Development and Preliminary Validation of an Equine Brief Pain Inventory for Owner Assessment of Chronic Pain Due to Osteoarthritis in Horses
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
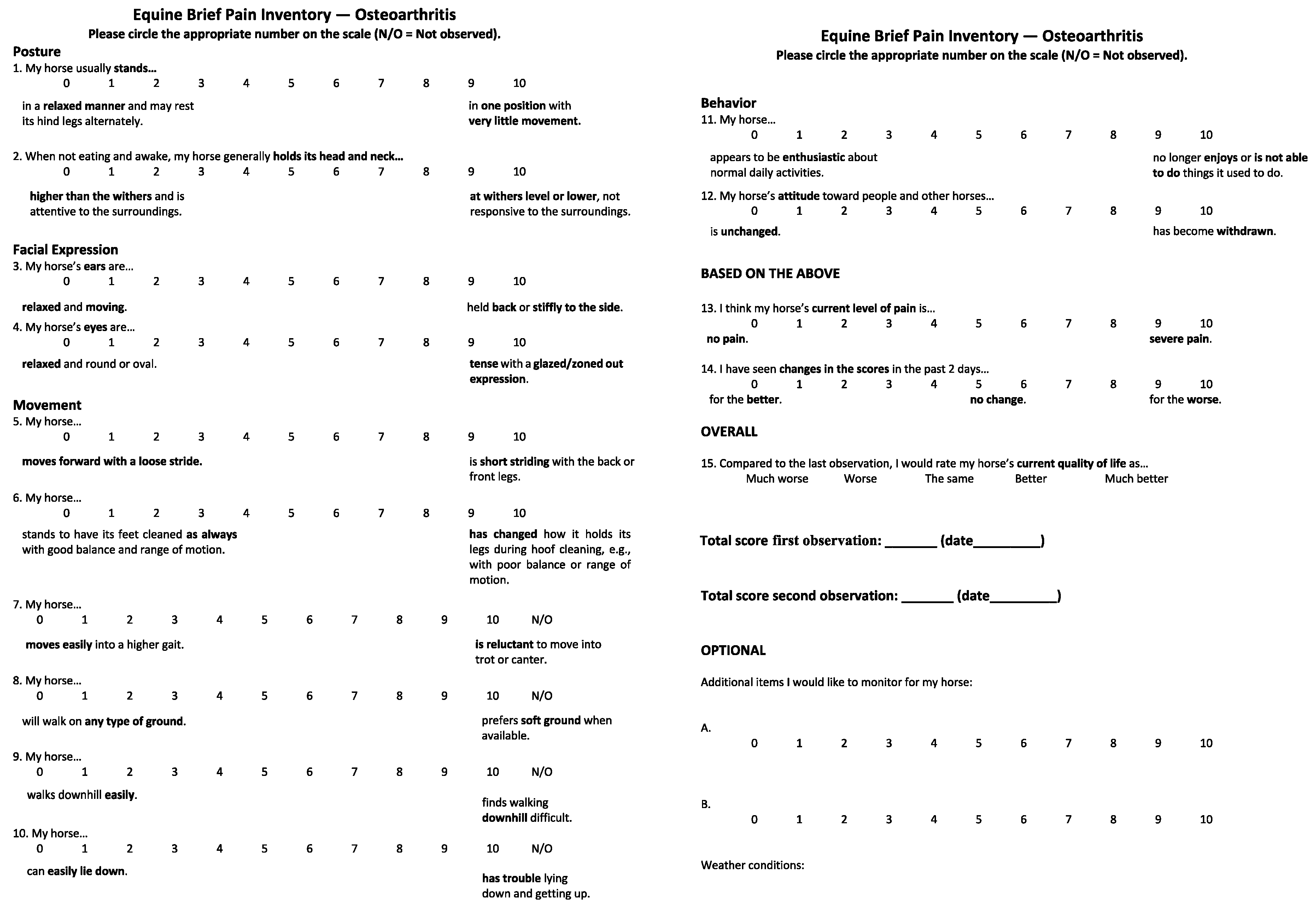
2.1. Step 1: Item Generation
2.1.1. Literature Review
2.1.2. Focus Group Discussions
2.2. Step 2: Initial Creation of Questionnaire
2.3. Step 3: Readability Evaluation
2.4. Step 4: Expert Evaluation and Questionnaire Revision
2.5. Step 5: Piloting the EBPI
Statistical Treatment
2.6. Step 6: Test–Retest for Scoring Reliability (Ongoing)
2.7. Step 7: Validity Assessment (Ongoing)
2.8. Step 8: Final Data Analysis and Preparation of Material for Publication
3. Results
3.1. Item Generation (Step 1)
3.2. Item Generation (Step 2)
3.3. Readability Evaluation (Step 3)
3.4. Expert Panel Evaluation (Step 4)
3.5. Pilot Study (Step 5)
3.6. Interim Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Karcioglu, O.; Topacoglu, H.; Dikme, O.; Dikme, O. A systematic review of the pain scales in adults: Which to use? Am. J. Emerg. Med. 2018, 36, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Hesselgard, K.; Larsson, S.; Romner, B.; Strömblad, L.G.; Reinstrup, P. Validity and reliability of the Behavioural Observational Pain Scale for postoperative pain measurement in children 1–7 years of age. Pediatr. Crit. Care Med. 2007, 8, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Fuchs-Lacelle, S.; Hadjistavropoulos, T. Development and preliminary validation of the pain assessment checklist for seniors with limited ability to communicate (PACSLAC). Pain Manag. Nurs. 2004, 5, 37–49. [Google Scholar] [CrossRef] [PubMed]
- National Research Council (US) Committee on Recognition and Alleviation of Pain in Laboratory Animals. Recognition and Alleviation of Pain in Laboratory Animals; National Academies Press: Washington, DC, USA, 2009. Available online: https://www.ncbi.nlm.nih.gov/books/NBK32658/ (accessed on 4 December 2023).
- McLennan, K.; Mahmoud, M. Development of an Automated Pain Facial Expression Detection System for Sheep (Ovis aries). Animals 2019, 9, 196. [Google Scholar] [CrossRef] [PubMed]
- Gleerup, K.; Andersen, P.; Munksgaard, L.; Forkman, B. Pain evaluation in dairy cattle. Appl. Anim. Behav. Sci. 2015, 171, 25–32. [Google Scholar] [CrossRef]
- Mathews, K.; Kronen, P.W.; Lascelles, D.; Nolan, A.; Robertson, S.; Steagall, P.V.; Wright, B.; Yamashita, K. Guidelines for recognition, assessment and treatment of pain: WSAVA Global Pain Council members and co-authors of this document. J. Small Anim. Pract. 2014, 55, E10–E68. [Google Scholar] [CrossRef] [PubMed]
- Wiseman-Orr, M.L.; Nolan, A.M.; Reid, J.; Scott, E.M. Development of a questionnaire to measure the effects of chronic pain on health-related quality of life in dogs. Am. J. Vet. Res. 2004, 65, 1077–1084. [Google Scholar] [CrossRef]
- Hielm-Björkman, A.K.; Rita, H.; Tulamo, R.M. Psychometric testing of the Helsinki chronic pain index by completion of a questionnaire in Finnish by owners of dogs with chronic signs of pain caused by osteoarthritis. Am. J. Vet. Res. 2009, 70, 727–734. [Google Scholar] [CrossRef]
- Brown, D.C.; Boston, R.C.; Coyne, J.C.; Farrar, J.T. Development and psychometric testing of an instrument designed to measure chronic pain in dogs with osteoarthritis. Am. J. Vet. Res. 2007, 68, 631–637. [Google Scholar] [CrossRef]
- Epstein, M. Assessing Chronic Pain in Dogs. Today’s Veterinary Practice. 2013, pp. 23–35. Available online: https://todaysveterinarypractice.com/wp-content/uploads/sites/4/2016/09/T1309F04.pdf (accessed on 4 December 2023).
- Allweiller, S. Recognition and Assessment of Pain in Animals. Merck Manual Veterinary Manual. 2023. Available online: https://www.merckvetmanual.com/management-and-nutrition/pain-assessment-and-management/recognition-and-assessment-of-pain-in-animals (accessed on 4 December 2023).
- Bussières, G.; Jacques, C.; Lainay, O.; Beauchamp, G.; Leblond, A.; Cadoré, J.L.; Desmaizières, L.M.; Cuvelliez, S.G.; Troncy, E. Development of a composite orthopaedic pain scale in horses. Res. Vet. Sci. 2008, 85, 294–306. [Google Scholar] [CrossRef]
- van Loon, J.; Back, W.; Hellebrekers, L.; van Weeren, P. Application of a composite pain scale to objectively monitor horses with somatic and visceral pain under hospital conditions. J. Equine Vet. Sci. 2010, 30, 641–649. [Google Scholar] [CrossRef]
- Graubner, C.; Gerber, V.; Doherr, M.; Spadavecchia, C. Clinical Application and Reliability of a Post Abdominal Surgery Pain Assessment Scale (PASPAS) in Horses. Vet. J. 2011, 188, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Sutton, G.A.; Dahan, R.; Turner, D.; Paltiel, O. A Behaviour-Based Pain Scale for Horses with Acute Colic: Scale Construction. Vet. J. 2013, 196, 394–401. [Google Scholar] [CrossRef]
- van Loon, J.; Jonckheer-Sheeny, V.; Back, W.; van Weeren, P.; Hellebrekers, L. Monitoring Equine Visceral Pain with a Composite Pain Scale Score and Correlation with Survival after Emergency Gastrointestinal Surgery. Vet. J. 2014, 200, 109–115. [Google Scholar] [CrossRef] [PubMed]
- van Loon, J.P.A.M.; Van Dierendonck, M.C. Monitoring Acute Equine Visceral Pain with the Equine Utrecht University Scale for Composite Pain Assessment (EQUUS-COMPASS) and the Equine Utrecht University Scale for Facial Assessment of Pain (EQUUS-FAP): A Scale-Construction Study. Vet. J. 2015, 206, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Taffarel, M.O.; Luna, S.P.L.; de Oliveira, F.A.; Cardoso, G.S.; de Moura Alonso, J.; Pantoja, J.C.; Brondani, J.T.; Love, E.; Taylor, P.; White, K.; et al. Refinement and Partial Validation of the UNESP-Botucatu Multidimensional Composite Pain Scale for Assessing Postoperative Pain in Horses. BMC Vet. Res. 2015, 11, 83. [Google Scholar] [CrossRef] [PubMed]
- Maskato, Y.; Dugdale, A.; Singer, E.; Kelmer, G.; Sutton, G. Prospective Feasibility and Revalidation of the Equine Acute Abdominal Pain Scale (EAAPS) in Clinical Cases of Colic in Horses. Animals 2020, 10, 2242. [Google Scholar] [CrossRef]
- Dalla Costa, E.; Minero, M.; Lebelt, D.; Stucke, D.; Canali, E.; Leach, M.C. Development of the Horse Grimace Scale (HGS) as a Pain Assessment Tool in Horses Undergoing Routine Castration. PLoS ONE 2014, 9, e92281. [Google Scholar] [CrossRef]
- van Loon, J.P.A.M.; Van Dierendonck, M.C. Monitoring Equine Head-Related Pain with the Equine Utrecht University Scale for Facial Assessment of Pain (EQUUS-FAP). Vet. Res. 2017, 220, 88–90. [Google Scholar] [CrossRef]
- Torcivia, C.; McDonnell, S. Equine Discomfort Ethogram. Animals 2021, 11, 580. [Google Scholar] [CrossRef]
- van Loon, J.P.A.M.; Macri, L. Objective Assessment of Chronic Pain in Horses Using the Horse Chronic Pain Scale (HCPS): A Scale-Construction Study. Animals 2021, 11, 1826. [Google Scholar] [CrossRef] [PubMed]
- van Weeren, P.R.; de Grauw, J.C. Pain in Osteoarthritis. Vet. Clin. N. Am. Equine Pract. 2010, 26, 619–642. [Google Scholar] [CrossRef] [PubMed]
- Ireland, J.L.; Clegg, P.D.; McGowan, C.M.; McKane, S.A.; Chandler, K.J.; Pinchbeck, G.L. Comparison of Owner-Reported Health Problems with Veterinary Assessment of Geriatric Horses in the United Kingdom. Equine Vet. J. 2011, 44, 94–100. [Google Scholar] [CrossRef] [PubMed]
- van Weeren, P.R.; Back, W. Musculoskeletal Disease in Aged Horses and Its Management. Vet. Clin. N. Am. Equine Pract. 2016, 32, 229–247. [Google Scholar] [CrossRef]
- Steiner, N.; Norman, G. Health Measurement Scales: A Practical Guide to Their Development and Use, 4th ed.; Oxford University Press: Oxford, UK, 2009. [Google Scholar]
- Hutchinson, N.; Baird, G.L.; Garg, M. Examining the Reading Level of Internet Medical Information for Common Internal Medicine Diagnoses. Am. J. Med. 2016, 129, 637–639. [Google Scholar] [CrossRef]
- Stossel, L.M.; Segar, N.; Gliatto, P.; Fallar, R.; Karani, R. Readability of Patient Education Materials Available online the Point of Care. J. Gen. Intern. Med. 2012, 27, 1165–1170. [Google Scholar] [CrossRef]
- Readable. Flesch Reading Ease and the Flesch Kincaid Grade Level. 2023. Available online: https://readable.com/readability/flesch-reading-ease-flesch-kincaid-grade-level/ (accessed on 4 December 2023).
- Readable. The SMOG Index. 2023. Available online: https://readable.com/readability/smog-index/ (accessed on 4 December 2023).
- Pollard, D.; Wylie, C.E.; Verheyen, K.L.P.; Newton, J.R. Assessment of Horse Owners’ Ability to Recognise Equine Laminitis: A Cross-Sectional Study of 93 Veterinary Diagnosed Cases in Great Britain. Equine Vet. J. 2017, 49, 759–766. [Google Scholar] [CrossRef]
- Mullard, J.; Berger, J.M.; Ellis, A.D.; Dyson, S. Development of an Ethogram to Describe Facial Expressions in Ridden Horses (FEReq). J. Vet. Behav. 2017, 18, 7–12. [Google Scholar] [CrossRef]
- Dyson, S.; Berger, J.M.; Ellis, A.D.; Mullard, J. Can the Presence of Musculoskeletal Pain Be Determined from the Facial Expressions of Ridden Horses (FEReq)? J. Vet. Beh. 2017, 19, 78–89. [Google Scholar] [CrossRef]
- Caron, J. Osteoarthritis. In Diagnosis and Management of Lameness in the Horse, 2nd ed.; Ross, M., Dyson, S., Eds.; Elsevier Saunders: St. Louis, MO, USA, 2011; pp. 655–667. [Google Scholar]
- Cleeland, C.S.; Ryan, K.M. Pain assessment: Global use of the Brief Pan Inventory. Ann. Acad. Med. Singap. 1994, 23, 129–138. [Google Scholar]
- Dixon, W.G.; Beukenhorst, A.L.; Yimer, B.B.; Cook, L.; Gasparrini, A.; El-Hay, T.; Hellman, B.; James, B.; Vicedo-Cabrera, A.M.; Maclure, M.; et al. How the Weather Affects the Pain of Citizen Scientists Using a Smartphone App. npj Digit. Med. 2019, 2, 105. [Google Scholar] [CrossRef] [PubMed]
- Gingerich, D.A.; Strobel, J.D. Use of client-specific outcome measures to assess treatment effects in geriatric, arthritic dogs: Controlled clinical evaluation of a nutraceutical. Vet Ther. 2003, 4, 376–386. [Google Scholar] [PubMed]
- Lascelles, B.D.X.; Hansen, B.D.; Roe, S.; DePuy, V.; Thomson, A.; Pierce, C.C.; Smith, E.S.; Rowinski, E. Evaluation of Client-Specific Outcome Measures and Activity Monitoring to Measure Pain Relief in Cats with Osteoarthritis. J. Vet. Intern. Med. 2007, 21, 410–416. [Google Scholar] [CrossRef] [PubMed]
- British Council. What Can a B1-Level Learner of English Do? Available online: https://learnenglish.britishcouncil.org/english-levels/understand-your-english-level/b1-intermediate (accessed on 4 December 2023).
- Olcoz, M.; Cabezas, M.Á.; Rocca, G.D.; Gómez de Segura, I.A. Translation to Spanish and linguistic validation of the Canine Brief Pain Inventory. Front. Vet. Sci. 2023, 10, 1203453. [Google Scholar] [CrossRef]
- Cronbach, L. Coefficient alpha and the internal structure of tests. Psychometrika 1951, 16, 297–334. [Google Scholar] [CrossRef]
- Taber, K.S. The Use of Cronbach’s Alpha When Developing and Reporting Research Instruments in Science Education. Res. Sci. Ed. 2018, 48, 1273–1296. [Google Scholar] [CrossRef]
- Torcivia, C.; McDonnell, S. In-Person Caretaker Visits Disrupt Ongoing Discomfort Behavior in Hospitalized Equine Orthopedic Surgical Patients. Animals 2020, 10, 210. [Google Scholar] [CrossRef]
- Stewart, D.; Shamdasani, P.; Rook, D. Focus Groups, 2nd ed.; Sage Publishing: Thousand Oaks, CA, USA, 2011. [Google Scholar]
- Cohn, N. Who in the World Is Still Answering Pollsters’ Phone Calls. The New York Times. 12 October 2022. Available online: https://www.nytimes.com/2022/10/12/upshot/midterms-polling-phone-calls.html (accessed on 4 December 2023).
- Lusin, N. (Head of Research, Modern Language Association, New York City, NY, USA). Email Communication, 2023.
| Item Type/No. | Administration 1 | Administration 2 | ||
|---|---|---|---|---|
| Median | Range | Median | Range | |
| Posture | ||||
| 1 | 3 | 0–9 | 3 | 0–9 |
| 2 | 3 | 0–8 | 3 | 0–8 |
| Facial expression | ||||
| 3 | 2 | 0–5 | 2 | 0–5 |
| 4 | 2 | 0–7 | 3 | 0–7 |
| Movement | ||||
| 5 | 7 | 2–10 | 7 | 2–10 |
| 6 | 5 | 0–10 | 5 | 0–10 |
| 7 | 7 | 0–10 | 7 | 0–10 |
| 8 | 7 | 0–10 | 7 | 0–10 |
| 9 | 7 | 0–10 | 7 | 0–10 |
| 10 | 2 | 0–10 | 3 | 0–10 |
| Behavior | ||||
| 11 | 3 | 0–8 | 3 | 0–8 |
| 12 | 1 | 0–7 | 1 | 0–7 |
| Item Type/No. | Administration 1 | Administration 2 | ||
|---|---|---|---|---|
| Median | Range | Median | Range | |
| Posture | ||||
| 1 | 0 | 0–3 | 0 | 0–3 |
| 2 | 0 | 0–3 | 0 | 0–3 |
| Facial expression | ||||
| 3 | 2 | 0–5 | 1 | 0–5 |
| 4 | 1 | 0–5 | 1 | 0–4 |
| Movement | ||||
| 5 | 2 | 0–4 | 2 | 0–4 |
| 6 | 0 | 0–2 | 1 | 0–2 |
| 7 | 3 | 0–3 | 3 | 0–3 |
| 8 | 2.5 | 0–5 | 2.5 | 0–5 |
| 9 | 2.5 | 0–3 | 1.5 | 0–3 |
| 10 | 1 | 0–2 | 1 | 0–2 |
| Behavior | ||||
| 11 | 2 | 0–3 | 2 | 0–3 |
| 12 | 0 | 0–5 | 0 | 0–4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Howard, D.L.; Lancaster, B.; de Grauw, J. Development and Preliminary Validation of an Equine Brief Pain Inventory for Owner Assessment of Chronic Pain Due to Osteoarthritis in Horses. Animals 2024, 14, 181. https://doi.org/10.3390/ani14020181
Howard DL, Lancaster B, de Grauw J. Development and Preliminary Validation of an Equine Brief Pain Inventory for Owner Assessment of Chronic Pain Due to Osteoarthritis in Horses. Animals. 2024; 14(2):181. https://doi.org/10.3390/ani14020181
Chicago/Turabian StyleHoward, Diane L., Bryony Lancaster, and Janny de Grauw. 2024. "Development and Preliminary Validation of an Equine Brief Pain Inventory for Owner Assessment of Chronic Pain Due to Osteoarthritis in Horses" Animals 14, no. 2: 181. https://doi.org/10.3390/ani14020181
APA StyleHoward, D. L., Lancaster, B., & de Grauw, J. (2024). Development and Preliminary Validation of an Equine Brief Pain Inventory for Owner Assessment of Chronic Pain Due to Osteoarthritis in Horses. Animals, 14(2), 181. https://doi.org/10.3390/ani14020181






