Simple Summary
Ex vivo, mouse, and human studies have pointed to the gut microbiota as playing roles in many diseases, including asthma susceptibility and severity. Equine asthma shares many similarities with human asthma, and gut microbiota could also be critical components in the pathophysiology of the disease. The purpose of this review was to describe the current knowledge on the potential role of the understudied gut–lung axis in the pathophysiology of equine asthma.
Abstract
Both microbe–microbe and host–microbe interactions can have effects beyond the local environment and influence immunological responses in remote organs such as the lungs. The crosstalk between the gut and the lungs, which is supported by complex connections and intricate pathways, is defined as the gut–lung axis. This review aimed to report on the potential role of the gut–lung gut–lung axis in the development and persistence of equine asthma. We summarized significant determinants in the development of asthma in horses and humans. The article discusses the gut–lung axis and proposes an integrative view of the relationship between gut microbiota and asthma. It also explores therapies for modulating the gut microbiota in horses with asthma. Improving our understanding of the horse gut–lung axis could lead to the development of techniques such as fecal microbiota transplants, probiotics, or prebiotics to manipulate the gut microbiota specifically for improving the management of asthma in horses.
1. Introduction
Equine asthma is a chronic and complex disease characterized by airway inflammation, mucus hypersecretion, and bronchoconstriction in response to inhaled antigens. It affects up to 15% of adult horses in its severe form and can greatly impact a horse’s athletic performance and quality of life [1,2]. Inhalation of environmental allergens such as antigens from mites and fungi, pollens, and endotoxins can trigger clinical exacerbations. During these exacerbations, airway hyperresponsiveness (AHR) leads to respiratory obstruction and clinical signs such as increased respiratory rate and effort, cough, and wheezing [3,4]. Although the role of aerosolized antigens in the pathophysiology of equine asthma exacerbations is well established, the factors contributing to the development and persistence of the disease remain largely uncertain. Recent research in rodents and humans has highlighted the significance of the gut–lung axis in the development of asthma [5,6,7].
In recent years, advancements in next-generation sequencing and bioinformatics have greatly enhanced our knowledge of equine gastrointestinal microbiota [8,9,10]. However, due to significant intra- and inter-host variability, it remains challenging to establish clear criteria for defining healthy microbiota. However, it is now understood that functional gut microbiota play a variety of physiological roles, such as producing short-chain fatty acids (SCFAs), enhancing local mucosal immunity, and promoting immunotolerance [6]. Fecal microbiota are greatly influenced by an individual’s diet and environment, and the intestinal microbiota of horses with and without asthma adapt differently to environmental changes, highlighting the possible role of altered gut microbiota in asthma [11,12]. In this review, the terms microbiota, referring to the microbes colonizing body surfaces, and microbiome, defined as the genome of these microbes, were employed according to their uses in the original references cited. Although microbiota include fungi, parasites, and viruses, this review mainly focused on bacterial microbiota.
The literature on the gut–lung axis and host–microbial community interactions in horses is sparse as current research on microbiota in horses is mostly descriptive and focuses on either the gut or the respiratory microbes separately [13]. Addressing the causality conundrum of the chicken or the egg concerning the role of the microbiota in equine asthma was a challenge. This review aimed to evaluate the potential impact of the gut–lung axis on the development and persistence of equine asthma. In this article, we first summarize the major determinants in the pathogenesis of horses and humans with asthma. We then discuss the gut–lung axis and propose an integrative view of the interactions between gut microbiota and asthma. Finally, we review therapies targeting the modulation of gut microbiota in horses.
2. Methodology
Publications were selected if they were in English and reported original research or reviewed literature on asthma or gut microbiota in horses. Studies on humans and laboratory animals and ex vivo studies were included if the results were relevant. The following keywords were used to conduct most of the literature searches using different databases (e.g., Pubmed, ScienceDirect, and Scopus): mild to moderate and severe equine asthma, inflammatory airway disease, heaves, recurrent airway obstruction, equine gut microbiota, equine gut microbiome, equine gut dysbiosis, equine probiotics, gut–lung axis, asthma, and dysbiosis. The most recent literature search was carried out in December 2023.
3. Determinants in the Pathogenesis of Asthma: A Focus on the Impact of Gut Microbiota
3.1. Role of the Environmental Microbiome
3.1.1. Inhaled Antigens as Causative Agents
The environment is a key factor in the development of equine asthma exacerbations, as demonstrated by the favorable response of affected horses to hay avoidance [2,14]. Horses that are kept in stables are cumulatively exposed to high levels of aerosolized particles such as fungi, endotoxins, mites, inorganic dust, and beta-D-glucans [15,16,17]. In young Thoroughbred and Standardbred racehorses, both tracheal mucus and bronchoalveolar lavage fluid (BALF) eosinophilia and neutrophilia are associated with respirable particle exposure [18,19,20]. Furthermore, older horses with asthma develop BALF neutrophilia when exposed to high levels of dust extracts and endotoxins [21]. These findings support the evidence that inhaled antigens from the environment are key factors in the pathophysiology of asthma. The impact of other environmental factors on airway inflammation such as exposure to pollutants, variations in environmental temperature, and pollen are also likely contributors to the disease severity but to a lesser extent [19,22,23].
3.1.2. Antigens as Protective Agents
Inhaled antigens play a role in the initiation of asthma exacerbations, but exposure to antigens at an early age may also be protective against the disease. According to Strachan’s hygiene hypothesis, as proposed in 1989, the escalating incidence of allergic diseases such as asthma could be, in part, caused by reduced exposure to environmental antigens that have resulted from higher standards of cleanliness and limited contact with animals [24]. In a mouse model of allergic asthma, the intranasal administration of grass arabinogalactan, an extract from cowshed dust, which is a source of immuno-modulating substances, prevented mice from developing allergic airway inflammation and AHR [25]. Studies evaluating the incidence of asthma in children raised on farms or in urban settings have also highlighted the protective effect of the environment on the development of allergic diseases [26,27,28]. Supporting Strachan’s hygiene hypothesis, hay fever and atopic asthma in children are inversely correlated with endotoxin (a bacterial wall component) concentrations in their mattresses [29]. The environmental microbiome could even have an impact on asthma susceptibility before birth as offspring from pregnant mice exposed to a cowshed-derived bacterium were protected from asthma [30]. Similar findings were observed in humans as maternal exposure to an agricultural environment was associated with the increased protection of the children against allergic illnesses such as asthma [31,32]. These studies and others support the following two main hypotheses that are not mutually exclusive: early antigen exposure could induce immune tolerance to those antigens later in life, and exposure to a microbe-rich environment could make the gastro-intestinal, skin, and respiratory microbiota richer and more diverse, which has indirect and, in general, beneficial effect on a host’s immune system, which is discussed later in this article. Whether exposure to stables with higher levels of aerosolized particles, microbe-rich environments, or use/overuse of antimicrobials during pregnancy or early in life have protective or detrimental effects on asthma susceptibility in horses has not been investigated. Nevertheless, one abstract reported that there is a significant association between the diversity of gut bacteria at one month of age and the risk of several adverse health outcomes later in life [33].
3.2. Role of Airway Microbiota and Pathogens
The lungs have traditionally been thought of as a sterile environment. The presence of a respiratory tract microbiome is now well-recognized, in part due to the development of next-generation sequencing [34]. In horses, tracheal and pulmonary microbiota have been studied with culture-free methods, such as qPCR and next-generation sequencing [35,36,37]. Lower respiratory tract microbiota are thought to originate from the oropharynx following mucosal dispersion and micro-aspirations, which result in lower airway diversity and richness compared to the upper airways in humans and horses [32,36,38,39]. These findings have contributed to the hypothesis that, rather than microbial persistence and growth within the lower respiratory tract, the bacterial microbiota in the lungs of healthy individuals are determined by the state of balance between the migration of bacteria from the upper airway and their removal [40]. The role of respiratory microbes in preserving a healthy lung milieu, notably by regulating the immune system and preventing the spread of respiratory pathogens, is now acknowledged. As in other systems, they can produce metabolites, compete with potential pathogens, and contribute to maintaining homeostasis (Figure 1) [41]. To date, some mechanistic studies have demonstrated the relative importance of the airway microbiota in maintaining a healthy lung environment. One of them showed that human-derived oral commensal bacteria administered in the tracheas of mice induced T-helper cell type 17 responses and increased resistance to a common bacteria pathogen (Streptococcus pneumoniae) [42]. Furthermore, altered respiratory microbiota have been proposed as the cause of the persistence and perpetuation of airway inflammation [43].
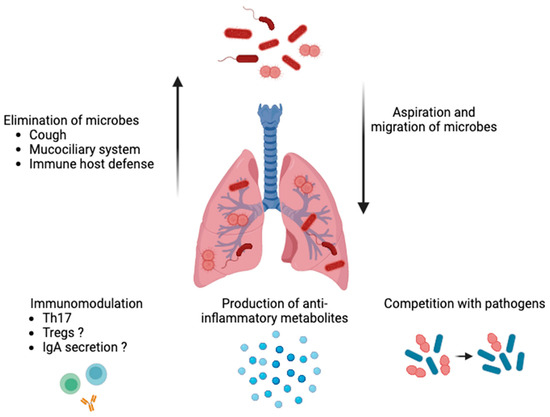
Figure 1.
Role of airway microbiota in healthy lungs. The bacterial microbiota are determined by the state of balance between the micro-aspirations from the upper airways and migration of bacteria along the mucosal surfaces and their clearance (e.g., via coughing, the mucociliary system, and a host’s immune defenses). Respiratory microbiota can produce metabolites, compete with potential pathogens for space and nutrients, contribute to maintaining homeostasis (e.g., pH and oxygen tension levels), and promote immunomodulation (by inducing a T-helper cell-type 17 response and, possibly, regulatory T cells (Tregs) and immunoglobulins (Ig) A). The image was created using BioRender.com, accessed on 16 December 2023.
In horses, most data have been observational. The lower airway microbiota of healthy horses and horses with asthma markedly differ from one another according to recent studies [35,37]. Manguin et al. found that sport horses with asthma had decreased abundances of the commensal bacteria Corynebacterium spp. as well as lower overall bacterial loads based on 16S rRNA gene qPCR [35]. This suggested that bacterial overgrowth is not a prominent feature of asthma in middle-aged horses, which contradicted past findings in racehorses, which are typically younger. The association between bacterial and viral pathogens, tracheal mucus, tracheal inflammation, and respiratory disease in racehorses has also been observational [44,45,46]. Environmental contamination and increased micro aspirations during strenuous exercise could play roles in mild-to-moderate asthma, as could common viruses such as equine rhinitis and herpes, but it is difficult to conclude on their contributions since they are ubiquitous [47,48]. Furthermore, the findings of past studies investigating lower airway microbiota and asthma need to be interpreted with caution as the environment and corticosteroid therapy both affect lower airway microbiota compositions in horses. Indeed, the tracheal microbiota in healthy and asthmatic horses are modified following systemic and nebulized dexamethasone administration [36].
In humans, asthma severity, prevalence, phenotype, AHR, and response to treatment are associated with airway dysbiosis. For example, certain bacteria (i.e., Moraxella) have been associated with increased abundances of neutrophils in the sputum samples of subjects with asthma, and a reduced response to corticosteroid therapy is linked to the presence of Haemophilus [49,50,51]. The influence of airway microbiota on asthma susceptibility may start very early in life. Upper airway microbes are acquired at birth, and the mode of delivery influences the composition and richness of the respiratory microbial populations. This could explain why offspring born via Caesarian section are at higher risk of developing asthma [52,53]. The influence of the mode of delivery on microbiota is further discussed in Section 4.1. The respiratory microbiota diversity and composition during the first months of life may have a significant impact on an infant’s susceptibility to asthma. Bisgaard et al. showed that the presence of certain bacteria such as Streptococcus pneumoniae in the hypopharynx of one-month old babies could predict diagnoses of asthma 5 years later [54]. Similarly, the nasopharyngeal colonization of babies with Streptococcus pneumoniae along with Haemophilus influenzae and Moraxella catarrhalis has been associated with asthma at 7 years of age [55]. Therefore, the importance of the respiratory microbiome at a young age has been highlighted in human medicine, but long-term longitudinal studies on horses are rare [33]. Since Caesarean sections are uncommon in mares, it is unlikely that a potential effect of the mode of delivery on the prevalence of equine asthma could be studied in a timely manner. However, other factors that could influence respiratory microbiota diversity at an early age could play roles, such as being born in a crowded barn versus in a field, being exposed to other foals, and being exposed to antimicrobials during pregnancy and in the first few months of life. Furthermore, the extent to which the fecal microbiota can impact the vaginal microbiota and, therefore, a foal’s colonization deserves better investigation [56]. Wild horses and horses receiving forage-only diets have higher diversity and different compositions [57,58].
3.3. Impact of Lifestyle on Microbiota and Asthma Susceptibility
3.3.1. Diet and Obesity
One of the ways digestive health could influence respiratory health and, specifically, asthma prevalence and severity, could be via poor diet, altered microbiota, and obesity. Studies in the human literature suggest that poor diet quality, obesity, and sedentary lifestyles increase asthma susceptibility, worsen prognosis, and influence asthma phenotypes [59,60]. In addition to the mechanical interference of adipose tissue with the movements of the rib cage and diaphragm, the link between obesity and asthma could be, in part, explained by leptin, an inflammatory mediator secreted by adipose tissue that promotes T cell proliferation and activation, as well as macrophages recruitment [61]. In lean mice sensitized with ovalbumin (OVA), AHR was enhanced following leptin infusion, and microbiota-depleted mice had enhanced leptin sensitivity [62,63]. Dysbiosis could, therefore, theoretically increase leptin sensitivity, and its inflammatory effects could promote AHR. Leptin levels are increased in overweight horses, but leptin’s association with asthma has not yet been studied [64]. Not only does obesity increase the likelihood of developing asthma, but it also makes the control of the disease more challenging. When evaluating the relationship between body mass index and response to fluticasone (an inhaled corticosteroid) with or without salmeterol (a long-acting β agonist), Boulet and Franssen found decreased likelihoods of achieving asthma control in class 3 obese patients (6% of those receiving fluticasone or a combination of fluticasone and salmeterol) when compared to lean patients (78% of those receiving the same treatments) [65]. The link between the gut microbiome and obesity is described in the next paragraphs.
The literature evaluating the association between asthma and obesity in horses is sparse. Limited evidence has suggested that obesity is a risk factor for equine asthma [66,67]. This is particularly interesting in the face of the proposed mechanism described above because obesity in horses is often a consequence of inadequate exercise and “poor diet”, such as high-energy diets rich in non-structural carbohydrates and low in soluble fibers. However, mild and moderate asthma are frequent in performance horses and racehorses who are typically not obese. The link between obesity, microbiota, and equine asthma remains unexplored, for now, but the association between obesity, endocrine diseases, and gut microbiota compositions in horses has been investigated. Biddle et al. found that both richness and diversity were increased in obese horses whereas Elzinga et al. identified decreased diversity in horses affected by equine metabolic syndrome (i.e., a predisposing factor to obesity) [68,69]. A possible explanation for the conflicting results may have stemmed from the fact that diet was not controlled in either study. In a diet-controlled study with a more homogenous population, fecal microbiome diversity and Bacteroidetes abundance were increased in obese horses [70]. In addition, murine macrophages exposed to fecal extracts from obese horses exhibited increased expressions of inflammatory markers such as IL-1β, TNF-α, and IL-6 when compared to those exposed to fecal extracts from non-obese horses [71].
Diet and the gut microbiome could also contribute to the connection between obesity and asthma. As they are usually low in soluble fibers, obesogenic diets are associated with altered microbiomes, decreased metabolites production by gut bacteria, such as SCFA which have anti-inflammatory and immunomodulatory properties [72]. Major SCFA producers such as Bacteroidetes are reduced in obese patients [73]. The impact of low- and high-fiber diets on SCFA concentrations and asthma was elegantly shown in mice, where low concentrations of propionate (SCFA) were associated with increased allergic airway inflammation levels and AHR [7]. Short-chain fatty acid administration (i.e., propionate and acetate) also attenuated or inhibited the development of allergic airway inflammation in this study. Microbiota metabolites and diet could, therefore, both contribute to the dysregulation of inflammatory homeostasis occurring in asthma and in gut–lung axis crosstalk (detailed in Section 3). Notably, the relative abundance of Fibrobacter, an SCFA-producer, was increased in healthy horses eating hay but not in horses with asthma on the same diet, which suggested that this crosstalk could also apply to horses [12]. However, SCFAs were not measured in that study, and so this remains speculative.
3.3.2. Exercise
Although it is clear that a lack of physical activity can contribute to human obesity and, consequently, to asthma, increased asthma prevalence and worsened asthma control have been associated with physical inactivity even in non-obese patients, suggesting that other factors are involved [74]. Improvements in AHR and cellular airway composition have been shown with regular physical activity, though the exact mechanisms by which asthma outcomes are improved are not fully understood [75]. Interestingly, decreases in eosinophils and total cells in the sputum and BALF of humans and ovalbumin-sensitized mice, respectively, have been observed following exercise [76,77,78]. Metabolomic pathways can also be affected by physical activity, with high concentrations of butyrate being associated with good cardiorespiratory fitness and mitigation of the negative impacts of a high-fat diet on the gastrointestinal microbiome [79,80].
The association between asthma and physical inactivity has not been investigated in horses, although there is limited evidence suggesting that intense training can transiently modify the gut microbiota composition [81]. In one study, blood metabolomics (including alanine and valine) before an endurance race were associated with gut microbiota but not with performance [82]. However, the 1H nuclear magnetic resonance approach used in this study only detected metabolites with high concentrations, and the interpretation of metabolite peaks can be ambiguous with this technique. The stress associated with intense exercise could also induce lower-airway inflammation as stress-related behaviors (touching a rubber tie-cord) are correlated with tracheal inflammation [83]. Yet, such behaviors are also correlated with a decreased frequency of head lowering, which may also affect tracheal inflammation. Because moderate exercise following transport increases intestinal permeability and systemic inflammation biomarkers (i.e., serum amyloid A and lipopolysaccharide) in horses, it can be hypothesized that bacterial translocation from the gastrointestinal tract to the respiratory tract could result in lower airway inflammation and/or be involved in asthma pathophysiology [84].
3.3.3. Antibiotic Exposure
Antibiotic exposure is yet another variable affecting the microbiome, and consequently, it has the potential to increase asthma susceptibility. Microbiome composition modifications following antimicrobial administration in horses are now being recognized. For example, fecal microbiota were modified shortly after the initiation of antibiotic treatment in healthy horses, and the alterations in the bacterial communities took at least 25 days to recover from [8]. Bacterial species diversity and richness were significantly decreased following trimethoprim sulfadiazine administration [8,85], and the relative abundance of Bacteroidetes decreased after ceftiofur administration [86]. A decrease in SCFA-producing bacteria such as Bacteroidetes could increase asthma susceptibility, but to date, convincing evidence that the fecal microbiota modifications observed following antibiotic administration in horses contributes to airway inflammation is lacking.
In humans, a systematic review by Baron et al. concluded that there is a moderate amount of evidence for an association between early life exposure to antibiotics and childhood asthma [87]. Furthermore, in a cohort of 143,000 children, asthma was associated with antibiotic administration in the first year of life [88]. In agreement with those findings, early alterations in the microbiome following antibiotic administration can also affect immune function and IgA responses, which are associated with an increased susceptibility to human allergic diseases [89]. Dysbiosis resulting from antibiotic administration therefore appears to increase susceptibility to asthma, but mostly later in life. This could explain why exacerbations are not typically observed clinically immediately following antibiotics administration, in horses with asthma.
3.3.4. Sex
Before puberty, asthma is more prevalent in boys. However, after puberty, asthma is more common in females, and women experience more asthma-related morbidity and mortality. The reasons for this are likely multifactorial, but sex hormones appear to play a role, as testosterone appears to have a protective effect against asthma [90]. In horses with asthma, there have been reports of a predisposition for mares [91], but this is controversial [1]. Applying what we know from human medicine, we can hypothesize that the protective effect of having more testosterone is lost in male horses because most males are geldings. This could explain why differences are not consistently observed between males and females. According to Mshelia et al., there were significant differences in the fecal microbiome of mares and stallions [92]. However, the study did not include any geldings. In mice, the microbiome may contribute to sex differences observed in airway hyperresponsiveness, as these sex differences disappear when the mice are treated with antibiotics to ablate the gut microbiome [93]. In humans, airway microbiome differ between males and females, and there is an association between airway microbial markers, asthma, and sex [94]. However, to the authors’ knowledge, there are no known sex-specific patterns in the gut microbiota of humans associated with asthma.
4. Gut–Lung Axis
Host–microbe interactions can exert impacts beyond their local environments and influence immunological responses in remote organs. Both microbe–microbe and host–microbe interactions can have long-reaching effects, and the crosstalk between the gut and the lungs is defined as the gut–lung axis. The gut–lung axis concept is supported by complex connections and intricate pathways involving both the gut and lung microbiota [95]. As of now, most recognized pathways are in the gut-to-lung direction.
4.1. Short-Chain Fatty Acids: Chemical Messengers
Short-chain fatty acids such as acetate, butyrate, and propionate are produced through the fermentation of fibers by fibrolytic bacteria such as members of the phyla Bacteroidetes and Fibrobacter. They regulate the barrier function of the gut by stimulating intestinal epithelial cells to secrete mucus and antimicrobial peptides, and they also upregulate tight junction proteins [96]. SCFAs can also attenuate inflammatory and allergic responses in the lungs by communicating with pulmonary antigen-presenting cells [96]. For example, diets with high-fiber contents increase circulating SCFAs, which modulate dendritic cell function in the lungs, as was demonstrated in a mice model of asthma [7]. In that study, propionate administration enhanced dendritic cells’ phagocytic activities and decreased their capacity to induce T2 inflammation responses. SCFAs can also alter cytokine and chemokine production and inflammatory cell proliferation and affect local and systemic immunity by promoting regulatory T cells (Tregs) [96]. The role of SCFAs in local and systemic immunity is illustrated in Figure 2.
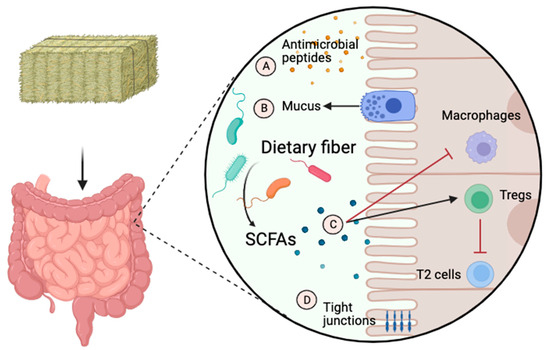
Figure 2.
The role of short-chain fatty acids in local and systemic immunity. Short-chain fatty acids (SCFAs) such as acetate, butyrate, and propionate are produced through the fermentation of fibers by fibrolytic bacteria. They regulate the gut’s barrier function by stimulating intestinal epithelial cells to secrete antimicrobial peptides (A) and mucus (B). They attenuate inflammatory and allergic responses in the lungs (C) by communicating with regulatory T cells (Tregs) and antigen-presenting cells such as macrophages. They also upregulate tight junction proteins (D). T2, type 2 inflammation. The image was created using BioRender.com, accessed on 16 December 2023.
4.2. Regulatory T Cells and T2 Responses
Tregs play central roles in antigen tolerance and immune homeostasis, especially in allergic diseases. The production of local and systemic mediators by gut anaerobic bacterial fermentation, primarily SCFAs, can regulate the generation of Tregs, which modulate T2 responses. Germ-free mice colonized with microbiota of lower diversity were more susceptible to developing T2 responses, atopy, and asthma [6,97]. Such increased T2 activity can lead to a hyperreactive response against commensal bacteria, which is normally considered inoffensive. The increased mRNA expression of both IL-4 and IL-5 in the pulmonary lymphocytes of asthmatic horses suggested that the dysregulation of T2 responses contributed to the pathophysiology of the disease [98].
The role of Tregs is not limited to T2 response modulation, and it includes the regulation of mucosal antibody production (i.e., IgA). Tregs reduce systemic inflammation and CD4+ T cell activation by modulating the secretion of IgA to eliminate microbial ligands [99]. The proportion of Tregs depends on the gut microbiota composition, as members of the genera Clostridium, Lactobacillus, and Bifidobacterium enhance their proliferation [100]. Theoretically, gut dysbiosis could alter the Tregs’ regulation of IgA secretion and contribute to asthma susceptibility. For example, lower levels of IgA-bound bacteria in children increased the risk of developing asthma [89].
4.3. Innate Lymphoid Cells and Mucosal Immunity
Innate lymphoid cells (ILCs) lack typical lymphocyte surface markers but can release cytokines and express genes such as T helper cells. They are particularly abundant in the gut and lung mucosa and have receptors for cytokines released from damaged tissues [101]. ILCs play a crucial role in mucosal immunity, notably, by inhibiting viral and bacterial infections through the secretion of interferon gamma (IFN-y) and IL-22 [102]. Through MHC (major histocompatibility complex)-II mediated antigen presentation, ILCs also contribute to antigen tolerance. The gut microbiome can promote IL-22 secretion by ILC3s, and this induces the production of antimicrobial peptides by the epithelium and enhances epithelial barrier integrity. However, when inappropriately activated, certain lineages such as ILC2s can have detrimental effects and induce allergic diseases [102]. ILC2s can initiate and promote allergic airway inflammation by stimulating the secretion of IL-13 and the T2 differentiation of dendritic cells [103]. The proportion of ILC2s was increased in the peripheral blood samples of asthmatic adults when compared to controls, and it could be used as a biomarker to predict eosinophilic airway inflammation [104]. Not only are there increased numbers of ILC2s in patients with asthma, their functions and reactivity were also altered. For example, the ILC2s from patients with asthma secreted more IL-5 and IL-13 compared to the ILC2s derived from healthy controls [105]. Interestingly, ILC2s can be recruited from the gut and migrate to the lungs in response to inflammation and gut dysbiosis [106].
5. Integrative View of Gut Dysbiosis and Asthma
5.1. Gut Dysbiosis and Asthma in Humans
The hygiene hypothesis is supported by an association between early exposure to environmental antigens and reduced asthma susceptibility [107,108]. A theory called the ‘microbiota hypothesis’ (originally, the microflora hypothesis) has recently emerged and suggests that alterations in gut microbiota occurring early in life can promote allergic diseases and asthma by depleting the microbial communities responsible for immunological tolerance [100,109]. The period of life in which alterations in microbial communities can promote the later development of diseases is called the ‘window of opportunity’.
Evidence of gut dysbiosis, illustrated by an increase in Clostridium spp. and a decrease in Lachnospira spp., was observed in a population of asthmatic children [110]. Similarly, colonization by Clostridium (now Clostridioides) difficile at one month of age was associated with asthma in childhood [111]. Another study showed that decreases in the relative abundances of Lachnospira and fecal SCFAs (acetate) in 3-month-old infants were associated with increased risks of developing childhood asthma [112]. The same group found no significant differences in the gut microbiota compositions between older children with asthma and atopy and healthy children [113]. These results highlighted the importance of the ‘window of opportunity’ in young infants for modulating susceptibility to allergic diseases. As discussed in Section 2, the mode of delivery can impact respiratory microbiota in newborns. There is also evidence that it can affect gut microbiota and thereby influence asthma susceptibility [53]. Stokholm et al. found that babies born via Caesarian section were at increased risk of developing asthma if their gut microbiota compositions remained similar to their microbiota profiles at birth [114]. While Caesarean sections are associated with decreased abundances of and diversity in Bacteroidetes and increased abundances of Firmicutes during the first three months of life, the impact of mode of delivery on gut microbiota colonization and diversity appears to lessen after 6 months [53]. While these studies illustrated the effects of early gut microbiota disturbances on asthma susceptibility, the minimal proportion of birth via Caesarean section in horses and the high prevalence of severe equine asthma (15%) suggests that other factors predominate.
The fecal microbiota of asthmatic patients differ from those of healthy subjects. For example, a relationship between allergen sensitization and fecal microbiota structure with decreased Bacteroidetes: Firmicutes ratios was observed in patients with asthma [115]. Beyond alterations in gut microbiota composition, its metabolites are also modified in asthma. Significant decreases in fecal SCFAs, including acetate, propionate, and butyrate, were detected in patients with asthma in two studies [116,117]. Gut microbiota can also have an impact on lung function in other diseases. For instance, in patients with chronic obstructive pulmonary disease (COPD), worsening lung function was also associated with lower fecal Firmicutes while stable function was associated with higher fecal Bacteroidetes [118]. In another study, Prevotella was overrepresented in a cluster of patients with reduced lung function [115]. However Prevotella relative abundances were reduced in asthmatic patients in another study, [119]. The exact pathways by which gut microbiota influence asthma susceptibility and persistence remain uncertain (Figure 3).
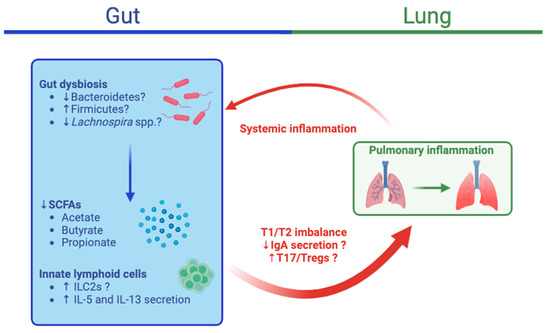
Figure 3.
Integrative view of the gut–lung axis in asthma. Gut dysbiosis can lead to the decreased production of short-chain fatty acids (SCFAs) due to decreases in the relative abundances of Bacteroidetes and Lachnospira spp or an increase in Firmicutes. Furthermore, innate lymphoid cells 2 (ILC2s) can stimulate the secretion of interleukin (IL)-5 and IL-13 and the T2 differentiation of dendritic cells, which can initiate and promote allergic airway inflammation. The dysregulation of IgA secretion, T1/T2 imbalances, and increased T17/Treg responses resulting from these changes can lead to airway inflammation, as has been observed in asthma. The crosstalk from the lungs to the gut remains unclear, but resulting systemic inflammation could further contribute to gut dysbiosis and inflammation. IL, interleukin; T1, type 1 inflammation; T2, type 2 inflammation. The image was created using BioRender.com, accessed on 19 December 2023.
5.2. Gut Dysbiosis and Asthma in Horses
The current research on microbiota in horses is mostly descriptive and has focused on either gut or respiratory microbes separately. To the authors’ knowledge, there is only one study investigating both the gut microbiota and asthma in horses. Leclere and Costa found that the intestinal microbiota differed between healthy and asthmatic horses [12]. Healthy horses transitioning from pasture to hay diets had increases in fecal Fibrobacter, and this was not observed in the horses with asthma. While differences were observed between the asthmatic horses in remission and the controls, they were less marked, which may suggest that gut microbiota alterations mostly occur in exacerbation. Some of the differences observed between the controls and the asthmatic horses in that study were similar to those seen in adult humans with asthma. For example, 8 of the 15 overrepresented genera in the horses with asthma belonged to the phylum of Firmicutes. Prevotella was also increased in horses in exacerbation compared to the controls. Because the sample size of this study was small and asthma exacerbation is inherently associated with diet change, it is difficult to conclude if the gut microbiota changed due to the disease or to the diet modifications. Evaluating the fecal microbiota in horses in which exacerbation was provoked without modifying the diet would greatly improve our understanding of the causality between the gut microbiome and equine asthma.
6. Microbiota-Directed Therapies and Modulation of the Gut–Lung Axis
With the increasing knowledge on how microbiota can contribute to asthma, strategies to restore microbial homeostasis have gained growing interest. However, the literature evaluating the efficacy of microbiota manipulation techniques in horses is sparse, and it outlines inconsistent results. In this section, we summarize the different techniques for gut microbiota manipulation including prebiotics, probiotics, and postbiotics. The efficacy of those techniques for the prevention or treatment of equine asthma has not been evaluated, and therefore, a few examples from human medicine are listed.
Prebiotics are substrates utilized by host microorganisms that exert benefits for the host. The most commonly used prebiotics are oligosaccharides, such as fructo-oligosaccharides (FOS) or mannan-oligosaccharides (MOS) [120]. They can prevent colonization by pathogens, stimulate the growth of probiotics, and undergo fermentation, resulting in the increased production of SCFAs [121,122,123]. Studies evaluating the effects of prebiotics on equine gut microbiota are limited, and the ones evaluating their impact on asthma are simply lacking. The effects of an oligosaccharide-rich diet on pregnant mares and their foals were investigated by Lindenberg et al., and they found that the supplemented foals had significantly higher relative abundances of Akkermansia spp. [124]. Interestingly, Akkermansia mucinophila abundance was decreased in the guts of children with allergic asthma [125]. While a four-week treatment with symbiotics (prebiotics and probiotics) did not alter bronchial inflammation in human patients with asthma, significant decreases in the systemic production of T2-cytokines such as IL-5 were observed in one study [126]. A prebiotic containing galactooligosaccharides was administered for 3 weeks in adults with asthma in another study, and decreased AHR associated with hyperpnea-induced bronchoconstriction was recorded [127]. These results hint at a potential for prebiotics in asthma management.
Probiotics are referred to living microorganisms with the capacity to restore microbial imbalances by preventing colonization by pathogens [120]. By upregulating tight junction proteins, they can enhance gut barrier integrity. They also have immunomodulatory properties by regulating the expression of Treg cells and decreasing T17 responses [121,128]. Developing effective probiotics for horses is challenging because the ideal healthy microbiota have not been determined. Currently published studies do not provide conclusive evidence of their benefits and yield contradictory results. Yeast probiotics have various advantages over probiotics containing mainly bacteria, such as their resistance to acidic environments and antimicrobials, which are often used in ill patients with concomitant dysbiosis [129]. Saccharomyces cerevisiae or boulardii are two nearly identical strains of non-pathogenic yeasts that can release proteases to degrade C. difficile toxins A and B [129]. The effects of Saccharomyces in horses with asthma have yet to be examined, but in mice models of asthma sensitized to OVA, significant reductions in AHR and airway inflammation were observed in the mice treated with S. cerevisiae [130,131].
In foals, probiotics may be useful in modifying their gut microbiota compositions because the ‘window of opportunity’ to permanently alter the gut microbiota is thought to be between birth and 50 days of age [132]. Alas, studies investigating the administration of probiotics in foals have not assessed the effects on microbiota compositions or asthma susceptibility, but rather, they primarily outline gastrointestinal clinicopathologic findings, which are beyond the scope of this review. In newborn mice, the administration of Lactobacillus rhamnosus and Bifidobacterium lactis during OVA sensitization and challenge suppressed airway reactivity and pulmonary eosinophilia [133]. In contrast, a meta-analysis of clinical trials assessing the effects of probiotic supplementation on atopy and asthma concluded that the evidence supporting their use in children to prevent asthma is currently insufficient [134]. It is important to note that probiotic administration in foals is considered generally safe, but it can lead to adverse effects, such as an increased incidence of diarrhea requiring veterinary intervention [135]. Therefore, the use of probiotics in foals to alter the gut microbiota requires further investigation.
Postbiotics are soluble products and metabolites secreted by gut microbial communities known for their protective effects on intestinal epithelium, immunomodulation functions, and selective cytotoxicity against tumors. The most well-known example of postbiotics are SCFAs. Propionate-supplemented water was given to mice sensitized to house-dust-mites in a model of allergic asthma [7]. Inflammatory cellular infiltration was reduced in the airways of the supplemented mice, and overall inflammatory responses were also decreased. Likewise, children were less likely to have asthma between 3 and 6 years of age if their fecal levels of butyrate and propionate were high [136]. These findings suggest that postbiotics such as SCFAs may decrease asthma susceptibility, but this awaits further clarification.
To the authors’ knowledge, fecal microbiota transplants have not been investigated for the treatment or prevention of either equine or human asthma.
7. Conclusions
The purpose of this review was to describe the role of the understudied gut–lung axis in the pathophysiology of equine asthma. Ex vivo, mouse, and human studies have pointed to gut microbiota as important components in asthma susceptibility and severity. While equine asthma shares many similarities with human asthma, further research is certainly needed to understand the implications of gut microbiota compositions and functions on equine asthma. The horse has the potential to serve as a model for human asthma across its lifespan. By focusing on the large-scale data integration of longitudinal equine health records from foals to adulthood, researchers could investigate key aspects of the effects of disease and antimicrobial administration during the “window of opportunity” on equine asthma susceptibility. If promising trends emerge, the horse could serve as a model for microbiota manipulation during the susceptible “window of opportunity” to reduce the risk of developing asthma in adulthood. Improving our understanding of the horse’s gut–lung axis could also lead to the development of techniques to manipulate the gut microbiome for the treatment of asthma in horses.
Author Contributions
L.L. contributed to writing the original draft, reviewing the manuscript, and submitting the manuscript. M.C. and M.L. supervised, edited, and revised the article. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data-sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hotchkiss, J.W.; Reid, S.W.J.; Christley, R.M. A survey of horse owners in Great Britain regarding horses in their care. Part 2: Risk factors for recurrent airway obstruction. Equine Vet. J. 2007, 39, 301–308. [Google Scholar] [CrossRef]
- Couëtil, L.L.; Cardwell, J.M.; Gerber, V.; Lavoie, J.-P.; Léguillette, R.; Richard, E.A. Inflammatory Airway Disease of Horses—Revised Consensus Statement. J. Vet. Intern. Med. 2016, 30, 503–515. [Google Scholar] [CrossRef]
- Robinson, N.E.; Chairperson, W. International Workshop on Equine Chronic Airway Disease Michigan State University 16–18 June 2000. Equine Vet. J. 2001, 33, 5–19. [Google Scholar] [CrossRef] [PubMed]
- Leclere, M.; Lavoie-Lamoureux, A.; Lavoie, J.-P. Heaves, an asthma-like disease of horses. Respirology 2011, 16, 1027–1046. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Li, S.; Wang, N.; Tan, H.-Y.; Zhang, Z.; Feng, Y. The Cross-Talk Between Gut Microbiota and Lungs in Common Lung Diseases. Front. Microbiol. 2020, 11, 301. [Google Scholar] [CrossRef] [PubMed]
- Frati, F.; Salvatori, C.; Incorvaia, C.; Bellucci, A.; Di Cara, G.; Marcucci, F.; Esposito, S. The Role of the Microbiome in Asthma: The Gut–Lung Axis. Int. J. Mol. Sci. 2019, 20, 123. [Google Scholar] [CrossRef]
- Trompette, A.; Gollwitzer, E.S.; Yadava, K.; Sichelstiel, A.K.; Sprenger, N.; Ngom-Bru, C.; Blanchard, C.; Junt, T.; Nicod, L.P.; Harris, N.L.; et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat. Med. 2014, 20, 159–166. [Google Scholar] [CrossRef]
- Costa, M.C.; Stämpfli, H.R.; Arroyo, L.G.; Allen-Vercoe, E.; Gomes, R.G.; Weese, J.S. Changes in the equine fecal microbiota associated with the use of systemic antimicrobial drugs. BMC Vet. Res. 2015, 11, 19. [Google Scholar] [CrossRef]
- Costa, M.C.; Weese, J.S. The equine intestinal microbiome. Anim. Health Res. Rev. 2012, 13, 121–128. [Google Scholar] [CrossRef]
- Costa, M.C.; Weese, J.S. Understanding the Intestinal Microbiome in Health and Disease. Vet. Clin. N. Am. Equine Pract. 2018, 34, 1–12. [Google Scholar] [CrossRef]
- Garber, A.; Hastie, P.; McGuinness, D.; Malarange, P.; Murray, J.-A. Abrupt dietary changes between grass and hay alter faecal microbiota of ponies. PLoS ONE 2020, 15, e0237869. [Google Scholar] [CrossRef] [PubMed]
- Leclere, M.; Costa, M.C. Fecal microbiota in horses with asthma. J. Vet. Intern. Med. 2020, 34, 996–1006. [Google Scholar] [CrossRef] [PubMed]
- Simões, J.; Batista, M.; Tilley, P. The Immune Mechanisms of Severe Equine Asthma—Current Understanding and What Is Missing. Animals 2022, 12, 744. [Google Scholar] [CrossRef] [PubMed]
- Couetil, L.; Cardwell, J.M.; Leguillette, R.; Mazan, M.; Richard, E.; Bienzle, D.; Bullone, M.; Gerber, V.; Ivester, K.; Lavoie, J.-P.; et al. Equine Asthma: Current Understanding and Future Directions. Front. Vet. Sci. 2020, 7, 450. [Google Scholar] [CrossRef] [PubMed]
- Ivester, K.M.; Couëtil, L.L.; Zimmerman, N.J. Investigating the link between particulate exposure and airway inflammation in the horse. J. Vet. Intern. Med. 2014, 28, 1653–1665. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, F.S.; Gruntman, A.; Couetil, L.L. A comparison of total, respirable, and real-time airborne particulate sampling in horse barns. J. Occup. Environ. Hyg. 2006, 3, 599–605. [Google Scholar] [CrossRef]
- Whittaker, A.G.; Hughes, K.J.; Parkin, T.D.H.; Love, S. Concentrations of dust and endotoxin in equine stabling. Vet. Rec. 2009, 165, 293–295. [Google Scholar] [CrossRef]
- Ivester, K.M.; Couëtil, L.L.; Moore, G.E.; Zimmerman, N.J.; Raskin, R.E. Environmental exposures and airway inflammation in young thoroughbred horses. J. Vet. Intern. Med. 2014, 28, 918–924. [Google Scholar] [CrossRef]
- Millerick-May, M.L.; Karmaus, W.; Derksen, F.J.; Berthold, B.; Holcombe, S.J.; Robinson, N.E. Local airborne particulate concentration is associated with visible tracheal mucus in Thoroughbred racehorses. Equine Vet. J. 2013, 45, 85–90. [Google Scholar] [CrossRef]
- Ivester, K.M.; Couëtil, L.L.; Moore, G.E. An observational study of environmental exposures, airway cytology, and performance in racing thoroughbreds. J. Vet. Intern. Med. 2018, 32, 1754–1762. [Google Scholar] [CrossRef]
- Pirie, R.S.; Collie, D.D.S.; Dixon, P.M.; McGorum, B.C. Evaluation of nebulised hay dust suspensions (HDS) for the diagnosis and investigation of heaves. 2: Effects of inhaled HDS on control and heaves horses. Equine Vet. J. 2002, 34, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Bullone, M.; Murcia, R.Y.; Lavoie, J.-P. Environmental heat and airborne pollen concentration are associated with increased asthma severity in horses. Equine Vet. J. 2016, 48, 479–484. [Google Scholar] [CrossRef]
- Davis, M.S.; Malayer, J.R.; Vandeventer, L.; Royer, C.M.; McKenzie, E.C.; Williamson, K.K. Cold weather exercise and airway cytokine expression. J. Appl. Physiol. 2005, 98, 2132–2136. [Google Scholar] [CrossRef] [PubMed]
- Strachan, D.P. Hay fever, hygiene, and household size. BMJ 1989, 299, 1259–1260. [Google Scholar] [CrossRef]
- Peters, M.; Kauth, M.; Scherner, O.; Gehlhar, K.; Steffen, I.; Wentker, P.; von Mutius, E.; Holst, O.; Bufe, A. Arabinogalactan isolated from cowshed dust extract protects mice from allergic airway inflammation and sensitization. J. Allergy Clin. Immunol. 2010, 126, 648–656.e4. [Google Scholar] [CrossRef]
- Ege, M.J.; Mayer, M.; Normand, A.-C.; Genuneit, J.; Cookson, W.O.C.M.; Braun-Fahrländer, C.; Heederik, D.; Piarroux, R.; von Mutius, E.; GABRIELA Transregio 22 Study Group. Exposure to environmental microorganisms and childhood asthma. N. Engl. J. Med. 2011, 364, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Jie, Y.; Isa, Z.M.; Jie, X.; Ju, Z.L.; Ismail, N.H. Urban vs. Rural Factors That Affect Adult Asthma. Rev. Environ. Contam. Toxicol. 2013, 226, 33–63. [Google Scholar] [CrossRef] [PubMed]
- Illi, S.; Depner, M.; Genuneit, J.; Horak, E.; Loss, G.; Strunz-Lehner, C.; Büchele, G.; Boznanski, A.; Danielewicz, H.; Cullinan, P.; et al. Protection from childhood asthma and allergy in Alpine farm environments-the GABRIEL Advanced Studies. J. Allergy Clin. Immunol. 2012, 129, 1470–1477.e6. [Google Scholar] [CrossRef]
- Braun-Fahrländer, C.; Riedler, J.; Herz, U.; Eder, W.; Waser, M.; Grize, L.; Maisch, S.; Carr, D.; Gerlach, F.; Bufe, A.; et al. Environmental exposure to endotoxin and its relation to asthma in school-age children. N. Engl. J. Med. 2002, 347, 869–877. [Google Scholar] [CrossRef]
- Debarry, J.; Garn, H.; Hanuszkiewicz, A.; Dickgreber, N.; Blümer, N.; von Mutius, E.; Bufe, A.; Gatermann, S.; Renz, H.; Holst, O.; et al. Acinetobacter lwoffii and Lactococcus lactis strains isolated from farm cowsheds possess strong allergy-protective properties. J. Allergy Clin. Immunol. 2007, 119, 1514–1521. [Google Scholar] [CrossRef]
- Loss, G.; Bitter, S.; Wohlgensinger, J.; Frei, R.; Roduit, C.; Genuneit, J.; Pekkanen, J.; Roponen, M.; Hirvonen, M.-R.; Dalphin, J.-C.; et al. Prenatal and early-life exposures alter expression of innate immunity genes: The PASTURE cohort study. J. Allergy Clin. Immunol. 2012, 130, 523–530.e9. [Google Scholar] [CrossRef] [PubMed]
- Douwes, J.; Cheng, S.; Travier, N.; Cohet, C.; Niesink, A.; McKenzie, J.; Cunningham, C.; Le Gros, G.; von Mutius, E.; Pearce, N. Farm exposure in utero may protect against asthma, hay fever and eczema. Eur. Respir. J. 2008, 32, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Chris, P. Results of a Cohort Study of Foals from Birth to 3 Years Old; Microbiome, Immune Status and Health Outcomes. In Proceedings of the European Equine Health and Nutrition Congress, Ghent, Belgium, 23–25 March 2023; pp. 39–40. [Google Scholar]
- Diao, Z.; Han, D.; Zhang, R.; Li, J. Metagenomics next-generation sequencing tests take the stage in the diagnosis of lower respiratory tract infections. J. Adv. Res. 2022, 38, 201–212. [Google Scholar] [CrossRef]
- Manguin, E.; Pépin, E.; Boivin, R.; Leclere, M. Tracheal microbial populations in horses with moderate asthma. J. Vet. Intern. Med. 2020, 34, 986–995. [Google Scholar] [CrossRef] [PubMed]
- Bond, S.L.; Timsit, E.; Workentine, M.; Alexander, T.; Léguillette, R. Upper and lower respiratory tract microbiota in horses: Bacterial communities associated with health and mild asthma (inflammatory airway disease) and effects of dexamethasone. BMC Microbiol. 2017, 17, 184. [Google Scholar] [CrossRef]
- Fillion-Bertrand, G.; Dickson, R.P.; Boivin, R.; Lavoie, J.-P.; Huffnagle, G.B.; Leclere, M. Lung Microbiome Is Influenced by the Environment and Asthmatic Status in an Equine Model of Asthma. Am. J. Respir. Cell Mol. Biol. 2019, 60, 189–197. [Google Scholar] [CrossRef]
- Dickson, R.P.; Erb-Downward, J.R.; Huffnagle, G.B. Homeostasis and its disruption in the lung microbiome. Am. J. Physiol. Lung Cell Mol. Physiol. 2015, 309, L1047–L1055. [Google Scholar] [CrossRef]
- Charlson, E.S.; Bittinger, K.; Haas, A.R.; Fitzgerald, A.S.; Frank, I.; Yadav, A.; Bushman, F.D.; Collman, R.G. Topographical continuity of bacterial populations in the healthy human respiratory tract. Am. J. Respir. Crit. Care Med. 2011, 184, 957–963. [Google Scholar] [CrossRef]
- Heul, A.V.; Planer, J.; Kau, A.L. The human microbiota and asthma. Clin. Rev. Allergy Immunol. 2019, 57, 350–363. [Google Scholar] [CrossRef]
- Barcik, W.; Boutin, R.C.T.; Sokolowska, M.; Finlay, B.B. The Role of Lung and Gut Microbiota in the Pathology of Asthma. Immunity 2020, 52, 241–255. [Google Scholar] [CrossRef]
- Wu, B.G.; Sulaiman, I.; Tsay, J.-C.J.; Perez, L.; Franca, B.; Li, Y.; Wang, J.; Gonzalez, A.N.; El-Ashmawy, M.; Carpenito, J.; et al. Episodic Aspiration with Oral Commensals Induces a MyD88-dependent, Pulmonary T-Helper Cell Type 17 Response that Mitigates Susceptibility to Streptococcus pneumoniae. Am. J. Respir. Crit. Care Med. 2021, 203, 1099–1111. [Google Scholar] [CrossRef] [PubMed]
- Segal, L.N.; Rom, W.N.; Weiden, M.D. Lung microbiome for clinicians. New discoveries about bugs in healthy and diseased lungs. Ann. Am. Thorac. Soc. 2014, 11, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Christley, R.M.; Hodgson, D.R.; Rose, R.J.; Wood, J.L.; Reids, S.W.; Whitear, K.G.; Hodgson, J.L. A case-control study of respiratory disease in Thoroughbred racehorses in Sydney, Australia. Equine Vet. J. 2001, 33, 256–264. [Google Scholar] [CrossRef]
- Chapman, P.S.; Green, C.; Main, J.P.; Taylor, P.M.; Cunningham, F.M.; Cook, A.J.; Marr, C.M. Retrospective study of the relationships between age, inflammation and the isolation of bacteria from the lower respiratory tract of thoroughbred horses. Vet. Rec. 2000, 146, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Cardwell, J.M.; Smith, K.C.; Wood, J.L.N.; Newton, J.R. Infectious risk factors and clinical indicators for tracheal mucus in British National Hunt racehorses. Equine Vet. J. 2014, 46, 150–155. [Google Scholar] [CrossRef]
- Doubli-Bounoua, N.; Richard, E.A.; Léon, A.; Pitel, P.-H.; Pronost, S.; Fortier, G. Multiple molecular detection of respiratory viruses and associated signs of airway inflammation in racehorses. Virol. J. 2016, 13, 197. [Google Scholar] [CrossRef]
- Houtsma, A.; Bedenice, D.; Pusterla, N.; Pugliese, B.; Mapes, S.; Hoffman, A.M.; Paxson, J.; Rozanski, E.; Mukherjee, J.; Wigley, M.; et al. Association between inflammatory airway disease of horses and exposure to respiratory viruses: A case control study. Multidiscip. Respir. Med. 2015, 10, 33. [Google Scholar] [CrossRef]
- Durack, J.; Lynch, S.V.; Nariya, S.; Bhakta, N.R.; Beigelman, A.; Castro, M.; Dyer, A.-M.; Israel, E.; Kraft, M.; Martin, R.J.; et al. Features of the bronchial bacterial microbiome associated with atopy, asthma, and responsiveness to inhaled corticosteroid treatment. J. Allergy Clin. Immunol. 2017, 140, 63–75. [Google Scholar] [CrossRef]
- Green, B.J.; Wiriyachaiporn, S.; Grainge, C.; Rogers, G.B.; Kehagia, V.; Lau, L.; Carroll, M.P.; Bruce, K.D.; Howarth, P.H. Potentially pathogenic airway bacteria and neutrophilic inflammation in treatment resistant severe asthma. PLoS ONE 2014, 9, e100645. [Google Scholar] [CrossRef]
- Taylor, S.L.; Leong, L.E.X.; Choo, J.M.; Wesselingh, S.; Yang, I.A.; Upham, J.W.; Reynolds, P.N.; Hodge, S.; James, A.L.; Jenkins, C.; et al. Inflammatory phenotypes in patients with severe asthma are associated with distinct airway microbiology. J. Allergy Clin. Immunol. 2018, 141, 94–103.e15. [Google Scholar] [CrossRef]
- Gürdeniz, G.; Ernst, M.; Rago, D.; Kim, M.; Courraud, J.; Stokholm, J.; Bønnelykke, K.; Björkbom, A.; Trivedi, U.; Sørensen, S.J.; et al. Neonatal metabolome of caesarean section and risk of childhood asthma. Eur. Respir. J. 2022, 59, 2102406. [Google Scholar] [CrossRef] [PubMed]
- Rutayisire, E.; Huang, K.; Liu, Y.; Tao, F. The mode of delivery affects the diversity and colonization pattern of the gut microbiota during the first year of infants’ life: A systematic review. BMC Gastroenterol. 2016, 16, 86. [Google Scholar] [CrossRef]
- Bisgaard, H.; Hermansen, M.N.; Buchvald, F.; Loland, L.; Halkjaer, L.B.; Bønnelykke, K.; Brasholt, M.; Heltberg, A.; Vissing, N.H.; Thorsen, S.V.; et al. Childhood asthma after bacterial colonization of the airway in neonates. N. Engl. J. Med. 2007, 357, 1487–1495. [Google Scholar] [CrossRef] [PubMed]
- Thorsen, J.; Li, X.J.; Peng, S.; Sunde, R.B.; Shah, S.A.; Bhattacharyya, M.; Poulsen, C.S.; Poulsen, C.E.; Leal Rodriguez, C.; Widdowson, M.; et al. The airway microbiota of neonates colonized with asthma-associated pathogenic bacteria. Nat. Commun. 2023, 14, 6668. [Google Scholar] [CrossRef] [PubMed]
- Husso, A.; Jalanka, J.; Alipour, M.J.; Huhti, P.; Kareskoski, M.; Pessa-Morikawa, T.; Iivanainen, A.; Niku, M. The composition of the perinatal intestinal microbiota in horse. Sci. Rep. 2020, 10, 441. [Google Scholar] [CrossRef]
- Metcalf, J.L.; Song, S.J.; Morton, J.T.; Weiss, S.; Seguin-Orlando, A.; Joly, F.; Feh, C.; Taberlet, P.; Coissac, E.; Amir, A.; et al. Evaluating the impact of domestication and captivity on the horse gut microbiome. Sci. Rep. 2017, 7, 15497. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, K.A.; Rogers, C.W.; Gee, E.K.; Kittelmann, S.; Bolwell, C.F.; Bermingham, E.N.; Biggs, P.J.; Thomas, D.G. Resilience of Faecal Microbiota in Stabled Thoroughbred Horses Following Abrupt Dietary Transition between Freshly Cut Pasture and Three Forage-Based Diets. Animals 2021, 11, 2611. [Google Scholar] [CrossRef]
- Kim, J.-H.; Ellwood, P.E.; Asher, M.I. Diet and asthma: Looking back, moving forward. Respir. Res. 2009, 10, 49. [Google Scholar] [CrossRef]
- Sutherland, E.R. Linking obesity and asthma. Ann. N. Y. Acad. Sci. 2014, 1311, 31–41. [Google Scholar] [CrossRef]
- Sierra-Honigmann, M.R.; Nath, A.K.; Murakami, C.; García-Cardeña, G.; Papapetropoulos, A.; Sessa, W.C.; Madge, L.A.; Schechner, J.S.; Schwabb, M.B.; Polverini, P.J.; et al. Biological action of leptin as an angiogenic factor. Science 1998, 281, 1683–1686. [Google Scholar] [CrossRef]
- Shore, S.A.; Schwartzman, I.N.; Mellema, M.S.; Flynt, L.; Imrich, A.; Johnston, R.A. Effect of leptin on allergic airway responses in mice. J. Allergy Clin. Immunol. 2005, 115, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Heiss, C.N.; Mannerås-Holm, L.; Lee, Y.S.; Serrano-Lobo, J.; Håkansson Gladh, A.; Seeley, R.J.; Drucker, D.J.; Bäckhed, F.; Olofsson, L.E. The gut microbiota regulates hypothalamic inflammation and leptin sensitivity in Western diet-fed mice via a GLP-1R-dependent mechanism. Cell Rep. 2021, 35, 109163. [Google Scholar] [CrossRef] [PubMed]
- Kearns, C.F.; McKeever, K.H.; Roegner, V.; Brady, S.M.; Malinowski, K. Adiponectin and leptin are related to fat mass in horses. Vet. J. 2006, 172, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Boulet, L.-P.; Franssen, E. Influence of obesity on response to fluticasone with or without salmeterol in moderate asthma. Respir. Med. 2007, 101, 2240–2247. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.J.; de Solis, C.N.; Coleman, M.C. Case-Control Study of Risk Factors for Equine Asthma in Texas. J. Equine Vet. Sci. 2021, 103, 103644. [Google Scholar] [CrossRef] [PubMed]
- Bond, S.; Léguillette, R.; Richard, E.A.; Couetil, L.; Lavoie, J.-P.; Martin, J.G.; Pirie, R.S. Equine asthma: Integrative biologic relevance of a recently proposed nomenclature. J. Vet. Intern. Med. 2018, 32, 2088–2098. [Google Scholar] [CrossRef] [PubMed]
- Biddle, A.S.; Tomb, J.-F.; Fan, Z. Microbiome and Blood Analyte Differences Point to Community and Metabolic Signatures in Lean and Obese Horses. Front. Vet. Sci. 2018, 5, 225. [Google Scholar] [CrossRef]
- Elzinga, S.E.; Weese, J.S.; Adams, A.A. Comparison of the Fecal Microbiota in Horses with Equine Metabolic Syndrome and Metabolically Normal Controls Fed a Similar All-Forage Diet. J. Equine Vet. Sci. 2016, 44, 9–16. [Google Scholar] [CrossRef]
- Morrison, P.K.; Newbold, C.J.; Jones, E.; Worgan, H.J.; Grove-White, D.H.; Dugdale, A.H.; Barfoot, C.; Harris, P.A.; Argo, C.M. The Equine Gastrointestinal Microbiome: Impacts of Age and Obesity. Front. Microbiol. 2018, 9, 3017. [Google Scholar] [CrossRef]
- Roth, P.; Stanley, J.; Chamoun-Emanuelli, A.; Whitfield-Cargile, C.; Coleman, M. Fecal extract from obese horses induces an inflammatory response by murine macrophages in vitro. Am. J. Vet. Res. 2022, 83, 419–425. [Google Scholar] [CrossRef]
- Marsland, B.J.; Gollwitzer, E.S. Host–microorganism interactions in lung diseases. Nat. Rev. Immunol. 2014, 14, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef]
- Kuder, M.M.; Nyenhuis, S.M. Optimizing lifestyle interventions in adult patients with comorbid asthma and obesity. Ther. Adv. Respir. Dis. 2020, 14, 1753466620906323. [Google Scholar] [CrossRef] [PubMed]
- Eichenberger, P.A.; Diener, S.N.; Kofmehl, R.; Spengler, C.M. Effects of exercise training on airway hyperreactivity in asthma: A systematic review and meta-analysis. Sports Med. 2013, 43, 1157–1170. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, M.; Creel, A.; Estell, K.; Davis, I.C.; Schwiebert, L.M. Acute exercise decreases airway inflammation, but not responsiveness, in an allergic asthma model. Am. J. Respir. Cell Mol. Biol. 2009, 40, 83–89. [Google Scholar] [CrossRef]
- França-Pinto, A.; Mendes, F.A.R.; de Carvalho-Pinto, R.M.; Agondi, R.C.; Cukier, A.; Stelmach, R.; Saraiva-Romanholo, B.M.; Kalil, J.; Martins, M.A.; Giavina-Bianchi, P.; et al. Aerobic training decreases bronchial hyperresponsiveness and systemic inflammation in patients with moderate or severe asthma: A randomised controlled trial. Thorax 2015, 70, 732–739. [Google Scholar] [CrossRef] [PubMed]
- Mendes, F.A.R.; Almeida, F.M.; Cukier, A.; Stelmach, R.; Jacob-Filho, W.; Martins, M.A.; Carvalho, C.R.F. Effects of aerobic training on airway inflammation in asthmatic patients. Med. Sci. Sports Exerc. 2011, 43, 197–203. [Google Scholar] [CrossRef]
- Estaki, M.; Pither, J.; Baumeister, P.; Little, J.P.; Gill, S.K.; Ghosh, S.; Ahmadi-Vand, Z.; Marsden, K.R.; Gibson, D.L. Cardiorespiratory fitness as a predictor of intestinal microbial diversity and distinct metagenomic functions. Microbiome 2016, 4, 42. [Google Scholar] [CrossRef]
- Welly, R.J.; Liu, T.-W.; Zidon, T.M.; Rowles, J.L.; Park, Y.-M.; Smith, T.N.; Swanson, K.S.; Padilla, J.; Vieira-Potter, V.J. Comparison of Diet versus Exercise on Metabolic Function and Gut Microbiota in Obese Rats. Med. Sci. Sports Exerc. 2016, 48, 1688–1698. [Google Scholar] [CrossRef]
- De Almeida, M.L.M.; Feringer Júnior, W.H.; Carvalho, J.R.G.; Rodrigues, I.M.; Jordão, L.R.; Fonseca, M.G.; de Rezende, A.S.C.; Neto, A.d.Q.; Weese, J.S.; da Costa, M.C.; et al. Intense Exercise and Aerobic Conditioning Associated with Chromium or L-Carnitine Supplementation Modified the Fecal Microbiota of Fillies. PLoS ONE 2016, 11, e0167108. [Google Scholar] [CrossRef]
- Plancade, S.; Clark, A.; Philippe, C.; Helbling, J.-C.; Moisan, M.-P.; Esquerré, D.; Le Moyec, L.; Robert, C.; Barrey, E.; Mach, N. Unraveling the effects of the gut microbiota composition and function on horse endurance physiology. Sci. Rep. 2019, 9, 9620. [Google Scholar] [CrossRef] [PubMed]
- Padalino, B.; Raidal, S.L.; Knight, P.; Celi, P.; Jeffcott, L.; Muscatello, G. Behaviour during transportation predicts stress response and lower airway contamination in horses. PLoS ONE 2018, 13, e0194272. [Google Scholar] [CrossRef] [PubMed]
- McGilloway, M.; Manley, S.; Aho, A.; Heeringa, K.N.; Lou, Y.; Squires, E.J.; Pearson, W. The combination of trailer transport and exercise increases gastrointestinal permeability and markers of systemic inflammation in horses. Equine Vet. J. 2023, 55, 853–861. [Google Scholar] [CrossRef]
- Di Pietro, R.; Arroyo, L.G.; Leclere, M.; Costa, M.C. Species-Level Gut Microbiota Analysis after Antibiotic-Induced Dysbiosis in Horses. Animals 2021, 11, 2859. [Google Scholar] [CrossRef] [PubMed]
- Liepman, R.S.; Swink, J.M.; Habing, G.G.; Boyaka, P.N.; Caddey, B.; Costa, M.; Gomez, D.E.; Toribio, R.E. Effects of Intravenous Antimicrobial Drugs on the Equine Fecal Microbiome. Animals 2022, 12, 1013. [Google Scholar] [CrossRef]
- Baron, R.; Taye, M.; der Vaart, I.B.; Ujčič-Voortman, J.; Szajewska, H.; Seidell, J.C.; Verhoeff, A. The relationship of prenatal antibiotic exposure and infant antibiotic administration with childhood allergies: A systematic review. BMC Pediatr. 2020, 20, 312. [Google Scholar] [CrossRef]
- Pitter, G.; Ludvigsson, J.F.; Romor, P.; Zanier, L.; Zanotti, R.; Simonato, L.; Canova, C. Antibiotic exposure in the first year of life and later treated asthma, a population based birth cohort study of 143,000 children. Eur. J. Epidemiol. 2016, 31, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Dzidic, M.; Abrahamsson, T.R.; Artacho, A.; Björkstén, B.; Collado, M.C.; Mira, A.; Jenmalm, M.C. Aberrant IgA responses to the gut microbiota during infancy precede asthma and allergy development. J. Allergy Clin. Immunol. 2017, 139, 1017–1025.e14. [Google Scholar] [CrossRef]
- Han, Y.Y.; Forno, E.; Celedón, J.C. Sex Steroid Hormones and Asthma in a Nationwide Study of U.S. Adults. Am. J. Respir. Crit. Care Med. 2020, 201, 158–166. [Google Scholar] [CrossRef]
- Couëtil, L.L.; Ward, M.P. Analysis of risk factors for recurrent airway obstruction in North American horses: 1,444 cases (1990–1999). J. Am. Vet. Med. Assoc. 2003, 223, 1645–1650. [Google Scholar] [CrossRef]
- Mshelia, E.S.; Adamu, L.; Wakil, Y.; Turaki, U.A.; Gulani, I.A.; Musa, J. The association between gut microbiome, sex, age and body condition scores of horses in Maiduguri and its environs. Microb. Pathog. 2018, 118, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Abu-Ali, G.; Tashiro, H.; Brown, T.A.; Osgood, R.S.; Kasahara, D.I.; Huttenhower, C.; Shore, S.A. Sex Differences in Pulmonary Responses to Ozone in Mice. Role of the Microbiome. Am. J. Respir. Cell Mol. Biol. 2019, 60, 198–208. [Google Scholar] [CrossRef]
- Chen, R.; Wang, L.; Koch, T.; Curtis, V.; Yin-DeClue, H.; Handley, S.A.; Shan, L.; Holtzman, M.J.; Castro, M.; Wang, L. Sex effects in the association between airway microbiome and asthma. Ann. Allergy Asthma Immunol. 2020, 125, 652–657.e3. [Google Scholar] [CrossRef]
- Enaud, R.; Prevel, R.; Ciarlo, E.; Beaufils, F.; Wieërs, G.; Guery, B.; Delhaes, L. The Gut-Lung Axis in Health and Respiratory Diseases: A Place for Inter-Organ and Inter-Kingdom Crosstalks. Front. Cell. Infect. Microbiol. 2020, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; McKenzie, C.; Potamitis, M.; Thorburn, A.N.; Mackay, C.R.; Macia, L. The role of short-chain fatty acids in health and disease. Adv. Immunol. 2014, 121, 91–119. [Google Scholar] [CrossRef] [PubMed]
- Gensollen, T.; Blumberg, R.S. Correlation between early-life regulation of the immune system by microbiota and allergy development. J. Allergy Clin. Immunol. 2017, 139, 1084–1091. [Google Scholar] [CrossRef]
- Cordeau, M.-E.; Joubert, P.; Dewachi, O.; Hamid, Q.; Lavoie, J.-P. IL-4, IL-5 and IFN-γ mRNA expression in pulmonary lymphocytes in equine heaves. Vet. Immunol. Immunopathol. 2004, 97, 87–96. [Google Scholar] [CrossRef]
- Cong, Y.; Feng, T.; Fujihashi, K.; Schoeb, T.R.; Elson, C.O. A dominant, coordinated T regulatory cell-IgA response to the intestinal microbiota. Proc. Natl. Acad. Sci. USA 2009, 106, 19256–19261. [Google Scholar] [CrossRef]
- Valverde-Molina, J.; García-Marcos, L. Microbiome and Asthma: Microbial Dysbiosis and the Origins, Phenotypes, Persistence, and Severity of Asthma. Nutrients 2023, 15, 486. [Google Scholar] [CrossRef]
- Salter, B.M.; Aw, M.; Sehmi, R. The role of type 2 innate lymphoid cells in eosinophilic asthma. J. Leukoc. Biol. 2019, 106, 889–901. [Google Scholar] [CrossRef]
- Panda, S.K.; Colonna, M. Innate Lymphoid Cells in Mucosal Immunity. Front. Immunol. 2019, 10, 861. [Google Scholar] [CrossRef] [PubMed]
- Halim, T.Y.F.; Steer, C.A.; Mathä, L.; Gold, M.J.; Martinez-Gonzalez, I.; McNagny, K.M.; McKenzie, A.N.J.; Takei, F. Group 2 Innate Lymphoid Cells Are Critical for the Initiation of Adaptive T Helper 2 Cell-Mediated Allergic Lung Inflammation. Immunity 2014, 40, 425–435. [Google Scholar] [CrossRef]
- Liu, T.; Wu, J.; Zhao, J.; Wang, J.; Zhang, Y.; Liu, L.; Cao, L.; Liu, Y.; Dong, L. Type 2 innate lymphoid cells: A novel biomarker of eosinophilic airway inflammation in patients with mild to moderate asthma. Respir. Med. 2015, 109, 1391–1396. [Google Scholar] [CrossRef] [PubMed]
- Bartemes, K.R.; Kephart, G.M.; Fox, S.J.; Kita, H. Enhanced innate type 2 immune response in peripheral blood from patients with asthma. J. Allergy Clin. Immunol. 2014, 134, 671–678.e4. [Google Scholar] [CrossRef] [PubMed]
- Pu, Q.; Lin, P.; Gao, P.; Wang, Z.; Guo, K.; Qin, S.; Zhou, C.; Wang, B.; Wu, E.; Khan, N.; et al. Gut Microbiota Regulate Gut–Lung Axis Inflammatory Responses by Mediating ILC2 Compartmental Migration. J. Immunol. 2021, 207, 257–267. [Google Scholar] [CrossRef]
- Durack, J.; Boushey, H.A.; Lynch, S.V. Airway Microbiota and the Implications of Dysbiosis in Asthma. Curr. Allergy Asthma Rep. 2016, 16, 52. [Google Scholar] [CrossRef]
- Martinez, F.D.; Guerra, S. Early Origins of Asthma. Role of Microbial Dysbiosis and Metabolic Dysfunction. Am. J. Respir. Crit. Care Med. 2018, 197, 573–579. [Google Scholar] [CrossRef]
- Hu, T.; Dong, Y.; Yang, C.; Zhao, M.; He, Q. Pathogenesis of Children’s Allergic Diseases: Refocusing the Role of the Gut Microbiota. Front. Physiol. 2021, 12, 749544. [Google Scholar] [CrossRef]
- Stiemsma, L.T.; Arrieta, M.-C.; Dimitriu, P.A.; Cheng, J.; Thorson, L.; Lefebvre, D.L.; Azad, M.B.; Subbarao, P.; Mandhane, P.; Becker, A.; et al. Shifts in Lachnospira and Clostridium sp. in the 3-month stool microbiome are associated with preschool age asthma. Clin. Sci. 2016, 130, 2199–2207. [Google Scholar] [CrossRef]
- van Nimwegen, F.A.; Penders, J.; Stobberingh, E.E.; Postma, D.S.; Koppelman, G.H.; Kerkhof, M.; Reijmerink, N.E.; Dompeling, E.; van den Brandt, P.A.; Ferreira, I.; et al. Mode and place of delivery, gastrointestinal microbiota, and their influence on asthma and atopy. J. Allergy Clin. Immunol. 2011, 128, 948–955.e3. [Google Scholar] [CrossRef]
- Arrieta, M.-C.; Stiemsma, L.T.; Dimitriu, P.A.; Thorson, L.; Russell, S.; Yurist-Doutsch, S.; Kuzeljevic, B.; Gold, M.J.; Britton, H.M.; Lefebvre, D.L.; et al. Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci. Transl. Med. 2015, 7, 307ra152. [Google Scholar] [CrossRef] [PubMed]
- Arrieta, M.-C.; Sadarangani, M.; Brown, E.M.; Russell, S.L.; Nimmo, M.; Dean, J.; Turvey, S.E.; Chan, E.S.; Finlay, B.B. A humanized microbiota mouse model of ovalbumin-induced lung inflammation. Gut Microbes 2016, 7, 342–352. [Google Scholar] [CrossRef]
- Stokholm, J.; Thorsen, J.; Blaser, M.J.; Rasmussen, M.A.; Hjelmsø, M.; Shah, S.; Christensen, E.D.; Chawes, B.L.; Bønnelykke, K.; Brix, S.; et al. Delivery mode and gut microbial changes correlate with an increased risk of childhood asthma. Sci. Transl. Med. 2020, 12, eaax9929. [Google Scholar] [CrossRef] [PubMed]
- Begley, L.; Madapoosi, S.; Opron, K.; Ndum, O.; Baptist, A.; Rysso, K.; Erb-Downward, J.R.; Huang, Y.J. Gut microbiota relationships to lung function and adult asthma phenotype: A pilot study. BMJ Open Respir. Res. 2018, 5, e000324. [Google Scholar] [CrossRef] [PubMed]
- Zolnikova, O.Y.; Potskhverashvili, N.D.; Kokina, N.I.; Trukhmanov, A.S.; Ivashkin, V.T. Intestinal Short-Chain Fatty Acids in Patients with Bronchial Asthma. Russ. J. Gastroenterol. Hepatol. Coloproctol. 2019, 29, 53–59. [Google Scholar] [CrossRef]
- Ivashkin, V.; Zolnikova, O.; Potskherashvili, N.; Trukhmanov, A.; Kokina, N.; Dzhakhaya, N.; Sedova, A.; Bueverova, E. Metabolic activity of intestinal microflora in patients with bronchial asthma. Clin. Pract. 2019, 9, 1126. [Google Scholar] [CrossRef] [PubMed]
- Chiu, Y.-C.; Lee, S.-W.; Liu, C.-W.; Lan, T.-Y.; Wu, L.S.-H. Relationship between gut microbiota and lung function decline in patients with chronic obstructive pulmonary disease: A 1-year follow-up study. Respir. Res. 2022, 23, 10. [Google Scholar] [CrossRef]
- Hilty, M.; Burke, C.; Pedro, H.; Cardenas, P.; Bush, A.; Bossley, C.; Davies, J.; Ervine, A.; Poulter, L.; Pachter, L.; et al. Disordered microbial communities in asthmatic airways. PLoS ONE 2010, 5, e8578. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef]
- Fong, W.; Li, Q.; Yu, J. Gut microbiota modulation: A novel strategy for prevention and treatment of colorectal cancer. Oncogene 2020, 39, 4925–4943. [Google Scholar] [CrossRef]
- Shoaf, K.; Mulvey, G.L.; Armstrong, G.D.; Hutkins, R.W. Prebiotic galactooligosaccharides reduce adherence of enteropathogenic Escherichia coli to tissue culture cells. Infect. Immun. 2006, 74, 6920–6928. [Google Scholar] [CrossRef] [PubMed]
- Monteagudo-Mera, A.; Rastall, R.A.; Gibson, G.R.; Charalampopoulos, D.; Chatzifragkou, A. Adhesion mechanisms mediated by probiotics and prebiotics and their potential impact on human health. Appl. Microbiol. Biotechnol. 2019, 103, 6463–6472. [Google Scholar] [CrossRef] [PubMed]
- Lindenberg, F.C.; Lützhøft, D.O.; Krych, L.; Fielden, J.; Kot, W.; Frøkiær, H.; van Galen, G.; Nielsen, D.S.; Hansen, A.K. An Oligosaccharide Rich Diet Increases Akkermansia spp. Bacteria in the Equine Microbiota. Front. Microbiol. 2021, 12, 666039. [Google Scholar] [CrossRef] [PubMed]
- Demirci, M.; Tokman, H.B.; Uysal, H.K.; Demiryas, S.; Karakullukcu, A.; Saribas, S.; Cokugras, H.; Kocazeybek, B.S. Reduced Akkermansia muciniphila and Faecalibacterium prausnitzii levels in the gut microbiota of children with allergic asthma. Allergol. Immunopathol. 2019, 47, 365–371. [Google Scholar] [CrossRef] [PubMed]
- van de Pol, M.A.; Lutter, R.; Smids, B.S.; Weersink, E.J.M.; van der Zee, J.S. Synbiotics reduce allergen-induced T-helper 2 response and improve peak expiratory flow in allergic asthmatics. Allergy 2011, 66, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Williams, N.C.; Johnson, M.A.; Shaw, D.E.; Spendlove, I.; Vulevic, J.; Sharpe, G.R.; Hunter, K.A. A prebiotic galactooligosaccharide mixture reduces severity of hyperpnoea-induced bronchoconstriction and markers of airway inflammation. Br. J. Nutr. 2016, 116, 798–804. [Google Scholar] [CrossRef]
- Klaenhammer, T.R.; Kleerebezem, M.; Kopp, M.V.; Rescigno, M. The impact of probiotics and prebiotics on the immune system. Nat. Rev. Immunol. 2012, 12, 728–734. [Google Scholar] [CrossRef]
- Czerucka, D.; Piche, T.; Rampal, P. Review article: Yeast as probiotics—Saccharomyces boulardii. Aliment. Pharmacol. Ther. 2007, 26, 767–778. [Google Scholar] [CrossRef]
- Fonseca, V.M.B.; Milani, T.M.S.; Prado, R.; Bonato, V.L.D.; Ramos, S.G.; Martins, F.S.; Vianna, E.O.; Borges, M.d.C. Oral administration of Saccharomyces cerevisiae UFMG A-905 prevents allergic asthma in mice. Respirology 2017, 22, 905–912. [Google Scholar] [CrossRef]
- Milani, T.M.S.; Sandy, C.M.; Calazans, A.P.C.T.; Silva, R.Q.; Fonseca, V.M.B.; Martins, F.S.; Borges, M.C. Dose-Response Effect of Saccharomyces cerevisiae UFMG A-905 on the Prevention of Asthma in an Animal Model. Probiotics Antimicrob. Proteins 2022. Online ahead of print. [Google Scholar] [CrossRef]
- Lindenberg, F.; Krych, L.; Kot, W.; Fielden, J.; Frøkiær, H.; van Galen, G.; Nielsen, D.S.; Hansen, A.K. Development of the equine gut microbiota. Sci. Rep. 2019, 9, 14427. [Google Scholar] [CrossRef]
- Feleszko, W.; Jaworska, J.; Rha, R.-D.; Steinhausen, S.; Avagyan, A.; Jaudszus, A.; Ahrens, B.; Groneberg, D.A.; Wahn, U.; Hamelmann, E. Probiotic-induced suppression of allergic sensitization and airway inflammation is associated with an increase of T regulatory-dependent mechanisms in a murine model of asthma. Clin. Exp. Allergy 2007, 37, 498–505. [Google Scholar] [CrossRef]
- Elazab, N.; Mendy, A.; Gasana, J.; Vieira, E.R.; Quizon, A.; Forno, E. Probiotic administration in early life, atopy, and asthma: A meta-analysis of clinical trials. Pediatrics 2013, 132, e666–e676. [Google Scholar] [CrossRef]
- Schoster, A.; Staempfli, H.R.; Abrahams, M.; Jalali, M.; Weese, J.S.; Guardabassi, L. Effect of a probiotic on prevention of diarrhea and Clostridium difficile and Clostridium perfringens shedding in foals. J. Vet. Intern. Med. 2015, 29, 925–931. [Google Scholar] [CrossRef]
- Roduit, C.; Frei, R.; Ferstl, R.; Loeliger, S.; Westermann, P.; Rhyner, C.; Schiavi, E.; Barcik, W.; Rodriguez-Perez, N.; Wawrzyniak, M.; et al. High levels of butyrate and propionate in early life are associated with protection against atopy. Allergy 2019, 74, 799–809. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).