Dietary Intervention of Benzoic Acid for Intestinal Health and Growth of Nursery Pigs
Abstract
Simple Summary
Abstract
1. Introduction
2. The Importance of Dietary Intervention for Intestinal Health and Growth of Nursery Pigs
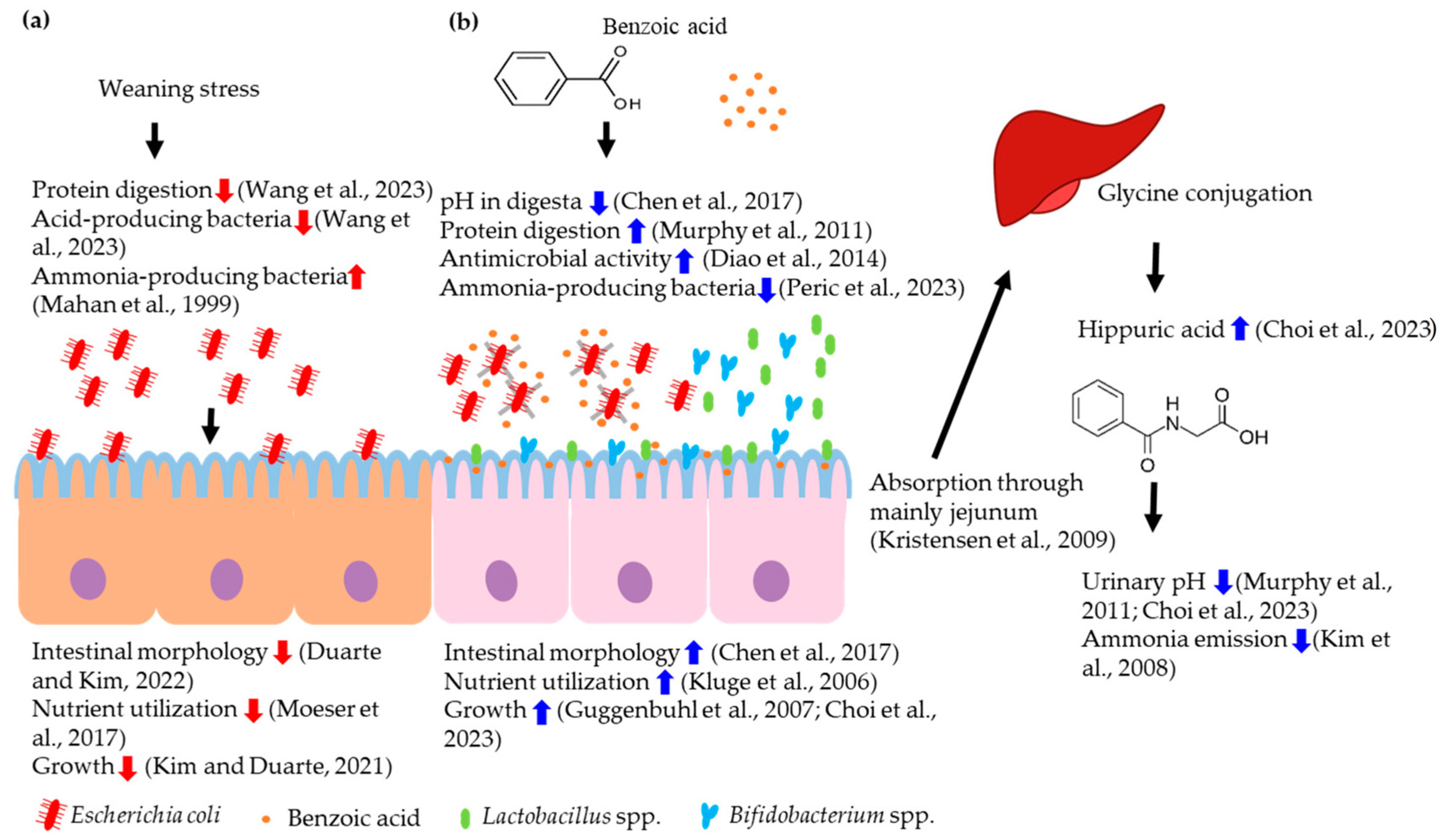
3. Exploring Dietary Intervention of Benzoic Acid on Intestinal Microbiota, Intestinal Health, and Growth of Nursery Pigs
3.1. Characteristic and Mechanism of Action of Benzoic Acid in Pigs
3.2. Lowering Digesta pH in Pigs
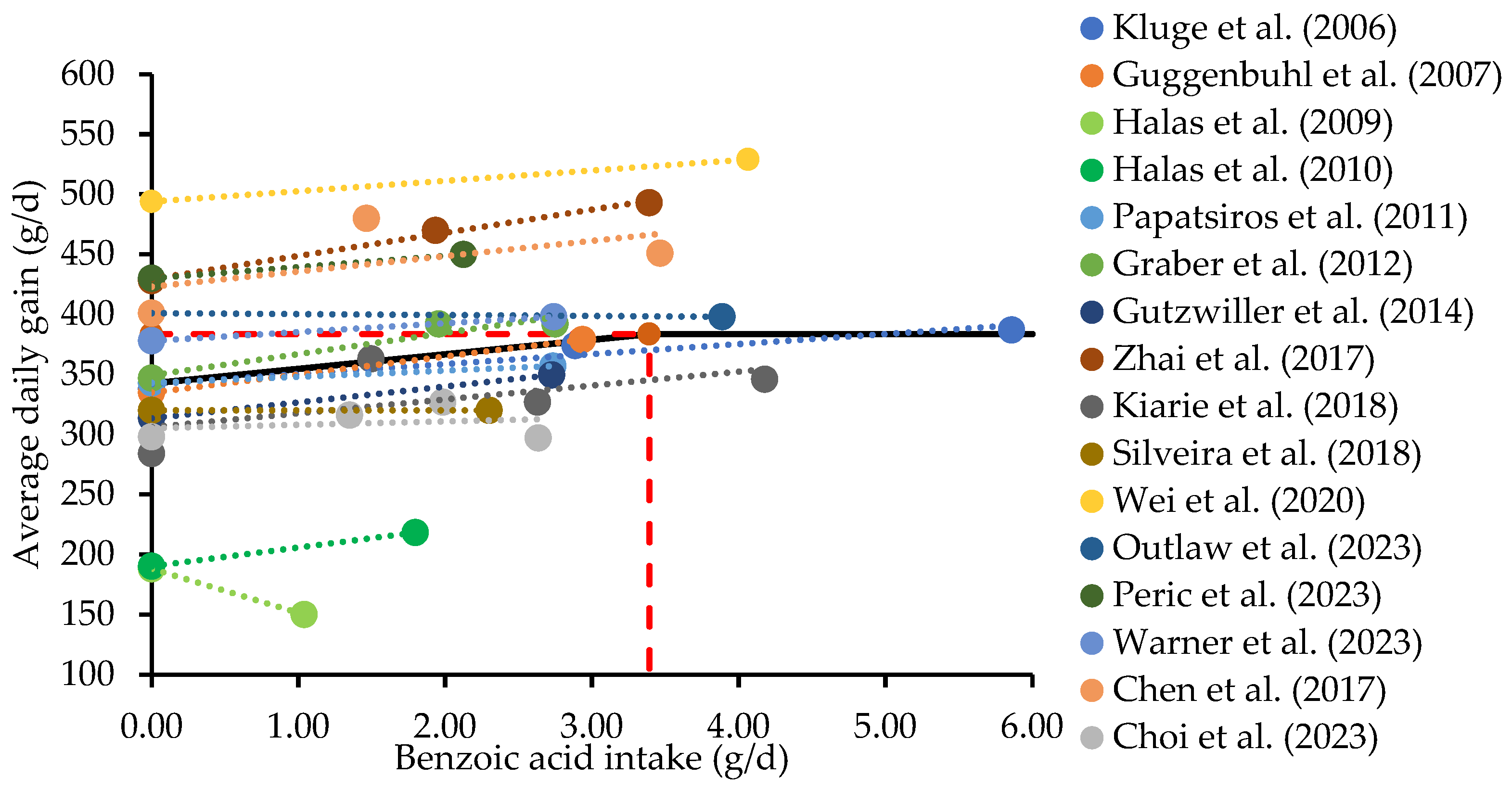
3.3. Modulation of Intestinal Microbiota and Positive Impact on Intestinal Health, Nutrient Utilization, and Growth of Pigs
| IBW 2 (kg) | Experimental Period (d) | BA (%) | Growth Performance 3 (% Change 4) | Reference | ||
|---|---|---|---|---|---|---|
| ADG | ADFI | G:F | ||||
| 7.3 | 35 | 0.50 | 10.7 | 8.5 | 2.0 | [27] |
| 1.00 | 14.5 ** | 10.2 | 3.9 | |||
| 7.4 | 32 | 0.50 | 13.1 ** | 6.3 | 6.5 ** | [35] |
| 5.0 | 21 | 0.50 | −20.2 | −17.5 | −3.3 | [15] |
| 6.0 | 21 | 0.50 | 14.7 ** | 10.9 ** | 3.4 | [18] |
| 7.9 | 35 | 0.50 | 4.1 ** | 2.6 ** | 1.4 | [57] |
| 6.5 | 42 | 0.35 | 13.0 ** | 10.9 ** | 1.9 | [28] |
| 0.50 | 13.0 ** | 9.1 ** | 3.5 | |||
| 9.7 | 42 | 0.50 | 11.5 * | 13.2 ** | −1.5 | [44] |
| 6.7 | 42 | 0.20 | 19.7 ** | 13.7 ** | 5.3 ** | [33] |
| 0.50 | 12.5 | 7.6 ** | 4.5 ** | |||
| 7.1 5 | 28 | 0.30 | 9.8 | 5.9 | 3.7 | [58] |
| 0.50 | 15.2 | 11.3 | 3.5 | |||
| 6.2 | 42 | 0.50 | 7.1 | 0.4 | 6.7 ** | [59] |
| 6.4 6 | 42 | 0.25 | 27.8 | 17.7 | 8.6 | [19] |
| 0.50 | 15.1 | 3.5 | 11.2 | |||
| 0.75 | 21.8 | 9.6 | 11.1 | |||
| 4.7 | 40 | 0.50 | 0.0 | −4.2 | 4.6 | [60] |
| 8.7 | 21 | 0.60 | −0.7 | 5.9 | −6.3 | [56] |
| 6.8 | 42 | 0.25 | 4.7 | −2.3 | 7.1 ** | [14] |
| 6.2 | 38 | 0.50 | 5.3 ** | 4.0 ** | 1.3 * | [61] |
| 6.9 7 | 41 | 0.25 | 6.0 | 5.1 | 0.9 | [13] |
| 0.35 | 9.7 | 10.3 | −0.5 | |||
| 0.50 | −0.3 | 2.5 | −2.8 | |||
| Average | 9.5 | 6.1 | 3.2 | |||
| Item | IBW 1 (kg) or Age (d) | Experimental Period (d) | BA (%) | Results 2 | Reference |
|---|---|---|---|---|---|
| Luminal microbiota | 7.3 kg | 35 | 1.00 | Decreased gram-negative bacteria in duodenum and total aerobic bacteria in ileum | [27] |
| 6.8 kg | 42 | 0.25 | Decreased Enterococcus spp. in duodenum, decreased Escherichia coli in jejunum, increased Clostridium perfringens in ileum, increased Lactobacillus spp. in ileum, and increrased Lactobacillus spp. in cecum | [14] | |
| 7.4 kg | 32 | 0.50 | Decreased total lactic acid bacteria in the stomach, decreased Escherichia coli in cecum | [35] | |
| 8.9 kg | 28 | 0.50 | Increased biodiversity in ileum | [68] | |
| 6.0 kg | 14 and 42 | 0.50 | Increased Bifidobacterium and decreased Escherichia coli in ileum on d 14 tended to decrease Escherichia coli in cecum on d 14, increased Bacillus and decreased Escherichia coli in the ileum on d 42 and decreased Escherichia coli in the cecum on d 42 | [12] | |
| 6.5 kg | 14 and 42 | 0.20 | Increased Bacillus in the ileal digesta, decreased Escherichia coli in the cecal digesta on d 14, increased Lactobacillus, Bifidobacterium, decreased Escherichia coli in the ileal digesta on d 42, increased Bifidobacterium, decreased Escherichia coli in the cecal digesta on d 42 | [33] | |
| 0.50 | Increased Lactobacillus and Bacillus in the ileal digesta on d 14, decreased Escherichia coli in the ileal digesta on d 42, decreased Escherichia coli in the cecal digesta on d 42 | [33] | |||
| Intestinal health and nutrient utilization | 6.8 kg | 42 | 0.25 | Increased villus height, decreased crypt depth, and increased VH:CD in the small intestine, including duodenum and jejunum | [14] |
| 6.0 kg | 42 | 0.50 | Increased VH:CD in the small intestine, including duodenum, jejunum, and ileum on d 14 and d 42 | [12] | |
| 5.9 kg | 21 | 0.50 | Increased villus height in the ileum and increased AID of CP | [18] | |
| 7.3 kg | 35 | 0.50 | Increased N retention | [27] | |
| 6.2 kg | 42 | 0.50 | Increased ATTD of CP | [59] | |
| 7.4 kg | 32 | 0.50 | Increased ATTD of CP and energy | [35] | |
| 6.5 kg | 42 | 0.50 | Increased N retention | [28] | |
| 6.5 kg | 14 and 42 | 0.20 | Increased villus height, decreased crypt depth, and increased VH:CD in the jejunum on d 14 and increased villus height and VH:CD in the jejunum on d 42 | [33] | |
| 0.50 | Decreased crypt depth, increased VH:CD in the jejunum on d 14, and increased VH:CD in the jejunum on d 42 | ||||
| 21 d | 35 | 0.50 and 1.00 | Increased ATTD of N | [34] | |
| 18.8 kg | 14 | 0.50 | Decreased crypt depth, increased VH:CD in the jejunum, trypsin, lipase, and amylase activities in the jejunum, and increased ATTD of CP, dry matter, ether extract, energy, and ash | [12] | |
| 33.1 kg | 53 | 0.50 | Increased N retention | [48] | |
| 26.0 kg | 84 | 1.00 | Increased ATTD of N | [47] | |
| 64.0 kg | 14 | 1.00, 2.00, and 3.00 | Linearly decreased total N excretion and tended to linearly increase N retained/intake | [16] | |
| 45.0 kg | 5 | 2.00 | Increased ATTD of Ca and P | [50] | |
| 29.9 kg | 6 | 1.00 | Increased retained N of absorbed (%) | [52] |
| Item | IBW 1 (kg) | Inclusion Rate (%) | ADG 2 (% Changes) | No. Exp | Reference | |
|---|---|---|---|---|---|---|
| Average | Range | |||||
| Benzoic acid | 5.0 to 9.7 | 0.20 to 1.00 | 9.5 | −20.2 to 27.8 | 16 | Table 2 |
| Citric acid | 4.2 to 9.6 | 0.50 to 3.00 | 3.0 | −8.1 to 12.2 | 12 | [55,71,72,73,74,75,76,77,78,79,80] |
| Fumaric acid | 5.7 to 9.6 | 0.20 to 4.00 | 5.0 | −11.8 to 21.1 | 15 | [38,55,71,72,73,76,79,81,82,83,84] |
| Formic acid | 5.7 to 9.1 | 0.20 to 2.40 | 7.1 | −15.1 to 26.9 | 7 | [54,78,81,84,85,86,87,88] |
| Formate salts | 5.6 to 10.0 | 0.40 to 2.80 | 6.9 | −9.4 to 22.9 | 9 | [27,68,89,90,91] |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Campbell, J.M.; Crenshaw, J.D.; Polo, J. The biological stress of early weaned piglets. J. Anim. Sci. Biotechnol. 2013, 4, 19. [Google Scholar] [CrossRef] [PubMed]
- Pluske, J.R. Invited review: Aspects of gastrointestinal tract growth and maturation in the pre-and postweaning period of pigs. J. Anim. Sci. 2016, 94, 399–411. [Google Scholar] [CrossRef]
- Gresse, R.; Chaucheyras-Durand, F.; Fleury, M.A.; Van de Wiele, T.; Forano, E.; Blanquet-Diot, S. Gut microbiota dysbiosis in postweaning piglets: Understanding the keys to health. Trends Microbiol. 2017, 25, 851–873. [Google Scholar] [CrossRef]
- Kim, S.W.; Duarte, M.E. Understanding intestinal health in nursery pigs and the relevant nutritional strategies. Anim. Biosci. 2021, 34, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Moeser, A.J.; Pohl, C.S.; Rajput, M. Weaning stress and gastrointestinal barrier development: Implications for lifelong gut health in pigs. Anim. Nutr. 2017, 3, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Lallès, J.-P.; Boudry, G.; Favier, C.; Le Floc’h, N.; Luron, I.; Montagne, L.; Oswald, I.P.; Pié, S.; Piel, C.; Sève, B. Gut function and dysfunction in young pigs: Physiology. Anim. Res. 2004, 53, 301–316. [Google Scholar] [CrossRef]
- Van der Fels-Klerx, H.J.; Puister-Jansen, L.F.; Van Asselt, E.D.; Burgers, S.L.G.E. Farm factors associated with the use of antibiotics in pig production. J. Anim. Sci. 2011, 89, 1922–1929. [Google Scholar] [CrossRef]
- Duarte, M.E.; Stahl, C.H.; Kim, S.W. Intestinal damages by F18+ Escherichia coli and its amelioration with an antibacterial bacitracin fed to nursery pigs. Antioxidants 2023, 12, 1040. [Google Scholar] [CrossRef]
- Casewell, M.; Friis, C.; Marco, E.; McMullin, P.; Phillips, I. The European ban on growth-promoting antibiotics and emerging consequences for human and animal health. J. Antimicrob. Chemother. 2003, 52, 159–161. [Google Scholar] [CrossRef]
- Tiseo, K.; Huber, L.; Gilbert, M.; Robinson, T.P.; Van Boeckel, T.P. Global trends in antimicrobial use in food animals from 2017 to 2030. Antibiotics 2020, 9, 918. [Google Scholar] [CrossRef]
- Gebhardt, J.T.; Tokach, M.D.; Dritz, S.S.; DeRouchey, J.M.; Woodworth, J.C.; Goodband, R.D.; Henry, S.C. Postweaning mortality in commercial swine production. I: Review of non-infectious contributing factors. Transl. Anim. Sci. 2020, 4, 462–484. [Google Scholar] [CrossRef]
- Diao, H.; Zheng, P.; Yu, B.; He, J.; Mao, X.B.; Yu, J.; Chen, D.W. Effects of dietary supplementation with benzoic acid on intestinal morphological structure and microflora in weaned piglets. Livest. Sci. 2014, 167, 249–256. [Google Scholar] [CrossRef]
- Choi, H.; Chen, Y.; Longo, F.; Kim, S.W. Comparative effects of benzoic acid and sodium benzoate in diets for nursery pigs on growth performance and acidification of digesta and urine. J. Anim. Sci. 2023, 101, skad116. [Google Scholar] [CrossRef] [PubMed]
- Perić, D.; Barea, R.; Nešić, S.; Makivić, L.; Janjić, J.; Šefer, D.; Marković, R. Effects of dietary supplementation with benzoic acid and chelated copper, zinc and manganese sources on production performance in piglets. Acta. Veterinaria. 2023, 73, 355–373. [Google Scholar] [CrossRef]
- Halas, D.; Hansen, C.F.; Hampson, D.J.; Mullan, B.P.; Wilson, R.H.; Pluske, J.R. Effect of dietary supplementation with inulin and/or benzoic acid on the incidence and severity of post-weaning diarrhoea in weaner pigs after experimental challenge with enterotoxigenic Escherichia coli. Arch. Anim. Nutr. 2009, 63, 267–280. [Google Scholar] [CrossRef] [PubMed]
- Murphy, D.P.; O’Doherty, J.V.; Boland, T.M.; O’shea, C.J.; Callan, J.J.; Pierce, K.M.; Lynch, M.B. The effect of benzoic acid concentration on nitrogen metabolism, manure ammonia and odour emissions in finishing pigs. Anim. Feed Sci. Technol. 2011, 163, 194–199. [Google Scholar] [CrossRef]
- De Vries, J.W.; Melse, R.W. Comparing environmental impact of air scrubbers for ammonia abatement at pig houses: A life cycle assessment. Biosyst. Eng. 2017, 161, 53–61. [Google Scholar] [CrossRef]
- Halas, D.; Hansen, C.F.; Hampson, D.J.; Mullan, B.P.; Kim, J.C.; Wilson, R.H.; Pluske, J.R. Dietary supplementation with benzoic acid improves apparent ileal digestibility of total nitrogen and increases villous height and caecal microbial diversity in weaner pigs. Anim. Feed Sci. Technol. 2010, 160, 137–147. [Google Scholar] [CrossRef]
- Silveira, H.; Amaral, L.G.d.M.; Garbossa, C.A.P.; Rodrigues, L.M.; Silva, C.C.d.; Cantarelli, V.d.S. Benzoic acid in nursery diets increases the performance from weaning to finishing by reducing diarrhoea and improving the intestinal morphology of piglets inoculated with Escherichia coli K88+. J. Anim. Physiol. Anim. Nutr. 2018, 102, 1675–1685. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Vaughn, J.O.; Monson, D.A. Effects of dietary supplementation of benzoic acid, formic, and lactic acids on growth and health of pigs. J. Anim. Sci. 2008, 86 (Suppl. S2), 100. [Google Scholar]
- Shu, Y.; Yu, B.; He, J.; Yu, J.; Zheng, P.; Yuan, Z.; Chen, D.; Mao, X. Excess of dietary benzoic acid supplementation leads to growth retardation, hematological abnormality and organ injury of piglets. Livest. Sci. 2016, 190, 94–103. [Google Scholar] [CrossRef]
- Xu, X.; Duarte, M.E.; Kim, S.W. Postbiotic effects of Lactobacillus fermentate on intestinal health, mucosa-associated microbiota, and growth efficiency of nursery pigs challenged with F18+ Escherichia coli. J. Anim. Sci. 2022, 100, skac210. [Google Scholar] [CrossRef]
- Duarte, M.E.; Kim, S.W. Intestinal microbiota and its interaction to intestinal health in nursery pigs. Anim. Nutr. 2022, 8, 169–184. [Google Scholar] [CrossRef] [PubMed]
- Arpaia, N.; Campbell, C.; Fan, X.; Dikiy, S.; Van Der Veeken, J.; Deroos, P.; Liu, H.; Cross, J.R.; Pfeffer, K.; Coffer, P.J. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 2013, 504, 451–455. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, Y.; Liu, S.; Huang, J.; Zhai, Z.; He, C.; Ding, J.; Wang, J.; Wang, H.; Fan, W. The dynamic distribution of porcine microbiota across different ages and gastrointestinal tract segments. PloS ONE 2015, 10, e0117441. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, B.; Kim, S.W.; Kwon, Y.M. Characterization of microbiota associated with digesta and mucosa in different regions of gastrointestinal tract of nursery pigs. Int. J. Mol. Sci. 2019, 20, 1630. [Google Scholar] [CrossRef]
- Kluge, H.; Broz, J.; Eder, K. Effect of benzoic acid on growth performance, nutrient digestibility, nitrogen balance, gastrointestinal microflora and parameters of microbial metabolism in piglets. J. Anim. Physiol. Anim. Nutr. 2006, 90, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Gräber, T.; Kluge, H.; Hirche, F.; Brož, J.; Stangl, G.I. Effects of dietary benzoic acid and sodium-benzoate on performance, nitrogen and mineral balance and hippuric acid excretion of piglets. Arch. Anim. Nutr. 2012, 66, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, N.B.; Nørgaard, J.V.; Wamberg, S.; Engbaek, M.; Fernández, J.A.; Zacho, H.D.; Poulsen, H.D. Absorption and metabolism of benzoic acid in growing pigs. J. Anim. Sci. 2009, 87, 2815–2822. [Google Scholar] [CrossRef][Green Version]
- Foegeding, P.M.; Busta, F.F. Chemical Food Preservatives: Disinfection, Sterilization and Preservation; Lea & Febiger: Philadelphia, PA, USA, 1991. [Google Scholar]
- Wang, L.F.; Bergstrom, J.R.; Hahn, J.D.; Young, M.G.; Zijlstra, R.T. Acid-binding capacity of feed in swine nutrition. Anim. Feed Sci. Technol. 2023, 295, 115519. [Google Scholar] [CrossRef]
- Mahan, D.C.; Wiseman, T.D.; Weaver, E.; Russell, L. Effect of supplemental sodium chloride and hydrochloric acid added to initial starter diets containing spray-dried blood plasma and lactose on resulting performance and nitrogen digestibility of 3-week-old weaned pigs. J. Anim. Sci. 1999, 77, 3016–3021. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, J.L.; Zheng, P.; Zhang, C.; Yu, B.; He, J.; Yu, J.; Luo, J.Q.; Mao, X.B.; Huang, Z.Q.; Chen, D.W. Benzoic acid beneficially affects growth performance of weaned pigs which was associated with changes in gut bacterial populations, morphology indices and growth factor gene expression. J. Anim. Physiol. Anim. Nutr. 2017, 101, 1137–1146. [Google Scholar] [CrossRef]
- Min, B.J.; Monson, D.A.; Vaughn, J.O.; Kim, S.W. Effects of dietary supplementation of benzoic, formic, and lactic acids on nitrogen balance of pigs. J. Anim. Sci. 2008, 86, 576. [Google Scholar]
- Guggenbuhl, P.; Séon, A.; Quintana, A.P.; Nunes, C.S. Effects of dietary supplementation with benzoic acid (VevoVitall®) on the zootechnical performance, the gastrointestinal microflora and the ileal digestibility of the young pig. Livest. Sci. 2007, 108, 218–221. [Google Scholar] [CrossRef]
- Kim, S.W.; Vaughn, J.O.; Monson, D.A. Effects of dietary supplementation of benzoic, formic, and lactic acids to pig diets on ammonia emission from manure and urine pH. J. Anim. Sci. 2008, 86 (Suppl. S1), 3. [Google Scholar]
- Bertschinger, H.U.; Nief, V.; Tschäpe, H. Active oral immunization of suckling piglets to prevent colonization after weaning by enterotoxigenic Escherichia coli with fimbriae F18. Vet. Microbiol. 2000, 71, 255–267. [Google Scholar] [CrossRef]
- Lawlor, P.G.; Lynch, P.B.; Caffrey, P.J. Effect of creep feeding, dietary fumaric acid and level of dairy product in the diet on post-weaning pig performance. Irish J. Agr. Food Res. 2005, 44, 45–55. [Google Scholar]
- Stas, E.B.; Tokach, M.D.; DeRouchey, J.M.; Goodband, R.D.; Woodworth, J.C.; Gebhardt, J.T. Evaluation of the acid-binding capacity of ingredients and complete diets commonly used for weanling pigs. Transl. Anim. Sci. 2022, 6, txac104. [Google Scholar] [CrossRef]
- Britton, J.R.; Koldovsky, O. Development of luminal protein digestion: Implications for biologically active dietary polypeptides. J. Pediatr. Gastroenterol. Nutr. 1989, 9, 144–162. [Google Scholar] [CrossRef] [PubMed]
- Kil, D.Y.; Kwon, W.B.; Kim, B.G. Dietary acidifiers in weanling pig diets: A review. Rev. Colomb. Cienc. Pecu. 2011, 24, 231–247. [Google Scholar]
- Tugnoli, B.; Giovagnoni, G.; Piva, A.; Grilli, E. From acidifiers to intestinal health enhancers: How organic acids can improve growth efficiency of pigs. Animals 2020, 10, 134. [Google Scholar] [CrossRef]
- Schokker, D.; Zhang, J.; Vastenhouw, S.A.; Heilig, H.G.; Smidt, H.; Rebel, J.M.; Smits, M.A. Long-lasting effects of early-life antibiotic treatment and routine animal handling on gut microbiota composition and immune system in pigs. PloS ONE 2015, 10, e0116523. [Google Scholar] [CrossRef] [PubMed]
- Gutzwiller, A.; Schlegel, P.; Guggisberg, D.; Stoll, P. Effects of benzoic acid and dietary calcium: Phosphorus ratio on performance and mineral metabolism of weanling pigs. Asian-Australas. J. Anim. Sci. 2014, 27, 530. [Google Scholar] [CrossRef]
- Diao, H.; Gao, Z.; Yu, B.; Zheng, P.; He, J.; Yu, J.; Huang, Z.; Chen, D.; Mao, X. Effects of benzoic acid (VevoVitall®) on the performance and jejunal digestive physiology in young pigs. J. Anim. Sci. Biotechnol. 2016, 7, 32. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.K.; Choi, Y.H.; Jin, Y.H.; Kim, Y.Y. Effect of dietary benzoic acid on beneficial microflora and immune response in the intestine of weaning pigs. J. Life Sci. 2012, 22, 1307–1315. [Google Scholar] [CrossRef]
- Bühler, K.; Wenk, C.; Broz, J.; Gebert, S. Influence of benzoic acid and dietary protein level on performance, nitrogen metabolism and urinary pH in growing-finishing pigs. Arch. Anim. Nutr. 2006, 60, 382–389. [Google Scholar] [CrossRef]
- Humphrey, D.C.; Bergstrom, J.R.; Calvo, E.P.; Trabue, S.L.; Scoggin, K.D.; Greiner, L.L. The effect of benzoic acid with or without a direct-fed microbial on the nutrient metabolism and gas emissions of growing pigs. J. Anim. Sci. 2022, 100, skac296. [Google Scholar] [CrossRef]
- Sauer, W.; Cervantes, M.; Yanez, J.; Araiza, B.; Murdoch, G.; Morales, A.; Zijlstra, R.T. Effect of dietary inclusion of benzoic acid on mineral balance in growing pigs. Livest. Sci. 2009, 122, 162–168. [Google Scholar] [CrossRef]
- Nørgaard, J.V.; Fernández, J.A.; Eriksen, J.; Olsen, O.H.; Carlson, D.; Poulsen, H.D. Urine acidification and mineral metabolism in growing pigs fed diets supplemented with dietary methionine and benzoic acid. Livest. Sci. 2010, 134, 113–115. [Google Scholar] [CrossRef]
- Nørgaard, J.V.; Fernández, J.A.; Sørensen, K.U.; Wamberg, S.; Poulsen, H.D.; Kristensen, N.B. Effect of benzoic acid supplementation on acid–base status and mineral metabolism in catheterized growing pigs. Livest. Sci. 2010, 134, 116–118. [Google Scholar] [CrossRef]
- Patráš, P.; Nitrayová, S.; BreSteNSký, M.; Heger, J. The effects of benzoic acid and protein level on urine ph and ammonia emission of pigs. Slovak J. Anim. 2014, 47, 100–104. [Google Scholar]
- Duarte, M.E.; Garavito-Duarte, Y.; Kim, S.W. Impacts of F18+ Escherichia coli on intestinal health of nursery pigs and dietary interventions. Animals 2023, 13, 2791. [Google Scholar] [CrossRef]
- Eckel, B.; Kirchgessner, M.; Roth, F.X. Influence of formic acid on daily weight gain, feed intake, feed conversion rate and digestibility. 1. Investigations about the nutritive efficacy of organic acids in the rearing of piglets. J. Anim. Physiol. Anim. Nutr. 1992, 67, 93–100. [Google Scholar] [CrossRef]
- Risley, C.R.; Kornegay, E.T.; Lindemann, M.D.; Weakland, S.M. Effects of organic acids with and without a microbial culture on performance and gastrointestinal tract measurements of weanling pigs. Anim. Feed Sci. Technol. 1991, 35, 259–270. [Google Scholar] [CrossRef]
- Outlaw, A.; Gachman, A.; Kim, H.; Xu, X.; Tan, Z.; Qin, Z.; Peng, X.; Rudar, M. Evaluation of protected benzoic acid on growth performance, nutrient digestibility, and gut health indices in starter pigs. Transl. Anim. Sci. 2023, 7, txad111. [Google Scholar] [CrossRef]
- Papatsiros, V.; Tassis, P.; Tzika, E.; Papaioannou, D.; Petridou, E.; Alexopoulos, C.; Kyriakis, S. Effect of benzoic acid and combination of benzoic acid with a probiotic containing Bacillus Cereus var. toyoi in weaned pig nutrition. Pol. J. Vet. Sci. 2011, 14, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Zhai, H.; Ren, W.; Wang, S.; Wu, J.; Guggenbuhl, P.; Kluenter, A.-M. Growth performance of nursery and grower-finisher pigs fed diets supplemented with benzoic acid. Anim. Nutr. 2017, 3, 232–235. [Google Scholar] [CrossRef]
- Kiarie, E.; Voth, C.; Wey, D.; Zhu, C.; Vingerhoeds, P.; Borucki, S.; Squires, E.J. Comparative efficacy of antibiotic growth promoter and benzoic acid on growth performance, nutrient utilization, and indices of gut health in nursery pigs fed corn–soybean meal diet. Can. J. Anim. Sci. 2018, 98, 868–874. [Google Scholar] [CrossRef]
- Wei, X.; Bottoms, K.A.; Stein, H.H.; Blavi, L.; Bradley, C.L.; Bergstrom, J.; Knapp, J.; Story, R.; Maxwell, C.; Tsai, T. Dietary organic acids modulate gut microbiota and improve growth performance of nursery pigs. Microorganisms 2021, 9, 110. [Google Scholar] [CrossRef]
- Warner, A.J.; DeRouchey, J.M.; Tokach, M.D.; Woodworth, J.C.; Goodband, R.D.; Gebhardt, J.T. Effect of added calcium carbonate without and with benzoic acid on weanling pig growth performance, fecal dry matter, and blood Ca and P concentrations. Transl. Anim. Sci. 2023, 7, txad055. [Google Scholar] [CrossRef]
- Brul, S.; Coote, P. Preservative agents in foods: Mode of action and microbial resistance mechanisms. Int. J. Food Microbiol. 1999, 50, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Kovanda, L.; Zhang, W.; Wei, X.; Luo, J.; Wu, X.; Atwill, E.R.; Vaessen, S.; Li, X.; Liu, Y. In vitro antimicrobial activities of organic acids and their derivatives on several species of gram-negative and gram-positive bacteria. Molecules 2019, 24, 3770. [Google Scholar] [CrossRef] [PubMed]
- Hancock, R.E.W. The bacterial outer membrane as a drug barrier. Trends Microbiol. 1997, 5, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Russell, J.B.; Diez-Gonzalez, F. The effects of fermentation acids on bacterial growth. Adv. Microb. Physiol. 1997, 39, 205–234. [Google Scholar] [CrossRef]
- Ruhal, R.; Kataria, R. Biofilm patterns in gram-positive and gram-negative bacteria. Microbiol. Res. 2021, 251, 126829. [Google Scholar] [CrossRef] [PubMed]
- Bentley, S.D.; Aanensen, D.M.; Mavroidi, A.; Saunders, D.; Rabbinowitsch, E.; Collins, M.; Donohoe, K.; Harris, D.; Murphy, L.; Quail, M.A. Genetic analysis of the capsular biosynthetic locus from all 90 pneumococcal serotypes. PLoS Genet. 2006, 2, e31. [Google Scholar] [CrossRef] [PubMed]
- Torrallardona, D.; Badiola, I.; Broz, J. Effects of benzoic acid on performance and ecology of gastrointestinal microbiota in weanling piglets. Livest. Sci. 2007, 108, 210–213. [Google Scholar] [CrossRef]
- Hazan, R.; Levine, A.; Abeliovich, H. Benzoic acid, a weak organic acid food preservative, exerts specific effects on intracellular membrane trafficking pathways in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2004, 70, 4449–4457. [Google Scholar] [CrossRef]
- Heo, J.M.; Kim, J.C.; Yoo, J.; Pluske, J.R. A between-experiment analysis of relationships linking dietary protein intake and post-weaning diarrhea in weanling pigs under conditions of experimental infection with an enterotoxigenic strain of E scherichia coli. Anim. Sci. J. 2015, 86, 286–293. [Google Scholar] [CrossRef]
- Falkowski, J.F.; Aherne, F.X. Fumaric and citric acid as feed additives in starter pig nutrition. J. Anim. Sci. 1984, 58, 935–938. [Google Scholar] [CrossRef]
- Edmonds, M.S.; Izquierdo, O.A.; Baker, D.H. Feed additive studies with newly weaned pigs: Efficacy of supplemental copper, antibiotics and organic acids. J. Anim. Sci. 1985, 60, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Giesting, D.W.; Easter, R.A. Response of starter pigs to supplementation of corn-soybean meal diets with organic acids. J. Anim. Sci. 1985, 60, 1288–1294. [Google Scholar] [CrossRef]
- Broz, J.; Schulze, J. Efficacy of citric acid as a feed additive in early weaned piglets. J. Anim. Physiol. Anim. Nutr. 1987, 58, 215–223. [Google Scholar] [CrossRef]
- Burnell, T.W.; Cromwell, G.L.; Stahly, T.S. Effects of dried whey and copper sulfate on the growth responses to organic acid in diets for weanling pigs. J. Anim. Sci. 1988, 66, 1100–1108. [Google Scholar] [CrossRef] [PubMed]
- Krause, D.O.; Harrison, P.C.; Easter, R.A. Characterization of the nutritional interactions between organic acids and inorganic bases in the pig and chick. J. Anim. Sci. 1994, 72, 1257–1262. [Google Scholar] [CrossRef][Green Version]
- Radcliffe, J.S.; Zhang, Z.; Kornegay, E.T. The effects of microbial phytase, citric acid, and their interaction in a corn-soybean meal-based diet for weanling pigs. J. Anim. Sci. 1998, 76, 1880–1886. [Google Scholar] [CrossRef][Green Version]
- Ravindran, V.; Kornegay, E.T. Acidification of weaner pig diets: A review. J. Sci. Food Agric. 1993, 62, 313–322. [Google Scholar] [CrossRef]
- Scipioni, R.; Zaghini, G.; Biavati, B. Use of acidified diets for early weaning of piglets. Zootec. Nutr. Anim. 1981, 4, 201–218. [Google Scholar]
- Radecki, S.V.; Juhl, M.R.; Miller, E.R. Fumaric and citric acids as feed additives in starter pig diets: Effect on performance and nutrient balance. J. Anim. Sci. 1988, 66, 2598–2605. [Google Scholar] [CrossRef]
- Kil, D.Y.; Piao, L.G.; Long, H.F.; Lim, J.S.; Yun, M.S.; Kong, C.S.; Ju, W.S.; Lee, H.B.; Kim, Y.Y. Effects of organic or inorganic acid supplementation on growth performance, nutrient digestibility and white blood cell counts in weanling pigs. Asian-Australas. J. Anim. Sci. 2006, 19, 252–261. [Google Scholar] [CrossRef]
- Giesting, D.W.; Roos, M.A.; Easter, R.A. Evaluation of the effect of fumaric acid and sodium bicarbonate addition on performance of starter pigs fed diets of different types. J. Anim. Sci. 1991, 69, 2489–2496. [Google Scholar] [CrossRef]
- Thacker, P.A.; Campbell, G.L.; Grootwassink, J. The effect of organic acids and enzyme supplementation on the performance of pigs fed barley-based diets. Can. J. Anim. Sci. 1992, 72, 395–402. [Google Scholar] [CrossRef]
- Eidelsburger, U.; Kirchgessner, M.; Roth, F.X. Influence of fumaric acid, hydrochloric acid, sodium formate, tylosin and toyocerin on daily weight gain, feed intake, feed conversion rate and digestibility. 11. Investigations about the nutritive efficacy of organic acids in the rearing of piglets. J. Anim. Physiol. Anim. Nutr. 1992, 68, 82–92. [Google Scholar] [CrossRef]
- Manzanilla, E.G.; Perez, J.F.; Martin, M.; Kamel, C.; Baucells, F.; Gasa, J. Effect of plant extracts and formic acid on the intestinal equilibrium of early-weaned pigs. J. Anim. Sci. 2004, 82, 3210–3218. [Google Scholar] [CrossRef]
- Luise, D.; Motta, V.; Salvarani, C.; Chiappelli, M.; Fusco, L.; Bertocchi, M.; Mazzoni, M.; Maiorano, G.; Costa, L.N.; Van Milgen, J. Long-term administration of formic acid to weaners: Influence on intestinal microbiota, immunity parameters and growth performance. Anim. Feed Sci. Technol. 2017, 232, 160–168. [Google Scholar] [CrossRef]
- Dahmer, P.L.; Harrison, O.L.; Jones, C.K. Effects of formic acid and glycerol monolaurate on weanling pig growth performance, fecal consistency, fecal microbiota, and serum immunity. Transl. Anim. Sci. 2022, 6, txac145. [Google Scholar] [CrossRef] [PubMed]
- Bolduan, G.; Jung, H.; Schneider, R.; Block, J.; Klenke, B. Die Wirkung von Propion-und Ameisensäure in der Ferkelaufzucht. J. Anim. Physiol. Anim. Nutr. 1988, 59, 72–78. [Google Scholar] [CrossRef]
- Roth, F.X.; Kirchgessner, M. Organic acids as feed additives for young pigs: Nutritional and gastrointestinal effects. J. Anim. Feed Sci 1998, 7, 25–33. [Google Scholar] [CrossRef]
- Pallauf, J.; Hüter, J. Studies on the influence of calcium formate on growth, digestibility of crude nutrients, nitrogen balance and calcium retention in weaned piglets. Anim. Feed Sci. Technol. 1993, 43, 65–76. [Google Scholar] [CrossRef]
- Paulicks, B.R.; Roth, F.X.; Kirchgessner, M. Dose effects of potassium diformate (FormiTM LHS) on the performance of growing piglets. Agribiol. Res. 1996, 49, 318–326. [Google Scholar]
| IBW 2 (kg) or Age (d) | Experimental Period (d) | BA (%) | Acidification (% Change 3) | |||||
|---|---|---|---|---|---|---|---|---|
| pH in Stomach | pH in Jejunum | pH in Urine | HA 4 in Urine | BA in Urine | Reference | |||
| 6.5 kg | 42 | 0.35 | - | - | −7.98 ** | 681.1 ** | −35.14 | [28] |
| 0.50 | - | - | −10.24 ** | 745.3 ** | 86.49 | |||
| 7.3 kg | 35 | 0.50 | −4.32 | −0.65 | −7.79 | - | - | [27] |
| 1.00 | −4.32 | 0.16 | −13.83 ** | - | - | |||
| 6.8 kg | 42 | 0.25 | −15.63 | −7.12 ** | - | - | - | [14] |
| 9.7 kg | 42 | 0.50 | - | - | −8.83 ** | - | - | [44] |
| 6.0 kg | 14 | 0.50 | - | −6.93 | - | - | - | [12] |
| 6.0 kg | 42 | 0.50 | - | −5.47 | - | - | - | [12] |
| 6.5 kg | 14 | 0.20 | −10.15 | −4.23 ** | - | - | - | [33] |
| 0.50 | −17.54 ** | −5.48 ** | - | - | - | |||
| 6.5 kg | 42 | 0.20 | −10.59 | −1.25 | - | - | - | [33] |
| 0.50 | −26.61 ** | −8.31 | - | - | - | |||
| 18.8 kg | 14 | 0.50 | - | −8.06 * | - | - | - | [45] |
| 6.6 kg | 35 | 0.30 | −7.93 | - | −6.42 ** | - | - | [46] |
| 0.50 | −26.90 | - | −3.83 ** | - | - | |||
| 21 d | 35 | 1.00 | - | - | −15.74 ** | - | - | [36] |
| Nursery phase (average): | −13.78 | −4.73 | −9.33 | 713.20 | 25.68 | |||
| 26.0 kg | 42 | 1.00 | - | - | −11.78 ** | 886.3 ** | - | [47] |
| 26.0 kg | 84 | 1.00 | - | - | −12.32 ** | 1019.5 ** | - | [47] |
| 33.1 kg | 53 | 0.50 | - | - | −5.56 ** | - | - | [48] |
| 63.0 kg | N/A 7 | 1.00 | - | - | −28.82 ** | 2064.5 ** | 752.33 ** | [29] |
| 28.0 kg 5,6 | 21 | 1.00 | - | - | −10.52 | - | - | [49] |
| 2.00 | - | - | −27.32 | - | - | |||
| 64.0 kg 5 | 14 | 1.00 | - | - | −12.21 | - | - | [16] |
| 2.00 | - | - | −29.58 | - | - | |||
| 3.00 | - | - | −32.28 | - | - | |||
| 45.0 kg | 5 | 2.00 | - | - | −11.21 ** | 612.9 ** | 4868.75 ** | [50] |
| 50.0 kg | 10 | 1.00 | - | - | −12.14 ** | 1500.0 ** | 1341.18 ** | [51] |
| 2.00 | - | - | −24.56 ** | 2733.3 ** | 2594.12 ** | |||
| 29.9 kg | 6 | 1.00 | - | - | −12.35 ** | 1858.4 ** | - | [52] |
| Growing phase (average): | - | - | −17.74 | 1525.0 | 2389.1 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, H.; Kim, S.W. Dietary Intervention of Benzoic Acid for Intestinal Health and Growth of Nursery Pigs. Animals 2024, 14, 2394. https://doi.org/10.3390/ani14162394
Choi H, Kim SW. Dietary Intervention of Benzoic Acid for Intestinal Health and Growth of Nursery Pigs. Animals. 2024; 14(16):2394. https://doi.org/10.3390/ani14162394
Chicago/Turabian StyleChoi, Hyunjun, and Sung Woo Kim. 2024. "Dietary Intervention of Benzoic Acid for Intestinal Health and Growth of Nursery Pigs" Animals 14, no. 16: 2394. https://doi.org/10.3390/ani14162394
APA StyleChoi, H., & Kim, S. W. (2024). Dietary Intervention of Benzoic Acid for Intestinal Health and Growth of Nursery Pigs. Animals, 14(16), 2394. https://doi.org/10.3390/ani14162394





