Evaluation of Mud Worm (Polydora spp.) Infestation in Cupped (Crassostrea gigas) and Flat Oyster (Ostrea edulis) Broodstocks: Comparison between Magnetic Resonance Imaging and Computed Tomography
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Advanced Diagnostic Techniques
2.3. Macroscopic Findings
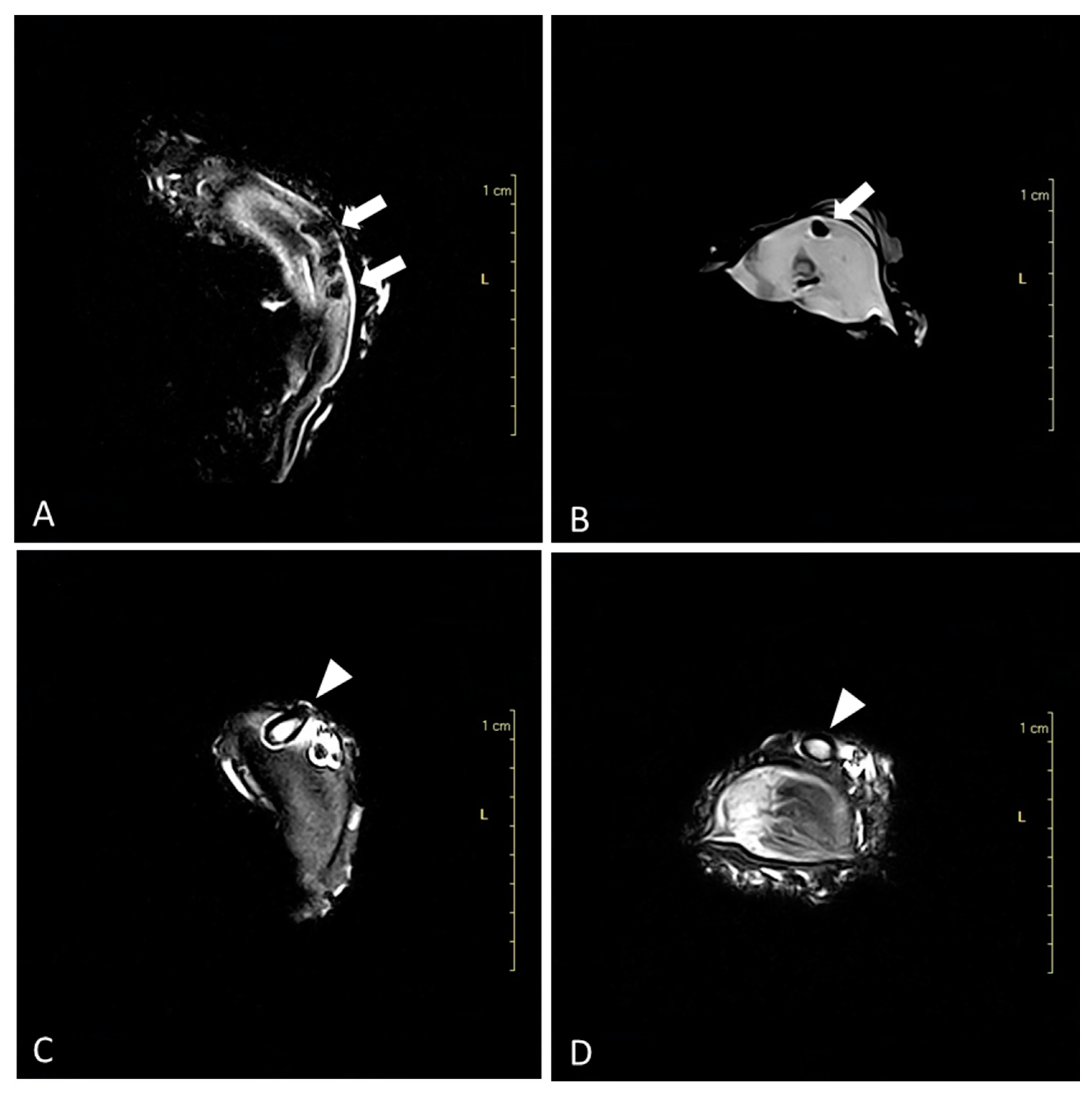
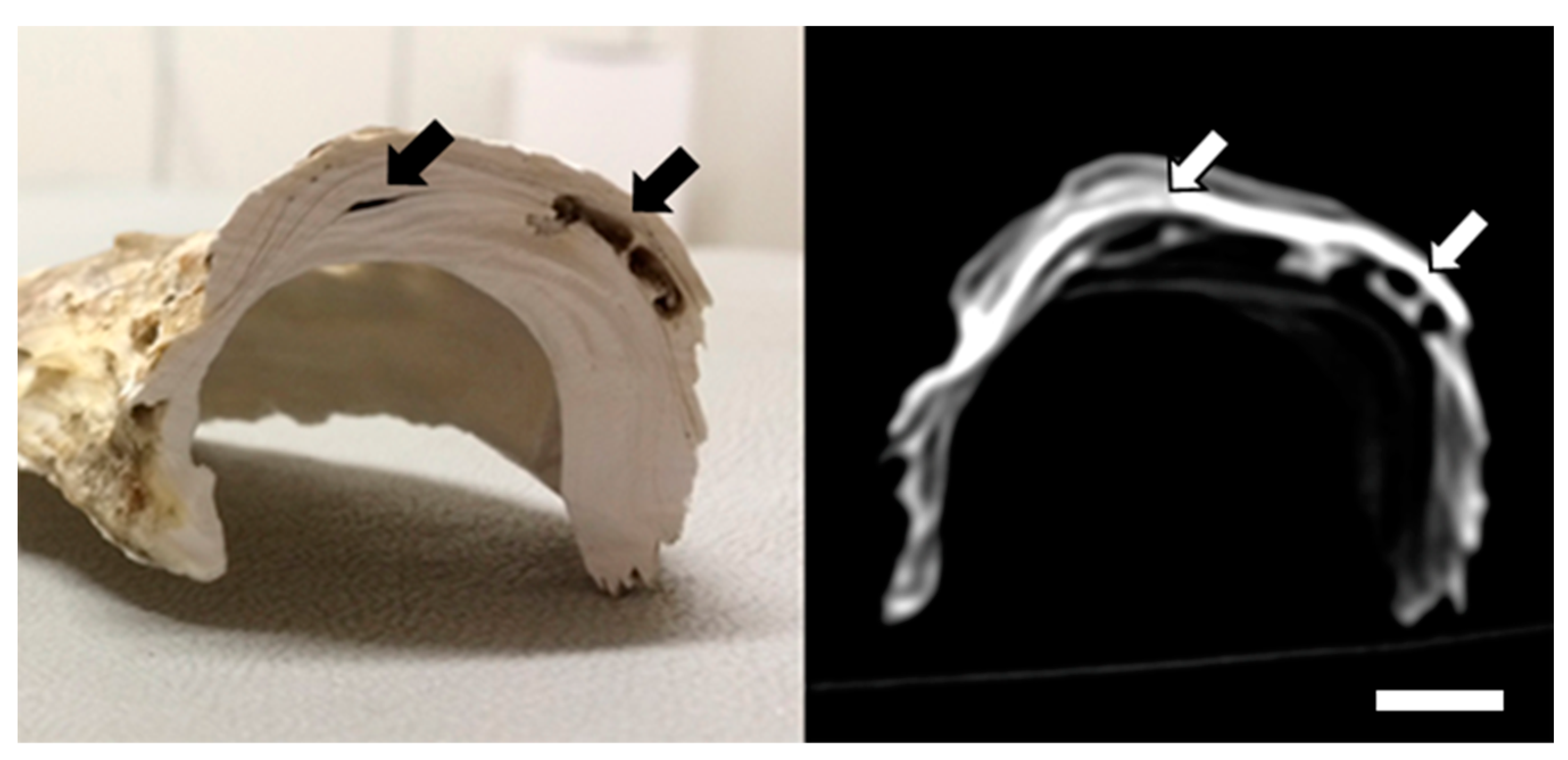
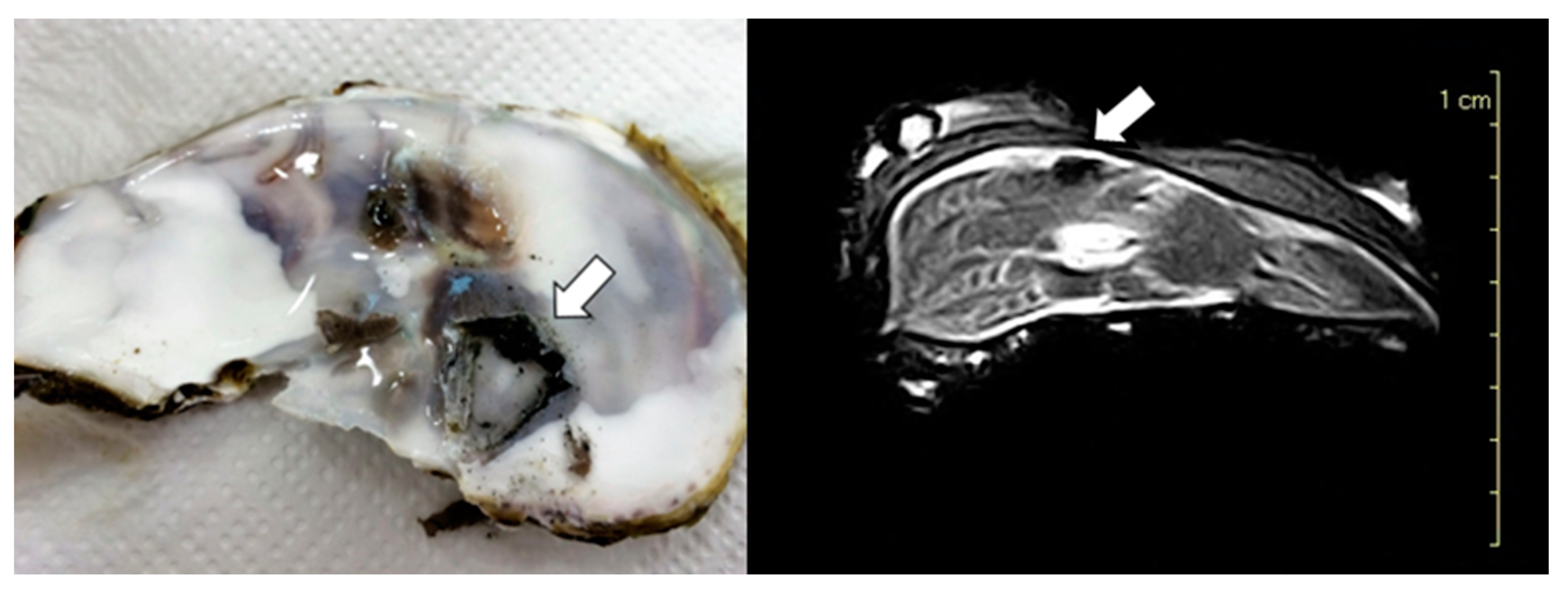
3. Results
3.1. Advanced Diagnostic Techniques
3.2. Macroscopic Findings
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roncarati, A.; Felici, A.; Magi, G.E.; Bilandžić, N.; Melotti, P. Growth and survival of cupped oysters (Crassostrea gigas) during nursery and pregrowing stages in open sea facilities using different stocking densities. Aquac. Int. 2017, 25, 1777–1785. [Google Scholar] [CrossRef]
- Summa, D.; Turolla, E.; Lanzoni, M.; Tamisari, E.; Castaldelli, G.; Tamburini, E. Life Cycle Assessment (LCA) of Two Different Oyster (Crassostrea gigas) Farming Strategies in the Sacca di Goro, Northern Adriatic Sea, Italy. Resources 2023, 12, 62. [Google Scholar] [CrossRef]
- Zottoli, R.A.; Carriker, M.R. Burrow morphology, tube formation, and microarchitecture of shell dissolution by the spionid polychaete Polydora websteri. Mar. Biol. 1974, 27, 307–316. [Google Scholar] [CrossRef]
- Handley, S.J.; Berquist, P.R. Spionid polychaete infestations of intertidal pacific oyster Crassostrea gigas (Thunberg), Mahurangi Harbour, northern New Zealand. Aquaculture 1997, 153, 191–205. [Google Scholar] [CrossRef]
- Caceres-Martìnez, J.; Tinoco, G.D.; Bustamante, M.U.U.; Gomez-Humaran, I.M. Relationship between the burrowing worm Polydora sp. and black clam Chione fluctifraga (Showerby). J. Shellfish Res. 1999, 18, 85–90. [Google Scholar]
- Liu, P.J.; Hsieh, H.L. Burrow architecture of the spionid polychaete Polydora villosa in the corals Montipora and Porites. Zool. Stud. 2000, 39, 47–54. [Google Scholar]
- Handley, S.J. Larval development of Boccardia knoxi, a shell-infesting spionid polychaete. N. Z. J. Mar. Freshw. Res. 2000, 34, 681–687. [Google Scholar] [CrossRef]
- Medcof, J.C. The mud-blister worm, Polydora, in Canadian oysters. J. Fish. Board Can. 1946, 6, 498–505. [Google Scholar] [CrossRef]
- Loosanoff, V.L.; Engle, J.B. Polydora in oysters suspended in the water. Biol. Bull. 1943, 85, 69–78. [Google Scholar] [CrossRef]
- Blake, J.A.; Evans, J.W. Polydora and related genera as borers in mollusk shell and other calcareous substrates (Polychaeta: Spionidae). Veliger 1973, 15, 235–249. [Google Scholar]
- Kent, R.M.L. The effect of Polydora ciliata on the shell strength of Mytilus edulis. ICES J. Mar. Sci. 1981, 39, 252–255. [Google Scholar] [CrossRef]
- Martinelli, J.C.; Lopes, H.M.; Hauser, L.; Jimenez-Hidalgo, I.; King, T.L.; Padilla-Gamiño, J.L.; Rawson, P.; Spencer, L.H.; Williams, J.D.; Wood, C.L. Confirmation of the shell-boring oyster parasite Polydora websteri (Polychaeta: Spionidae) in Washington State, USA. Sci. Rep. 2020, 10, 3961. [Google Scholar] [CrossRef]
- Brundu, G.; Loi, B.; Chindris, A.; Graham, P.; Bernabè, D.; Giménez Papiol, G. Rearing equipment on the quality aspects and growth performance of Crassostrea gigas (Thunberg, 1793): Implications for marketability. Aquact. Res. 2020, 51, 2936–2947. [Google Scholar] [CrossRef]
- Chambon, C.; Legeay, A.; Durrieu, G.; Gonzalez, P.; Ciret, P.; Massabuau, J.C. Influence of the parasite worm Polydora sp. on the behaviour of the oyster Crassostrea gigas: A study of the respiratory impact and associated oxidative stress. Mar. Biol. 2007, 152, 329–338. [Google Scholar] [CrossRef]
- Skeel, M.E. Shell boring worms (Spionidae: Polychaeta) infecting cultivated bivalves molluscs in Australia. Proc. World Maric. Soc. 1979, 10, 529–533. [Google Scholar] [CrossRef]
- Spiga, B.; Fenzi, G.; Salati, F. Treatment trials in Crassostrea gigas against Polydora ciliata infection. Ittiopatologia 2007, 4, 207–213. [Google Scholar]
- Fleury, P.G.; Goyard, E.; Mazurie, J.; Claude, S.; Bouget, J.F.; Langlade, A.; Le Coguic, Y. The assessing of Pacific oyster (Crassostrea gigas) rearing performances by the IFREMER/REMORA network: Method and first results (1993–98) in Brittany (France). Hydrobiologia 2001, 465, 195–208. [Google Scholar] [CrossRef]
- Chávez-Villalba, J.; Pommier, J.; Andriamiseza, J.; Pouvreau, S.; Barret, J.; Cochard, J.-C.; Le Pennec, M. Broodstock conditioning of the oyster Crassostrea gigas: Origin and temperature effect. Aquaculture 2022, 214, 115–130. [Google Scholar] [CrossRef]
- Dridi, S.; Romdhane, M.S.; Elcafsi, M. Seasonal variation in weight and biochemical composition of the Pacific oyster, Crassostrea gigas in relation to the gametogenic cycle and environmental conditions of the Bizert lagoon, Tunisia. Aquaculture 2007, 263, 238–248. [Google Scholar] [CrossRef]
- Davenel, A.; Quellec, S.; Pouvreau, S. Non invasive characterization of gonad maturation and determination of the sex of Pacific oysters by MRI. Magn. Reson. Imaging 2006, 24, 1103–1110. [Google Scholar] [CrossRef]
- Pouvreau, S.; Rambeau, M.; Cochard, J.C.; Robert, R. Investigation of marine bivalves morphology by in vivo MR imaging: First anatomical results of a promising technique. Aquaculture 2006, 259, 415–423. [Google Scholar] [CrossRef]
- Hatt, P.J.; Davenel, A.; Eliat, P.A.; Quellec, S. Magnetic resonance imaging as a means to assess the body growth and the gonad development of the oyster Crassostrea gigas. Aquat. Living Resour. 2009, 22, 331–339. [Google Scholar] [CrossRef]
- Davenel, A.; González, R.; Suquet, M.; Quellec, S.; Robert, R. Individual monitoring of gonad development in the European flat oyster Ostrea edulis by in vivo magnetic resonance imaging. Aquaculture 2010, 307, 165–169. [Google Scholar] [CrossRef]
- Cole, S.M.; Dorgan, K.M.; Walton, W.; Dzwonkowski, B.; Coogan, J. Seasonal and spatial patterns of mudblister worm Polydora websteri infestation of farmed oysters in the northern Gulf of Mexico. Aquacult. Environ. Interact. 2020, 12, 297–314. [Google Scholar] [CrossRef]
- Rusco, G.; Di Iorio, M.; Felici, A.; Galosi, L.; Iaffaldano, N.; Roncarati, A. Strategies to Improve the Post-Harvest Management of Flat Oyster (Ostrea edulis) from Aquaculture Using the Short-Term Storage and Package in an Innovative Closed-Circuit System. J. Food Sci. 2023; in print. [Google Scholar]
- Bower, S.M.; McGladdery, S.E.; Price, I.M. Synopsis of infectious diseases and parasites of commercially exploited shellfish. Annu. Rev. Fish Dis. 1994, 4, 1–199. [Google Scholar] [CrossRef]
- Ghode, G.S.; Kripa, V. Polydora infestation on Crassoslrea madrasensis: A study on the infestation rate and eradication methods. J. Mar. Biol. Assoc. India 2001, 43, 110–119. [Google Scholar]
- Bishop, M.J.; Hooper, P.J. Flow, stocking density and treatment against Polydora spp.: Influences on nursery growth and mortality of the oysters Crassostrea virginica and C. ariakensis. Aquaculture 2005, 246, 251–261. [Google Scholar] [CrossRef]
- Flahauw, E.; Quellec, S.; Davenel, A.; Degremont, L.; Lapegue, S.; Hatt, P.J. Gonad volume assessment in the oyster Crassostrea gigas: Comparison between a histological method and a magnetic resonance imaging (MRI) method. Aquaculture 2012, 370–371, 84–89. [Google Scholar] [CrossRef]
- Smith, P.T.; Reddy, N. Application of magnetic resonance imaging (MRI) to study the anatomy and reproductive condition of live Sydney rock oyster, Saccostrea glomerata (Gould). Aquaculture 2012, 334–337, 191–198. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galosi, L.; Dini, F.; Meligrana, M.C.T.; Gennari, L.; Tamburini, E.; Roncarati, A. Evaluation of Mud Worm (Polydora spp.) Infestation in Cupped (Crassostrea gigas) and Flat Oyster (Ostrea edulis) Broodstocks: Comparison between Magnetic Resonance Imaging and Computed Tomography. Animals 2024, 14, 242. https://doi.org/10.3390/ani14020242
Galosi L, Dini F, Meligrana MCT, Gennari L, Tamburini E, Roncarati A. Evaluation of Mud Worm (Polydora spp.) Infestation in Cupped (Crassostrea gigas) and Flat Oyster (Ostrea edulis) Broodstocks: Comparison between Magnetic Resonance Imaging and Computed Tomography. Animals. 2024; 14(2):242. https://doi.org/10.3390/ani14020242
Chicago/Turabian StyleGalosi, Livio, Fabrizio Dini, Marina C. T. Meligrana, Lorenzo Gennari, Elena Tamburini, and Alessandra Roncarati. 2024. "Evaluation of Mud Worm (Polydora spp.) Infestation in Cupped (Crassostrea gigas) and Flat Oyster (Ostrea edulis) Broodstocks: Comparison between Magnetic Resonance Imaging and Computed Tomography" Animals 14, no. 2: 242. https://doi.org/10.3390/ani14020242
APA StyleGalosi, L., Dini, F., Meligrana, M. C. T., Gennari, L., Tamburini, E., & Roncarati, A. (2024). Evaluation of Mud Worm (Polydora spp.) Infestation in Cupped (Crassostrea gigas) and Flat Oyster (Ostrea edulis) Broodstocks: Comparison between Magnetic Resonance Imaging and Computed Tomography. Animals, 14(2), 242. https://doi.org/10.3390/ani14020242







