Astaxanthin Improved the Quality of Hu Ram Semen by Increasing the Antioxidant Capacity and Mitochondrial Potential and Mitigating Free Radicals-Induced Oxidative Damage
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Semen Collection
2.2. Chemicals
2.3. Semen Processing, Supplementation and Preservation
2.4. Sperm Kinematics Parameters Evaluation
2.5. Sperm Viability Test
2.6. Plasma Membrane Integrity Evaluation of Spermatozoa
2.7. Acrosome Integrity Evaluation of Spermatozoa
2.8. Assessment of ROS in Semen Samples
2.9. Assessment of MDA Content of Spermatozoa
2.10. Assessment of T-AOC Content of Spermatozoa
2.11. Assessment of Mitochondrial Membrane Potential of Spermatozoa
2.12. Statistical Analysis
3. Results
3.1. Effect of AXT Supplementation on Sperm Viability
3.2. Effect of AXT Supplementation on Sperm Kinematic Index
3.3. Effect of AXT Supplementation on Sperm Plasma Membrane Integrity
3.4. Effect of AXT Supplementation on Sperm Acrosome Integrity
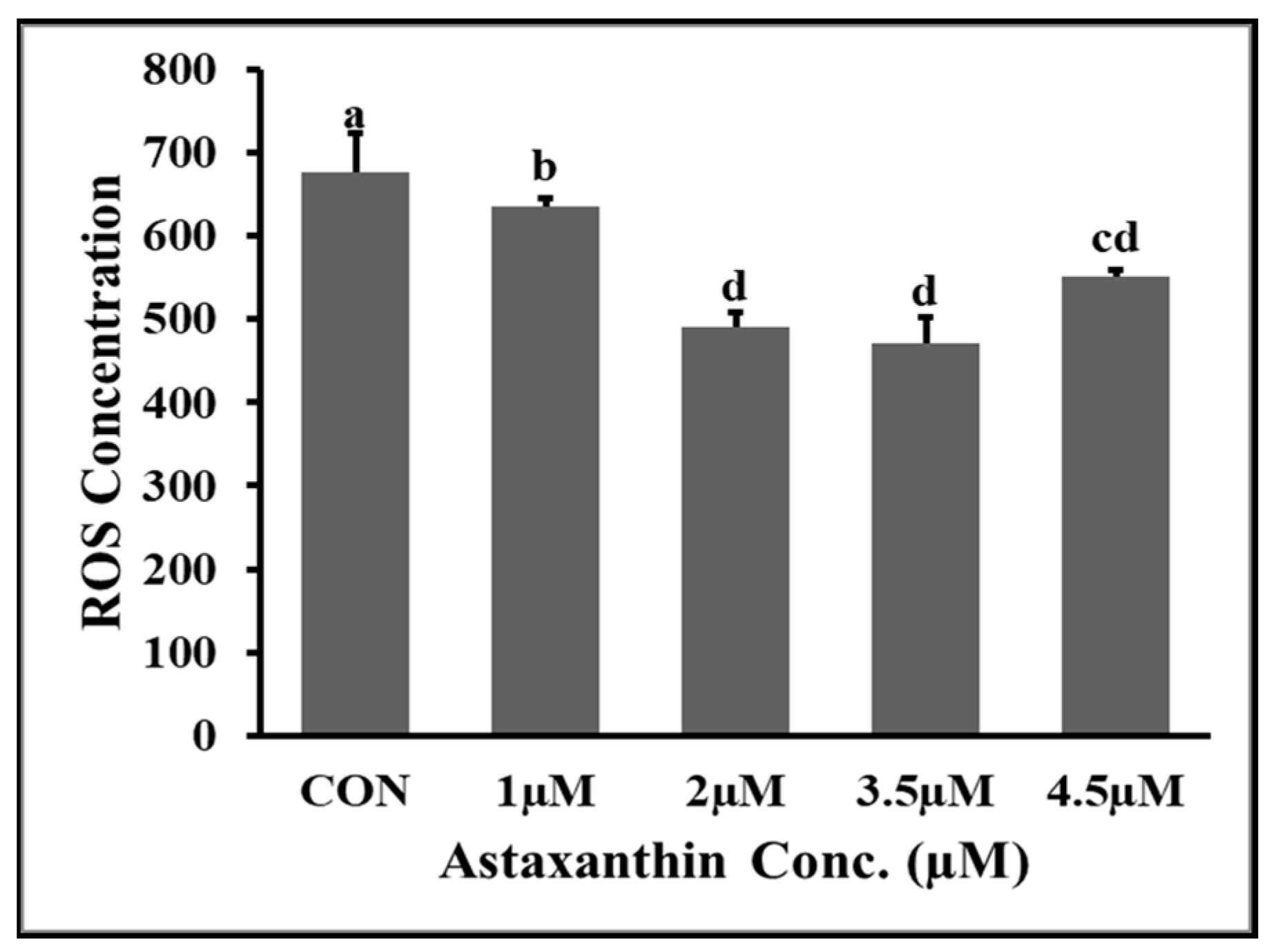
3.5. Effects of AXT Supplementations on ROS Content of Hu Ram Spermatozoa
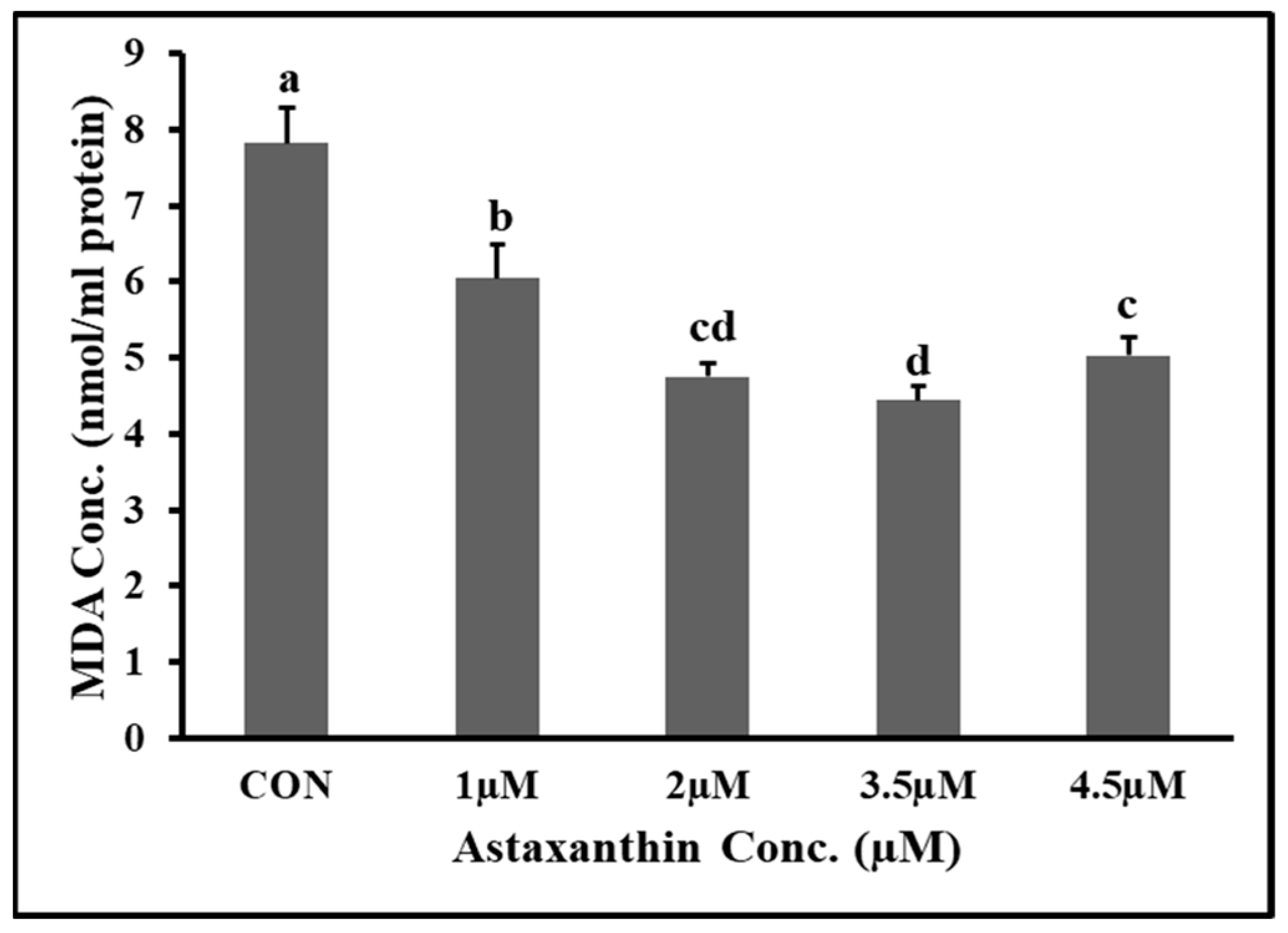
3.6. Effects of Astaxanthin Supplementations on MDA Content of Hu Ram Spermatozoa
3.7. Effect of AXT on T-AOC Capacity of Hu Rams Spermatozoa
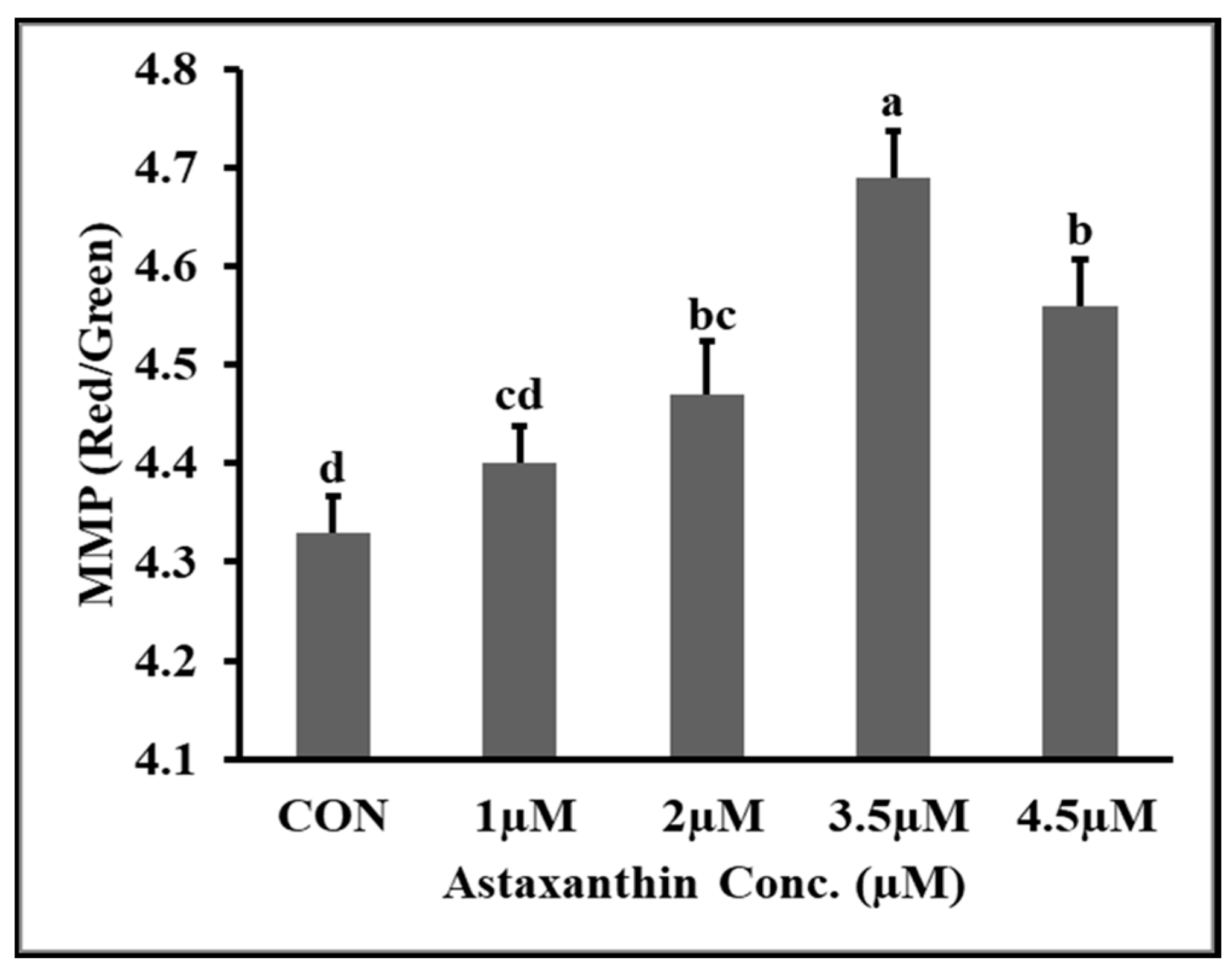
3.8. Effects of AXT on Mitochondrial Membrane Potential (MMP) of Hu Ram Spermatozoa
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eer, H.; Ma, L.; Xie, X.; Ma, J.; Ma, X.; Yue, C.; Ma, Q.; Liang, X.; Ding, W.; Li, Y. Genetic polymorphism association analysis of SNPs on the species conservation genes of Tan sheep and Hu sheep. Trop. Anim. Health Prod. 2020, 52, 915–926. [Google Scholar] [CrossRef]
- Chen, T.; Wang, L.; Li, Q.; Long, Y.; Lin, Y.; Yin, J.; Zeng, Y.; Huang, L.; Yao, T.; Abbasi, M.N.; et al. Functional probiotics of lactic acid bacteria from Hu sheep milk. BMC Microbiol. 2020, 20, 1–12. [Google Scholar] [CrossRef]
- Wu, C.; Dai, J.; Zhang, S.; Sun, L.; Liu, Y.; Zhang, D. Effect of Thawing Rates and Antioxidants on Semen Cryopreservation in Hu Sheep. Biopreserv. Biobank. 2021, 19, 204–209. [Google Scholar] [CrossRef]
- Shabbir, S.; Boruah, P.; Xie, L.; Kulyar, M.F.-E.; Nawaz, M.; Yousuf, S.; Liu, T.; Jabeen, F.; Miao, X. Genome-wide transcriptome profiling uncovers differential miRNAs and lncRNAs in ovaries of Hu sheep at different developmental stages. Sci. Rep. 2021, 11, 5865. [Google Scholar] [CrossRef]
- Rizkallah, N.; Chambers, C.G.; de Graaf, S.P.; Rickard, J.P. Factors Affecting the Survival of Ram Spermatozoa during Liquid Storage and Options for Improvement. Animals 2022, 12, 244. [Google Scholar] [CrossRef]
- Zhang, L.; Sohail, T.; Yanhu, W.; Yan, K.; Xuyang, W.; Xiaomei, S.; Yongjun, L. The Effect of Different Storage Temperature on Hu Ram Sperm Parameters. Kafkas Üniversitesi Vet. Fakültesi Derg. 2022, 28, 201–209. [Google Scholar]
- Bailey, J.L.; Blodeau, J.; Cormier, N. Semen Cryopreservation in Domestic Animals: A Damaging and Capacitating Phenomenon. J. Androl. 2000, 21, 1–7. [Google Scholar] [CrossRef]
- Chauhan, M.; Kapila, R.; Gandhi, K.; Anand, S. Acrosome damage and enzyme leakage of goat spermatozoa during dilution, cooling and freezing. Andrologia 1994, 26, 21–26. [Google Scholar] [CrossRef]
- Leboeuf, B.; Restall, B.; Salamon, S. Production and storage of goat semen for artificial insemination. Anim. Reprod. Sci. 2000, 62, 113–141. [Google Scholar] [CrossRef]
- Abdi-Benemar, H.; Jafaroghli, M.; Khalili, B.; Zamiri, M.; Ezazi, H.; Shadparvar, A. Effects of DHA supplementation of the extender containing egg yolk and α- tocopherol on the freezability and post-thawing fertility of ram semen. Small Rumin. Res. 2015, 130, 166–170. [Google Scholar] [CrossRef]
- Kaeoket, K.; Sang-Urai, P.; Thamniyom, A.; Chanapiwat, P.; Techakumphu, M. Effect of Docosahexaenoic Acid on Quality of Cryopreserved Boar Semen in Different Breeds. Reprod. Domest. Anim. 2010, 45, 458–463. [Google Scholar] [CrossRef]
- Aitken, R.J. Reactive oxygen species as mediators of sperm capacitation and pathological damage. Mol. Reprod. Dev. 2017, 84, 1039–1052. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Sohail, T.; Kang, Y.; Sun, X.; Li, Y. Chlorogenic Acid Improves Quality of Chilled Ram Sperm by Mitigating Oxidative Stress. Animals 2022, 12, 163. [Google Scholar] [CrossRef]
- Nguyen, T.M.D.; Duittoz, A.; Praud, C.; Combarnous, Y.; Blesbois, E. Calcium channels in chicken sperm regulate motility and the acrosome reaction. FEBS J. 2016, 283, 1902–1920. [Google Scholar] [CrossRef]
- Nili-Ahmadabadi, A.; Ali-Heidar, F.; Ranjbar, A.; Mousavi, L.; Ahmadimoghaddam, D.; Larki-Harchegani, A.; Ghafouri-Khosrowshahi, A. Protective effect of amlodipine on diazinon-induced changes on oxidative/antioxidant balance in rat hippocampus. Res. Pharm. Sci. 2018, 13, 368. [Google Scholar] [CrossRef]
- Navarro, B.; Kirichok, Y.; Chung, J.-J.; Clapham, D.E. Ion channels that control fertility in mammalian spermatozoa. Int. J. Dev. Biol. 2008, 52, 607. [Google Scholar] [CrossRef]
- Ezazi, H.; Abdi-Benemar, H.; Taghizadeh, A.; Khalili, B.; Seifdavati, J.; Jafaroghli, M.; Elghandour, M.M.; Salem, A.Z. The influence of dietary sunflower oil, rich in n-6 polyunsaturated fatty acids, in combination with vitamin C on ram semen parameters, sperm lipids and fertility. J. Sci. Food Agric. 2019, 99, 3803–3810. [Google Scholar] [CrossRef]
- Almbro, M.; Dowling, D.K.; Simmons, L.W. Effects of vitamin E and beta-carotene on sperm competitiveness. Ecol. Lett. 2011, 14, 891–895. [Google Scholar] [CrossRef]
- Wang, J.-Y.; Lee, Y.-J.; Chou, M.-C.; Chang, R.; Chiu, C.-H.; Liang, Y.-J.; Wu, L.-S. Astaxanthin Protects Steroidogenesis from Hydrogen Peroxide-Induced Oxidative Stress in Mouse Leydig Cells. Mar. Drugs 2015, 13, 1375–1388. [Google Scholar] [CrossRef]
- Tripathi, D.; Jena, G. Astaxanthin inhibits cytotoxic and genotoxic effects of cyclophosphamide in mice germ cells. Toxicology 2008, 248, 96–103. [Google Scholar] [CrossRef]
- Agarwal, A.; Sekhon, L.H. The role of antioxidant therapy in the treatment of male infertility. Hum. Fertil. 2010, 13, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Cheuquemán, C.; Arias, M.; Risopatrón, J.; Felmer, R.; Álvarez, J.; Mogas, T.; Sánchez, R. Supplementation of IVF medium with melatonin: Effect on sperm functionality and in vitro produced bovine embryos. Andrologia 2015, 47, 604–615. [Google Scholar] [CrossRef] [PubMed]
- Kurcer, Z.; Hekimoglu, A.; Aral, F.; Baba, F.; Sahna, E. Effect of melatonin on epididymal sperm quality after testicular ischemia/reperfusion in rats. Fertil. Steril. 2010, 93, 1545–1549. [Google Scholar] [CrossRef] [PubMed]
- Yue, D.; Yan, L.; Luo, H.; Xu, X.; Jin, X. Effect of Vitamin E supplementation on semen quality and the testicular cell membranal and mitochondrial antioxidant abilities in Aohan fine-wool sheep. Anim. Reprod. Sci. 2010, 118, 217–222. [Google Scholar] [CrossRef]
- Festa, R.; Giacchi, E.; Raimondo, S.; Tiano, L.; Zuccarelli, P.; Silvestrini, A.; Meucci, E.; Littarru, G.; Mancini, A. Coenzyme Q10 supplementation in infertile men with low-grade varicocele: An open, uncontrolled pilot study. Andrologia 2014, 46, 805–807. [Google Scholar] [CrossRef]
- Andersen, A.H.; Thinnesen, M.; Failing, K.; Goericke-Pesch, S. Effect of reduced glutathione (GSH) supplementation to Tris-egg yolk extender on chilled semen variables of dogs. Anim. Reprod. Sci. 2018, 198, 145–153. [Google Scholar] [CrossRef]
- Ambati, R.R.; Phang, S.-M.; Ravi, S.; Aswathanarayana, R.G. Astaxanthin: Sources, Extraction, Stability, Biological Activities and Its Commercial Applications—A Review. Mar. Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef]
- Fassett, R.G.; Coombes, J.S. Astaxanthin, oxidative stress, inflammation and cardiovascular disease. Future Cardiol. 2009, 5, 333–342. [Google Scholar] [CrossRef]
- Kuroki, T.; Ikeda, S.; Okada, T.; Maoka, T.; Kitamura, A.; Sugimoto, M.; Kume, S. Astaxanthin ameliorates heat stress-induced impairment of blastocyst development In Vitro: –astaxanthin colocalization with and action on mitochondria–. J. Assist. Reprod. Genet. 2013, 30, 623–631. [Google Scholar] [CrossRef]
- Fang, Y.; Zhong, R.; Chen, L.; Feng, C.; Sun, H.; Zhou, D. Effects of astaxanthin supplementation on the sperm quality and antioxidant capacity of ram semen during liquid storage. Small Rumin. Res. 2015, 130, 178–182. [Google Scholar] [CrossRef]
- Farzan, M.; Chamani, M.; Varnaseri, H. The antioxidant effect of astaxanthin on quantitative and qualitative parameters of bull sperm. Indian J. Foundamental Appl. Life Sci. 2014, 4, 425–430. [Google Scholar]
- Basioura, A.; Tsakmakidis, I.; Martinez, E.; Roca, J.; Li, J.; Molina, M.; Theodoridis, A.; Boscos, C.; Parrilla, I. Effect of astaxanthin in extenders on sperm quality and functional variables of frozen-thawed boar semen. Anim. Reprod. Sci. 2020, 218, 106478. [Google Scholar] [CrossRef] [PubMed]
- Basioura, A.; Boscos, C.; Parrilla, I.; Tsousis, G.; Tsakmakidis, I. Effect of astaxanthin on the quality of boar sperm stored at 17 C, incubated at 37 C or under in vitro conditions. Reprod. Domest. Anim. 2018, 53, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Andrisani, A.; Donà, G.; Tibaldi, E.; Brunati, A.M.; Sabbadin, C.; Armanini, D.; Alvisi, G.; Gizzo, S.; Ambrosini, G.; Ragazzi, E.; et al. Astaxanthin Improves Human Sperm Capacitation by Inducing Lyn Displacement and Activation. Mar. Drugs 2015, 13, 5533–5551. [Google Scholar] [CrossRef] [PubMed]
- Najafi, D.; Taheri, R.A.; Najafi, A.; Shamsollahi, M.; Alvarez-Rodriguez, M. Effect of astaxanthin nanoparticles in protecting the post-thawing quality of rooster sperm challenged by cadmium administration. Poult. Sci. 2020, 99, 1678–1686. [Google Scholar] [CrossRef] [PubMed]
- Goto, S.; Kogure, K.; Abe, K.; Kimata, Y.; Kitahama, K.; Yamashita, E.; Terada, H. Efficient radical trapping at the surface and inside the phospholipid membrane is responsible for highly potent antiperoxidative activity of the carotenoid astaxanthin. Biochim. Biophys. Acta Biomembr. 2001, 1512, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Donà, G.; Kožuh, I.; Brunati, A.M.; Andrisani, A.; Ambrosini, G.; Bonanni, G.; Ragazzi, E.; Armanini, D.; Clari, G.; Bordin, L. Effect of Astaxanthin on Human Sperm Capacitation. Mar. Drugs 2013, 11, 1909–1919. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Yang, Q.; Li, Y.; Li, P.; Wang, L.; Li, X. A mechanism by which Astragalus polysaccharide protects against ROS toxicity through inhibiting the protein dephosphorylation of boar sperm preserved at 4 °C. J. Cell. Physiol. 2018, 233, 5267–5280. [Google Scholar] [CrossRef]
- Kang, X.; Xie, Q.; Zhou, X.; Li, F.; Huang, J.; Liu, D.; Huang, T. Effects of Hepatitis B Virus S Protein Exposure on Sperm Membrane Integrity and Functions. PLoS ONE 2012, 7, e33471. [Google Scholar] [CrossRef]
- Li, Q.; Shaoyong, W.; Li, Y.; Chen, M.; Hu, Y.; Liu, B.; Yang, G.; Hu, J. Effects of oligomeric proanthocyanidins on quality of boar semen during liquid preservation at 17 °C. Anim. Reprod. Sci. 2018, 198, 47–56. [Google Scholar] [CrossRef]
- Zhang, J.; Kong, D.-L.; Xiao, B.; Yuan, H.-J.; Kong, Q.-Q.; Han, X.; Luo, M.-J.; Tan, J.-H. Restraint stress of male mice triggers apoptosis in spermatozoa and spermatogenic cells via activating the TNF-α system. Zygote 2020, 28, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Comhaire, F.; Garem, Y.E.; Mahmoud, A.; Eertmans, F.; Schoonjans, F. Combined conventional/antioxidant “Astaxanthin” treatment for male infertility: A double blind, randomized trial. Asian J. Androl. 2005, 7, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Mortazavi, M.; Salehi, I.; Alizadeh, Z.; Vahabian, M.; Roushandeh, A.M. Protective Effects of Antioxidants on Sperm Parameters and Seminiferous Tubules Epithelium in High Fat-fed Rats. J. Reprod. Infertil. 2014, 15, 22. [Google Scholar] [PubMed]
- Vahidinia, A.; Rahbar, A.R.; Mahmoodabadi, M.M.S. Effect of Astaxanthin, Vitamin E, and Vitamin C in Combination with Calorie Restriction on Sperm Quality and Quantity in Male Rats. J. Diet. Suppl. 2017, 14, 252–263. [Google Scholar] [CrossRef] [PubMed]
- McNulty, H.P.; Byun, J.; Lockwood, S.F.; Jacob, R.F.; Mason, R.P. Differential effects of carotenoids on lipid peroxidation due to membrane interactions: X-ray diffraction analysis. Biochim. Biophys. Acta Biomembr. 2007, 1768, 167–174. [Google Scholar] [CrossRef]
- Sterlie, M.; Bjerkeng, B.; Liaaen-Jensen, S. Plasma appearance and distribution of astaxanthin E/Z and R/S isomers in plasma lipoproteins of men after single dose administration of astaxanthin. J. Nutr. Biochem. 2000, 11, 482–490. [Google Scholar] [CrossRef]
- Pashkow, F.J.; Watumull, D.G.; Campbell, C.L. Astaxanthin: A Novel Potential Treatment for Oxidative Stress and Inflammation in Cardiovascular Disease. Am. J. Cardiol. 2008, 101, S58–S68. [Google Scholar] [CrossRef]
- Saberi, E.; Mohammadrezaei, F.M.; Jazayeri, O.; Fathi, N.; Moghadam, A.H. Astaxanthin Induces the Expression of CatSper1 Gene and Protects Sperms in Toxicity Induced by Cadmium in Mice. Drug Res. 2021, 71, 512–519. [Google Scholar] [CrossRef]
- Mansour, N.; McNiven, M.A.; Richardson, G.F. The effect of dietary supplementation with blueberry, α-tocopherol or astaxanthin on oxidative stability of Arctic char (Salvelinus alpinus) semen. Theriogenology 2006, 66, 373–382. [Google Scholar] [CrossRef]
- Tizkar, B.; Kazemi, R.; Alipour, A.; Seidavi, A.; Naseralavi, G.; Ponce-Palafox, J.T. Effects of dietary supplementation with astaxanthin and β-carotene on the semen quality of goldfish (Carassius auratus). Theriogenology 2015, 84, 1111–1117. [Google Scholar] [CrossRef]
- Min, Y.; Sun, T.; Niu, Z.; Liu, F. Vitamin C and vitamin E supplementation alleviates oxidative stress induced by dexamethasone and improves fertility of breeder roosters. Anim. Reprod. Sci. 2016, 171, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Sapanidou, V.; Taitzoglou, I.; Tsakmakidis, I.; Kourtzelis, I.; Fletouris, D.; Theodoridis, A.; Lavrentiadou, S.; Tsantarliotou, M. Protective effect of crocetin on bovine spermatozoa against oxidative stress during in vitro fertilization. Andrology 2016, 4, 1138–1149. [Google Scholar] [CrossRef] [PubMed]
- Sarıözkan, S.; Bucak, M.N.; Tuncer, P.B.; Büyükleblebici, S.; Cantürk, F. Influence of various antioxidants added to TCM-199 on post-thaw bovine sperm parameters, DNA integrity and fertilizing ability. Cryobiology 2014, 68, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Asadpour, R.; Pourseif, M.M.; Moghadam, G.; Jafari, R.; Tayefi, H.; Mahmodi, H. Effect of vitamin B12 addition to extenders on some physicochemical parameters of semen in crossbred rams. Afr. J. Biotechnol. 2012, 11, 11741–11745. [Google Scholar] [CrossRef]
- Tuncer, P.B.; Bucak, M.N.; Sarıözkan, S.; Sakin, F.; Yeni, D.; Çiğerci, İ.H.; Ateşşahin, A.; Avdatek, F.; Gündoğan, M.; Büyükleblebici, O. The effect of raffinose and methionine on frozen/thawed Angora buck (Capra hircus ancryrensis) semen quality, lipid peroxidation and antioxidant enzyme activities. Cryobiology 2010, 61, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Bucak, M.N.; Sarıözkan, S.; Tuncer, P.B.; Ulutaş, P.A.; Akçadağ, H.İ. Effect of antioxidants on microscopic semen parameters, lipid peroxidation and antioxidant activities in Angora goat semen following cryopreservation. Small Rumin. Res. 2009, 81, 90–95. [Google Scholar] [CrossRef]
- Abdi-Benemar, H.; Khalili, B.; Zamiri, M.; Ezazi, H.; Ardabili, G.S.; Moghadam, S.H.; Simanoor, N. Effects of astaxanthin supplementation on the freezability, lipid peroxidation, antioxidant enzyme activities and post-thawing fertility of ram semen. Small Rumin. Res. 2020, 192, 106213. [Google Scholar] [CrossRef]
- Wolf, A.M.; Asoh, S.; Hiranuma, H.; Ohsawa, I.; Iio, K.; Satou, A.; Ishikura, M.; Ohta, S. Astaxanthin protects mitochondrial redox state and functional integrity against oxidative stress. J. Nutr. Biochem. 2010, 21, 381–389. [Google Scholar] [CrossRef]

| Index (%) | Time (d) | Control | 1 µM | 2 µM | 3.5 µM | 4.5 µM |
|---|---|---|---|---|---|---|
| Viability (%) | 0 | 81.87 ± 1.7 | 82.12 ± 1.09 | 82.09 ± 1.1 | 82.77 ± 0.9 | 82.05 ± 0.76 |
| 1 | 67.89 ± 1.05 b | 68.08 ± 1.19 b | 72.74 ± 0.63 ab | 76.91 ± 0.11 a | 72.94 ± 1.46 ab | |
| 2 | 63.52 ± 0.46 c | 62.55 ± 1.41 c | 65.68 ± 0.82 b | 71.19 ± 0.98 a | 69.47 ± 0.74 ab | |
| 3 | 57.21 ± 1.86 b | 59.98 ± 2.76 ab | 62.01 ± 0.45 ab | 63.82 ± 0.75 a | 61.59 ± 1.05 ab | |
| 4 | 51.66 ± 0.95 c | 53.95 ± 0.93 b | 57.71 ± 0.20 a | 58.4 ± 0.58 a | 56.64 ± 0.56 ab | |
| 5 | 42.49 ± 1.43 c | 44.62 ± 0.97 c | 50.27 ± 0.93 b | 53.59 ± 0.53 a | 49.88 ± 0.93 b | |
| T.M (%) | 0 | 79.72 ± 0.86 | 78.98 ± 1.32 | 81.23 ± 0.63 | 82.10 ± 1.21 | 80.75 ± 0.55 |
| 1 | 64.24 ± 1.44 c | 65.21 ± 1.74 c | 70.5 ± 0.22 b | 75.99 ± 1.52 a | 70.77 ± 0.71 b | |
| 2 | 62.48 ± 0.38 b | 61.65 ± 0.66 b | 62.7 ± 0.57 b | 70.48 ± 0.88 a | 69.05 ± 1.04 a | |
| 3 | 52.13 ± 0.64 b | 53.79 ± 1.18 b | 53.99 ± 1.23 b | 62.57 ± 0.84 a | 60.98 ± 0.92 a | |
| 4 | 42.57 ± 0.71 b | 43.86 ± 1.33 b | 48.97 ± 0.57 a | 50.46 ± 2.19 a | 50.14 ± 1.8 a | |
| 5 | 29.1 ± 1.47 d | 35.27 ± 0.56 c | 38.44 ± 0.34 b | 45.24 ± 1 a | 37.27 ± 0.97 bc | |
| P.M (%) | 0 | 73.58 ± 0.32 | 71.67 ± 0.76 | 74.50 ± 1.10 | 75.43 ± 1.17 | 73.43 ± 0.91 |
| 1 | 56.31 ± 1.25 b | 57.49 ± 1.96 b | 63.56 ± 1.02 a | 67.91 ± 1.95 a | 62.84 ± 1.45 a | |
| 2 | 53.26 ± 0.93 b | 53.48 ± 0.89 b | 55.8 ± 0.7 b | 62.18 ± 1.14 a | 60.66 ± 0.59 a | |
| 3 | 45.54 ± 1.47 b | 48.18 ± 0.95 b | 46.79 ± 1.17 b | 53.9 ± 1.94 a | 54.01 ± 1.21 a | |
| 4 | 34.1 ± 0.44 c | 36.22 ± 1.57 bc | 39.46 ± 0.83 ab | 42.62 ± 0.94 a | 43.42 ± 1.96 a | |
| 5 | 21.83 ± 0.62 d | 28.69 ± 0.61 c | 30.84 ± 0.21 b | 36.7 ± 0.42 a | 30.21 ± 0.97 bc | |
| MAD (%) | 0 | 57.43 ± 1.30 | 56.34 ± 1.02 | 58.31 ± 0.54 | 60.22 ± 2.31 | 59.41 ± 2.01 |
| 1 | 52.82 ± 0.66 | 43.35 ± 1.67 | 51.61 ± 6.56 | 58.25 ± 7.95 | 51.06 ± 2.45 | |
| 2 | 37.57 ± 1.43 c | 38.66 ± 2.21 ab | 43.82 ± 3.44 ab | 49.98 ± 3.96 a | 49.1 ± 4.75 a | |
| 3 | 35.39 ± 1.61 b | 36.44 ± 0.81 b | 34.51 ± 2.85 b | 48.16 ± 3.91 a | 47.2 ± 2.91 a | |
| 4 | 29.69 ± 2.98 ab | 24.27 ± 1.63 b | 32.04 ± 3.35 ab | 32.66 ± 0.43 ab | 35.56 ± 3.12 a | |
| 5 | 16.72 ± 1.09 b | 24.14 ± 3 b | 25.86 ± 2.96 ab | 33.9 ± 2.38 a | 24.14 ± 3.93 b | |
| LIN (%) | 0 | 0.61 ± 0.03 | 0.60 ± 0.01 | 0.59 ± 0.02 | 0.59 ± 0.01 | 0.58 ± 0.02 |
| 1 | 0.59 ± 0.01 a | 0.58 ± 0.01 a | 0.57 ± 0.01 ab | 0.58 ± 0.01 a | 0.57 ± 0.01 ab | |
| 2 | 0.56 ± 0.01 | 0.55 ± 0.01 | 0.56 ± 0 | 0.55 ± 0.01 | 0.54 ± 0.01 | |
| 3 | 0.53 ± 0.02 | 0.53 ± 0.01 | 0.54 ± 0.01 | 0.53 ± 0.01 | 0.52 ± 0.01 | |
| 4 | 0.53 ± 0.02 | 0.54 ± 0.01 | 0.53 ± 0.01 | 0.53 ± 0.01 | 0.52 ± 0.02 | |
| 5 | 0.49 ± 0.01 c | 0.54 ± 0.02 a | 0.52 ± 0.01 ab | 0.53 ± 0.01 ab | 0.54 ± 0.01 a | |
| STR (%) | 0 | 0.87 ± 0.03 | 0.86 ± 0.02 | 0.85 ± 0.01 | 0.86 ± 0.04 | 0.85 ± 0.03 |
| 1 | 0.84 ± 0.01 a | 0.83 ± 0.01 a | 0.8 ± 0.02 ab | 0.83 ± 0.01 a | 0.81 ± 0.01 ab | |
| 2 | 0.79 ± 0.02 | 0.78 ± 0.01 | 0.8 ± 0.01 | 0.78 ± 0.02 | 0.77 ± 0.01 | |
| 3 | 0.74 ± 0.03 | 0.75 ± 0.01 | 0.76 ± 0.02 | 0.75 ± 0.01 | 0.73 ± 0.02 | |
| 4 | 0.76 ± 0.04 | 0.76 ± 0.02 | 0.75 ± 0.02 | 0.75 ± 0.01 | 0.74 ± 0.03 | |
| 5 | 0.67 ± 0.01 c | 0.75 ± 0.02 a | 0.73 ± 0.02 ab | 0.74 ± 0.01 ab | 0.76 ± 0.02 a |
| Index (%) | Time (d) | Control | 1 µM | 2 µM | 3.5 µM | 4.5 µM |
|---|---|---|---|---|---|---|
| Plasma membrane integrity | 0 | 61.34 ± 1.27 | 62.58 ± 0.96 | 62.48 ± 1.46 | 63.92 ± 1.72 | 62.68 ± 1.37 |
| 1 | 50.63 ± 1.68 b | 51.1 ± 1.96 b | 57.90 ± 1.94 a | 59.73 ± 1.69 a | 56.44 ± 1.76 ab | |
| 2 | 40.99 ± 1.45 b | 40.45 ± 1.15 b | 44.65 ± 1.35 ab | 47.07 ± 1.01 a | 46.98 ± 1.07 a | |
| 3 | 32.54 ± 1.27 c | 39.68 ± 0.86 b | 41.48 ± 1.86 ab | 44.92 ± 1.92 a | 41.68 ± 1.27 ab | |
| 4 | 29.67 ± 0.33 b | 29.92 ± 0.72 b | 36.79 ± 2.17 a | 37.93 ± 1.40 a | 34.13 ± 1.06 a | |
| 5 | 22.76 ± 0.89 c | 25.20 ± 1.08 c | 33.36 ± 0.61 a | 33.15 ± 1.29 a | 29.02 ± 1.54 b | |
| Acrosome Integrity | 0 | 91.2 ± 0.76 | 90.67 ± 0.45 | 91.66 ± 0.37 | 91.56 ± 0.85 | 90.89 ± 0.65 |
| 1 | 84.12 ± 0.84 c | 85.1 ± 0.33 bc | 87.4 ± 0.42 a | 88.11 ± 0.43 a | 86.14 ± 0.83 ab | |
| 2 | 83.31 ± 0.35 b | 84.87 ± 1.07 ab | 86.03 ± 0.49 a | 86.3 ± 0.82 a | 85.46 ± 0.42 ab | |
| 3 | 81.51 ± 1.13 b | 84 ± 0.45 a | 84.32 ± 0.12 a | 85.47 ± 0.77 a | 84.94 ± 0.41 a | |
| 4 | 80.29 ± 0.68 b | 82.06 ± 0.30 a | 83.18 ± 0.56 a | 83.48 ± 0.21 a | 81.88 ± 0.64 a | |
| 5 | 74.69 ± 0.52 b | 76.93 ± 1.08 ab | 79.53 ± 1.24 a | 79.8 ± 0.57 a | 78.91 ± 0.69 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sohail, T.; Zhang, L.; Wang, X.; Jiang, C.; Wang, J.; Sun, X.; Li, Y. Astaxanthin Improved the Quality of Hu Ram Semen by Increasing the Antioxidant Capacity and Mitochondrial Potential and Mitigating Free Radicals-Induced Oxidative Damage. Animals 2024, 14, 319. https://doi.org/10.3390/ani14020319
Sohail T, Zhang L, Wang X, Jiang C, Wang J, Sun X, Li Y. Astaxanthin Improved the Quality of Hu Ram Semen by Increasing the Antioxidant Capacity and Mitochondrial Potential and Mitigating Free Radicals-Induced Oxidative Damage. Animals. 2024; 14(2):319. https://doi.org/10.3390/ani14020319
Chicago/Turabian StyleSohail, Tariq, Liuming Zhang, Xuyang Wang, Caiyu Jiang, Jian Wang, Xiaomei Sun, and Yongjun Li. 2024. "Astaxanthin Improved the Quality of Hu Ram Semen by Increasing the Antioxidant Capacity and Mitochondrial Potential and Mitigating Free Radicals-Induced Oxidative Damage" Animals 14, no. 2: 319. https://doi.org/10.3390/ani14020319
APA StyleSohail, T., Zhang, L., Wang, X., Jiang, C., Wang, J., Sun, X., & Li, Y. (2024). Astaxanthin Improved the Quality of Hu Ram Semen by Increasing the Antioxidant Capacity and Mitochondrial Potential and Mitigating Free Radicals-Induced Oxidative Damage. Animals, 14(2), 319. https://doi.org/10.3390/ani14020319






