Chicken Secondary Lymphoid Tissues—Structure and Relevance in Immunological Research
Abstract
:Simple Summary
Abstract
1. Introduction
2. Secondary Lymphoid Organs
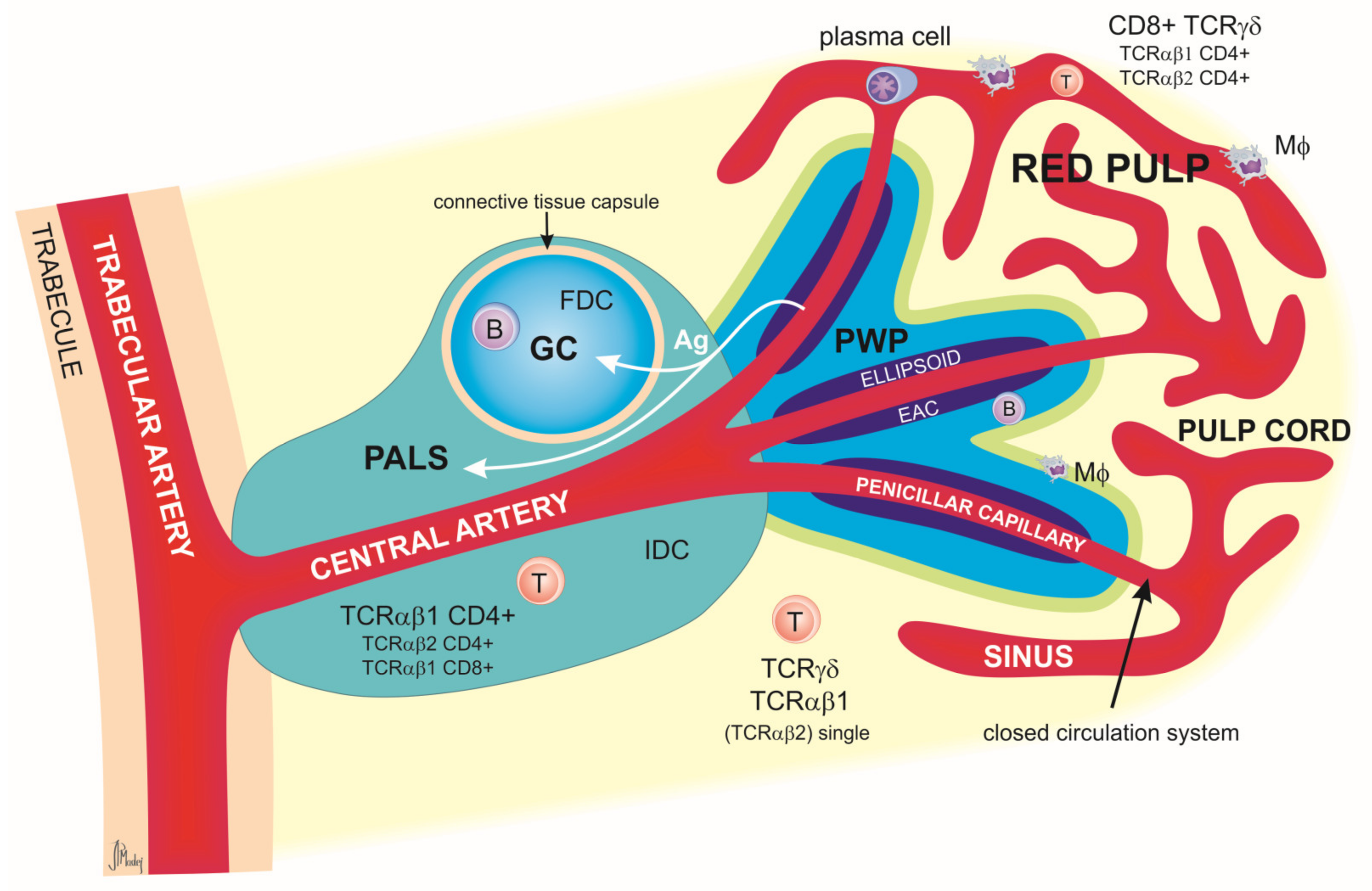
2.1. Spleen
Relevance of the Spleen in Immunological Research
2.2. Mucosa-Associated Lymphoid Tissue (MALT)
2.2.1. Nasal-Associated Lymphoid Tissue (NALT)
2.2.2. Tracheal Mucosal Immunity
2.2.3. Bronchus-Associated Lymphoid Tissue (BALT)
2.2.4. Gut-Associated Lymphoid Tissue (GALT)
Esophageal Tonsils
Pyloric Tonsils
Meckel’s Diverticulum (MD)
Peyer’s Patches (PPs)
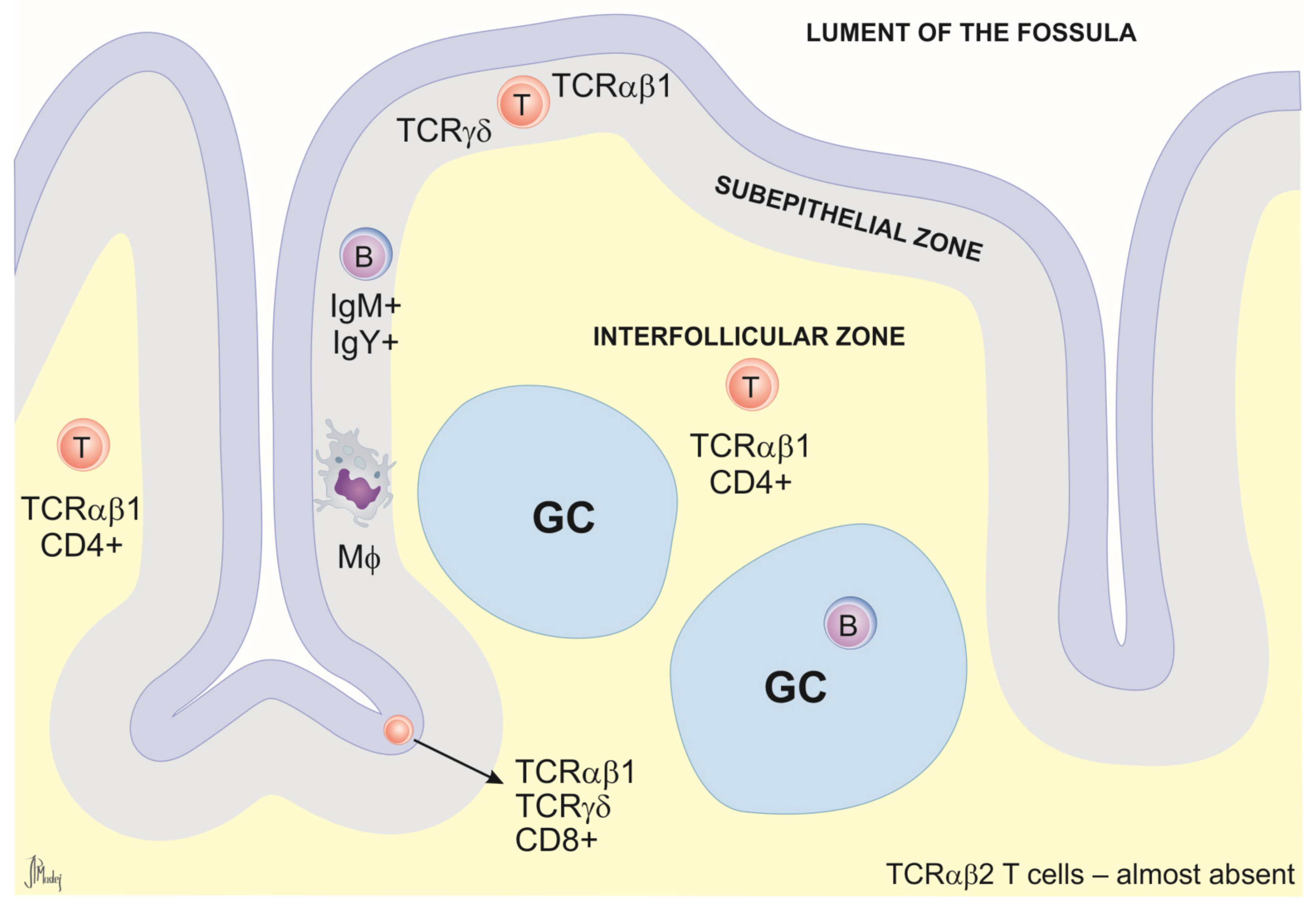
Cecal Tonsils (CTs)
2.2.5. Relevance of MALT in Immunological Research
2.3. Gonad-Associated Lymphoid Tissue (GOALT)
Relevance of GOALT in Immunological Research
2.4. Pineal Gland-Associated Lymphoid Tissue (PALT)
Relevance of PALT in Immunological Research
2.5. Skin-Associated Lymphoid Tissue
2.6. Mural Lymph Nodes (MLNs)
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lavelle, E.C.; Ward, R.W. Mucosal Vaccines—Fortifying the Frontiers. Nat. Rev. Immunol. 2022, 22, 236–250. [Google Scholar] [CrossRef]
- Nochi, T.; Jansen, C.A.; Toyomizu, M.; van Eden, W. The Well-Developed Mucosal Immune Systems of Birds and Mammals Allow for Similar Approaches of Mucosal Vaccination in Both Types of Animals. Front. Nutr. 2018, 5, 60. [Google Scholar] [CrossRef] [PubMed]
- Garcia, P.; Wang, Y.; Viallet, J.; Jilkova, Z.M. The Chicken Embryo Model: A Novel and Relevant Model for Immune-Based Studies. Front. Immunol. 2021, 12, 791081. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zeng, X.; Cui, P.; Yan, C.; Chen, H. Alarming Situation of Emerging H5 and H7 Avian Influenza and Effective Control Strategies. Emerg. Microbes Infect. 2023, 12, 2155072. [Google Scholar] [CrossRef] [PubMed]
- Bhuiyan, M.S.A.; Amin, Z.; Rodrigues, K.F.; Saallah, S.; Shaarani, S.M.; Sarker, S.; Siddiquee, S. Infectious Bronchitis Virus (Gammacoronavirus) in Poultry Farming: Vaccination, Immune Response and Measures for Mitigation. Vet. Sci. 2021, 8, 273. [Google Scholar] [CrossRef]
- Hafez, H.M.; Attia, Y.A. Challenges to the Poultry Industry: Current Perspectives and Strategic Future after the COVID-19 Outbreak. Front. Vet. Sci. 2020, 7, 516. [Google Scholar] [CrossRef]
- Van Asselt, M.; Poortvliet, P.M.; Ekkel, E.D.; Kemp, B.; Stassen, E.N. Risk Perceptions of Public Health and Food Safety Hazards in Poultry Husbandry by Citizens, Poultry Farmers and Poultry Veterinarians. Poult. Sci. 2018, 97, 607–619. [Google Scholar] [CrossRef]
- De Camargo, M.M.; Caetano, A.R.; Santos, I.K.F.d.M. Evolutionary Pressures Rendered by Animal Husbandry Practices for Avian Influenza Viruses to Adapt to Humans. iScience 2022, 25, 104005. [Google Scholar] [CrossRef]
- Markowska, M.; Majewski, P.M.; Skwarło-Sońta, K. Avian Biological Clock—Immune System Relationship. Dev. Comp. Immunol. 2017, 66, 130–138. [Google Scholar] [CrossRef]
- Shepon, A.; Wu, T.; Kremen, C.; Dayan, T.; Perfecto, I.; Fanzo, J.; Eshel, G.; Golden, C.D. Exploring Scenarios for the Food System-Zoonotic Risk Interface. Lancet Planet. Health 2023, 7, e329–e335. [Google Scholar] [CrossRef]
- Bovenhuis, H.; Berghof, T.V.L.; Visker, M.H.P.W.; Arts, J.A.J.; Visscher, J.; van der Poel, J.J.; Parmentier, H.K. Divergent Selection for Natural Antibodies in Poultry in the Presence of a Major Gene. Genet. Sel. Evol. 2022, 54, 24. [Google Scholar] [CrossRef]
- Kheimar, A.; Klinger, R.; Bertzbach, L.D.; Sid, H.; Yu, Y.; Conradie, A.M.; Schade, B.; Böhm, B.; Preisinger, R.; Nair, V.; et al. A Genetically Engineered Commercial Chicken Line Is Resistant to Highly Pathogenic Avian Leukosis Virus Subgroup J. Microorganisms 2021, 9, 1066. [Google Scholar] [CrossRef] [PubMed]
- Van der Most, P.J.; de Jong, B.; Parmentier, H.K.; Verhulst, S. Trade-off between Growth and Immune Function: A Meta-Analysis of Selection Experiments. Funct. Ecol. 2011, 25, 74–80. [Google Scholar] [CrossRef]
- Bertzbach, L.D.; Tregaskes, C.A.; Martin, R.J.; Deumer, U.S.; Huynh, L.; Kheimar, A.M.; Conradie, A.M.; Trimpert, J.; Kaufman, J.; Kaufer, B.B. The Diverse Major Histocompatibility Complex Haplotypes of a Common Commercial Chicken Line and Their Effect on Marek’s Disease Virus Pathogenesis and Tumorigenesis. Front. Immunol. 2022, 13, 908305. [Google Scholar] [CrossRef]
- Da Silva, A.P.; Gallardo, R.A. The Chicken MHC: Insights into Genetic Resistance, Immunity, and Inflammation Following Infectious Bronchitis Virus Infections. Vaccines 2020, 8, 637. [Google Scholar] [CrossRef] [PubMed]
- Erf, G.F.; Kong, H.R.; Falcon, D.M.; Byrne, K.A. Two-Window Approach to Monitor and Assess Cellular and Humoral Immune Responses in Poultry. Poultry 2023, 2, 82–97. [Google Scholar] [CrossRef]
- Hao, X.; Zhang, F.; Yang, Y.; Shang, S. The Evaluation of Cellular Immunity to Avian Viral Diseases: Methods, Applications, and Challenges. Front. Microbiol. 2021, 12, 794514. [Google Scholar] [CrossRef]
- Hammer, D.K. The Immune System in Chickens. Avian Pathol. 1974, 3, 65–78. [Google Scholar] [CrossRef]
- Null, M.; Arbor, T.C.; Agarwal, M. Anatomy, Lymphatic System. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Pishesha, N.; Harmand, T.J.; Ploegh, H.L. A Guide to Antigen Processing and Presentation. Nat. Rev. Immunol. 2022, 22, 751–764. [Google Scholar] [CrossRef] [PubMed]
- Kaiko, G.E.; Horvat, J.C.; Beagley, K.W.; Hansbro, P.M. Immunological Decision-Making: How Does the Immune System Decide to Mount a Helper T-Cell Response? Immunology 2008, 123, 326–338. [Google Scholar] [CrossRef] [PubMed]
- Jeurissen, S.H.M. Structure and Function of the Chicken Spleen. Res. Immunol. 1991, 142, 352–355. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Chen, B.; Yang, P.; Zhang, L.; Liu, Y.; Ullah, S.; Wu, L.; Waqas, Y.; Le, Y.; Chen, W.; et al. Identification and Structural Composition of the Blood-Spleen Barrier in Chickens. Vet. J. 2015, 204, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Peralta, M.F. Rediscovering the Importance of Mucosal Immune System (MIS) in Poultry. Acad. J. Biotechnol. 2016, 4, 91–95. [Google Scholar]
- Beheiry, R.R.; Ali, S.A.; Aref, M.; Emam, H. Harderian Gland of Flying and Non-Flying Birds: Morphological, Histological, and Histochemical Studies. J. Basic Appl. Zool. 2020, 81, 35. [Google Scholar] [CrossRef]
- Watanabe, T.; Takahashi, N.; Minaguchi, J.; Mochizuki, A.; Hiramatsu, K. Three-Dimensional Analysis of the Nasolacrimal Duct and Nasal Cavity and Arrangement of Mucosal Tissue in Chicken. J. Poult. Sci. 2020, 57, 303–309. [Google Scholar] [CrossRef]
- Sutton, K.M.; Morris, K.M.; Borowska, D.; Sang, H.; Kaiser, P.; Balic, A.; Vervelde, L. Characterization of Conventional Dendritic Cells and Macrophages in the Spleen Using the CSF1R-Reporter Transgenic Chickens. Front. Immunol. 2021, 12, 636436. [Google Scholar] [CrossRef]
- Zhang, Q.; Waqas, Y.; Yang, P.; Sun, X.; Liu, Y.; Ahmed, N.; Chen, B.; Li, Q.; Hu, L.; Huang, Y.; et al. Cytological Study on the Regulation of Lymphocyte Homing in the Chicken Spleen during LPS Stimulation. Oncotarget 2017, 8, 7405–7419. [Google Scholar] [CrossRef]
- Nanduri, B.; Gresham, C.R.; Hui, W.W.; Ou, M.; Bailey, R.H.; Edelmann, M.J. An Atlas of the Catalytically Active Liver and Spleen Kinases in Chicken Identified by Chemoproteomics. J. Proteom. 2020, 225, 103850. [Google Scholar] [CrossRef] [PubMed]
- Castelo-Soccio, L.; Kim, H.; Gadina, M.; Schwartzberg, P.L.; Laurence, A.; O’Shea, J.J. Protein Kinases: Drug Targets for Immunological Disorders. Nat. Rev. Immunol. 2023, 23, 787–806. [Google Scholar] [CrossRef]
- Liu, R.H.; Shi, W.; Zhang, Y.X.; Zhuo, M.; Li, X.H. Selective Inhibition of Adenylyl Cyclase Subtype 1 Reduces Inflammatory Pain in Chicken of Gouty Arthritis. Mol. Pain 2021, 17, 17448069211047863. [Google Scholar] [CrossRef]
- Mashaly, M.M.; Hendricks, G.L.; Kalama, M.A.; Gehad, A.E.; Abbas, A.O.; Patterson, P.H. Effect of Heat Stress on Production Parameters and Immune Responses of Commercial Laying Hens. Poult. Sci. 2004, 83, 889–894. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Jiang, R.; Su, A.; Tian, H.; Zhang, Y.; Li, W.; Tian, Y.; Li, K.; Sun, G.; Han, R.; et al. Identification of Genes Related to Effects of Stress on Immune Function in the Spleen in a Chicken Stress Model Using Transcriptome Analysis. Mol. Immunol. 2020, 124, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Hirakawa, R.; Nurjanah, S.; Furukawa, K.; Murai, A.; Kikusato, M.; Nochi, T.; Toyomizu, M. Heat Stress Causes Immune Abnormalities via Massive Damage to Effect Proliferation and Differentiation of Lymphocytes in Broiler Chickens. Front. Vet. Sci. 2020, 7, 46. [Google Scholar] [CrossRef] [PubMed]
- Su, A.; Zhou, Y.; Guo, Y.; Yang, X.; Zhang, Y.; Li, W.; Tian, Y.; Li, K.; Sun, G.; Jiang, R.; et al. Identification and Expression Analysis of MicroRNAs in Chicken Spleen in a Corticosterone-Induced Stress Model. Vet. Sci. 2021, 136, 287–296. [Google Scholar] [CrossRef]
- Nagy, N.; Oláh, I.; Vervelde, L. Structure of the Avian Lymphoid System. In Avian Immunology; Kaspers, B., Schat, K.A., Göbel, T.W., Vervelde, L., Eds.; Academic Press: London, UK, 2021; pp. 11–44. [Google Scholar]
- Peralta, M.F.; Magnoli, A.; Alustiza, F.; Nilson, A.; Miazzo, R.; Vivas, A. Gut-Associated Lymphoid Tissue: A Key Tissue Inside the Mucosal Immune System of Hens Immunized with Escherichia Coli F4. Front. Immunol. 2017, 8, 568. [Google Scholar] [CrossRef]
- Ohshima, K.; Hiramatsu, K. Distribution of T-Cell Subsets and Immunoglobulin-Containing Cells in Nasal-Associated Lymphoid Tissue (NALT) of Chickens. Histol. Histopathol. 2000, 15, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Omotainse, O.S.; Wawegama, N.K.; Kulappu Arachchige, S.N.; Coppo, M.J.C.; Vaz, P.K.; Woodward, A.P.; Kordafshari, S.; Bogeski, M.; Stevenson, M.; Noormohammadi, A.H.; et al. Tracheal Cellular Immune Response in Chickens Inoculated with Mycoplasma Synoviae Vaccine, MS-H or Its Parent Strain 86079/7NS. Vet. Immunol. Immunopathol. 2022, 251, 110472. [Google Scholar] [CrossRef] [PubMed]
- Fagerland, J.A.; Arp, L.H. Structure and Development of Bronchus-Associated Lymphoid Tissue in Conventionally Reared Broiler Chickens. Avian Dis. 1993, 37, 10–18. [Google Scholar] [CrossRef]
- Reese, S.; Dalamani, G.; Kaspers, B. The Avian Lung-Associated Immune System: A Review. Vet. Res. 2006, 37, 311–324. [Google Scholar] [CrossRef]
- Suárez, L.J.; Arboleda, S.; Angelov, N.; Arce, R.M. Oral Versus Gastrointestinal Mucosal Immune Niches in Homeostasis and Allostasis. Front. Immunol. 2021, 12, 705206. [Google Scholar] [CrossRef]
- Bar-Shira, E.; Sklan, D.; Friedman, A. Establishment of Immune Competence in the Avian GALT during the Immediate Post-Hatch Period. Dev. Comp. Immunol. 2003, 27, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Nagy, N.; Igyártó, B.; Magyar, A.; Gazdag, E.; Palya, V.; Oláh, I. Oesophageal Tonsil of the Chicken. Acta Vet. Hung. 2005, 53, 173–188. [Google Scholar] [CrossRef] [PubMed]
- Nagy, N.; Oláh, I. Pyloric Tonsil as a Novel Gut-Associated Lymphoepithelial Organ of the Chicken. J. Anat. 2007, 211, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Gofur, M.R. Meckel’s Diverticulum in Animals and Birds: An Immuno-Pathoclinical Perspective. Bangladesh J. Vet. Med. 2020, 18, 1–12. [Google Scholar] [CrossRef]
- Oláh, I.; Glick, B.; Taylor, R.L. Meckel’s Diverticulum. II. A Novel Lymphoepithelial Organ in the Chicken. Anat. Rec. 1984, 208, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Befus, A.D.; Johnston, N.; Leslie, G.A.; Bienenstock, J. Gut-Associated Lymphoid Tissue in the Chicken. I. Morphology, Ontogeny, and Some Functional Characteristics of Peyer’s Patches. J. Immunol. 1980, 125, 2626–2632. [Google Scholar] [CrossRef]
- Prados, A.; Onder, L.; Cheng, H.-W.; Mörbe, U.; Lütge, M.; Gil-Cruz, C.; Perez-Shibayama, C.; Koliaraki, V.; Ludewig, B.; Kollias, G. Fibroblastic Reticular Cell Lineage Convergence in Peyer’s Patches Governs Intestinal Immunity. Nat. Immunol. 2021, 22, 510–519. [Google Scholar] [CrossRef]
- Kitagawa, H.; Hiratsuka, Y.; Imagawa, T.; Uehara, M. Distribution of Lymphoid Tissue in the Caecal Mucosa of Chickens. J. Anat. 1998, 192 Pt 2, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Alqazlan, N.; Emam, M.; Nagy, É.; Bridle, B.; Sargolzaei, M.; Sharif, S. Transcriptomics of Chicken Cecal Tonsils and Intestine after Infection with Low Pathogenic Avian Influenza Virus H9N2. Sci. Rep. 2021, 11, 20462. [Google Scholar] [CrossRef]
- Gómez Del Moral, M.; Fonfría, J.; Varas, A.; Jiménez, E.; Moreno, J.; Zapata, A.G. Appearance and Development of Lymphoid Cells in the Chicken (Gallus gallus) Caecal Tonsil. Anat. Rec. 1998, 250, 182–189. [Google Scholar] [CrossRef]
- Aylward, R.B. Eradicating Polio: Today’s Challenges and Tomorrow’s Legacy. Ann. Trop. Med. Parasitol. 2006, 100, 401–413. [Google Scholar] [CrossRef] [PubMed]
- Dubois, V.; Locht, C. Mucosal Immunization Against Pertussis: Lessons From the Past and Perspectives. Front. Immunol. 2021, 12, 701285. [Google Scholar] [CrossRef] [PubMed]
- Ganapathy, K. Infectious Bronchitis Virus Infection of Chicken: The Essential Role of Mucosal Immunity. Avian Dis. 2021, 65, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Kiyono, H.; Yuki, Y.; Nakahashi-Ouchida, R.; Fujihashi, K. Mucosal Vaccines: Wisdom from Now and Then. Int. Immunol. 2021, 33, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Heinen, E. Place of Malt in the Immune Defence System. In In Vivo Immunology. Advances in Experimental Medicine and Biology; Heinen, E., Defresne, M.P., Boniver, J., Geenen, V., Eds.; Springer: Boston, MA, USA, 1994; pp. 303–307. [Google Scholar]
- Ruan, Y.; Wang, Y.; Guo, Y.; Xiong, Y.; Chen, M.; Zhao, A.; Liu, H. T Cell Subset Profile and Inflammatory Cytokine Properties in the Gut-Associated Lymphoid Tissues of Chickens during Infectious Bursal Disease Virus (IBDV) Infection. Arch. Virol. 2020, 165, 2249–2258. [Google Scholar] [CrossRef]
- Calzas, C.; Mao, M.; Turpaud, M.; Viboud, Q.; Mettier, J.; Figueroa, T.; Bessière, P.; Mangin, A.; Sedano, L.; Hervé, P.-L.; et al. Immunogenicity and Protective Potential of Mucosal Vaccine Formulations Based on Conserved Epitopes of Influenza A Viruses Fused to an Innovative Ring Nanoplatform in Mice and Chickens. Front. Immunol. 2021, 12, 772550. [Google Scholar] [CrossRef]
- Huang, M.; Zhang, M.; Zhu, H.; Du, X.; Wang, J. Mucosal Vaccine Delivery: A Focus on the Breakthrough of Specific Barriers. Acta Pharm. Sin. B 2022, 12, 3456–3474. [Google Scholar] [CrossRef]
- Feng, L.; Wang, Q.; Shan, C.; Yang, C.; Feng, Y.; Wu, J.; Liu, X.; Zhou, Y.; Jiang, R.; Hu, P.; et al. An Adenovirus-Vectored COVID-19 Vaccine Confers Protection from SARS-CoV-2 Challenge in Rhesus Macaques. Nat. Commun. 2020, 11, 4207. [Google Scholar] [CrossRef]
- Van Doremalen, N.; Lambe, T.; Spencer, A.; Belij-Rammerstorfer, S.; Purushotham, J.N.; Port, J.R.; Avanzato, V.A.; Bushmaker, T.; Flaxman, A.; Ulaszewska, M.; et al. ChAdOx1 nCoV-19 Vaccine Prevents SARS-CoV-2 Pneumonia in Rhesus Macaques. Nature 2020, 586, 578–582. [Google Scholar] [CrossRef]
- Wu, S.; Zhong, G.; Zhang, J.; Shuai, L.; Zhang, Z.; Wen, Z.; Wang, B.; Zhao, Z.; Song, X.; Chen, Y.; et al. A Single Dose of an Adenovirus-Vectored Vaccine Provides Protection against SARS-CoV-2 Challenge. Nat. Commun. 2020, 11, 4081. [Google Scholar] [CrossRef]
- Wu, S.; Huang, J.; Zhang, Z.; Wu, J.; Zhang, J.; Hu, H.; Zhu, T.; Zhang, J.; Luo, L.; Fan, P.; et al. Safety, Tolerability, and Immunogenicity of an Aerosolised Adenovirus Type-5 Vector-Based COVID-19 Vaccine (Ad5-nCoV) in Adults: Preliminary Report of an Open-Label and Randomised Phase 1 Clinical Trial. Lancet Infect. Dis. 2021, 21, 1654–1664. [Google Scholar] [CrossRef]
- Ascough, S.; Vlachantoni, I.; Kalyan, M.; Haijema, B.-J.; Wallin-Weber, S.; Dijkstra-Tiekstra, M.; Ahmed, M.S.; van Roosmalen, M.; Grimaldi, R.; Zhang, Q.; et al. Local and Systemic Immunity against Respiratory Syncytial Virus Induced by a Novel Intranasal Vaccine. A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Am. J. Respir. Crit. Care Med. 2019, 200, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Wang, Y.; Gonzalez, G.X.; Ma, Y.; Song, Y.; Wang, S.; Kang, S.-M.; Compans, R.W.; Wang, B.-Z. Intranasal Vaccination with Influenza HA/GO-PEI Nanoparticles Provides Immune Protection against Homo- and Heterologous Strains. Proc. Natl. Acad. Sci. USA 2021, 118, e2024998118. [Google Scholar] [CrossRef] [PubMed]
- Gurwith, M.; Lock, M.; Taylor, E.M.; Ishioka, G.; Alexander, J.; Mayall, T.; Ervin, J.E.; Greenberg, R.N.; Strout, C.; Treanor, J.J.; et al. Safety and Immunogenicity of an Oral, Replicating Adenovirus Serotype 4 Vector Vaccine for H5N1 Influenza: A Randomised, Double-Blind, Placebo-Controlled, Phase 1 Study. Lancet Infect. Dis. 2013, 13, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.; Qi, Y.; Wang, M.; Yu, N.; Nan, F.; Zhang, H.; Tian, M.; Li, C.; Lu, H.; Jin, N. mRNA Vaccines Encoding the HA Protein of Influenza A H1N1 Virus Delivered by Cationic Lipid Nanoparticles Induce Protective Immune Responses in Mice. Vaccines 2020, 8, 123. [Google Scholar] [CrossRef]
- Lindsay, K.E.; Vanover, D.; Thoresen, M.; King, H.; Xiao, P.; Badial, P.; Araínga, M.; Park, S.B.; Tiwari, P.M.; Peck, H.E.; et al. Aerosol Delivery of Synthetic mRNA to Vaginal Mucosa Leads to Durable Expression of Broadly Neutralizing Antibodies against HIV. Mol. Ther. 2020, 28, 805–819. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.-X.; Wheatley, A.K.; Esterbauer, R.; Jegaskanda, S.; Glass, J.J.; Masopust, D.; Rose, R.D.; Kent, S.J. Induction of Vaginal-Resident HIV-Specific CD8 T Cells with Mucosal Prime–Boost Immunization. Mucosal Immunol. 2018, 11, 994–1007. [Google Scholar] [CrossRef]
- Mai, Y.; Guo, J.; Zhao, Y.; Ma, S.; Hou, Y.; Yang, J. Intranasal Delivery of Cationic Liposome-Protamine Complex mRNA Vaccine Elicits Effective Anti-Tumor Immunity. Cell Immunol. 2020, 354, 104143. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Wang, R.; Liu, J.; Wang, W.; Chen, Y.; Cai, W. Effects of Novel Microecologics Combined with Traditional Chinese Medicine and Probiotics on Growth Performance and Health of Broilers. Poult. Sci. 2022, 101, 101412. [Google Scholar] [CrossRef]
- Trapp, S.; Saint-martin, V.; Que, P.; Guabiraba, R. Uncovering the Core Principles of the Gut-Lung Axis to Enhance Innate Immunity in the Chicken. Front. Immunol. 2022, 13, 956670. [Google Scholar] [CrossRef]
- Al-Khalaifah, H.S. Benefits of Probiotics and/or Prebiotics for Antibiotic-Reduced Poultry. Poult. Sci. 2018, 97, 3807–3815. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, M.; Bavananthasivam, J.; Shojadoost, B.; Astill, J.; Taha-Abdelaziz, K.; Alqazlan, N.; Boodhoo, N.; Shoja Doost, J.; Sharif, S. In Ovo and Oral Administration of Probiotic Lactobacilli Modulate Cell- and Antibody-Mediated Immune Responses in Newly Hatched Chicks. Front. Immunol. 2021, 12, 664387. [Google Scholar] [CrossRef] [PubMed]
- Arreguin-Nava, M.A.; Graham, B.D.; Adhikari, B.; Agnello, M.; Selby, C.M.; Hernandez-Velasco, X.; Vuong, C.N.; Solis-Cruz, B.; Hernandez-Patlan, D.; Latorre, J.D.; et al. In Ovo Administration of Defined Lactic Acid Bacteria Previously Isolated From Adult Hens Induced Variations in the Cecae Microbiota Structure and Enterobacteriaceae Colonization on a Virulent Escherichia Coli Horizontal Infection Model in Broiler Chickens. Front. Vet. Sci. 2020, 7, 489. [Google Scholar] [CrossRef]
- Fathima, S.; Shanmugasundaram, R.; Adams, D.; Selvaraj, R.K. Gastrointestinal Microbiota and Their Manipulation for Improved Growth and Performance in Chickens. Foods 2022, 11, 1401. [Google Scholar] [CrossRef] [PubMed]
- Siwek, M.; Slawinska, A.; Stadnicka, K.; Bogucka, J.; Dunislawska, A.; Bednarczyk, M. Prebiotics and Synbiotics—In Ovo Delivery for Improved Lifespan Condition in Chicken. BMC Vet. Res. 2018, 14, 402. [Google Scholar] [CrossRef]
- Wilson, K.M.; Rodrigues, D.R.; Briggs, W.N.; Duff, A.F.; Chasser, K.M.; Bielke, L.R. Evaluation of the Impact of in Ovo Administered Bacteria on Microbiome of Chicks through 10 Days of Age. Poult. Sci. 2019, 98, 5949–5960. [Google Scholar] [CrossRef]
- Madej, J.P.; Bednarczyk, M. Effect of in Ovo- Delivered Prebiotics and Synbiotics on the Morphology and Specific Immune Cell Composition in the Gut-Associated Lymphoid Tissue. Poult. Sci. 2016, 95, 19–29. [Google Scholar] [CrossRef]
- Szczypka, M.; Suszko-pawłowska, A.; Kuczkowski, M.; Gorczykowski, M.; Lis, M.; Kowalczyk, A.; Łukaszewicz, E.; Poradowski, D.; Zbyryt, I.; Bednarczyk, M.; et al. Effects of Selected Prebiotics or Synbiotics Administered in Ovo on Lymphocyte Subsets in Bursa of the Fabricius, Thymus, and Spleen in Non-immunized and Immunized Chicken Broilers. Animals 2021, 11, 476. [Google Scholar] [CrossRef]
- Romero-Calle, D.; Benevides, R.G.; Góes-Neto, A.; Billington, C. Bacteriophages as Alternatives to Antibiotics in Clinical Care. Antibiotics 2019, 8, 138. [Google Scholar] [CrossRef] [PubMed]
- Clavijo, V.; Morales, T.; Vives-Flores, M.J.; Muñoz, A.R. The Gut Microbiota of Chickens in a Commercial Farm Treated with a Salmonella Phage Cocktail. Sci. Rep. 2022, 12, 991. [Google Scholar] [CrossRef] [PubMed]
- Kuralkar, P.; Kuralkar, S.V. Role of Herbal Products in Animal Production—An Updated Review. J. Ethnopharmacol. 2021, 278, 114246. [Google Scholar] [CrossRef] [PubMed]
- Paradowska, M.; Dunislawska, A.; Siwek, M.; Slawinska, A. Avian Cell Culture Models to Study Immunomodulatory Properties of Bioactive Products. Animals 2022, 12, 670. [Google Scholar] [CrossRef] [PubMed]
- Durrani, F.R.; Ullah, S.; Chand, N.; Durrani, Z.; Akhtar, S. Using Aqueous Extract of Aloe Gel as Anticoccidial and Immunostimulant Agent in Broiler Production. Sarhad J. Agric. 2008, 24, 665–669. [Google Scholar]
- Varmuzova, K.; Matulova, M.E.; Gerzova, L.; Cejkova, D.; Gardan-Salmon, D.; Panhéleux, M.; Robert, F.; Sisak, F.; Havlickova, H.; Rychlik, I. Curcuma and Scutellaria Plant Extracts Protect Chickens against Inflammation and Salmonella Enteritidis Infection. Poult. Sci. 2015, 94, 2049–2058. [Google Scholar] [CrossRef]
- Bondar, A.; Horodincu, L.; Solcan, G.; Solcan, C. Use of Spirulina Platensis and Curcuma Longa as Nutraceuticals in Poultry. Agriculture 2023, 13, 1553. [Google Scholar] [CrossRef]
- Ahmad, R.; Chand, N.; Farmanullah, F.; Salim, M.; Hosseini, S.; Khan, M.; Rehman, I.; Khan, S.U.; Babar, A.; Sciences, M. Role of Feed Added Aniseed as Immunomodulant and Growth Promoter in Broiler Chicks. Biomed. J. Sci. Tech. Res. 2020, 30, 23105–23112. [Google Scholar] [CrossRef]
- Rahimi, D.; Yarahmadi, H.M.; Yaghobfar, A.; Fakhraei, J. Effects of Garlic Powder and Satureja Khuzestanica Essential Oil on Male Ross 308 Chickens Performance, Blood Lipid Profile, Immune Responses, Intestinal Microflora, and Morphology. Jundishapur J. Nat. Pharm. Prod. 2021, 16, e94567. [Google Scholar] [CrossRef]
- Mumtaz, S.; Akhtar, M.; Awais, M.M.; Anwar, M.I. Evaluation of Immunomodulatory, Growth Promoting and Protective Effects of Ficus Religiosa against Coccidiosis in Broilers. Pak. J. Agric. Sci. 2021, 58, 219–228. [Google Scholar]
- Travel, A.; Petit, A.; Barat, P.; Collin, A.; Bourrier-Clairat, C.; Pertusa, M.; Skiba, F.; Crochet, S.; Cailleau-Audouin, E.; Chartrin, P.; et al. Methodologies to Assess the Bioactivity of an Herbal Extract on Immunity, Health, Welfare and Production Performance in the Chicken: The Case of Melissa officinalis L. Extract. Front. Vet. Sci. 2021, 8, 759456. [Google Scholar] [CrossRef]
- Sigolo, S.; Milis, C.; Dousti, M.; Jahandideh, E.; Jalali, A.; Mirzaei, N.; Rasouli, B.; Seidavi, A.; Gallo, A.; Ferronato, G.; et al. Effects of Different Plant Extracts at Various Dietary Levels on Growth Performance, Carcass Traits, Blood Serum Parameters, Immune Response and Ileal Microflora of Ross Broiler Chickens. Ital. J. Anim. Sci. 2021, 20, 359–371. [Google Scholar] [CrossRef]
- Lau, A.T.Y.; Barbut, S.; Ross, K.; Diarra, M.S.; Balamurugan, S. The Effect of Cranberry Pomace Ethanol Extract on the Growth of Meat Starter Cultures, Escherichia Coli O157:H7, Salmonella Enterica Serovar Enteritidis and Listeria Monocytogenes. LWT 2019, 115, 108452. [Google Scholar] [CrossRef]
- Saied, A.M.; Attia, A.I.; El-Kholy, M.S.; Reda, F.M.; Nagar, A.G.E. Effect of Cinnamon Oil Supplementation into Broiler Chicken Diets on Growth, Carcass Traits, Haemato-Biochemical Parameters, Immune Function, Antioxidant Status and Caecal Microbial Count. J. Anim. Feed Sci. 2022, 31, 21–33. [Google Scholar] [CrossRef]
- Gazwi, H.S.S.; Mahmoud, M.E.; Toson, E.M.A. Analysis of the Phytochemicals of Coriandrum Sativum and Cichorium Intybus Aqueous Extracts and Their Biological Effects on Broiler Chickens. Sci. Rep. 2022, 12, 6399. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, A.A.; Tayeb, I.T. The Influence of Dietary Salvia and Lavender Powders on Productive Performance, Some Physiological Parameters, and Immunity of Broiler under Stocking Density Stress. Iraqi J. Agric. Sci. 2022, 53, 1280–1288. [Google Scholar] [CrossRef]
- Keerthirathne, T.; Ross, K.; Fallowfield, H.; Whiley, H. Reducing Risk of Salmonellosis through Egg Decontamination Processes. Int. J. Environ. Res. Public Health 2017, 14, 335. [Google Scholar] [CrossRef] [PubMed]
- Kusstatscher, P.; Cernava, T.; Liebminger, S.; Berg, G. Replacing Conventional Decontamination of Hatching Eggs with a Natural Defense Strategy Based on Antimicrobial, Volatile Pyrazines. Sci. Rep. 2017, 7, 13253. [Google Scholar] [CrossRef]
- Tayel, A.A.; El-Sedfy, M.A.; Ibrahim, A.I.; Moussa, S.H. Application of Quercus Infectoria Extract as a Natural Antimicrobial Agent for Chicken Egg Decontamination. Rev. Argent. Microbiol. 2018, 50, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Koslová, A.; Trefil, P.; Mucksová, J.; Reinišová, M.; Plachý, J.; Kalina, J.; Kučerová, D.; Geryk, J.; Krchlíková, V.; Lejčková, B.; et al. Precise CRISPR/Cas9 Editing of the NHE1 Gene Renders Chickens Resistant to the J Subgroup of Avian Leukosis Virus. Proc. Natl. Acad. Sci. USA 2020, 117, 2108–2112. [Google Scholar] [CrossRef]
- Khan, M.Z.; Hashimoto, Y.; Konno, A.; Kon, Y.; Iwanaga, T. Development of T-Lymphocyte Subpopulations in the Postnatal Chicken Oviduct. Cell Tissue Res. 1996, 284, 317–325. [Google Scholar] [CrossRef]
- Crinion, R.A.; Hofstad, M.S. Pathogenicity of Four Serotypes of Avian Infectious Bronchitis Virus for the Oviduct of Young Chickens of Various Ages. Avian Dis. 1972, 16, 351–363. [Google Scholar] [CrossRef]
- Crinion, R.A. Egg Quality and Production Following Infectious Bronchitis Virus Exposure at One Day Old. Poult. Sci. 1972, 51, 582–585. [Google Scholar] [CrossRef] [PubMed]
- Barua, A.; Yoshimura, Y. Effects of Aging and Sex Steroids on the Localization of T Cell Subsets in the Ovary of Chicken, Gallus domesticus. Gen. Comp. Endocrinol. 1999, 114, 28–35. [Google Scholar] [CrossRef]
- Nakamura, K.; Mitarai, Y.; Tanimura, N.; Hara, H.; Ikeda, A.; Shimada, J.; Isobe, T. Pathogenesis of Reduced Egg Production and Soft-Shelled Eggs in Laying Hens Associated with Leucocytozoon Caulleryi Infection. J. Parasitol. 1997, 83, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, Y. Significance of Local Immunity in Hen Reproductive Organs. Anim. Sci. J. 2004, 75, 183–191. [Google Scholar] [CrossRef]
- Feberwee, A.; Wit, J.J.D.; Landman, W.J.M. Induction of Eggshell Apex Abnormalities by Mycoplasma Synoviae: Field and Experimental Studies. Avian Pathol. 2009, 38, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Gantois, I.; Ducatelle, R.; Pasmans, F.; Haesebrouck, F.; Gast, R.; Humphrey, T.J.; Immerseel, F.V. Mechanisms of Egg Contamination by Salmonella Enteritidis: Review Article. FEMS Microbiol. Rev. 2009, 33, 718–738. [Google Scholar] [CrossRef] [PubMed]
- Nii, T.; Isobe, N.; Yoshimura, Y. Effects of Avian Infectious Bronchitis Virus Antigen on Eggshell Formation and Immunoreaction in Hen Oviduct. Theriogenology 2014, 81, 1129–1138. [Google Scholar] [CrossRef]
- Nii, T. Relationship between Mucosal Barrier Function of the Oviduct and Intestine in the Productivity of Laying Hens. J. Poult. Sci. 2022, 59, 105–113. [Google Scholar] [CrossRef]
- Chen, L.; Yang, M.; Zhu, W.; Su, Y.; Li, D.; Wang, T. Multi-Omics Analysis After Vaginal Administration of Bacteroides Fragilis in Chickens. Front. Microbiol. 2022, 13, 846011. [Google Scholar] [CrossRef] [PubMed]
- Nii, T.; Shinkoda, T.; Isobe, N.; Yoshimura, Y. Intravaginal Injection of Lactobacillus Johnsonii May Modulates Oviductal Microbiota and Mucosal Barrier Function of Laying Hens. Poult. Sci. 2023, 102, 102699. [Google Scholar] [CrossRef]
- Turkowska, E.; Majewski, P.M.; Rai, S.; Skwarlo-Sonta, K. Pineal Oscillator Functioning in the Chicken—Effect of Photoperiod and Melatonin. Chronobiol. Int. 2014, 31, 134–143. [Google Scholar] [CrossRef]
- Mosenson, J.A.; McNulty, J.A. Characterization of Lymphocyte Subsets over a 24-Hour Period in Pineal-Associated Lymphoid Tissue (PALT) in the Chicken. BMC Immunol. 2006, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Huang, J.; Quan, S.; Yang, Y. Light Regimen on Health and Growth of Broilers: An Update Review. Poult. Sci. 2022, 101, 101545. [Google Scholar] [CrossRef] [PubMed]
- Helva, B.; Akşit, M.; Yalcin, S. Effects of Monochromatic Light on Growth Performance, Welfare and Hormone Levels in Broiler Chickens. Eur. Poult. Sci. 2019, 83, 1–12. [Google Scholar] [CrossRef]
- House, G.M.; Sobotik, E.B.; Nelson, J.R.; Archer, G.S. Effect of the Addition of Ultraviolet Light on Broiler Growth, Fear, and Stress Response. J. Appl. Poult. Res. 2020, 29, 402–408. [Google Scholar] [CrossRef]
- James, C.; Wiseman, J.; Asher, L. The Effect of Supplementary Ultraviolet Wavelengths on the Performance of Broiler Chickens. Poult. Sci. 2020, 99, 5517–5525. [Google Scholar] [CrossRef]
- Tuell, J.R.; Park, J.Y.; Wang, W.; Cheng, H.W.; Kim, Y.H.B. Functional/Physicochemical Properties and Oxidative Stability of Ground Meat from Broilers Reared under Different Photoperiods. Poult. Sci. 2020, 99, 3761–3768. [Google Scholar] [CrossRef] [PubMed]
- Couteaudier, M.; Denesvre, C. Marek’s Disease Virus and Skin Interactions. Vet. Res. 2014, 45, 36. [Google Scholar] [CrossRef]
- Zavala, G.; Jackwood, M.W.; Hilt, D.A. Polymerase Chain Reaction for Detection of Avian Leukosis Virus Subgroup J in Feather Pulp. Avian Dis. 2002, 46, 971–978. [Google Scholar] [CrossRef]
- Davidson, I.; Artzi, N.; Shkoda, I.; Lublin, A.; Loeb, E.; Schat, K.A. The Contribution of Feathers in the Spread of Chicken Anemia Virus. Virus Res. 2008, 132, 152–159. [Google Scholar] [CrossRef]
- King, A.S.; McLelland, J. Birds Their Structure & Function-Lymphatic System; Bailliere, T., Ed.; Bailliere Tindall: Eastbourne, UK, 1984; pp. 228–236. [Google Scholar]
- Oláh, I.; Glick, B. Avian Lymph Node: Light and Electron Microscopic Study. Anat. Rec. 1983, 205, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Lawn, A.M.; Rose, M.E. Presence of a Complete Endothelial Barrier between Lymph and Lymphoid Tissue in the Lumbar Lymph Nodes of the Duck (Anas platyrhynchos). Res. Vet. Sci. 1981, 30, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Biggs, P.M. The Association of Lymphoid Tissue with the Lymph Vessels in the Domestic Chicken (Gallus domesticus). Acta Anat. 1957, 29, 36–47. [Google Scholar] [CrossRef] [PubMed]
- McCorkle, F.M.; Stinson, R.S.; Olah, I.; Glick, B. The Chicken’s Femoral-Lymph Nodules: T and B Cells and the Immune Response. J. Immunol. 1979, 123, 667–669. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.Y.; Porter, R.E.; Hester, P.Y. Delayed-Type Hypersensitivity Reaction Induced in Broilers via Trachea Inoculation of Killed Staphylococcus Aureus. Poult. Sci. 1999, 78, 1711–1716. [Google Scholar] [CrossRef]
- Oladele, O.A.; Emikpe, B.O.; Esan, O.O. Comparative Assessment of Innate Humoral and Cellular Immunity of Exotic and Nigerian Indigenous Breeds of Chickens. J. Commonw. Vet. Assoc. 2010, 26, 16–22. [Google Scholar]

| Plant-Derived Substances | Major Effects | References |
|---|---|---|
| Aloe vera extract | Anticoccidial properties and enhancement of growth performance | [86] |
| Curcuma longa, Scutellaria baicalensis, Spirulina platensis | Anti-inflammatory effect | [87,88] |
| Aniseed extract | Positive influence on immune responses, lipid profile, and overall animal performance | [89] |
| Garlic powder and Satureja khuzestanica essential oil | Performance improvement, reduction in serum concentrations of lipid profiles, prebiotic effect, morphological changes (increased villus length to crypt depth and villus area) | [90] |
| Ficus religiosa L. extracts | Immunomodulatory effect and protective properties against coccidiosis | [91] |
| Melissa officinalis | Beneficial effects on the redox balance and improved performance during the growth phase | [92] |
| Thyme extract | Improvements in growth performance, carcass traits, blood serum parameters, immune responses, and ileal microflora | [93] |
| Fruit-derived phenolic compounds | Antimicrobial, antioxidant, immunostimulatory, and growth-enhancing effects | [94] |
| Cinnamon oil | Immunostimulatory, antimicrobial, prebiotic, and growth-enhancing effects | [95] |
| Coriandrum sativum and Cichorium intybus extracts | Positive effects on growth performance, carcass characteristics, liver function, serum lipid profile, and antioxidant status | [96] |
| Salvia and lavender powders | Immunostimulatory and growth-enhancing effects | [97] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ceccopieri, C.; Madej, J.P. Chicken Secondary Lymphoid Tissues—Structure and Relevance in Immunological Research. Animals 2024, 14, 2439. https://doi.org/10.3390/ani14162439
Ceccopieri C, Madej JP. Chicken Secondary Lymphoid Tissues—Structure and Relevance in Immunological Research. Animals. 2024; 14(16):2439. https://doi.org/10.3390/ani14162439
Chicago/Turabian StyleCeccopieri, Cassandra, and Jan P. Madej. 2024. "Chicken Secondary Lymphoid Tissues—Structure and Relevance in Immunological Research" Animals 14, no. 16: 2439. https://doi.org/10.3390/ani14162439
APA StyleCeccopieri, C., & Madej, J. P. (2024). Chicken Secondary Lymphoid Tissues—Structure and Relevance in Immunological Research. Animals, 14(16), 2439. https://doi.org/10.3390/ani14162439





