The Effects of Harvest Maturity of Eragrostis tef ‘Moxie’ Hay and Supplemental Energy Source on Forage Utilization in Beef Heifers
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Animals and Diets
2.2. Measurements
2.3. Laboratory Analyses
2.4. Calculations
2.5. Statistical Analysis
3. Results
3.1. Dietary Chemical Composition
3.2. Nutrient Intake and Apparent Total-Tract Digestibility
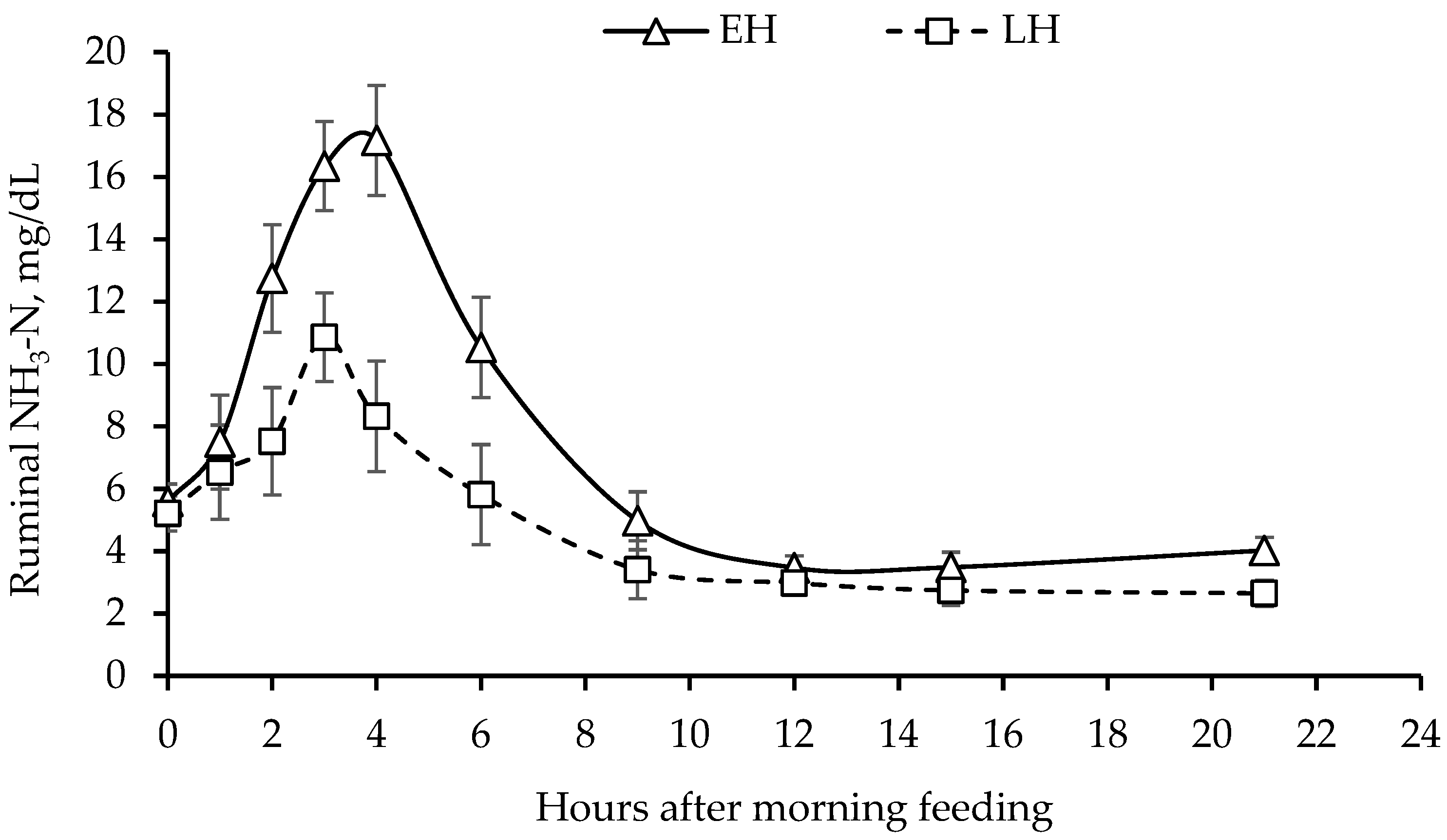
3.3. Ruminal Fermentation Characteristics
3.4. Nitrogen Utilization
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kunkle, W.E.; Johns, J.T.; Poore, M.H.; Herd, D.B. Designing supplementation programs for beef cattle fed forage-based diets. J. Anim. Sci. 2000, 77, 1–11. [Google Scholar] [CrossRef]
- Miller, D. Teff Grass: Crop Overview and Forage Production Guide, 2nd ed. 2011. Available online: https://kingsagriseeds.com/wp-content/uploads/2014/12/Teff-Grass-Management-Guide.pdf (accessed on 8 September 2021).
- Ream, C.N.; Stevens, A.V.; Hall, J.B.; Chibisa, G.E. Harvest maturity of Eragrostis tef ‘Moxie’: Effects on ruminal fermentation, total-tract nutrient digestibility, and growth performance in backgrounding beef cattle. Appl. Anim. Sci. 2020, 36, 600–609. [Google Scholar] [CrossRef]
- Staniar, W.B.; Bussard, J.R.; Repard, N.M.; Hall, M.H.; Burk, A.O. Voluntary intake and digestibility of teff hay fed to horses. J. Anim. Sci. 2010, 88, 3296–3303. [Google Scholar] [CrossRef] [PubMed]
- Vinyard, J.R.; Hall, J.B.; Sprinkle, J.E.; Chibisa, G.E. Effects of maturity at harvest on the nutritive value and ruminal digestion of Eragrostis tef (cv. Moxie) when fed to beef cattle. J. Anim. Sci. 2018, 96, 3420–3432. [Google Scholar] [CrossRef] [PubMed]
- Suggs, J.D.; Sarturi, J.O.; Henry, D.D.; Ciriaco, F.M.; West, C.P.; Ballou, M.A.; Hoffmann, C.A. Ruminal degradation kinetics, intake, digestibility, and feeding behavior of beef steers offered annual or perennial grass-hay with or without supplementation. J. Anim. Sci. 2021, 99, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hersom, M.J. Opportunities to enhance performance and efficiency through nutrient synchrony in forage-fed ruminants. J. Anim. Sci. 2008, 86, E306–E317. [Google Scholar] [CrossRef]
- NASEM. Nutrient Requirements of Beef Cattle, 8th ed.; The National Academies Press: Washington, DC, USA, 2016. [Google Scholar] [CrossRef]
- Penner, G.B.; Beauchemin, K.A.; Mutsvangwa, T. An evaluation of the accuracy and precision of a stand-alone submersible continuous ruminal pH measurement system. J. Dairy Sci. 2006, 89, 2132–2140. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 15th ed.; AOAC: Gaithersburg, MD, USA, 1990. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Valente, T.N.P.; Detmann, E.; de Queiroz, A.C.; Valadares Filho, S.C.; Gomes, D.I.; Figueiras, J.F. Evaluation of ruminal degradation profiles of forages using bags made from different textiles. Rev. Bras. Zootec. 2011, 40, 2565–2573. [Google Scholar] [CrossRef]
- Broderick, G.A.; Kang, J.H. Automated simultaneous determination of ammonia and total amino acids in ruminal fluid and in vitro media. J. Dairy Sci. 1980, 63, 64–75. [Google Scholar] [CrossRef]
- Coats, E.R.; Ibrahim, I.; Briones, A.; Brinkman, C.K. Methane production on thickened, pre-fermented manure. Bioresour. Technol. 2012, 107, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Stentoft, C.; Vestergaard, M.; Løvendahl, P.; Kristensen, N.B.; Moorby, J.M.; Jensen, S.K. Simultaneous quantification of purine and pyrimidine bases, nucleosides and their degradation products in bovine blood plasma by high performance liquid chromatography tandem mass spectrometry. J. Chromatogr. 2014, A1356, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Valadares, R.F.; Broderick, G.A.; Valadares Filho, S.C.; Clayton, M.K. Effect of replacing alfalfa silage with high moisture corn on ruminal protein synthesis estimated from excretion of total purine derivatives. J. Dairy Sci. 1999, 82, 2686–2696. [Google Scholar] [CrossRef] [PubMed]
- Tedeschi, L.O.; Molle, G.; Menendez, H.M.; Cannas, A.; Fonseca, M.A. The assessment of supplementation requirements for grazing ruminants using nutrition models. Transl. Anim. Sci. 2019, 3, 812–828. [Google Scholar] [CrossRef]
- Horn, G.W.; McCollum, F.T. Energy supplementation of grazing ruminants. In Proceedings of the Grazing Livestock Nutrition Conference, Laramie, WY, USA, 23–24 July 1987; pp. 125–136. [Google Scholar]
- Moore, J.E.; Brant, M.H.; Kunkle, W.E.; Hopkins, D.I. Effects of supplementation on voluntary forage intake, diet digestibility, and animal performance. J. Anim. Sci. 1999, 77, 122–135. [Google Scholar] [CrossRef] [PubMed]
- Bowman, J.G.; Sanson, D.W. Starch- or fiber-based energy supplements of grazing ruminants. Proc. West. Sect. Am. Soc. Anim. Sci. 1996, 47 (Suppl. 1), 118–135. [Google Scholar]
- Russell, J.B.; Wilson, D.B. Why are ruminal cellulolytic bacteria unable to digest cellulose at low pH? J. Dairy Sci. 1996, 79, 1503–1509. [Google Scholar] [CrossRef]
- Pulido, R.G.; Muñoz, R.; Lemarie, P.; Wittwer, F.; Orellana, P.; Waghorn, G.C. Impact of increasing grain feeding frequency on production of dairy cows grazing pasture. Livest. Sci. 2009, 125, 109–114. [Google Scholar] [CrossRef]
- Canozzi, M.E.A.; Banchero, G.; Fernandez, E.; La Manna, A.; Clariget, J.M. Individual feed intake and performance of finishing steers on ryegrass pasture supplemented with increasing amounts of corn using an automated feeding system. Livest. Sci. 2023, 269, 105169. [Google Scholar] [CrossRef]
- Sanson, D. Effects of increasing levels of corn or beet pulp on utilization of low-quality crested wheatgrass hay by lambs and in vitro dry matter disappearance of forages. J. Anim. Sci. 1993, 71, 1615–1622. [Google Scholar] [CrossRef]
- France, J.; Dijkstra, J. (Eds.) Volatile fatty acid production. In Quantitative Aspects of Ruminant Digestion and Metabolism, 2nd ed.; CABI: Wallingford, UK, 2005; pp. 157–175. [Google Scholar]
- Johnson, T.R.; Cecava, M.J.; Sheiss, E.B.; Cunningham, K.D. Additions of ruminally degradable crude protein and branched-chain volatile fatty acids to diets containing hydrolyzed feather meal and blood meal for lactating cows. J. Dairy Sci. 1994, 77, 3676–3682. [Google Scholar] [CrossRef] [PubMed]
- Harvatine, K.J.; Allen, M.S. Effects of fatty acid supplements on ruminal and total tract nutrient digestion in lactating dairy cows. J. Dairy Sci. 2006, 89, 1092–1103. [Google Scholar] [CrossRef] [PubMed]
- Heldt, J.S.; Cochran, R.C.; Stokka, G.L.; Farmer, C.G.; Mathis, C.P.; Titgemeyer, E.C.; Nagaraja, T.G. Effects of different supplemental sugars and starch fed in combination with degradable intake protein on low-quality forage use by beef steers. J. Anim. Sci. 1999, 77, 2793–2802. [Google Scholar] [CrossRef] [PubMed]
- Hoover, W.H.; Stokes, S.R. Balancing carbohydrates and proteins for optimum rumen microbial yield. J. Dairy Sci. 1991, 74, 3630–3644. [Google Scholar] [CrossRef] [PubMed]
- Russell, J.B.; Sniffen, C.J.; Van Soest, P.J. Effect of carbohydrate limitation on degradation and utilization of casein by mixed rumen bacteria. J. Dairy Sci. 1983, 66, 763–775. [Google Scholar] [CrossRef]
- Russell, J.B.; Strobel, H.J. Concentration of ammonia across cell membranes of mixed rumen bacteria. J. Dairy Sci. 1987, 70, 970–976. [Google Scholar] [CrossRef]
- Hristov, A.N.; Ropp, J.K.; Grandeen, K.L.; Abedi, S.; Etter, R.P.; Melgar, A.; Foley, A.E. Effect of carbohydrate source on ammonia utilization in lactating dairy cows. J. Anim. Sci. 2015, 83, 408–421. [Google Scholar] [CrossRef]
- Hristov, A.N.; Jouany, J.-P. Factors affecting the efficiency of nitrogen utilization in the rumen. In Nitrogen and Phosphorus Nutrition of Cattle: Reducing the Environmental Impact of Cattle Operations; Pfeffer, E., Hristov, A.N., Eds.; CABI International: Cambridge, MA, USA, 2005; pp. 117–166. [Google Scholar] [CrossRef]
- Reynal, S.M.; Broderick, G.A. Effect of dietary level of rumen-degraded protein on production and nitrogen metabolism in lactating dairy cows. J. Dairy Sci. 2005, 88, 4045–4064. [Google Scholar] [CrossRef]
| Item | Feedstuff 1 | |||
|---|---|---|---|---|
| EH Teff Hay | LH Teff Hay | Corn Grain | Beet Pulp | |
| Chemical analysis | ||||
| Dry matter | 92.5 ± 0.46 | 92.7 ± 0.30 | 86.5 ± 1.60 | 91.8 ± 0.20 |
| Organic matter | 89.3 ± 0.58 | 86.7 ± 1.32 | 98.7 ± 0.17 | 86.0 ± 0.43 |
| Crude protein | 14.1 ± 0.22 | 12.4 ± 0.32 | 8.6 ± 0.37 | 6.9 ± 0.09 |
| Acid detergent fiber | 30.7 ± 0.67 | 31.6 ± 0.01 | 1.6 ± 0.06 | 30.7 ± 0.25 |
| Neutral detergent fiber | 62.9 ± 0.87 | 63.4 ± 1.28 | 9.9 ± 0.58 | 45.3 ± 0.49 |
| Starch | 0.06 ± 0.014 | 1.05 ± 0.071 | 73.3 ± 5.8 | 0.90 ± 0.07 |
| Water soluble carbohydrates | 4.60 ± 0.141 | 3.45 ± 0.071 | - | - |
| Variable | Harvest Maturity | SEM | Supplement | SEM | p-Value 1 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| EH | LH | CON | Beet Pulp | Corn Grain | HM | SP | HM × SP | |||
| Intake 2, kg/d | ||||||||||
| DM | 12.2 | 11.3 | 0.37 | 9.8 a | 12.6 b | 12.8 b | 0.47 | 0.13 | <0.01 | 0.90 |
| OM | 10.7 | 10.2 | 0.36 | 8.6 a | 11.0 b | 11.6 b | 0.44 | 0.34 | <0.01 | 0.90 |
| NDF | 7.37 | 6.73 | 0.255 | 6.70 | 7.73 | 6.72 | 0.312 | 0.11 | 0.07 | 0.89 |
| ADF | 3.73 | 3.35 | 0.124 | 3.31 a | 4.12 b | 3.20 a | 0.151 | 0.055 | <0.01 | 0.91 |
| CP | 1.56 x | 1.32 y | 0.050 | 1.32 | 1.45 | 1.55 | 0.059 | <0.01 | 0.06 | 0.90 |
| ATTD 3, % | ||||||||||
| DM | 55.2 x | 43.1 y | 2.61 | 42.6 | 52.8 | 52.1 | 3.20 | <0.01 | 0.09 | 0.80 |
| OM | 59.0 x | 48.8 y | 2.40 | 48.1 | 56.7 | 57.0 | 2.94 | 0.01 | 0.096 | 0.84 |
| NDF | 60.2 x | 48.8 y | 2.25 | 54.6 | 56.5 | 52.4 | 2.75 | <0.01 | 0.58 | 0.88 |
| ADF | 57.4 x | 47.3 y | 2.55 | 51.1 | 57.0 | 48.9 | 3.12 | 0.02 | 0.23 | 0.91 |
| CP | 58.7 x | 44.2 y | 3.10 | 52.0 | 53.4 | 49.1 | 3.80 | <0.01 | 0.72 | 0.93 |
| Variable | Harvest Maturity | SEM | Supplement | SEM | p-Value 1 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| EH | LH | CON | Beet Pulp | Corn Grain | HM | SP | HM × SP | |||
| Intake, kg/d | ||||||||||
| Total | 12.2 | 11.3 | 0.37 | 9.8 a | 12.6 b | 12.8 b | 0.47 | 0.13 | <0.01 | 0.90 |
| Forage | 9.83 | 8.88 | 0.348 | 9.83 | 8.88 | 9.35 | 0.427 | 0.08 | 0.33 | 0.85 |
| pH | ||||||||||
| Mean | 6.38 | 6.37 | 0.048 | 6.46 a | 6.41 a | 6.25 b | 0.046 | 0.79 | 0.04 | 0.60 |
| Minimum | 5.91 | 5.96 | 0.088 | 6.06 | 6.02 | 5.73 | 0.090 | 0.74 | 0.09 | 0.53 |
| Maximum | 6.75 | 6.78 | 0.036 | 6.83 | 6.75 | 6.72 | 0.044 | 0.55 | 0.29 | 0.50 |
| DUR 2 | ||||||||||
| <6.2 | 319 | 304 | 93.7 | 146 a | 239 a | 551 b | 93.8 | 0.92 | 0.049 | 0.36 |
| <5.8 | 30.4 | 82.9 | 28.71 | 8.1 | 36.7 | 125.2 | 40.13 | 0.25 | 0.13 | 0.14 |
| AUC 3 | ||||||||||
| <6.2 | 57.2 | 89.1 | 28.48 | 23.1 | 50.0 | 146.6 | 33.30 | 0.43 | 0.08 | 0.18 |
| <5.8 | 3.59 | 16.03 | 6.176 | 0.46 | 6.10 | 22.87 | 7.783 | 0.17 | 0.15 | 0.13 |
| Acidosis index 4 | 0.275 | 1.321 | 0.5113 | 0.045 | 0.483 | 1.867 | 0.6518 | 0.17 | 0.15 | 0.14 |
| SCFA 5 | ||||||||||
| Total, mmol/L | 80.1 | 75.7 | 2.15 | 77.3 | 77.9 | 78.4 | 3.49 | 0.22 | 0.95 | 0.74 |
| Acetate, % | 77.6 | 77.0 | 0.63 | 78.9 a | 78.2 a | 74.9 b | 0.65 | 0.54 | <0.01 | 0.71 |
| Propionate, % | 15.4 | 14.3 | 0.47 | 14.5 | 15.0 | 15.2 | 0.58 | 0.12 | 0.67 | 0.97 |
| Butyrate, % | 5.27 x | 6.83 y | 0.317 | 4.84 a | 5.29 a | 8.01 b | 0.334 | 0.03 | <0.01 | 0.44 |
| Valerate, % | 0.412 | 0.475 | 0.0379 | 0.455 | 0.389 | 0.488 | 0.0464 | 0.27 | 0.35 | 0.72 |
| Isobutyrate, % | 0.662 | 0.687 | 0.0371 | 0.674 | 0.624 | 0.724 | 0.0350 | 0.66 | 0.12 | 0.79 |
| Isovalerate, % | 0.584 | 0.655 | 0.0361 | 0.616 a | 0.510 a | 0.733 b | 0.0367 | 0.24 | <0.01 | 0.53 |
| Total BCFA 6, % | 1.24 | 1.34 | 0.073 | 1.29 a | 1.13 b | 1.46 a | 0.064 | 0.40 | <0.01 | 0.51 |
| A:P ratio 7 | 5.04 | 5.42 | 0.184 | 5.48 | 5.22 | 5.01 | 0.225 | 0.18 | 0.37 | 0.96 |
| Variable | Harvest Maturity | SEM | Supplement | SEM | p-Value 1 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| EH | LH | CON | Beet Pulp | Corn Grain | HM | SP | HM × SP | |||
| Intake | ||||||||||
| N, g/d | 249 x | 212 y | 8.8 | 211 a | 232 ab | 249 b | 11.1 | <0.01 | 0.048 | 0.90 |
| Fecal excretion | ||||||||||
| DM, kg/d | 5.42 | 6.30 | 0.315 | 5.58 | 5.88 | 6.12 | 0.386 | 0.07 | 0.62 | 0.92 |
| N, g/d | 103 | 117 | 8.5 | 99 | 106 | 126 | 9.6 | 0.30 | 0.08 | 0.96 |
| N, % of N intake | 41.3 x | 55.6 y | 3.84 | 48.0 | 46.6 | 50.7 | 4.87 | 0.02 | 0.77 | 0.94 |
| Urinary excretion | ||||||||||
| Total output, kg/d | 17.1 | 12.8 | 1.75 | 16.5 | 15.1 | 13.3 | 2.22 | 0.095 | 0.58 | 0.82 |
| N, g/d | 178 | 145 | 17.3 | 179 | 165 | 139 | 22.0 | 0.19 | 0.42 | 0.50 |
| Urea-N, g/d | 118 | 91 | 12.5 | 116 | 108 | 90 | 15.8 | 0.13 | 0.47 | 0.57 |
| Urea-N, % urine N | 66.7 | 62.0 | 2.23 | 64.6 | 64.2 | 64.3 | 2.83 | 0.15 | 0.99 | 0.93 |
| Total N, % N intake | 71.8 | 70.9 | 8.76 | 86.0 | 71.7 | 56.3 | 11.11 | 0.94 | 0.18 | 0.51 |
| Allantoin, mmol/d | 199 x | 119 y | 17.2 | 167 | 154 | 156 | 21.9 | <0.01 | 0.90 | 0.50 |
| Uric acid, mmol/d | 55.0 x | 22.8 y | 4.18 | 36.6 | 32.8 | 47.3 | 5.30 | <0.01 | 0.16 | 0.051 |
| Total PD 2, mmol/dL | 254 x | 142 y | 18.8 | 203 | 187 | 210 | 23.9 | <0.01 | 0.83 | 0.26 |
| Microbial N, g/d | 117.2 x | 50.4 y | 11.40 | 86.7 | 77.2 | 87.5 | 13.3 | <0.01 | 0.83 | 0.29 |
| Total N excretion, | ||||||||||
| g/d | 281 | 261 | 20.3 | 278 | 271 | 264 | 25.7 | 0.49 | 0.92 | 0.54 |
| % N of intake | 113 | 126 | 11.0 | 134 | 118 | 107 | 12.4 | 0.39 | 0.37 | 0.58 |
| N retention, g/d | −31.9 | −48.9 | 22.96 | −67.2 | −38.9 | −14.9 | 29.1 | 0.60 | 0.43 | 0.67 |
| Rumen NH3-N, mg/dL | 10.04 | 7.98 | 0.816 | 9.21 | 9.67 | 8.16 | 1.000 | 0.095 | 0.56 | 0.87 |
| Plasma urea-N, mg/dL | 13.6 | 13.5 | 0.04 | 13.5 | 13.7 | 13.5 | 0.05 | 0.22 | 0.11 | 0.27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stevens, A.V.; Myers, C.A.; Hall, J.B.; Chibisa, G.E. The Effects of Harvest Maturity of Eragrostis tef ‘Moxie’ Hay and Supplemental Energy Source on Forage Utilization in Beef Heifers. Animals 2024, 14, 254. https://doi.org/10.3390/ani14020254
Stevens AV, Myers CA, Hall JB, Chibisa GE. The Effects of Harvest Maturity of Eragrostis tef ‘Moxie’ Hay and Supplemental Energy Source on Forage Utilization in Beef Heifers. Animals. 2024; 14(2):254. https://doi.org/10.3390/ani14020254
Chicago/Turabian StyleStevens, Allison V., Cheyanne A. Myers, John B. Hall, and Gwinyai E. Chibisa. 2024. "The Effects of Harvest Maturity of Eragrostis tef ‘Moxie’ Hay and Supplemental Energy Source on Forage Utilization in Beef Heifers" Animals 14, no. 2: 254. https://doi.org/10.3390/ani14020254
APA StyleStevens, A. V., Myers, C. A., Hall, J. B., & Chibisa, G. E. (2024). The Effects of Harvest Maturity of Eragrostis tef ‘Moxie’ Hay and Supplemental Energy Source on Forage Utilization in Beef Heifers. Animals, 14(2), 254. https://doi.org/10.3390/ani14020254





