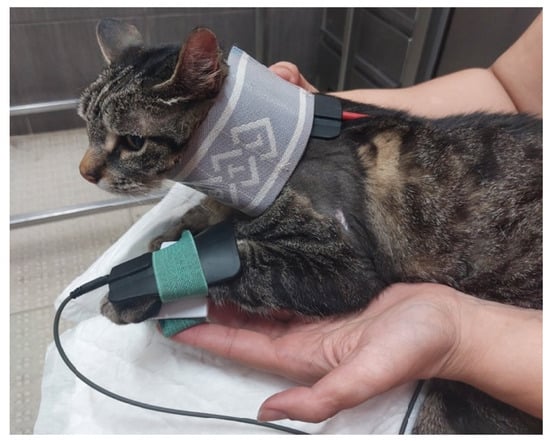
Figure 8.
Functional electrical stimulation of the radial nerve for carpal extension (one electrode at C7, C8, T1 and T2 and the other one on the dorsal region of the paw).
4th–6th Week of INRP
The laser therapy program continued, along with the radial nerve FES (both triceps muscle and extension of the carpus), range-of-motion exercises, postural standing, ultrasounds and, in some cases, FES of the pelvic limb. All these therapies were followed by locomotor training on the land treadmill (10–20 min), with bicycle movements performed by a CCRP veterinarian or nurse. This training was repeated twice a day, six days/week. Bicycle movements were performed without limb stretching and with direct contact between the digits and the treadmill belt to stimulate mechanoreceptors and proprioceptors (Figure 9). There was no incline, and the speed was increased until it reached 1.8 km/h.
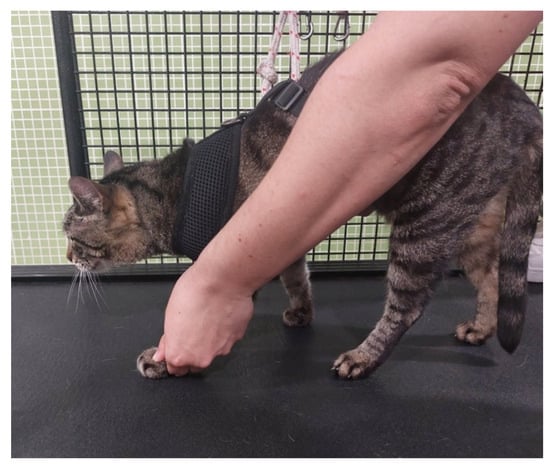
Figure 9.
Locomotor training on a land treadmill, with bicycle movements performed on the affected limb.
Around the 4th week, when the possibility of neural regeneration increased, underwater treadmill (UWTM) training was introduced and undertaken once a day for a maximum of 10 min, with the water level at the lateral epicondyle of the femur, at a temperature of 24–26 °C, with no incline and a maximum speed of 2.2 km/h (Figure 10). UWTM training was performed after the rehabilitation modalities of laser, ultrasound and FES, allowing physiological muscle contraction based on the agonist/antagonist rule.

Figure 10.
Locomotor training on the underwater treadmill, with bicycle movements performed on the affected limb.
In this exercise, as in all types of exercises, the cats had to be in a controlled, calm and quiet environment and were motivated by positive reinforcements. The rehabilitator was inside the UWTM, performing rhythmic bicycle movements.
Furthermore, in this neurological phase, postural standing exercises on top of a balance board were added (2 min, 4 min rest), followed by walking on different floor surfaces (2 min, 6 min rest) and stepping over cavaletti rails (1–2 min), always with the corrective splint applied in order to facilitate elbow flexion.
If cats developed wounds, cleaning was performed at the end of the day with a 3% chlorhexidine solution and class IV laser therapy was done to stimulate granulation tissue [50].
6th–8th Week of INRP
The same protocol was carried out, including components A + B + C + D + E (Table 3), with the addition of locomotor training on the land treadmill with a 10% incline and the application of a TheraBand to increase muscle strength and reduce neurogenic atrophy. The aim was to carry out these therapies for 30 min, twice a day; however, for patients that could not sustain this prolonged training, a comprehensive circuit was substituted, with the following components: stimulation while standing on a central pad (2 min), rest (2 min), walking on different floor types (2 min), rest (2 min), walking up/down stairs (2 min), rest (2 min), walking up/down ramps (2 min), rest (2 min), stepping over cavaletti poles (2 min), rest (2 min), walking in figures of eight, rest (2 min) and, lastly, free time (playing with other cats or with toys).
In the last two weeks, the frequency of application of modalities such as laser and ultrasound were reduced to every 48 h and the laser was applied only to the interphalangeal and carpal regions of the joints. Additionally, the frequency of FES protocols was reduced to once a day, 5–6 times/week.
Neuropathic Pain
In addition, cats with signs of neuropathic pain were given pharmacological support in the form of pregabalin (2 mg/kg BID) for four weeks, which was then decreased to SID for the next four weeks. If any of these cats showed signs of pain, such as excessive licking, interferential transcutaneous electrical nerve stimulation (TENS) was prescribed for the carpal and digits regions. This technique used two different channels crossing each other at a 90◦ angle: firstly, two channels at 80–150 Hz, 0.5–1 mA, pulse duration 50 µs for 10 min; secondly, two channels at 1–10 Hz, 0.5–1 mA, pulse duration 150 µs for 10 min [51].
2.4. Data Collection
Data collected from the 22 cats included all variables mentioned in Table 4.

Table 4.
Description and categorization of variables for statistical analysis.
At the follow-up time points the muscle mass (improved or not) and muscle weakness (improved or not) were also evaluated. The cats were considered ambulatory when they could rise and take at least 10 consecutive weight-bearing steps unassisted and without falling [52,53].
2.5. Statistical Analyses
Data were recorded using Microsoft Office Excel 365® (Microsoft Corporation, Redmond, WA, USA) and processed in IBM SPSS Statistics 25® (International Business Machines Corporation, Armonk, NY, USA) software. A Shapiro–Wilk normality test (for n < 50), arithmetic means, median, mode, variance, standard deviation (SD), minimum, maximum and standard error of mean (SEM), were calculated and recorded for the continuous variables age and weight. Descriptive statistics with frequency analysis were calculated for all categorical nominal variables. Chi-square tests were also performed to verify relevant relationships, which were considered significant at a p-value < 0.05.
3. Results
Out of 22 individuals observed in this study, 68.2% were males and 31.8% were females. The mean age and weight of the individuals treated were 4.86 and 4.73 kg, respectively (Table 5).

Table 5.
Characterization of study population (n = 22) at time of admission.
Only 86% of the cats tested positive for dermatomes; the remaining 14% tested negative (Table 5). Nevertheless, all the cats had dermatomes between the elbow and carpus and between the carpus and digits.
Regarding the presence of dermatomes at T0, there was no significant relationship with ambulation at discharge; however, the recovery of dermatomes during rehabilitation was significantly associated with ambulation recovery [X2 (1, n = 22) = 9.263, p = 0.002]. Of the 72.7% of cats that recovered ambulation, all had positive dermatomes at medical discharge. These same 16 cats recovered movement of all three joints during the INRP: 11 within 30 days, 3 cats within 30–45 days and only 2 within 45–60 days.
The evaluation of deep pain at T0 revealed the absence of DPP in the first four digits in 45.5% of the cats, while the remaining 54.5% had doubtful DPP. The relationship between DPP at admission and ambulation achievement was highly significant [X2 (1, n = 22) = 9.900, p = 0.002], with all cats with doubtful DPP achieving ambulation.
Deep pain in the fifth digit was present in 40.9% and doubtful in 59.1% and showed no significant relationship with ambulation recovery. However, a significant relationship [X2 (1, n = 22) = 4.701, p = 0.030] was observed regarding development of carpal contractures, with most contractures seen on cats with doubtful DPP in the 5° digit (n = 9).
Additionally, a strong relationship between the development of carpal contractures and time until medical discharge [X2 (1, n = 22) = 22.000, p < 0.001] was found, with all cats that presented this clinical sign being discharged at 60 days.
Among all treated cats, 72.7% achieved ambulation, whereas 27.3% did not recover. For these 16 cats, the median time to ambulation was 30 days, with 11 cats being medically discharged at day 30 and 5 cats discharged at day 60.
In total, 50% of the cats were medically discharged at day 30, and all were ambulatory, recovering DPP by 15 days (n = 9) or between 15 and 30 days (n = 2). All showed the knuckling position during the INRP, which was followed by total recovery of reflexes, dermatomes and joint movement, with absence of carpal contracture, although there was diminished extension of the carpal joint.
Of the remaining 50% that were medically discharged by day 60, only 5 cats recovered total DPP and developed a knuckling position followed by ambulation, although all presented positive changes in their reflexes and the development of carpal contractures. There were six cats with incomplete recovery of DPP but with improvement in the fifth digit, and three of them recovered all dermatomes with joint movement until the elbow. These cats were treated with surgical arthrodesis. The other three recovered dermatomes only between the shoulder and the elbow and movement through the elbow, with amputation of the limb on later follow up (2 cats in F3 and 1 cat in F4).
Regarding neuropathic pain, 18.2% cats showed this clinical sign at admission. Later during the INRP, 50% cats showed development of neuropathic pain by day 15, which was associated with the development of carpal contractures [X2 (1, n = 22) = 18.333, p < 0.001]. These cats showed progressive improvement, and only six cats still showed this sign at medical discharge, these being the same cats that later required surgical arthrodesis or amputation.
The clinical problems that arose during the study period were wounds on the dorsal region of the digits that occurred due to neurological deficits and were recorded in 40.9% of the cats. No iatrogenic clinical problems were observed as a consequence of the INRP, including burns, pruritus or hyperemia.
Horner syndrome was diagnosed in 13.6 % of the cats, with total recovery in two cats by day 30, one that achieved ambulation and one needing arthrodesis. The cats that had not recovered from the Horner syndrome by day 60 ultimately required limb amputation at F3.
On follow up, there was an evident improvement in both muscle mass and muscle weakness (Figure 11). However, three cats showed only a slight improvement on the first follow up (3 months), with continued neuropathic pain. These cats later required limb amputation.
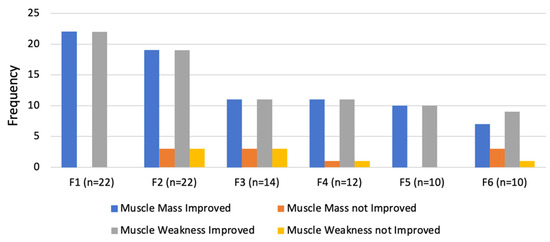
Figure 11.
Evaluation of outcomes regarding muscle mass and muscle weakness in the follow-up consultations. F1: 3 months; F2: 6 months; F3:1 year; F4: 2 years; F5: 3 years; F6: 4 years.
Compared to the contralateral limb, the affected limbs always had less muscle mass and more muscle weakness at all follow up visits until one year (F3). However, after two years (F4), seven of the cats showed no difference between the limbs, an improvement that was maintained until four years after treatment (F6) with resolution of neuropathic pain.
4. Discussion
In this study, 22 cats were referred to the neurorehabilitation center after PTBPI diagnosis due to road traffic accidents, which are common in both human patients and small animals [5,14,15,16,54]. All had acute non-progressive injury of the peripheral nervous system, and the aim was to begin nerve repair early to improve functional outcomes [10,55] and avoid muscle fibrosis and neurogenic atrophy. Our INRP implementation timeline was similar to those used in human medicine and began earlier than the median time of 14–13 days after injury that is described by Menchetti et al. (2020) [54]. Furthermore, the same study revealed a median duration of 60 days until recovery, with owners reporting improvement in five cats. By comparison, in our study, these 22 cats had a high probability of axonotmesis (equivalent to Sunderland grade II–IV) and the median duration until ambulation was half that seen in the previous study (30 days).
Protocols in human medicine are usually based on the beneficial role of locomotor training, which is one of the best approaches to treat peripheral nerve injury when administered in conjunction with other physical exercises and modalities, such as ES. The volume and intensity of training, with standardized and correctly selected frequencies of ES, can improve functionally and can be translated to veterinary medicine in the spirit of the “one health” concept [56,57,58,59].
Although differences can be seen in recovery according to age due to the decrease in depolarization and concomitant problems associated with older cats, there was no significant relationship between the final functional outcome and age in this study. This result can probably be explained by the fact that our sample of younger cats were similar in age, with a mean age of 4.86 (±0.467) years. The same was observed with weight, with a mean of 4.73 kg (SEM 0.239), and with sex and breed.
Many factors can influence functional recovery following nerve injury. Beyond the time elapsed between trauma and reparative treatment, there is the distance from the injury site to the target muscle and the regeneration rate, which is usually around 1 mm per day. The combination of a long distance and slow regeneration can result in atrophy of both the distal nerve and the effector muscles, which may make functional recovery impossible. Also, on admission (T0), 45.5% of cats were without DPP in the first four digits and 54.5% had doubtful DPP, an important fact given that the literature points to the absence of DPP as the single neurologic sign most strongly associated with the need for amputation of the affected limb [54,60,61].
The present study was performed in a rehabilitation clinical setting and was strictly guided by the neurorehabilitation treatment of these cats, with the aim of achieving functionality of the limb and ambulation (performing 10 consecutive steps without muscle weakness and falling). On admission, all of the cats had absent or doubtful DPP, making nerve biopsies a risk factor for neurological deterioration, that could compromise future functionality. This risk was the main reason that owners chose not to authorize the procedure.
Total ambulation recovery was 72.7% after the implementation of INRP, with a median time of 30 days: 11 cats discharged at day 30 and 5 cats at day 60. Thus, time to recovery was shorter than that previously described [52], although one cat recovered at the rate described by Santifort (2016) [62]. However, we cannot completely exclude the possibility that this fast recovery rate was due to spontaneous regeneration.
Sensorimotor evaluation with dermatomes recovery showed a clear, significant association with ambulation throughout the study (p = 0.002) and was related to resolution of joint contracturesm with 11 cats recovering shoulder, elbow, and carpus motion within 30 days. Therefore, these results illustrate that it is essential to avoid muscle and joint contractures, maintaining limb mobility with conservative treatment [62,63,64,65].
In our results, there was a significant relationship between DPP and ambulation (p = 0.002): all cats with doubtful DPP became ambulatory. This result may be related to the conservative nature of the INRP treatment, which ensured protection of the injury region, avoiding further damage to nerve structures with pain control and management of sensory deficits in the first 15 to 21 days. The ES programs can be used as an adjunctive tool with early implementation, with stimulation administered according to the neurological evaluation and range of motion of the joint in the affected limb.
Early recovery of DPP in the fifth digit showed a significant relationship with ambulation (p = 0.03), and 9 cats that developed carpus contractures due to faster re-innervation of the flexor muscles than of the extensor muscles exhibited a persistent knuckling position while walking [66,67]. Experimental studies on rats with peripheral lesions on the sciatic nerve suggest the implementation of passive range-of-motion and assisted active exercises, such as the use of a 45° net inside the cage to avoid prolonged muscle inactivation associated with incorrect positioning [68,69,70].
In human medicine, several non-surgical approaches, including pharmacological, electrical, cell-based and laser therapies have been used to promote re-myelination and enhance recovery in diseases of the peripheral nervous system [17,71,72,73,74]. The implementation of ES, particularly between the second and third week, may help muscles achieve passive contraction, promote normal electromyographic waves during treatment [75], reduce muscle atrophy and promote muscle reinnervation by increasing expression of structural protective proteins and neurotrophic factors [74].
Various authors [76,77,78] have suggested that it is possible to mimic the physiological wave of Ca2+ influx that generates a retrograde signal leading to subsequent activation of autonomous cellular mechanisms and thus initiating regeneration [79,80]. FES may in this way help to promote upregulation of BDNF, neurotrophins and TrK receptors [81,82], as well as glial-cell-like derived neurotrophic factor (GDNF) [74,83,84,85]. Additionally, studies have demonstrated earlier outgrowth of axons and Schwann cells, with accelerated reconnection to the target injury site. This result may be related to cellular mechanisms that increase the production of adenosine monophosphate with Ca2+ influx, regulating BDNF and TrkB expression [26,79,86,87,88].
In the present study, 50% of the cats recovered ambulation by day 30, but all showed progressive sensorimotor improvement. The cats required different ES programs, adapted to each case and their neurological evolution (i.e., presence of knuckling position). For example, FES for the extensor muscles of the carpus with a precise trapezoid current and parameters adjusted to DPP was used to generate an action potential strong enough for nerve depolarization and consequent muscle contraction [89]. Also, accurate placement of electrodes is crucial for opposing the low current that makes physiological contraction impossible under these conditions [90]. Cats with evident neurogenic atrophy need more time to recover; to avoid muscle fatigue in these cats, the programmed duty cycle should be 1:5, with a ramp-down time of 1–2 sec [91,92]. The trapezoid modulation, which is a triangular current with a duration of 200 μs, has shown better effects on denervated muscles than have other modulations [74,93].
Thus, in this study, FES was conducted with specific parameters and methods according to the daily neurological evaluation of each cat and consideration of the fact that high frequencies of ES can aggravate nerve damage [74,94], causing fatigue and neuropathic pain.
The authors cannot state that the obtained outcomes are a direct result of the implementation of FES; however, a multimodal approach with ES and locomotor training may have had a fundamental role in their recovery. It would be interesting to assess recovery, specifically, the extension movement of the carpus, with functional measures (i.e., kinematic and kinetic analysis), which would allow evaluation of progress in muscle strength. However, as this was a clinical study, limb functionality was evaluated using neurological examination, the dermatomes map and behavioral changes indicating possible paresthesias.
The INRP, which included ES and treadmill training, aimed to achieve synergetic BDNF upregulation [29,95,96,97,98]. Furthermore, the intensive locomotor exercise started after 21 days, when neural regeneration was higher, preventing muscle neurogenic atrophy and promoting improvement in strength [75,76]. After six weeks, the start of stretching maneuvers allowed to the cats to maintain muscular flexibility [75,99], and passive stretching promoted stimulation of mechanoreceptors, slowing protein degradation [100] and increasing the range of motion of the elbow and carpus. Among the other 50% of cats medically discharged at day 60, five recovered DPP and ambulation of the injured limb. However, six showed improved DPP only on the fifth digit, and three had dermatomes recovery with complete movement of the elbow, a perfect scenario for performing an arthrodesis. This treated improves functionality and quality of life and decreases neuropathic pain.
Some authors suggest that recovery after PTBPI requires more than three months (from 3 to 12 months) [17,54]. Our results showed that only three of the cats did not recover dermatomes in the forearm with joint movement to the elbow. These cats showed ongoing neuropathic pain and presented with wounds and discomfort throughout the long-term follow-ups. Ultimately, 13.6% of the cats required limb amputation, in agreement with [54,60].
In human patients with brachial plexus injury, neuropathic pain is described in 30 to 80% [101,102] and has a highly refractory presentation [103,104,105,106,107]. In our sample population, at admission, 18.2% of the cats had this clinical sign, increasing to 50% during the first 15 days. This pain was probably associated with carpal contracture (p < 0.001), and in 40.9%, it was related to wounds on the dorsal region of the digits that arose due to their knuckling position. Pain management involved pregabalin administration, and resolution of contracture and knuckling improved the outcomes.
In this investigation, there was high variability in sensory patterns and sympathetic innervation consistent with the evolution of Horner syndrome, as well as weakness. In some cases, these symptoms followed a positive trajectory. During the long-term follow ups, seven cats showed improvements in muscle mass and muscle weakness at F4 (two years). These improvements were sustained at the four-year follow-up, and these cats did not develop neuropathic pain.
Interferential TENS treatments can also be associated with progressive improvement in neuropathic pain, given their analgesic effects [75,108]; they can be used to control allodynia and hyperalgesia, according to the type of injury and the probability of recovery [75,109]. In addition, to treat pain, inflammation and edema, the INRP included class IV laser therapy, which acts via the upregulation of nitric oxide. Nitric oxide, like other free radicals that result from lipidic peroxidation of the central and peripheric nervous system, is associated with cell necrosis and has an especially important role on neuropathic pain compared to other human medicine peripheral neuropathies (e.g., diabetic polyneuropathy, multiple sclerosis, stroke) [101,110].
In our study, there were no observations of signs related to possible phantom limb pain, which is reported to occur in 54–85% of amputees in human medicine [98,111,112,113]. This phenomenon, interpreted as a result of cortical reorganization [109], is estimated to happen in veterinary patients within the first two years after amputation and can persist throughout life in up to 10% [114,115,116]. However, this phenomenon was not observed on long-term follow-ups up to four years for the three cats that underwent amputation. Additionally, no iatrogenic effects, including burns, pruritus or hyperemia, were observed during the INRP.
The main limitation of this study was the absence of EMG studies that can localize injuries and provide functional information and follow-up of the re-innervation process, although these studies require specific timing. Such timing is difficult to coordinate, mostly because, in axonal lesions, reduction of CMAPs requires several days, while electrical stimulation distal to the injury can be normal and signs of Wallerian degeneration take at least two to three weeks to appear. Subsequent research endeavors should prioritize the inclusion of EMG procedures as an integral component of INRP monitoring and early injury classification. As this was a clinical study, biopsies were considered an invasive procedure and were not allowed by the owners. However, this was a preliminary pilot study to verify the possibility of an intensive neurorehabilitation approach and was safe, repeatable and feasible. The intervention resulted in functional recovery, based on the neurorehabilitation examination. This study opens the possibility for future investigations with kinematic, kinetic and superficial electromyographic outcome measures.
The small sample size is another limitation that resulted from the strict criteria for inclusion, which were needed to reduce selection bias. Additionally, there was no control group, as the study was carried out in a clinical rehabilitation center. Thus, it is important to mention that these findings should be interpreted with caution.
5. Conclusions
Early INRP may have a role in promoting ambulation in cats diagnosed with partial traumatic brachial plexus injury and can be applied in a safe and repeatable way.
Appropriate FES parameters, depending on DPP, can be essential to improving sensory-motor recovery by allowing expression of structural protective proteins and neurotrophic factors. The strength of these effects increases with the synergic effect of locomotor training, which in this study, started after 21 days. This pilot study achieved 72.7% ambulation, with a median time of 30 days, although one must always consider the possibility of spontaneous neural reorganization. This investigation should be continued with further studies that include specific outcome measures, such as electromyography.
Author Contributions
Conceptualization Â.M.; methodology D.G.; validation Â.M., A.F. and A.C.M.; formal analysis, Â.M., A.F., A.S.P.V. and A.C.M.; investigation D.G., A.C. (Ana Cardoso), C.C., I.R., Ó.G. A.A., B.L., P.S., A.C. (André Coelho), M.M.B. and R.A.; writing—original draft preparation D.G.; writing—review and editing Â.M, A.F., A.S.P.V., R.A., A.C.M. and A.J.S.; supervision Â.M., A.C.M. and A.J.S.; project administration Â.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Prémios Santa Casa Neurociencias–Prize Melo e Castro for Spinal Cord Injury Research (MC-04/17; MC-18-2021). The author Rui D. Alvites acknowledges the Centro de Estudos de Ciência Animal (CECA), Instituto de Ciências, Tecnologias e Agroambiente (ICETA), Porto University (UP), and Fundação para a Ciência e Tecnologia (FCT) for the funding and availability of all technical, structural, and human resources necessary for the development of this work. The work was supported through the project UIDB/00211/2020 funded by FCT/MCTES through national funds. The authors acknowledges FCT for funding the project 2022.04501.PTDC (Olfabionerve—Olfactory Mucosa Mesenchymal Stem Cells and Biomaterials Promoting Peripheral Nerve Regeneration) and the PhD Scholarships Bruna Lopes (2021.05265.BD), Patrícia Sousa (2023.00246.BD), and André Coelho (2023.00428.BD).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Uemura, E.E. Motor system. In Fundamentals of Canine Neuroanatomy and Neurophysiology; Uemura, E.E., Ed.; John Wiley & Sons: Ames, IA, USA, 2015; pp. 257–288. [Google Scholar]
- Troupel, T.; Caenegem, N.V.; Jeandel, A.; Thibaud, J.; Nicolle, A.; Blot, S. Epidemiological, clinical, and electrophysiological findings in dogs and cats with traumatic brachial plexus injury: A retrospectove study of 226 cases. J. Vet. Intern. Med. 2021, 35, 2837–2845. [Google Scholar] [CrossRef] [PubMed]
- De Lahunta, A.; Glass, E.; Kent, M. Lower motor neuron: Spinal nerve, general somatic efferent system. In Veterinary Neuroanatomy and Clinical Neurology, 4th ed.; de Lahunta, A., Glass, E., Klent, M., Eds.; Elsevier: St. Louis, MO, USA, 2014; pp. 102–161. [Google Scholar]
- Wheeler, S.J.; Jones, D.G.; Wright, J.A. The diagnosis of brachial plexus disoreders in dogs: A review of twenty-two cases. J. Small Anim. Pract. 1986, 27, 147–157. [Google Scholar] [CrossRef]
- Soens, I.V.; Struys, M.M.; Polis, I.E.; Bhatti, S.F.; Meervenne, S.A.; Martlé, V.A.; Nollet, H.; Tshamala, M.; Vanhaesebrouck, A.E.; Ham, L.M. Magnetic stimulation of the radial nerve in dogs and cats with brachial plexus trauma: A report of 53 cases. Vet. J. 2009, 182, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, I.R.; Duncan, I.D.; Lawson, D.D. Avulsion of the brachial plexus-2. Clinical aspects. J. Small Anim. Pract. 1974, 15, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, H.S. Brachial plexus injuries and dysfunctions. Vet. Clin. N. Am. Small Anim. Pract. 1988, 18, 565–580. [Google Scholar] [CrossRef] [PubMed]
- Wood, M.D.; Kemp, S.W.; Weber, C.; Borschel, G.H.; Gordon, T. Outcome measures of peripheral nerve regeneration. Ann. Anat. 2011, 193, 321–333. [Google Scholar] [CrossRef] [PubMed]
- Anson, A.; Gil, F.; Laredo, F.G.; Soler, M.; Belda, E.; Ayala, M.D.; Agut, A. Correlative ultrasound anatomy of the feline brachial plexus and major nerves of the thoracic limb. Vet. Radiol. Ultrasound 2013, 54, 185–193. [Google Scholar] [CrossRef]
- Grinsell, D.; Keating, C.P. Peripheral nerve reconstruction after injury: A review of clinical and experimental therapies. Biomed Res. Int. 2014, 2014, 698256. [Google Scholar] [CrossRef]
- Effron, C.R.; Beasley, R.W. Compression neuropathies in the upper limb and electrophysiological studies. In Grabb and Smith’s Plastic Surgery; Thorne, C.H., Bartlett, S.P., Beasley, R.W., Aston, S.J., Gurtner, G.C., Spear, S.L., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2006; p. 86. [Google Scholar]
- Robinson, L.R. Traumatic injury to peripheral nerves. Muscle Nerve 2000, 23, 863–887. [Google Scholar] [CrossRef]
- Seddon, H.J. Three types of nerve injury. Brain 1943, 66, 237. [Google Scholar] [CrossRef]
- Menorca, R.M.; Fussell, T.S.; Elfar, J.C. Peripheral nerve trauma: Mechanisms of injury and recovery. Hand Clin. 2013, 29, 317–330. [Google Scholar] [CrossRef] [PubMed]
- Hussain, G.; Wang, J.; Rasul, A.; Anwar, H.; Qasim, M.; Zafar, S.; Aziz, N.; Razzaq, A.; Hussain, R.; Aguilar, J.; et al. Current status of therapeutic approaches against peripheral nerve injuries: A detailed story from injury to recovery. Int. J. Biol. Sci. 2020, 16, 116–134. [Google Scholar] [CrossRef] [PubMed]
- Houschyar, K.S.; Momeni, A.; Pyles, M.N.; Cha, J.Y.; Maan, Z.N.; Duscher, D.; Jew, O.S.; Siemers, F.; Schoonhoven, J. The role of current techniques and concepts in peripheral nerve repair. Plastic Surg. Int. 2016, 2016, 4175293. [Google Scholar] [CrossRef] [PubMed]
- Modrak, M.; Talukder, M.A.; Gurgenashvili, K.; Noble, M.; Elfar, J.C. Peripheral nerve injury and myelination: Potential therapeutic strategies. J. Neurosci. Res. 2020, 98, 780–795. [Google Scholar] [CrossRef] [PubMed]
- Pfister, B.J.; Gordon, T.; Loverde, J.R.; Kochar, A.S.; Mackinnon, S.E.; Cullen, D.K. Biomedical engineering strategies for peripheral nerve repair: Surgical application, atate of the art, and future challenges. Crit. Rev. Biomed. Eng. 2011, 39, 81–124. [Google Scholar] [CrossRef] [PubMed]
- De Lahunta, A. Feline neurology. Vet. Clin. N. Am. 1976, 6, 433–451. [Google Scholar] [CrossRef] [PubMed]
- Dessal, F. Fundamentos de Neuroanatomia. In Neurologia Felina; Dessal, F., Ed.; Editorial Inter-Médica: Buenos Aires, Argentina, 2020. [Google Scholar]
- Drum, M.G.; Bockstahler, B.; Levine, D.; Marcellin-Little, D.J. Feline rehabilitation. Vet. Clin. N. Am. Small Anim. Pract. 2015, 45, 185–201. [Google Scholar] [CrossRef]
- Willand, M.P. Electrical stimulation enhances reinnervation after nerve injury. Eur. J. Trans. Myol. 2015, 25, 243–248. [Google Scholar] [CrossRef]
- Kao, C.; Chen, J.; Hsu, Y.; Bau, D.; Yao, C.; Chen, Y. High-Frequency electrical stimulation can be a complementary therapy to promote nerve regeneration in diabetic rats. PLoS ONE 2013, 8, e79078. [Google Scholar] [CrossRef]
- Foecking, E.M.; Fargo, K.N.; Coughlin, L.M.; Kim, J.T.; Marzo, S.J.; Jones, K.J. Single session if brief electrical stimulation immeadiately following crush injury enhances functional recovery of rat facial nerve. J. Rehabil. Res. Dev. 2012, 49, 451–458. [Google Scholar] [CrossRef]
- Gigo-Benato, D.; Russo, T.L.; Geuna, S.; Domingues, N.; Salvini, T.F.; Parizotto, N.A. Electrical stimulation impairs early functional recovery and accentuates skeletal muscle atrophy after sciatic nerve crush injury in rats. Muscle Nerve 2010, 41, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Brushart, T.M.; Hoffman, P.N.; Royall, R.M.; Murinson, B.B.; Witzel, C.; Gordon, T. Electrical stimulation promotes motoneuron regeneration without increasing its speed or conditioning the neuron. J. Neurosci. 2002, 22, 6631–6638. [Google Scholar] [CrossRef] [PubMed]
- Brushart, T.M.; Jari, R.; Verge, V.; Rohde., C.; Gordon, T. Electrical stimulation restores the specificity of sensory axon regeneration. Exp. Neurol. 2005, 194, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Evans, G.R. Peripheral nerve injury: A review and approach to tissue engineered constructs. Anat. Rec. 2001, 263, 396–404. [Google Scholar] [CrossRef]
- Marqueste, T.; Alliez, J.R.; Alluin, O.; Jammes, Y.; Decherchi, P. Neuromuscular rehabilitation by treadmill running or electrical stimulation af- ter peripheral nerve injury and repair. J. Appl. Physiol. 2004, 96, 1988–1995. [Google Scholar] [CrossRef] [PubMed]
- Russo, T.L.; Peviani, S.M.; Durigan, J.L.; Salvini, T.F. Electrical stimulation increases matrix metalloproteinase-2 gene expression but does not change its activity in denervated rat muscle. Muscle Nerve 2008, 37, 593–600. [Google Scholar] [CrossRef]
- Varejão, A.S.; Cabrita, A.M.; Meek, M.F.; Bulas-Cruz, J.; Melo-Pinto, P.; Raimondo, S.; Geuna, S.; Giacobini-Robecchi, M.G. Functional and morphological assessment of a standardized rat sciatic nerve crush injury with a non-serrated clamp. J. Neurotrauma 2004, 21, 1652–1670. [Google Scholar] [CrossRef]
- Baptista, A.F.; Gomes, J.R.; Oliveira, J.T.; Santos, S.M.; Vannier-Santos, M.A.; Martinez, A.M. High- and low-frequency transcutaneous electrical nerve stimulation delay sciatic nerve regeneration after crush lesion in the mouse. J. Peripher. Nerv. Syst. 2008, 13, 71–80. [Google Scholar] [CrossRef]
- Kerns, J.M.; Lucchinetti, C. Electrical field effects on crushed nerve regeneration. Exp. Neurol. 1992, 117, 71–80. [Google Scholar] [CrossRef]
- Lundborg, G. Enhancing posttraumatic nerve regeneration. J. Peripher. Nerv. Syst. 2002, 7, 139–140. [Google Scholar] [CrossRef]
- Samii, M.; Carvalho, G.A.; Nikkhah, G.; Penkert, G. Surgical reconstruction of the musculocutaneous nerve in traumatic brachial plexus injuries. J. Neurosurg. 1997, 87, 881–886. [Google Scholar] [CrossRef]
- Mendonça, A.C.; Barbieri, C.H.; Mazzer, N. Directly applied low intensity direct electric current enhances peripheral nerve regeneration in rats. J. Neurosci. Methods 2003, 129, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Dow, D.E.; Cederna, P.S.; Hassett, C.A.; Kostrominova, T.Y.; Faulkner, J.A.; Dennis, R.G. Number of contractions to maintain mass and force of a denervated rat muscle. Muscle Nerve 2004, 30, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Denny, H.R.; Butterworth, S.J. Peripheral nerve injury. In A Guide to Canine and Feline Orthopaedic Surgery; Denny, H.R., Butterworth, S.J., Eds.; Blackwell Science Lds.: Oxford, UK, 2000; pp. 201–205. [Google Scholar]
- Añor, S. Monoparesis. In BSAVA Manual of Canine and Feline Neurology; Platt, S., Olby, N., Eds.; Brithish Small Animal Veterinary Association: Cheltenahm, UK, 2013; pp. 328–341. [Google Scholar]
- Levine, D.; Millis, D.L.; Marcellin-Little, D.J.; Taylor, R. (Eds.) Therapeutic exercise and manual therapy. In Reabilitaçāo e Fisioterapia na Prática de Pequenos Animais; Roca: São Paulo, Brasil, 2008; pp. 447–463. [Google Scholar]
- Levine, D.; Millis, D.L.; Marcellin-Little, D.J.; Taylor, R. (Eds.) Physical therapy for specific diagnoses. In Reabilitação e Fisioterapia na Prática de Pequenos Animais; Roca: São Paulo, Brasil, 2008; pp. 609–627. [Google Scholar]
- Shores, A.; Pearce, L. Traumatic and Neoplastic Diseases of the Brachial Plexus. In Mechanisms of Disease in Small Animal Surgery; Bojrab, M.J., Monnet, E., Eds.; Teton New Media Inc.: Jackson, WY, USA, 2010. [Google Scholar]
- Marcellin-Little, D.J.; Levine, D. Principles and application of range of motion and stretching in companion animals. Vet. Clin. N. Am. Small Anim. Pract. 2015, 45, 57–72. [Google Scholar] [CrossRef] [PubMed]
- Knecht, C.D.; Raffe, M.R. Diseases of the Brachial Plexus. In Textbook of Small Animal Orthopaedics; Newton, C.D., Nunamaker, D.M., Eds.; Lippincott: Philadelphia, PA, USA, 1985. [Google Scholar]
- Marcolino, A.M.; Barbosa, R.I.; Fonseca, M.C.; Mazzer, N.; Ellui, V.M. Reabilitação fisioterapêutica na lesaõ do plexo braquial: Relato de caso. Fisioter. Mov. 2008, 21, 53–60. [Google Scholar]
- Monte-Raso, V.V.; Barbieri, C.H.; Mazzer, N.; Fazan, V.P. Os efeitos do ultra- som terapêutico nas lesões por esmagamento do nervo ciático de ratos: Análise funcional da marcha. Rev. Bras. Fisioter. 2006, 10, 113–119. [Google Scholar] [CrossRef]
- Millis, D.L.; Levine, D. Basic science of veterinary rheabilitation. In Canine Rehabilitation and Physical Therapy; Millis, D.L., Levine, D., Eds.; W. B. Saunders Co., Ltd.: Philadelphia, PA, USA, 2014; pp. 79–153. [Google Scholar]
- Bocksthler, B.; Wittek, K. Passive Range of Motion Exercises and Stretching. In Essential Facts of Physical Medicine, Rehabilitation and Sports Medicine in Companion Animals; Bocksthler, B., Wittek, K., Eds.; VBS GmbH: Munich, Germany, 2019; p. 110. [Google Scholar]
- Sawaya, S.G.; Combet, D.; Chanoit, G.; Thiebault, J.J.; Levine, D.; Marcellin-Little, D.J. Assessment of impulse duration thresholds for electrical stimulation of muscles (chronaxy) in dogs. Am. J. Vet. Res. 2008, 69, 1305–1309. [Google Scholar] [CrossRef]
- Gouveia, D.; Fonseca, S.; Carvalho, C.; Cardoso, A.; Almeida, A.; Gamboa, Ó.; Canejo-Teixeira, R.; Ferreira, A.; Martins, Â. Clinical occurrences in the neurorehabilitation of dogs with severe spinal cord injury. Animals 2023, 13, 1164. [Google Scholar] [CrossRef]
- Martins, Â.; Gouveia, D.; Cardoso, A.; Viegas, I.; Gamboa, Ó.; Ferreira, A. A comparison between body weight-supported treadmill training and conventional over-ground training in dogs with incomplete spinal cord injury. Front. Vet. Sci. 2021, 8, 597949. [Google Scholar] [CrossRef]
- Martins, Â.; Gouveia, D.; Cardoso, A.; Carvalho, C.; Silva, C.; Coelho, T.; Gamboa, Ó.; Ferreira, A. Functional neurorehabilitation in dogs with an incomplete recovery 3 months following intervertebral disc surgery: A case series. Animals 2021, 11, 2442. [Google Scholar] [CrossRef]
- Lewis, M.J.; Jeffery, N.D.; Olby, N.J. Ambulation in dogs with absent pain perception after acute thoracolumbar Spinal Cord Injury. Front. Vet. Sci. 2020, 7, 560–572. [Google Scholar] [CrossRef] [PubMed]
- Menchetti, M.; Gandini, G.; Bravaccini, B.; Dondi, M.; Gagliardo, T.; Bianchi, E. Clinical and electrodiagnostic findings and quality of life of dogs and cats with brachial plexus injury. Vet. Sci. 2020, 7, 101. [Google Scholar] [CrossRef] [PubMed]
- Mackinnon, S.; Dellon, A.L. Surgery of the peripheral nerve. In Diagnosis of Nerve Injury; Thieme: New York, NY, USA, 1988; pp. 74–78. [Google Scholar]
- Alvites, R.; Rita Caseiro, A.; Santos Pedrosa, S.; Vieira Branquinho, M.; Ronchi, G.; Geuna, S.; Varejão, A.S.P.; Colette Maurício, A.; Spurkland, A. Peripheral nerve injury and axonotmesis: State of the art and recent advances. Cogent. Med. 2018, 5, 1466404. [Google Scholar] [CrossRef]
- Lopes, B.; Sousa, P.; Alvites, R.; Branquinho, M.; Sousa, A.C.; Mendonça, C.; Atayde, L.M.; Luís, A.L.; Varejão, A.S.P.; Maurício, A.C. Peripheral Nerve Injury Treatments and Advances: One Health Perspective. Int. J. Mol. Sci. 2022, 23, 918. [Google Scholar] [CrossRef] [PubMed]
- Vijayavenkataraman, S. Nerve guide conduits for peripheral nerve injury repair: A review on design, materials and fabrication methods. Acta Biomater. 2020, 106, 54–69. [Google Scholar] [CrossRef] [PubMed]
- Maugeri, G.; D’Agata, V.; Trovato, B.; Roggio, F.; Castorina, A.; Vecchio, M.; Di Rosa, M.; Musumeci, G. The role of exercise on peripheral nerve regeneration: From animal model to clinical application. Heliyon 2021, 7, e08281. [Google Scholar] [CrossRef] [PubMed]
- Franzblau, L.E.; Shauver, M.J.; Chung, K.C. Patient satisfaction and self-reported outcomes after complete brachial plexus avulsion injury. J. Hand Surg. 2014, 39, 948–955. [Google Scholar] [CrossRef]
- Welch, J.A. Peripheral nerve injury. Semin. Vet. Med. Surg. Small Anim. 1996, 11, 273–284. [Google Scholar] [CrossRef]
- Santifort, K.M. Return of function in a feline thoracic limb after suspected traumatic brachial plexus injury with loss of nociception. Vet. Rec. Case Rep. 2016, 4, e000334. [Google Scholar] [CrossRef]
- Smith, B.W.; Daunter, A.K.; Yang, L.J.S.; Wilson, T.J. An Update on the Management of Neonatal Brachial Plexus Palsy—Replacing Old Paradigms: A Review. JAMA Pediatr. 2018, 172, 585. [Google Scholar] [CrossRef]
- Rich, J.A.; Newell, A.; Williams, T. Traumatic brachial plexus injury rehabilitation using neuromuscular electrical muscle stimulation in a polytrauma patient. BMJ Case Rep. 2019, 12, e232107. [Google Scholar] [CrossRef] [PubMed]
- Helgeson, K.; Stoneman, P. Shoulder injuries in rugby players: Mechanisms, examination, and rehabilitation. Phys. Ther. Sport 2014, 15, 218. [Google Scholar] [CrossRef] [PubMed]
- Dijkstra, J.R.; Meek, M.; Robinson, P.H.; Gramsbergen, A. Methods to evaluate functional nerve recovery in adult rats: Walking track analysis, video analysis and the withdrawal reflex. J. Neurosci. Methods 2000, 96, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Meek, M.F.; Van Der Werff, J.F.A.; Nicolai, J.P.A.; Gramsbergen, A. Biodegradable p (DLLA-e-CL) Nerve guides versus autologous nerve grafts: Electromyographic amd video analysis. Muscle Nerve 2001, 24, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Watson, N.C.; Jejurikar, S.; Kalliainen, L.K.; Calderon, M.S.; Urbanchek, M.G.; Eguchi, T.; Kuzon, J.R. Range of motion physiotherapy reduces the force deficit in antagonists to denervated rat muscles. J. Surg. Res. 2001, 99, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Siegel, S.G.; Patton, B.; English, A.W. Ciliary neurotrophic factor is required for motoneuron sprouting. Exp. Neurol. 2000, 166, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Varejão, A.S.P.; Cabrita, A.M.; Geuna, S.; Melo-Pinto, P.; Filipe, V.M.; Gramsbergen, A.; Meek, M.F. Toe out angle: A functional index for the evaluation of sciatic nerve recovey in the rat model. Exp. Neurol. 2003, 183, 695–699. [Google Scholar] [CrossRef]
- Sullivan, R.; Dailey, T.; Duncan, K.; Abel, N.; Borlongan, C.V. Peripheral Nerve Injury: Stem Cell Therapy and Peripheral Nerve Transfer. Int. J. Mol. Sci. 2016, 17, 2101. [Google Scholar] [CrossRef]
- Faroni, A.; Mobasseri, S.A.; Kingham, P.J.; Reid, A.J. Peripheral nerve regeneration: Experimental strategies and future perspectives. Adv. Drug Deliv. Rev. 2015, 82–83, 160–167. [Google Scholar] [CrossRef]
- Magnaghi, V.; Procacci, P.; Tata, A.M. Chapter 15: Novel pharmacological approaches to Schwann cells as neuroprotective agents for peripheral nerve regeneration. Int. Rev. Neurobiol. 2009, 87, 295–315. [Google Scholar]
- Ni, L.; Yao, Z.; Zhao, Y.; Zhang, T.; Wang, J.; Li, S.; Chen, Z. Electrical stimulation therapy for peripheral nerve injury. Front. Neurol. 2023, 14, 1081458. [Google Scholar] [CrossRef] [PubMed]
- Belviso, I.; Palermi, S.; Sacco, A.M.; Romano, V.; Corrado, B.; Zappia, M.; Sirico, F. Brachial plexus injuries in sport medicine: Clinical evaluation, diagnostic approaches, treatment options and rehabilitative interventions. J. Funct. Morphol. Kinesiol. 2020, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- Javeed, S.; Faraji, A.H.; Dy, C.; Ray, W.Z.; MacEwan, M.R. Application of electrical stimulation for peripheral nerve regeneration: Stimulation parameters and future horizons. Interdiscip. Neurosurg. Adv. Tech. Case Manag. 2021, 24, 101117. [Google Scholar] [CrossRef]
- Gordon, T.; Udina, E.; Verge, V.M.K.; de Chaves, E.I.P. Brief electrical stimulation accelerates axon regeneration in the peripheral nervous system and promotes sensory axon regeneration in the central nervous system. Motor Control 2009, 13, 412–441. [Google Scholar] [CrossRef] [PubMed]
- Aglah, C.; Gordon, T.; de Chaves, E.I.P. cAMP promotes neurite outgrowth and extension through protein kinase A but independently of Erk activation in cultured rat motoneurons. Neuropharmacology 2008, 55, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Xu, Q.-G.; Franz, C.K.; Zhang, R.; Dalton, C.; Gordon, T.; Verge, V.M.K.; Midha, R.; Zochodne, D.W. Accelerated axon outgrowth, guidance, and target reinnervation across nerve transection gaps following a brief electrical stimulation paradigm: Laboratory investigation. J. Neurosurg. 2012, 116, 498–512. [Google Scholar] [CrossRef]
- McGregor, C.E.; English, A.W. The role of BDNF in peripheral nerve regeneration: Activity-dependent treatments and Val66Met. Front. Cell. Neurosci. 2019, 12, 522. [Google Scholar] [CrossRef]
- Hoffman, H. Acceleration and retardation of the process of axon-sprouting in partially denervated muscles. Aust. J. Exp. Biol. Med. Sci. 1952, 30, 541–566. [Google Scholar] [CrossRef]
- Nix, W.A.; Hopf, H.C. Electrical stimulation of regenerating nerve and its effect on motor recovery. Brain Res. 1983, 272, 21–25. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, Y.; Lu, L.; Hu, X.; Luo, Z. Electrical stimulation accelerates nerve regeneration and functional recovery in delayed peripheral nerve injury in rats. Eur. J. Neurosci. 2013, 38, 3691–3701. [Google Scholar] [CrossRef]
- Song, S.; McConnell, K.W.; Amores, D.; Levinson, A.; Vogel, H.; Quarta, M.; Rando, T.A.; George, P.M. Electrical stimulation of human neural stem cells via conductive polymer nerve guides enhances peripheral nerve recovery. Biomaterials 2021, 275, 120982. [Google Scholar] [CrossRef] [PubMed]
- Cobianchi, S.; Casals-Diaz, L.; Jaramillo, J.; Navarro, X. Differential effects of activity dependent treatments on axonal regeneration and neuropathic pain after peripheral nerve injury. Exp. Neurol. 2013, 240, 157–167. [Google Scholar] [CrossRef] [PubMed]
- English, A.W.; Meador, W.; Carrasco, D.I. Neurotrophin-4/5 is required for the early growth of regenerating axons in peripheral nerves. Eur. J. Neurosci. 2005, 21, 2624–2634. [Google Scholar] [CrossRef]
- Singh, B.; Krishnan, A.; Micu, I.; Koshy, K.; Singh, V.; Martinez, J.A.; Koshy, D.; Xu, F.; Chandrasekhar, A.; Dalton, C.; et al. Peripheral neuron plasticity is enhanced by brief electrical stimulation and overrides attenuated regrowth in experimental diabetes. Neurobiol. Dis. 2015, 83, 134–151. [Google Scholar] [CrossRef] [PubMed]
- Udina, E.; Furey, M.; Busch, S.; Silver, J.; Gordon, T.; Fouad, K. Electrical stimulation of intact peripheral sensory axons in rats promotes outgrowth of their central projections. Exp. Neurol. 2008, 210, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Richard, M.A.; Spaich, E.G.; Serrao, M.; Andersen, O.K. Stimulation site and phase modulation of the withdrawal reflex during gait initiaion. Clin. Neurophysiol. 2015, 126, 2282–2289. [Google Scholar] [CrossRef] [PubMed]
- Pilkar, R.B.; Yarossi, M.; Forrest, G. Empirical mode decomposition as a tool to remove the function electrical stimulation artifact from surface electromyograms: Preliminary investigation. In Proceedings of the 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Diego, CA, USA, 28 August–1 September 2012; pp. 1847–1850. [Google Scholar]
- Martins, A.; Gouveia, D.; Cardoso, A.; Gamboa, Ó.; Millis, D.; Ferreira, A. Nervous system modulation through electrical stimulation in companion animals. Acta Vet. Scand. 2021, 63, 22. [Google Scholar] [CrossRef]
- Levine, D.; Bockstahler, B. Electrical stimulation. In Canine Rehabilitation and Physical Therapy; Millis, D., Levine, D., Eds.; Elseviers: Philadelphia, PA, USA, 2014; pp. 342–356. [Google Scholar]
- Haastert-Talini, K.; Schmitte, R.; Korte, N.; Klode, D.; Ratzka, A.; Grothe, C. Electrical stimulation accelerates axonal and functional peripheral nerve regeneration across long gaps. J. Neurotrauma 2011, 28, 661–674. [Google Scholar] [CrossRef]
- Pieber, K.; Herceg, M.; Paternostro-Sluga, T.; Schuhfried, O. Optimizing stimulation parameters in functional electrical stimulation of denervated muscles: A cross-sectional study. J. Neuroeng. Rehabil. 2015, 12, 51. [Google Scholar] [CrossRef]
- Chiaramonte, R.; Pavone, V.; Testa, G.; Pesce, I.; Scaturro, D.; Musumeci, G.; Mauro, G.; Vecchio, M. The role of physical exercise and rehabilitative implication in the process of nerve repair in peripheral neuropathies: A systematic review. Diagnostics 2023, 13, 364. [Google Scholar] [CrossRef]
- Pachter, B.R.; Eberstein, A. Passive Exercise and Reinnervation of the Rat Denervated Extensor Digitorum Longus Muscle after Nerve Crush. Am. J. Phys. Med. Rehabil. 1989, 68, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Sabatier, M.J.; Redmon, N.; Schwartz, G.; English, A.W. Treadmill training promotes axon regeneration in injured peripheral nerves. Exp. Neurol. 2008, 211, 489–493. [Google Scholar] [CrossRef]
- Asensio-Pinilla, E.; Udina, E.; Jaramillo, J.; Navarro, X. Electrical stimulation combined with exercise increase axonal regeneration after peripheral nerve injury. Exp. Neurol. 2009, 219, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Hartley, R.A.; Kordecki, M.E. Rehabilitation of chronic brachial plexus neuropraxia and loss of cervical extension in a high school football player: A case report. Int. J. Sports Phys. Ther. 2018, 13, 1061–1072. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Blaha, C.; Moradkhan, R.; Gray, K.S.; Sinoway, L.I. Muscle sympathetic nerve activity responses to dynamic passive muscle stretch in humans. J. Physiol. 2006, 576, 625. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, M.J.; da Paz, M.G.D.S.; Bina, M.T.; Santos, S.N.; Raicher, I.; Galhardoni, R.; Fernandes, D.T.; Yeng, L.T.; Baptista, A.F.; de Andrade, D.C. Neuropathic pain after brachial plexus avulsion-central and peripheral mechanisms. BMC Neurol. 2015, 15, 73. [Google Scholar] [CrossRef] [PubMed]
- Treede, R.-D.; Jensen, T.S.; Campbell, J.N.; Cruccu, G.; Dostrovsky, J.O.; Griffin, J.W.; Hansson, P.; Hughes, R.; Nurmikko, T.; Serra, J. Neuropathic pain: Redefinition and a grading system for clinical and research purposes. Neurology 2008, 70, 1630–1635. [Google Scholar] [CrossRef]
- Vannier, J.L.; Belkheyar, Z.; Oberlin, C.; Montravers, P. Management of neuropathic pain after brachial plexus injury in adult patients: A report of 60 cases. Ann. Fr. Anesth. Reanim. 2008, 27, 890–895. [Google Scholar] [CrossRef]
- Abdel-Aziz, S.; Ghaleb, A.H. Cervical Spinal Cord Stimulation for the Management of Pain from Brachial Plexus Avulsion. Pain Med. 2014, 15, 712–714. [Google Scholar] [CrossRef][Green Version]
- Ciaramitaro, P.; Mondelli, M.; Logullo, F.; Grimaldi, S.; Battiston, B.; Sard, A.; Scarinzi, C.; Migliaretti, G.; Faccani, G.; Cocito, D.; et al. Traumatic peripheral nerve injuries: Epidemiological findings, neuropathic pain and quality of life in 158 patients. J. Peripher. Nerv. Syst. 2010, 15, 120–127. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, P.; Rui, J.; Zhao, X.; Lao, J. The clinical characteristics of neuropathic pain in patients with total brachial plexus avulsion: A 30-case study. Injury 2016, 47, 1719–1724. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, L.; Meng, C.; Zhou, Y.; Lao, J.; Zhao, X. A new model for the study of neuropathic pain after brachial plexus injury. Injury 2017, 48, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Lovaglio, A.; Socolovsky, M.; Di Masi, G.; Bonilla, G. Treatment of neuropathic pain after peripheral nerve and brachial plexus traumatic injury. Neurol. India 2019, 67, 32. [Google Scholar] [CrossRef] [PubMed]
- Emamhadi, M.; Andalib, S. Successful recovery of sensation loss in upper brachial plexus injuries. Acta Neurochir. 2018, 160, 2019–2023. [Google Scholar] [CrossRef] [PubMed]
- Sadosky, A.; McDermott, A. A review of the epidemiology of painful diabetic peripheral neuropathy, postherpetic neuralgia, and less commonly studied neuropathic pain conditions. Pain Pract. 2008, 8, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Ehde, D.M.; Czerniecki, J.M.; Smith, D.G.; Campbell, K.M.; Edwards, W.T.; Jensen, M.P.; Robinson, L.R. Chronic phantom sensations, phantom pain, residual limb pain, and other regional pain after lower limb amputation. Arch. Phys. Med. Rehabil. 2000, 81, 1039–1044. [Google Scholar] [CrossRef]
- Melzack, R. Phantom limbs. Sci. Am. 1992, 266, 120–126. [Google Scholar] [CrossRef]
- Jensen, T.S.; Krebs, B.; Nielsen, J.; Rasmussen, P. Immediate and long-term phantom limb pain in amputees: Incidence, clinical characteristics and relationship to pre-amputation limb pain. Pain 1985, 21, 267–278. [Google Scholar] [CrossRef]
- Menchetti, M.; Gandini, G.; Gallucci, A.; Della Rocca, G.; Matiasek, L.; Matiasek, K.; Gentilini, F.; Rosati, M. Approaching phantom complex after limb amputation in the canine species. J. Vet. Behav. 2017, 22, 24–28. [Google Scholar] [CrossRef]
- Probstner, D.; Thuler, L.C.; Ishikawa, N.M.; Alvarenga, R.M. Phantom limb phenomena in cancer amputees. Pain Pract. 2010, 10, 249–256. [Google Scholar] [CrossRef]
- Menchetti, M.; Rocca, G.D.; Tartari, I.; Gandini, G.; Di Salvo, A.; Rosati, M. Approaching phantom complex after limb amputation in cats. J. Vet. Behav. 2022, 50, 23–29. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).