Deciphering the Hearts: Geometric Morphometrics Reveals Shape Variation in Abatus Sea Urchins across Subantarctic and Antarctic Seas
Abstract
Simple Summary
Abstract
1. Introduction
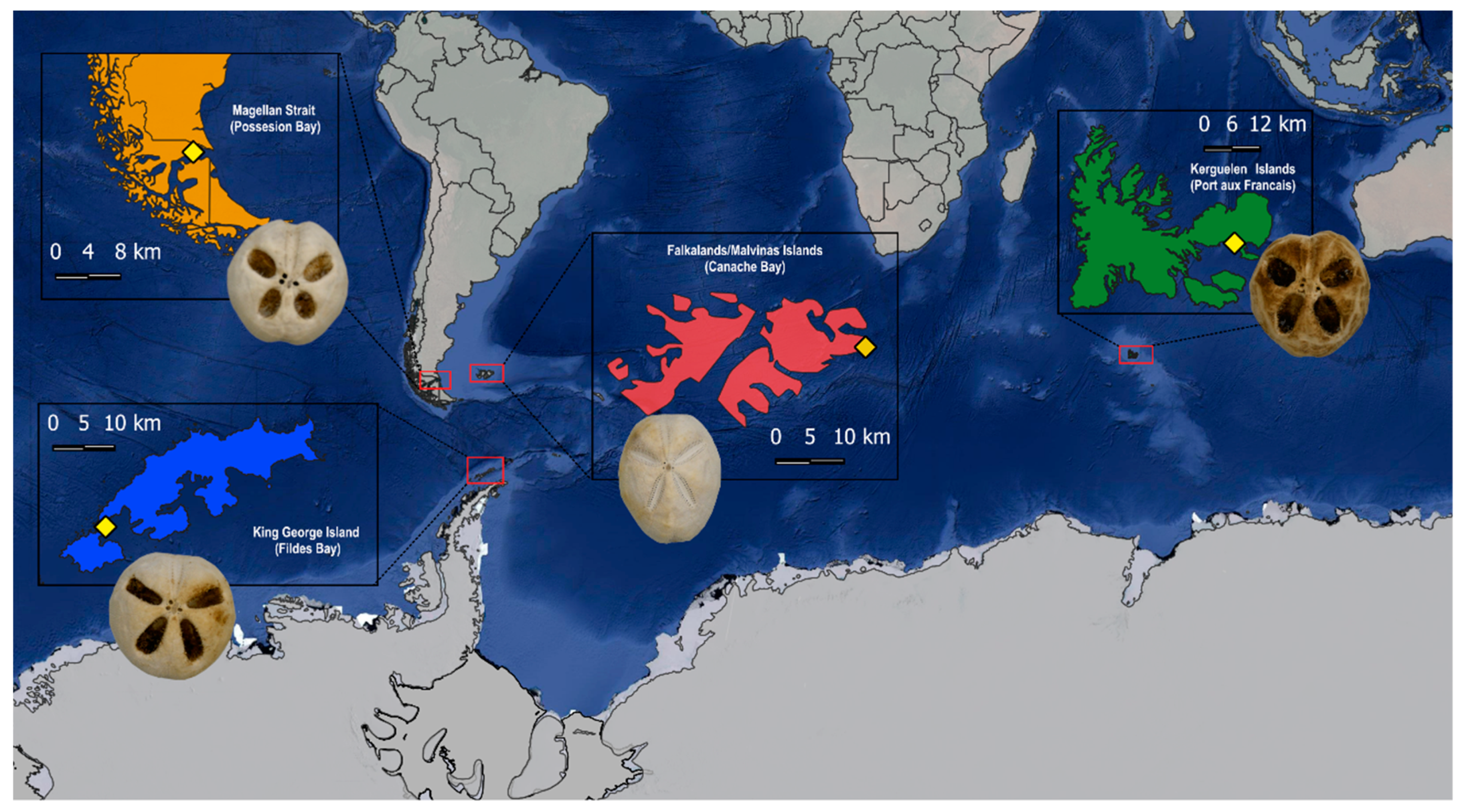
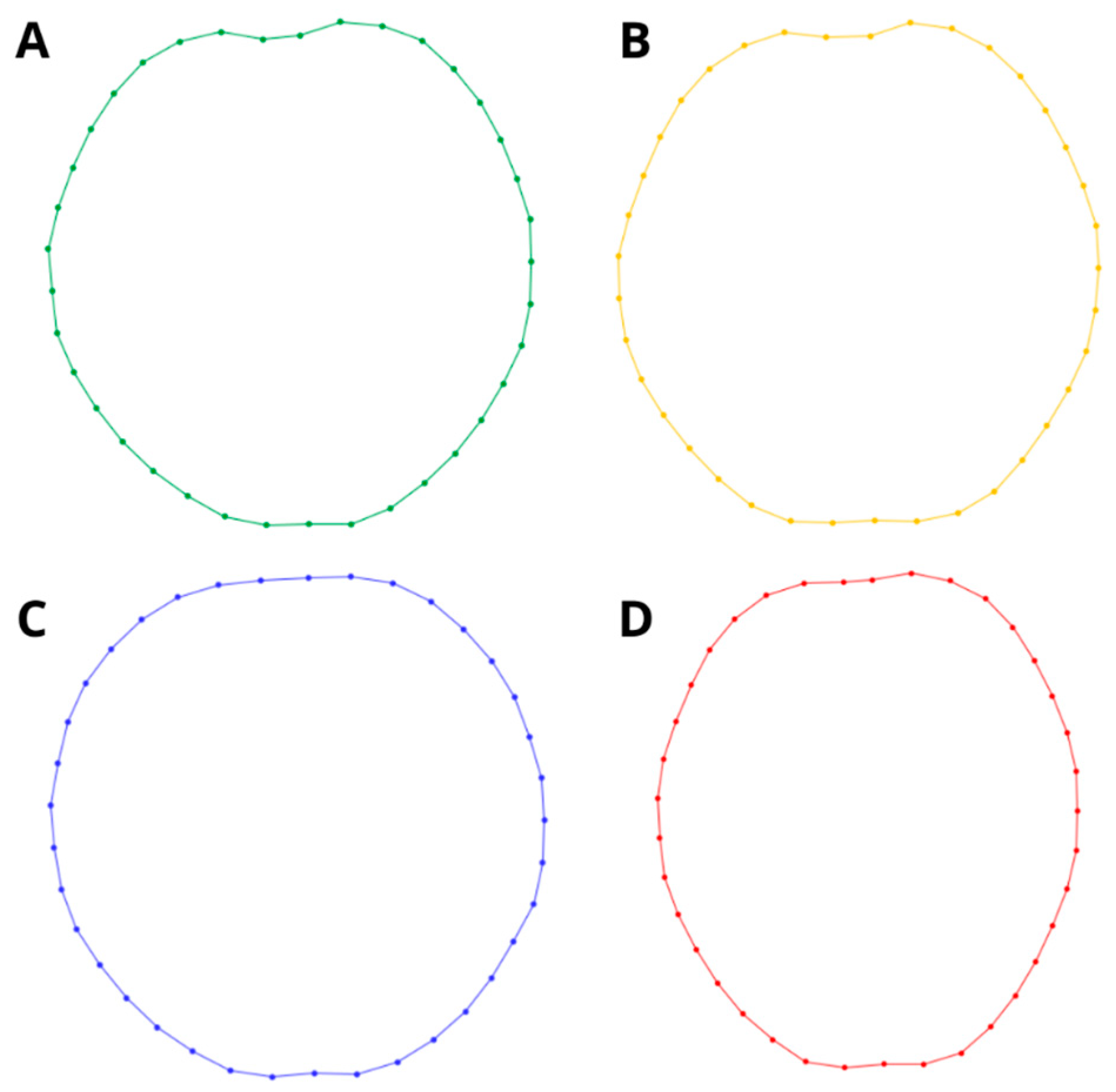
2. Materials and Methods
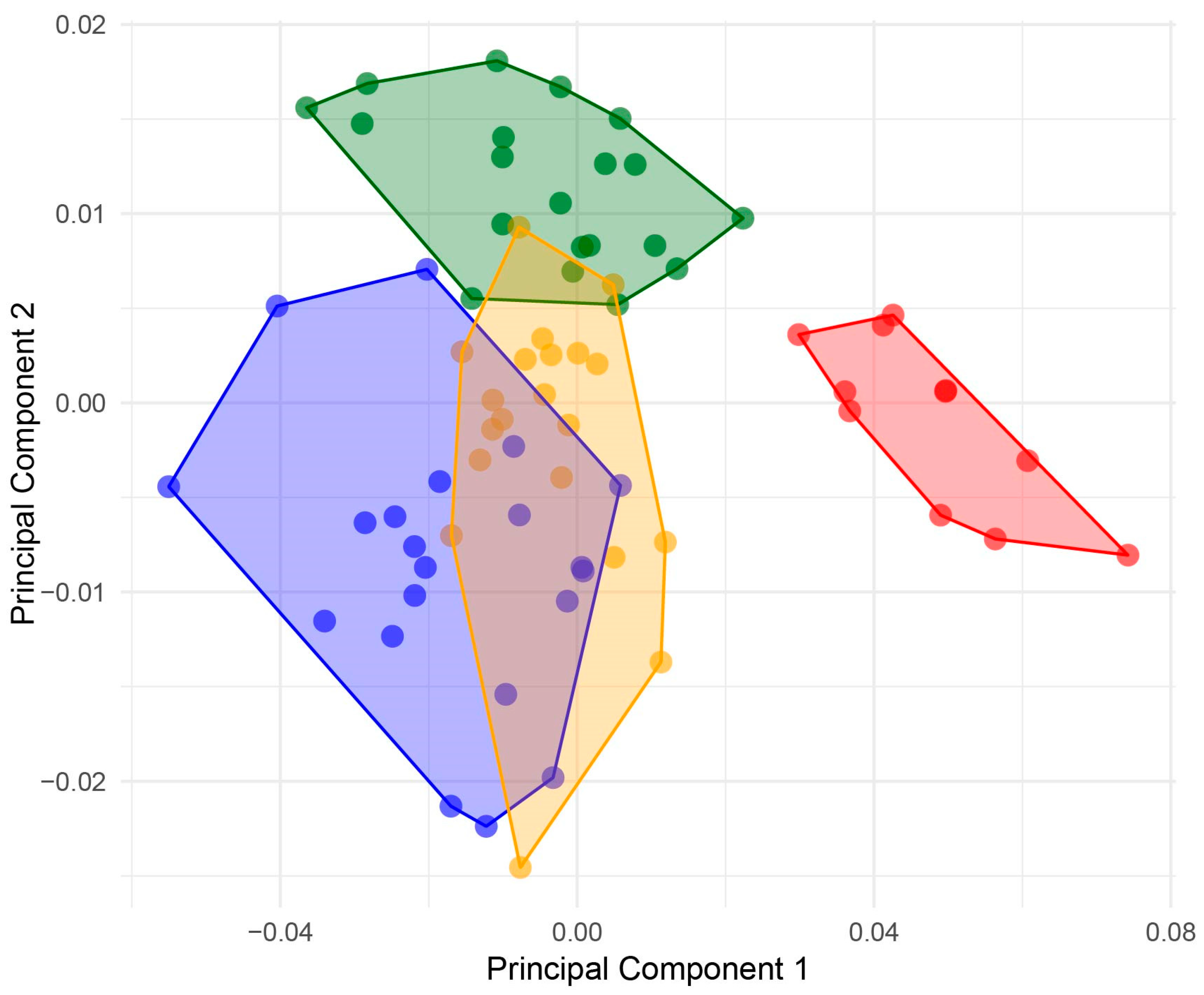
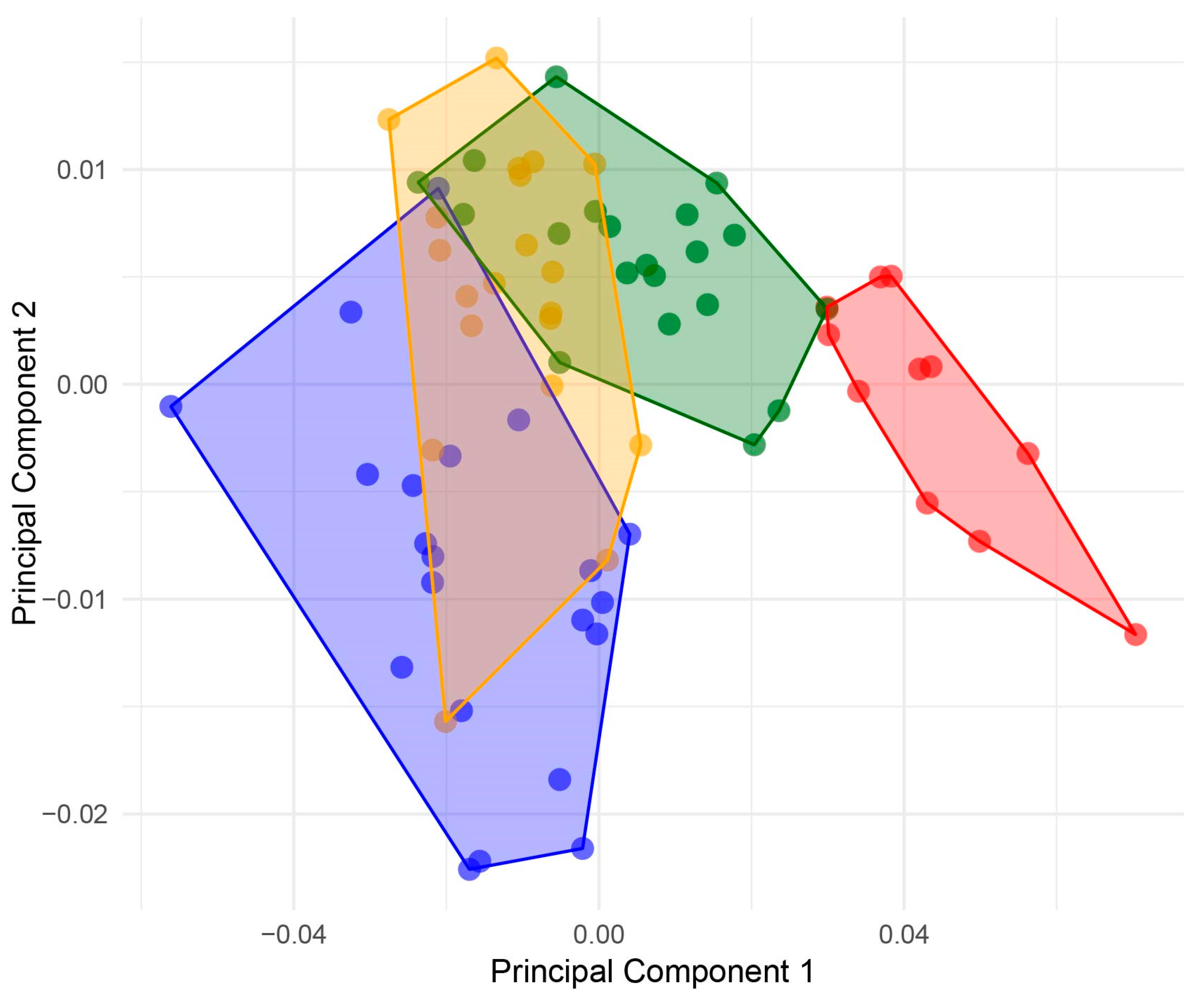
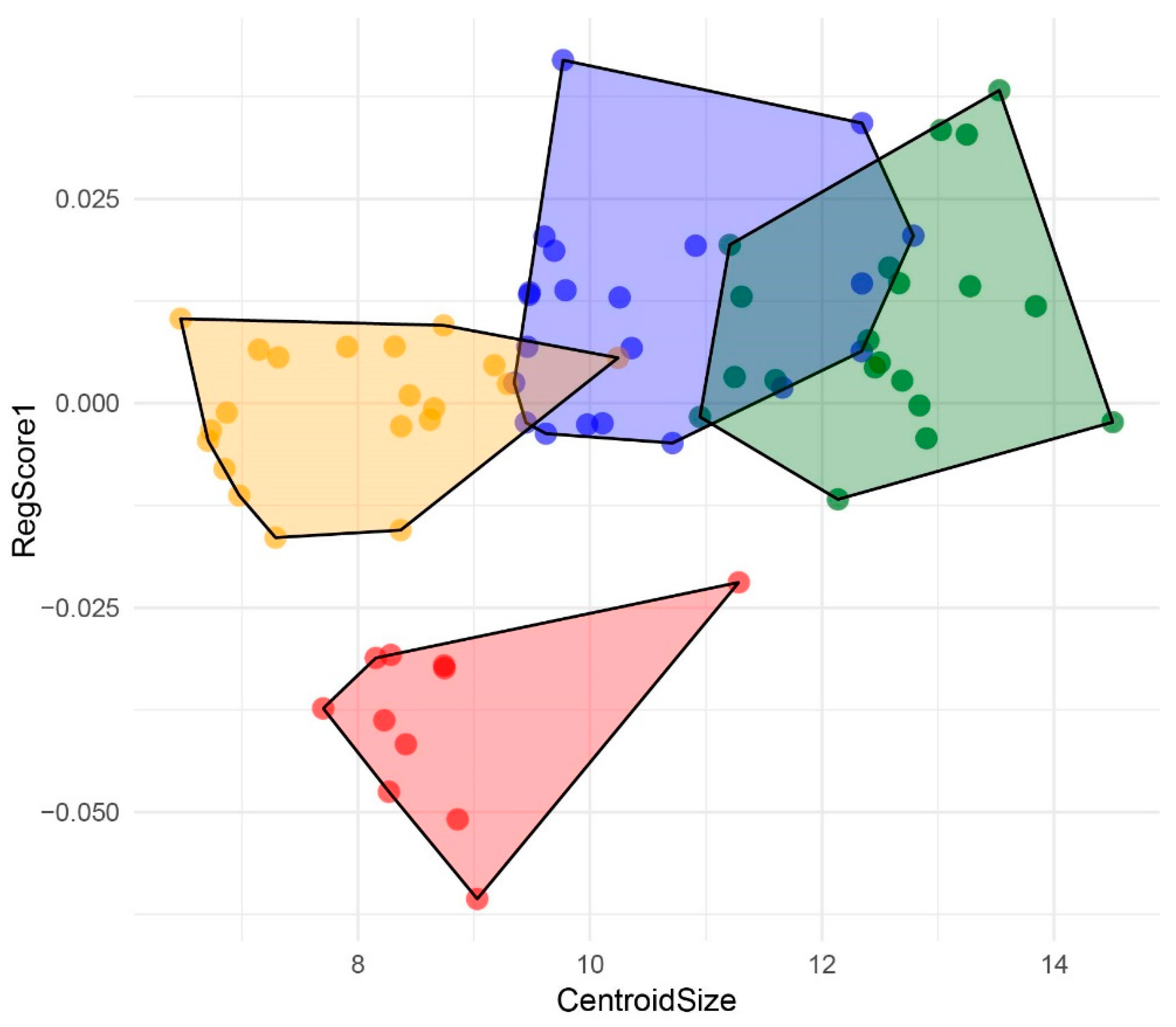
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- David, B.; Choné, T.; Mooi, R.; De Ridder, C. Antarctic echinoidea. In Synopses of the Antarctic Benthos; Koeltz Scientific Books: Königstein, Gemany, 2005. [Google Scholar]
- Dell, R.K. Antarctic benthos. Adv. Mar. Biol. 1972, 10, 1–216. [Google Scholar]
- Schatt, P.; Féral, J.-P. The brooding cycle of Abatus cordatus (Echinodermata: Spatangoida) at Kerguelen islands. Polar Biol. 1991, 11, 283–292. [Google Scholar] [CrossRef]
- Gil, D.G.; Zaixso, H.E.; Tolosano, J.A. Brooding of the sub-Antarctic heart urchin, Abatus cavernosus (Spatangoida: Schizasteridae), in southern Patagonia. Mar. Biol. 2009, 156, 1647–1657. [Google Scholar] [CrossRef]
- Flores, J.N.; Penchaszadeh, P.E.; Brogger, M.I. Heart urchins from the depths: Corparva lyrida gen. et sp. nov. (Palaeotropidae), and new records for the Southwestern Atlantic Ocean. Rev. Biol. Trop. 2021, 69, 14–33. [Google Scholar] [CrossRef]
- Díaz, A.; González-Wevar, C.A.; Maturana, C.S.; Palma, A.T.; Poulin, E.; Gerard, K. Restricted geographic distribution and low genetic diversity of the brooding sea urchin Abatus agassizii (Spatangoidea: Schizasteridae) in the South Shetland Islands: A bridgehead population before the spread to the northern Antarctic Peninsula? Rev. Chil. Hist. Nat. 2012, 85, 457–468. [Google Scholar] [CrossRef][Green Version]
- Poulin, E.; Féral, J.-P. Pattern of spatial distribution of a brood-protecting schizasterid echinoid, Abatus cordatus, endemic to the Kerguelen Islands. Mar. Ecol. Prog. Ser. 1995, 118, 179–186. [Google Scholar] [CrossRef]
- Pascal, P.Y.; Reynaud, Y.; Poulin, E.; De Ridder, C.; Saucede, T. Feeding in spatangoids: The case of Abatus cordatus in the Kerguelen Islands (Southern Ocean). Polar Biol. 2021, 44, 795–808. [Google Scholar] [CrossRef]
- Lohrer, A.M.; Thrush, S.F.; Gibbs, M.M. Bioturbators enhance ecosystem function through complex biogeochemical interactions. Nature 2004, 431, 1092–1095. [Google Scholar] [CrossRef]
- Pearse, J.S.; McClintock, J.B. A comparison of reproduction by the brooding spatangoid echinoids Abatus shackletoni and A. nimrodi in McMurdo Sound, Antarctica. Invertebr. Reprod. Dev. 1990, 17, 181–191. [Google Scholar] [CrossRef]
- Maturana, C.S. Estrategias de Reproducción en la Antártica: Estacionalidad Reproductiva y Patrón de Apareamiento en el Erizo Incubante, Abatus agassizii (Mortensen 1910). Master’s Thesis, Facultad de Ciencias, Universidad de Chile, Santiago, Chile, 2011. [Google Scholar]
- David, B.; Saucède, T.; Chenuil, A.; Steimetz, E.; De Ridder, C. The taxonomic challenge posed by the Antarctic echinoids Abatus bidens and Abatus cavernosus (Schizasteridae, Echinoidea). Polar Biol. 2016, 39, 897–912. [Google Scholar] [CrossRef]
- Guzzi, A.; Alvaro, M.C.; Cecchetto, M.; Schiaparelli, S. Echinoids and Crinoids from Terra Nova Bay (Ross Sea) Based on a Reverse Taxonomy Approach. Divers 2023, 15, 875. [Google Scholar] [CrossRef]
- Mortensen, T. The Echinoidea of the Swedish South Polar Expedition. Wissenschaftliche Ergebnisse der Schwedischen Südpolar-Expedition 1901–1903; Lithographisches Institut des Generalstabs: Stockholm, Sweden, 1910. [Google Scholar]
- David, B.; Choné, T.; Mooi, R.; De Ridder, C. Biodiversity of Antarctic echinoids: A comprehensive and interactive database. Sci. Mar. 2005, 69, 201–203. [Google Scholar] [CrossRef][Green Version]
- Schinner, G.O.; McClintock, J.B. Form and function of brood pouches of the Antarctic heart urchins Abatus nimrodi and Abatus shackletoni. In Echinoderms through Time; David, B., Guille, A., Féral, J.-P., Roux, M., Eds.; A.A. Balkema: Rotterdam, The Netherlands, 1994; p. 872. [Google Scholar]
- Gil, D.G.; Zaixso, H.E.; Tolosano, J.A. Sex-specific differences in gonopore and gonadal growth trajectories in the brooding sea urchin, Abatus cavernosus (Spatangoida). Invertebr. Biol. 2020, 139, e12278. [Google Scholar] [CrossRef]
- Adams, D.C.; Rohlf, F.J.; Slice, D.E. Geometric morphometrics: Ten years of progress following the ‘revolution’. Ital. J. Zool. 2004, 71, 5–16. [Google Scholar] [CrossRef]
- Zelditch, M.; Swiderski, D.L.; Sheets, H.D. Geometric Morphometrics for Biologists: A Primer, 2nd ed.; Academic Press: San Diego, CA, USA, 2012. [Google Scholar]
- Márquez-Borrás, F.; Solís-Marín, F.A.; Mejía-Ortiz, L.M. Troglomorphism in the brittle star Ophionereis commutabilis Bribiesca-Contreras et al., 2019 (Echinodermata, Ophiuroidea, Ophionereididae). Subterr. Biol. 2020, 33, 87–108. [Google Scholar] [CrossRef]
- Swisher, R.E. Convergent discoidal sand dollars from isolated regions: A geometric morphometric analyses of Dendraster and Arachnoides. Terr. Atmos. Ocean. Sci. 2021, 32, 1117–1130. [Google Scholar] [CrossRef]
- Hernández-Díaz, Y.Q.; Solis, F.; Beltrán-López, R.G.; Benítez, H.A.; Díaz-Jaimes, P.; Paulay, G. Integrative species delimitation in the common ophiuroid Ophiothrix angulata (Echinodermata: Ophiuroidea): Insights from COI, ITS2, arm coloration, and geometric morphometrics. PeerJ 2023, 11, e15655. [Google Scholar] [CrossRef] [PubMed]
- Martín-Ledo, R.; Sands, C.J.; López-González, P.J. A new brooding species of brittle star (Echinodermata: Ophiuroidea) from Antarctic waters. Polar Biol. 2013, 36, 115–126. [Google Scholar] [CrossRef]
- De-los-Palos-Peña, M.; Solís-Marín, F.A.; Laguarda-Figueras, A.; Durán-González, A. Ontogenetic variation of the odontophore of Luidia superba (Asteroidea: Paxillosida) and its taxonomic implications. Rev. Biol. Trop. 2021, 69, 89–100. [Google Scholar] [CrossRef]
- Klingenberg, C.P. Evolution and development of shape: Integrating quantitative approaches. Nat. Rev. Genet. 2010, 11, 623–635. [Google Scholar] [CrossRef]
- Barría, E.M.; Benítez, H.A.; Hernández, C.E. Evolvability in the cephalothoracic structural complexity of Aegla araucaniensis (Crustacea: Decapoda) determined by a developmental system with low covariational constraint. Biology 2022, 11, 958. [Google Scholar] [CrossRef]
- Stige, L.C.; David, B.; Alibert, P. On hidden heterogeneity in directional asymmetry–can systematic bias be avoided? J. Evol. Biol. 2006, 19, 492–499. [Google Scholar] [CrossRef] [PubMed]
- GBIF. GBIF Backbone Taxonomy. 2023. Available online: https://www.gbif.org/species/3249617 (accessed on 22 July 2024).
- Rohlf, F.J. The tps series of software. Hystrix 2015, 26, 9–12. [Google Scholar] [CrossRef]
- Benítez, H.A.; Lemic, D.; Villalobos-Leiva, A.; Bažok, R.; Órdenes-Claveria, R.; Pajač Živković, I.; Mikac, K.M. Breaking symmetry: Fluctuating asymmetry and geometric morphometrics as tools for evaluating developmental instability under diverse agroecosystems. Symmetry 2020, 12, 1789. [Google Scholar] [CrossRef]
- Rohlf, F.J. TpsDig; Version 2.31; Department of Ecology and Evolution, State University of New York at Stony Brook: New York, NY, USA, 2017. [Google Scholar]
- Rohlf, F.J.; Slice, D. Extensions of the procrustes method for the optimal superimposition of landmarks. Syst. Biol. 1990, 39, 40–59. [Google Scholar] [CrossRef]
- Drake, A.G.; Klingenberg, C.P. The pace of morphological change: Historical transformation of skull shape in St Bernard dogs. Proc. Biol. Sci. 2008, 275, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Klingenberg, C.P. MorphoJ: An integrated software package for geometric morphometrics. Mol. Ecol. Resour. 2011, 11, 353–357. [Google Scholar] [CrossRef]
- Baken, E.K.; Collyer, M.L.; Kaliontzopoulou, A.; Adams, D.C. geomorph v4. 0 and gmShiny: Enhanced analytics and a new graphical interface for a comprehensive morphometric experience. Methods Ecol. Evol. 2021, 12, 2355–2363. [Google Scholar] [CrossRef]
- Faulkes, Z. Morphological Adaptations for Digging and Burrowing. In Functional Morphology and Diversity; Watling, L., Thiel, M., Eds.; Oxford University Press: New York, NY, USA, 2012; pp. 276–295. [Google Scholar]
- Mespoulhé, P. Morphologie d’un Echinide Irregulier Subantarctique de L’archipel des Kerguelen: Ontogenese, Dimorphisme Sexual et Variabilite. Ph.D. Thesis, Université de Bourgogne, Dijon, France, 1992. [Google Scholar]
- Ferber, I.; Lawrence, J.M. Distribution, substratum preference and burrowing behaviour of Lovenia elongata (Gray)(Echinoidea: Spatangoida) in the Gulf of Elat (‘Aqaba), Red Sea. J. Exp. Mar. Bio. Ecol. 1976, 22, 207–225. [Google Scholar] [CrossRef]
- Saitoh, M.; Kanazawa, K.I. Adaptative morphology for living in shallow water environments in spatangoid echinoids. Zoosymposia 2012, 7, 255–265. [Google Scholar] [CrossRef]
- West-Eberhard, M.J. Developmental Plasticity and Evolution; Oxford University Press: New York, NY, USA, 2003. [Google Scholar]
- González-Wevar, C.A.; Segovia, N.I.; Rosenfeld, S.; Noll, D.; Maturana, C.S.; Hüne, M.; Naretto, J.; Gérard, K.; Díaz, A.; Spencer, H.G.; et al. Contrasting biogeographical patterns in Margarella (Gastropoda: Calliostomatidae: Margarellinae) across the Antarctic polar front. Mol. Phylogenet. Evol. 2021, 156, 107039. [Google Scholar] [CrossRef] [PubMed]
- González-Wevar, C.A.; de Aranzamendi, M.C.; Segovia, N.I.; Rosenfeld, S.; Maturana, C.S.; Molina, C.R.; Brickle, P.; Gardenal, C.N.; Bastida, R.; Poulin, E. Genetic footprints of Quaternary glacial cycles over the patterns of population diversity and structure in three Nacella (Patellogastropoda: Nacellidae) species across the Magellan province in southern South America. Front. Mar. Sci. 2023, 10, 1154755. [Google Scholar] [CrossRef]
- Ledoux, J.B.; Tarnowska, K.; Gérard, K.; Lhuillier, E.; Jacquemin, B.; Weydmann, A.; Féral, J.-P.; Chenuil, A. Fine-scale spatial genetic structure in the brooding sea urchin Abatus cordatus suggests vulnerability of the Southern Ocean marine invertebrates facing global change. Polar Biol. 2012, 35, 611–623. [Google Scholar] [CrossRef]
- Leese, F.; Kop, A.; Wägele, J.-W.; Held, C. Cryptic speciation in a benthic isopod from Patagonian and Falkland Island waters and the impact of glaciations on its population structure. Front. Zool. 2008, 5, 19. [Google Scholar] [CrossRef] [PubMed]
- Mesphoulhé, P.; David, B. Stratégie de croissance d’un oursin subantarctique: Abatus cordatus des îles Kerguelen. C. R. Acad. Sci. Paris Ser. III 1992, 314, 205–211. [Google Scholar]
- Glazier, D.S.; Hirst, A.G.; Atkinson, D. Shape shifting predicts ontogenetic changes in metabolic scaling in diverse aquatic invertebrates. Proc. R. Soc. Lond. B Biol. Sci. 2015, 282, 20142302. [Google Scholar] [CrossRef]
- Smith, E.; Son, E. The role of animal morphology in adaptation and survival. J. Zool. Res. 2024, 5, 1–8. [Google Scholar]
| Centroid Size | |||||
|---|---|---|---|---|---|
| Effect | SS | MS | df | F | p |
| Localities | 238.214 | 79.404681 | 3 | 73.83 | <0.0001 |
| Sex | 0.57326 | 0.57326 | 1 | 0.53 | 0.4679 |
| Individual | 72.059 | 1.075507 | 67 | ||
| Shape | |||||
| Localities | 0.03835 | 0.0003653 | 105 | 46.39 | <0.0001 |
| Sex | 0.00011 | 0.000003278 | 35 | 0.42 | 0.999 |
| Individual | 0.01847 | 0.000007874 | 2345 | 2.77 | <0.0001 |
| A. agassizii | A. cordatus | A. cavernosus (F/M) | |
| A. cordatus | 12.1293 * | ||
| A. cavernosus (F/M) | 13.4967 * | 9.5791 * | |
| A. cavernosus (P) | 10.5548 * | 7.9832 * | 10.1073 * |
| A. agassizii | A. cordatus | A. cavernosus (F/M) | |
| A. cordatus | 0.0244 * | ||
| A. cavernosus (F/M) | 0.0657 * | 0.0537 * | |
| A. cavernosus (P) | 0.0191 * | 0.0171 * | 0.0527 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moya, F.; Hernández, J.; Suazo, M.J.; Saucède, T.; Brickle, P.; Poulin, E.; Benítez, H.A. Deciphering the Hearts: Geometric Morphometrics Reveals Shape Variation in Abatus Sea Urchins across Subantarctic and Antarctic Seas. Animals 2024, 14, 2376. https://doi.org/10.3390/ani14162376
Moya F, Hernández J, Suazo MJ, Saucède T, Brickle P, Poulin E, Benítez HA. Deciphering the Hearts: Geometric Morphometrics Reveals Shape Variation in Abatus Sea Urchins across Subantarctic and Antarctic Seas. Animals. 2024; 14(16):2376. https://doi.org/10.3390/ani14162376
Chicago/Turabian StyleMoya, Fernando, Jordan Hernández, Manuel J. Suazo, Thomas Saucède, Paul Brickle, Elie Poulin, and Hugo A. Benítez. 2024. "Deciphering the Hearts: Geometric Morphometrics Reveals Shape Variation in Abatus Sea Urchins across Subantarctic and Antarctic Seas" Animals 14, no. 16: 2376. https://doi.org/10.3390/ani14162376
APA StyleMoya, F., Hernández, J., Suazo, M. J., Saucède, T., Brickle, P., Poulin, E., & Benítez, H. A. (2024). Deciphering the Hearts: Geometric Morphometrics Reveals Shape Variation in Abatus Sea Urchins across Subantarctic and Antarctic Seas. Animals, 14(16), 2376. https://doi.org/10.3390/ani14162376








