Effect of High Dietary Iron on Fat Deposition and Gut Microbiota in Chickens
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Sample Collection
2.3. Determination of Serum Biochemical Parameters
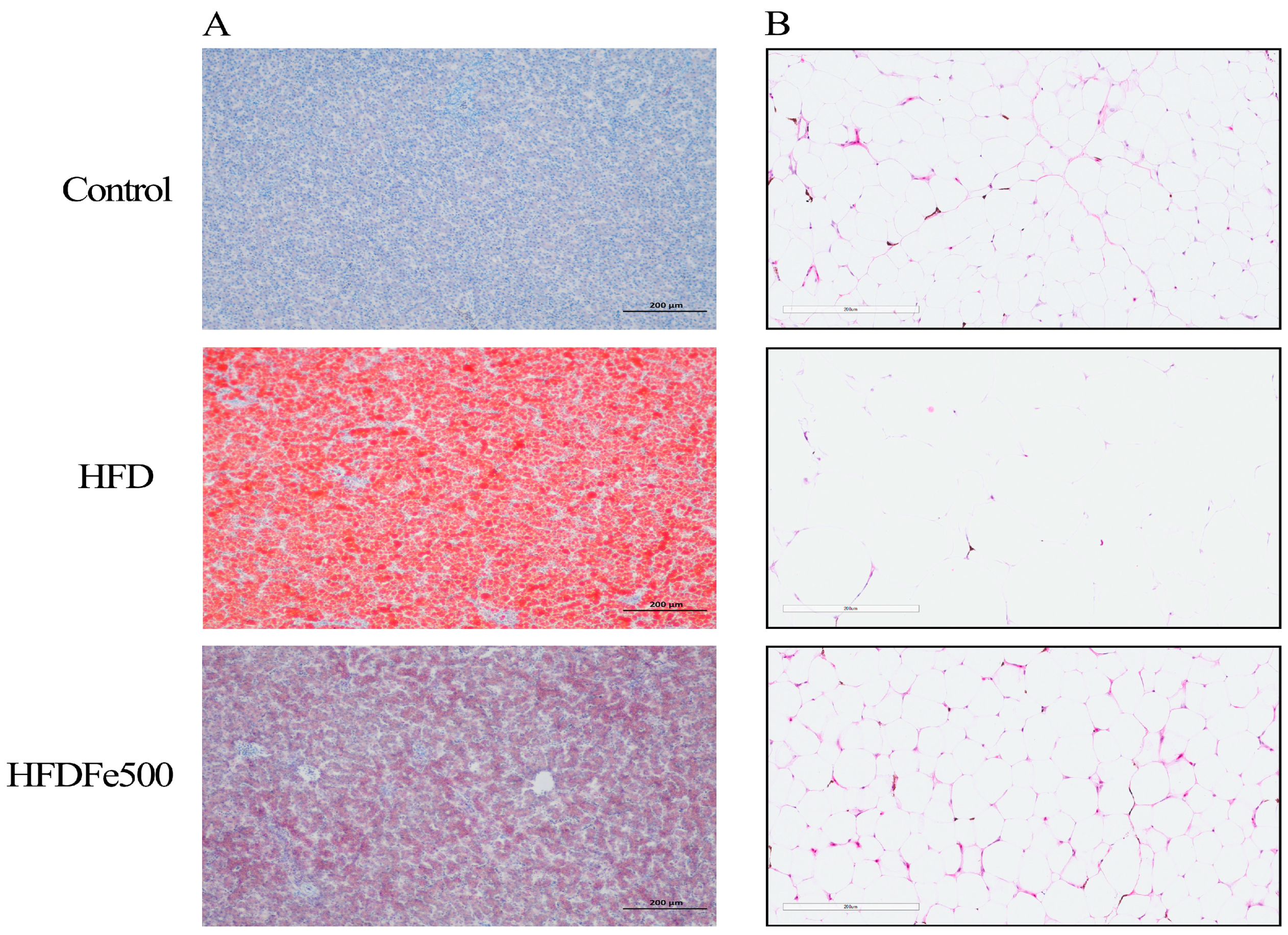
2.4. Oil Red O Staining
2.5. HE Staining
2.6. DNA Extraction, Library Preparation, and 16S rDNA Gene Sequence
2.7. Analysis of 16S rDNA Gene Sequence
2.8. Statistical Analysis
3. Results
3.1. Growth Performance
3.2. Serum Biochemical Parameters
3.3. Lipid Deposition and Adipose Tissue Development
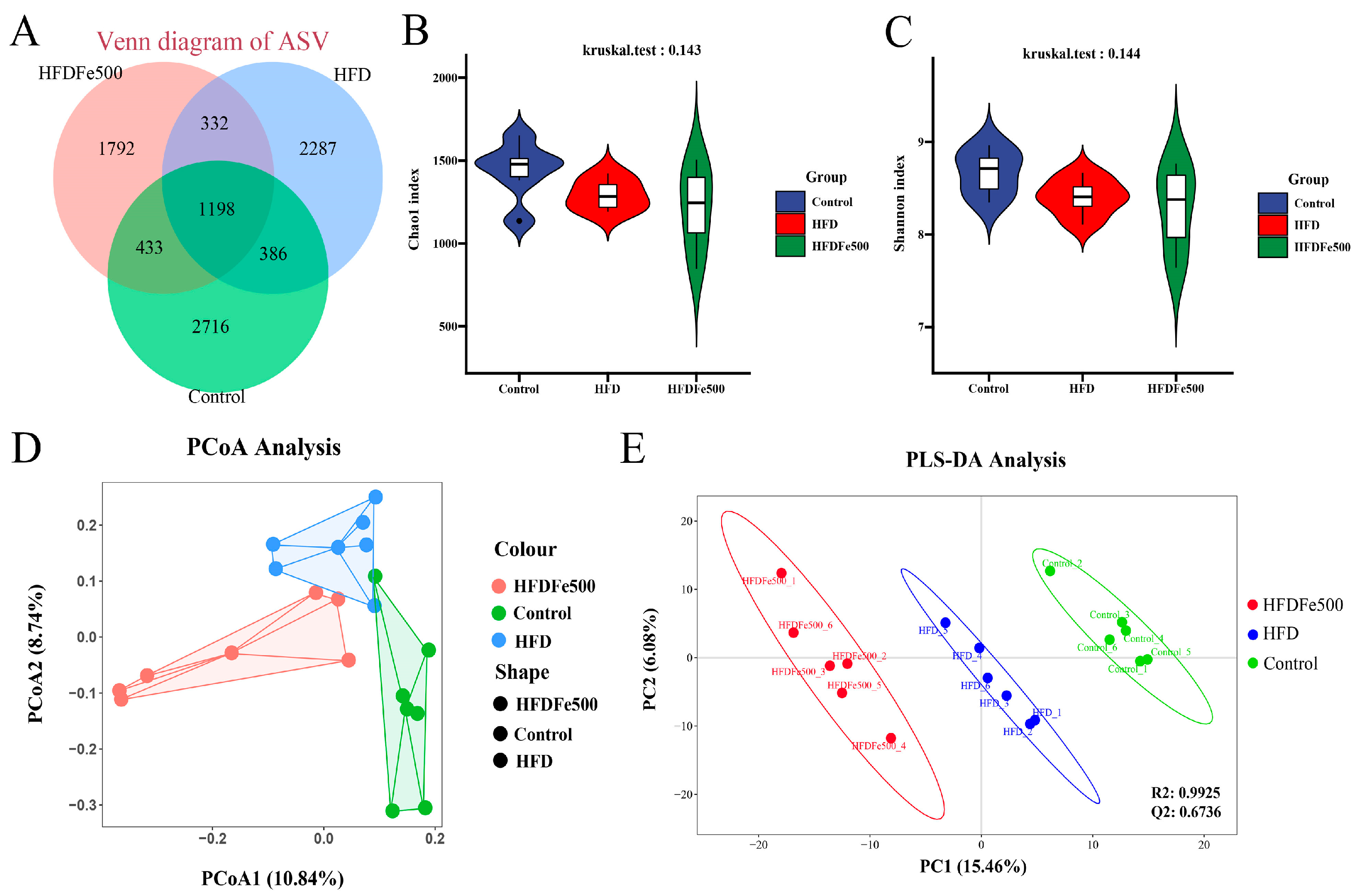
3.4. Alpha Diversity and Beta Diversity Analysis of Gut Microbiota
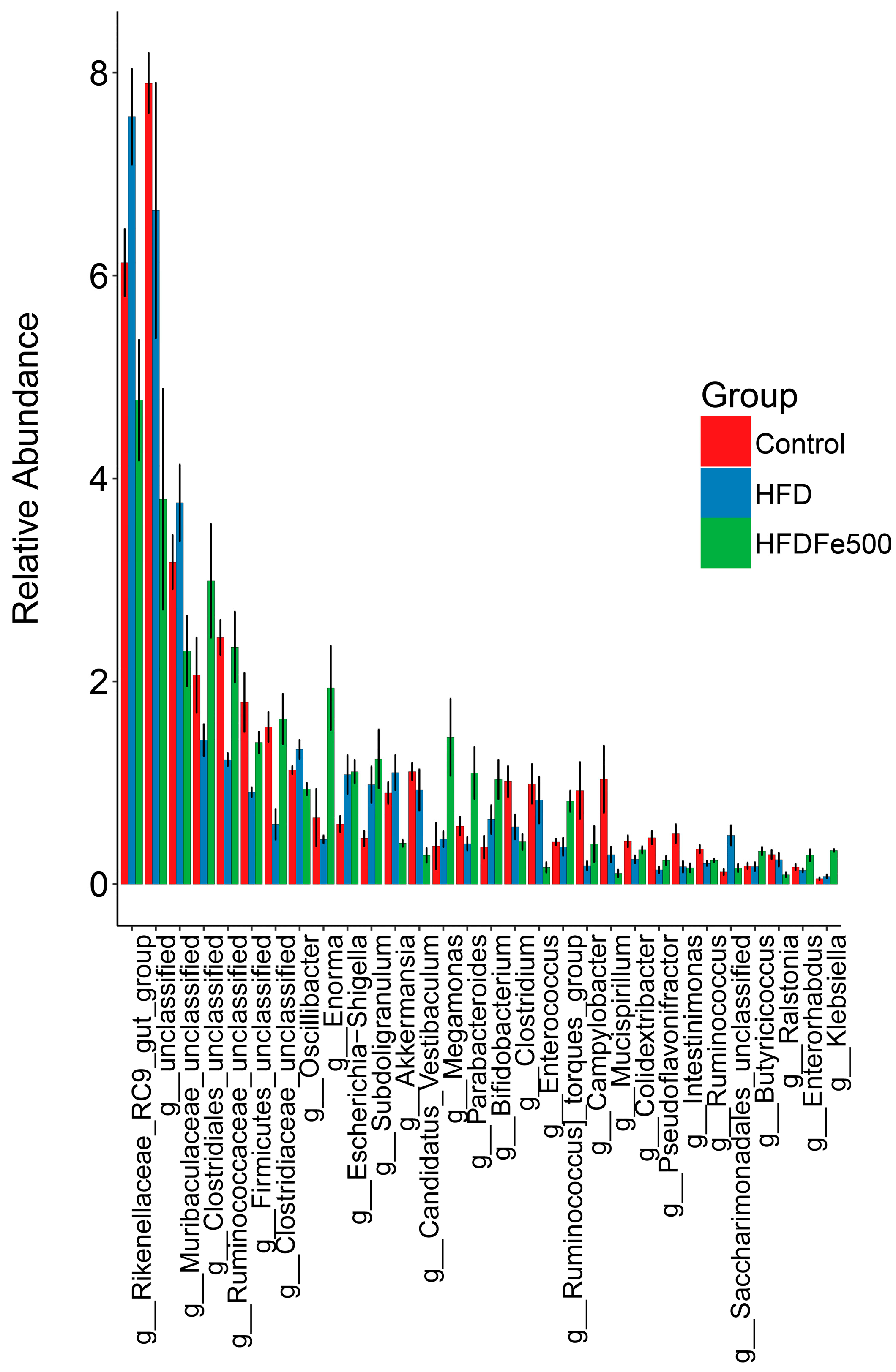
3.5. Analysis of Gut Microbiota Community Structure
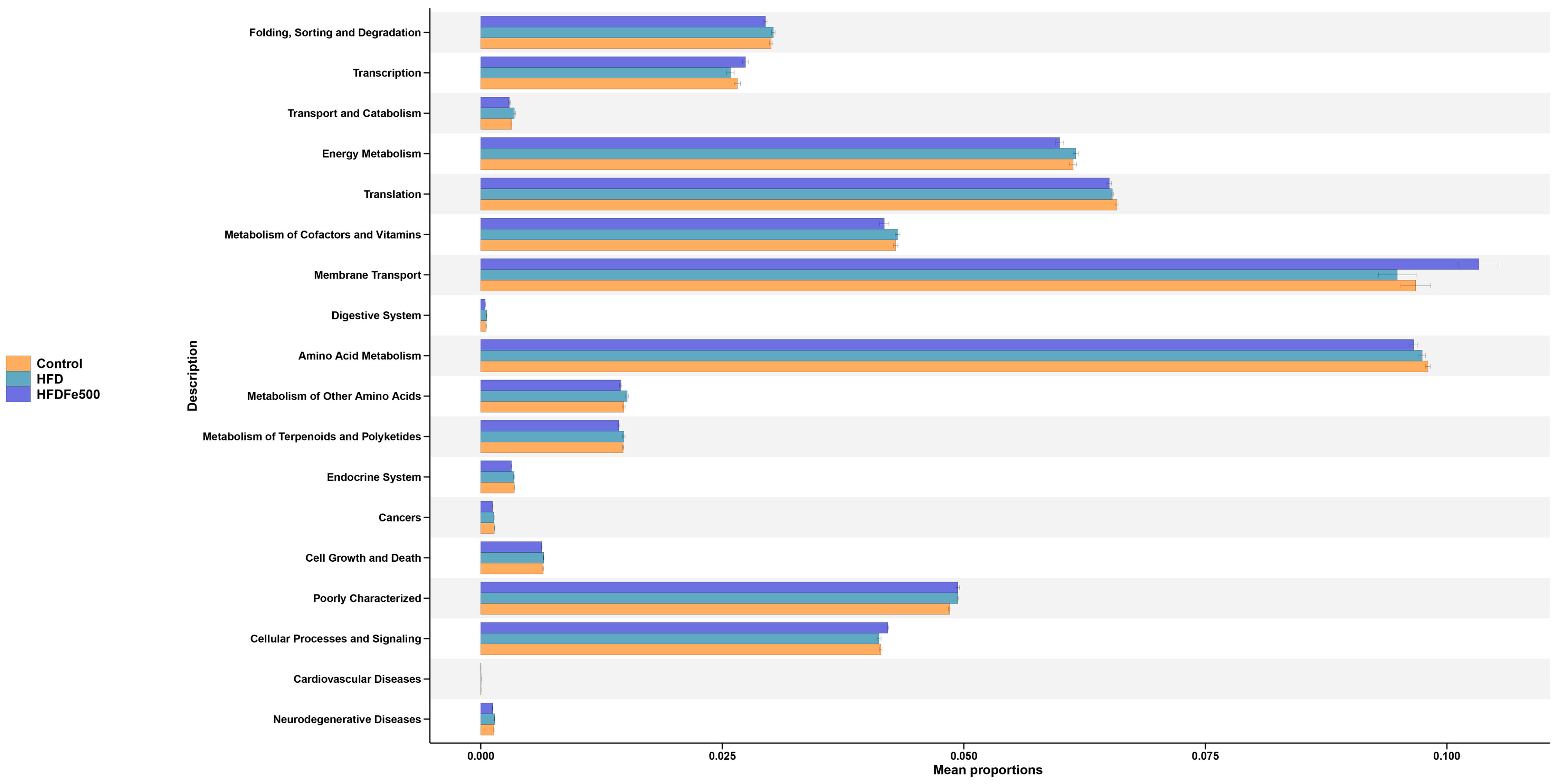
3.6. Functional Prediction of the Differential Gut Microbiota
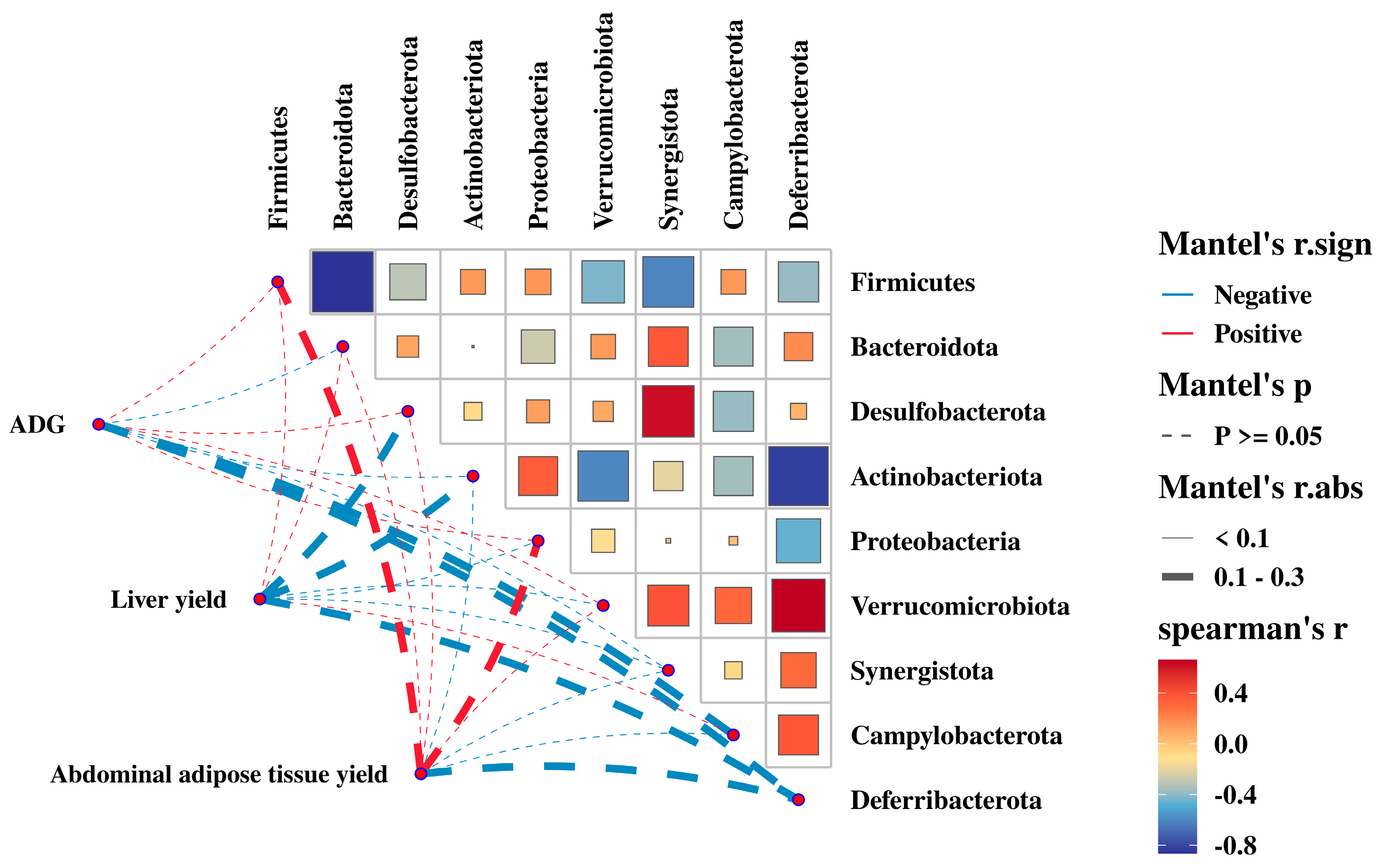
3.7. Relationship between the Gut Microbiota with ADG, Liver Yield, and Abdominal Adipose Tissue Yield
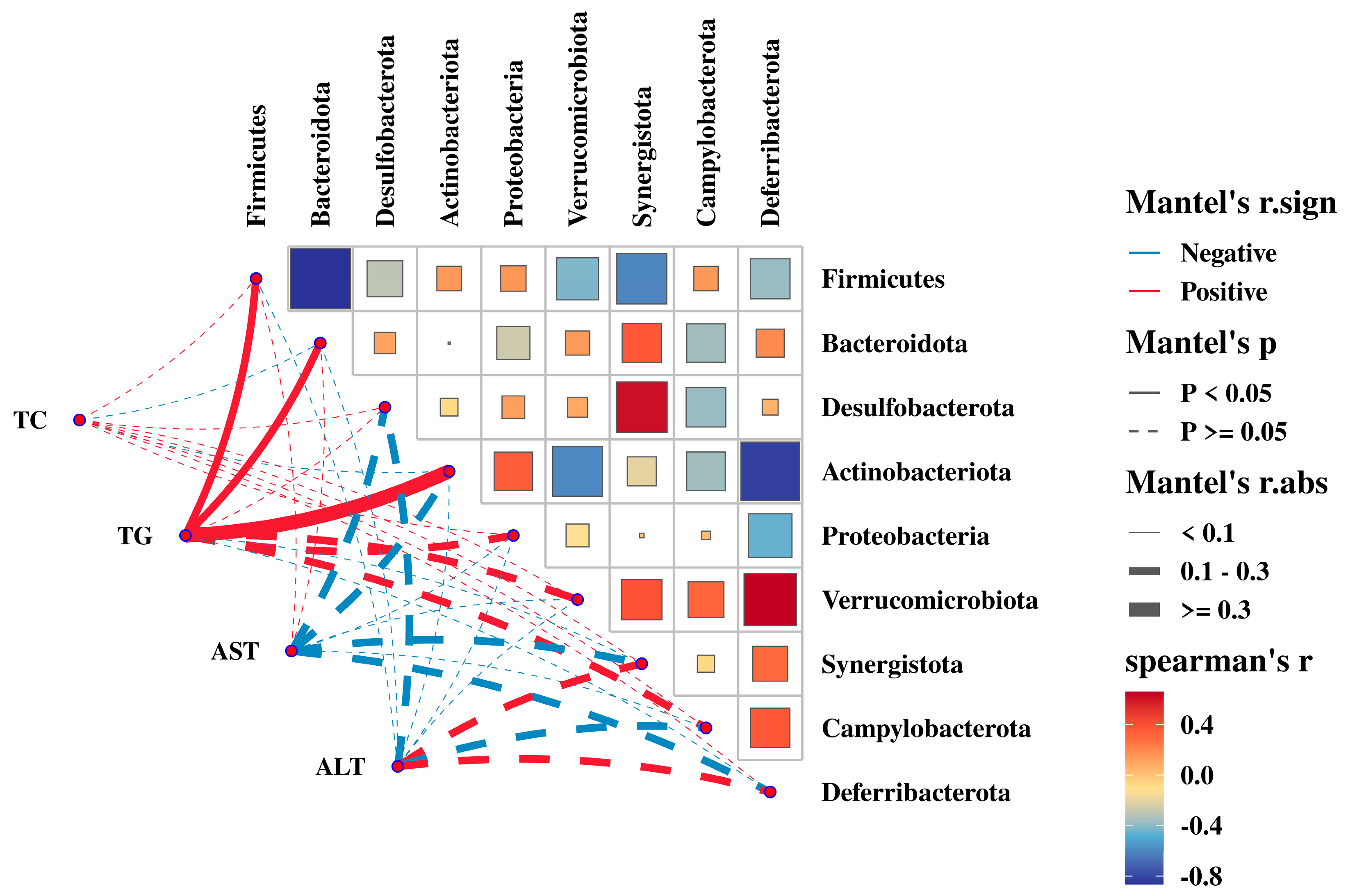
3.8. Relationship between the Gut Microbiota with Serum Biochemical Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gouda, A.; Tolba, S.; Mahrose, K.; Felemban, S.G.; Khafaga, A.F.; Khalifa, N.E.; Jaremko, M.; Moustafa, M.; Alshaharni, M.O.; Algopish, U.; et al. Heat shock proteins as a key defense mechanism in poultry production under heat stress conditions. Poult. Sci. 2024, 103, 103537. [Google Scholar] [CrossRef] [PubMed]
- Korver, D.R. Review: Current challenges in poultry nutrition, health, and welfare. Animal 2023, 17, 100755. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.Q.; Wang, L.M.; Sivendiran, T.; Bohrer, B.M. Review: Amino acid concentration of high protein food products and an overview of the current methods used to determine protein quality. Crit. Rev. Food Sci. 2018, 58, 2673–2678. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Z.; Hu, X.X.; Wang, Z.P.; Li, X.C.; Wang, Q.G.; Wang, Y.X.; Tang, Z.Q.; Li, H. Quantitative trait loci associated with body weight and abdominal fat traits on chicken chromosomes 3, 5 and 7. Genet. Mol. Res. 2012, 11, 956–965. [Google Scholar] [CrossRef] [PubMed]
- Nematbakhsh, S.; Pei, C.P.; Selamat, J.; Nordin, N.; Idris, L.H.; Razis, A.F.A. Molecular Regulation of Lipogenesis, Adipogenesis and Fat Deposition in Chicken. Genes 2021, 12, 414. [Google Scholar] [CrossRef] [PubMed]
- Posey, J.E.; Gherardini, F.C. Lack of a role for iron in the Lyme disease pathogen. Science 2000, 288, 1651–1653. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Kim, H.K.; Chung, J.H.; Lim, B.O.; Yamada, K.; Lim, Y.; Kang, S.A. Increased expression of hypothalamic NADPH-diaphorase neurons in mice with iron supplement. Biosci. Biotechnol. Biochem. 2005, 69, 1978–1981. [Google Scholar] [CrossRef]
- Stangl, G.I.; Kirchgessner, M. Different degrees of moderate iron deficiency modulate lipid metabolism of rats. Lipids 1998, 33, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; Koh, I.U.; Lee, H.J.; Kim, W.H.; Song, J. Effects of excess dietary iron and fat on glucose and lipid metabolism. J. Nutr. Biochem. 2013, 24, 1634–1644. [Google Scholar] [CrossRef]
- Bai, S.P.; Luo, W.Q.; Liu, H.; Zhang, K.Y.; Wang, J.P.; Ding, X.M.; Zeng, Q.F.; Peng, H.W.; Bai, J.; Xuan, Y.; et al. Effects of high dietary iron on the lipid metabolism in the liver and adipose tissue of male broiler chickens. Anim. Feed Sci. Technol. 2021, 282, 115131. [Google Scholar] [CrossRef]
- De Vadder, F.; Kovatcheva-Datchary, P.; Goncalves, D.; Vinera, J.; Zitoun, C.; Duchampt, A.; Bäckhed, F.; Mithieux, G. Microbiota-Generated Metabolites Promote Metabolic Benefits via Gut-Brain Neural Circuits. Cell 2014, 156, 84–96. [Google Scholar] [CrossRef] [PubMed]
- Li, H.F.; Zhao, L.Q.; Liu, S.; Zhang, Z.H.; Wang, X.J.; Lin, H. Propionate inhibits fat deposition via affecting feed intake and modulating gut microbiota in broilers. Poult. Sci. 2021, 100, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Torres-Fuentes, C.; Schellekens, H.; Dinan, T.G.; Cryan, J.F. The microbiota-gut-brain axis in obesity. Lancet Gastroenterol. 2017, 2, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Bäckhed, F.; Turnbaugh, P.; Lozupone, C.A.; Knight, R.D.; Gordon, J.I. Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. USA 2005, 102, 11070–11075. [Google Scholar] [CrossRef] [PubMed]
- Ridaura, V.K.; Faith, J.J.; Rey, F.E.; Cheng, J.Y.; Duncan, A.E.; Kau, A.L.; Griffin, N.W.; Lombard, V.; Henrissat, B.; Bain, J.R.; et al. Gut Microbiota from Twins Discordant for Obesity Modulate Metabolism in Mice. Science 2013, 341, 1241214. [Google Scholar] [CrossRef] [PubMed]
- Kalavathy, R.; Abdullah, N.; Jalaludin, S.; Ho, Y.W. Effects of cultures on growth performance, abdominal fat deposition, serum lipids and weight of organs of broiler chickens. Br. Poult. Sci. 2003, 44, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.S.; Ni, X.Q.; Qing, X.D.; Zeng, D.; Luo, M.; Liu, L.; Li, G.Y.; Pan, K.C.; Jing, B. Live probiotic Lactobacillus johnsonii BS15 promotes growth performance and lowers fat deposition by improving lipid metabolism, intestinal development, and gut microflora in broilers. Front. Microbiol. 2017, 8, 1073. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.L.; Yan, W.; Sun, C.J.; Ji, C.L.; Zhou, Q.Q.; Zhang, D.X.; Zheng, J.X.; Yang, N. The gut microbiota is largely independent of host genetics in regulating fat deposition in chickens. ISME J. 2019, 13, 1422–1436. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.L.; Mao, P.C.; Tian, X.X.; Guo, Q.; Meng, L. Effects of dietary supplementation of alfalfa meal on growth performance, carcass characteristics, meat and egg quality, and intestinal microbiota in Beijing-you chicken. Poult. Sci. 2019, 98, 2250–2259. [Google Scholar] [CrossRef]
- Ussar, S.; Griffin, N.W.; Bezy, O.; Fujisaka, S.; Vienberg, S.; Softic, S.; Deng, L.X.; Bry, L.; Gordon, J.I.; Kahn, C.R. Interactions between Gut Microbiota, Host Genetics and Diet Modulate the Predisposition to Obesity and Metabolic Syndrome. Cell Metab. 2015, 22, 516–530. [Google Scholar] [CrossRef]
- Kelly, J.R.; Borre, Y.; Brien, C.O.; Patterson, E.; El Aidy, S.; Deane, J.; Kennedy, P.J.; Beers, S.; Scott, K.; Moloney, G.; et al. Transferring the blues: Depression-associated gut microbiota induces neurobehavioural changes in the rat. J. Psychiatr. Res. 2016, 82, 109–118. [Google Scholar] [CrossRef]
- Wang, X.; Teng, D.; Tian, F.; Guan, Q.; Wang, J. Comparison of Three DNA Extraction Methods for Feed Products and Four Amplification Methods for the 5′-Junction Fragment of Roundup Ready Soybean. J. Agric. Food Chem. 2012, 60, 4586–4595. [Google Scholar] [CrossRef] [PubMed]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Ankra-Badu, G.A.; Shriner, D.; Le Bihan-Duval, E.; Mignon-Grasteau, S.; Pitel, F.; Beaumont, C.; Duclos, M.J.; Simon, J.; Porter, T.E.; Vignal, A.; et al. Mapping main, epistatic and sex-specific QTL for body composition in a chicken population divergently selected for low or high growth rate. BMC Genom. 2010, 11, 107. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Chi, X.; Wang, J.; Shen, Z.; Li, S.; Xu, S. High fat induces activation of the tryptophan-ERK-CREB pathway and promotes bone absorption in cage layers. Poult. Sci. 2021, 100, 101149. [Google Scholar] [CrossRef] [PubMed]
- Stevanović, Z.D.; Bošnjak-Neumüller, J.; Pajić-Lijaković, I.; Raj, J.; Vasiljević, M. Essential Oils as Feed Additives—Future Perspectives. Molecules 2018, 23, 1717. [Google Scholar] [CrossRef]
- Bozkurt, M.; Küçükyilmaz, K.; Çabuk, M.; Çath, A.U. The Long Term Effects of Fairly Low-level of Supplemental Fat on the Productive Performance of Commercial Layers. Asian Australas. J. Anim. 2012, 25, 524–530. [Google Scholar] [CrossRef]
- Geissler, C.; Singh, M. Iron, Meat and Health. Nutrients 2011, 3, 283–316. [Google Scholar] [CrossRef]
- Dongiovanni, P.; Ruscica, M.; Rametta, R.; Recalcati, S.; Steffani, L.; Gatti, S.; Girelli, D.; Cairo, G.; Magni, P.; Fargion, S.; et al. Dietary Iron Overload Induces Visceral Adipose Tissue Insulin Resistance. Am. J. Pathol. 2013, 182, 2254–2263. [Google Scholar] [CrossRef]
- Kim, S.L.; Shin, S.; Yang, S.J. Iron Homeostasis and Energy Metabolism in Obesity. Clin. Nutr. Res. 2022, 11, 316–330. [Google Scholar] [CrossRef]
- Hamid, H.; Zhang, J.Y.; Li, W.X.; Liu, C.; Li, M.L.; Zhao, L.H.; Ji, C.; Ma, Q.G. Interactions between the cecal microbiota and non-alcoholic steatohepatitis using laying hens as the model. Poult. Sci. 2019, 98, 2509–2521. [Google Scholar] [CrossRef] [PubMed]
- Tremaroli, V.; Bäckhed, F. Functional interactions between the gut microbiota and host metabolism. Nature 2012, 489, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Sobhonslidsuk, A.; Chanprasertyothin, S.; Pongrujikorn, T.; Kaewduang, P.; Promson, K.; Petraksa, S.; Ongphiphadhanakul, B. The Association of Gut Microbiota with Nonalcoholic Steatohepatitis in Thais. BioMed Res. Int. 2018, 2018, 9340316. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Z.; Thomsen, M.; Vitetta, L. Interaction of gut microbiota with dysregulation of bile acids in the pathogenesis of nonalcoholic fatty liver disease and potential therapeutic implications of probiotics. J. Cell. Biochem. 2019, 120, 2713–2720. [Google Scholar] [CrossRef] [PubMed]
- van der Stel, A.X.; Wösten, M.M.S.M. Regulation of Respiratory Pathways in Campylobacterota: A Review. Front. Microbiol. 2019, 10, 1719. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, A.; Andree, K.B.; Furones, D.; Holhorea, P.G.; Calduch-Giner, J.A.; Viñas, M.; Pérez-Sánchez, J.; Gisbert, E. Modulation of gut microbiota and intestinal immune response in gilthead seabream (Sparus aurata) by dietary bile salt supplementation. Front. Microbiol. 2023, 14, 1123716. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.B.; Zhang, X.X.; Xu, D.; Zhang, D.Y.; Zhang, Y.K.; Song, Q.Z.; Li, X.L.; Zhao, Y.; Zhao, L.M.; Li, W.X.; et al. Relationship between rumen microbial differences and traits among Hu sheep, Tan sheep, and Dorper sheep. J. Anim. Sci. 2022, 100, skac261. [Google Scholar] [CrossRef] [PubMed]
- Daniel, H.; Gholami, A.M.; Berry, D.; Desmarchelier, C.; Hahne, H.; Loh, G.; Mondot, S.; Lepage, P.; Rothballer, M.; Walker, A.; et al. High-fat diet alters gut microbiota physiology in mice. ISME J. 2014, 8, 295–308. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.T.; Kong, Q.M.; Li, X.; Zhao, J.X.; Zhang, H.; Chen, W.; Wang, G. A High-Fat Diet Increases Gut Microbiota Biodiversity and Energy Expenditure Due to Nutrient Difference. Nutrients 2020, 12, 3197. [Google Scholar] [CrossRef]
- Li, J.; Pan, X.; Pan, G.; Song, Z.; He, Y.; Zhang, S.; Ye, X.; Yang, X.; Xie, E.; Wang, X.; et al. Transferrin Receptor 1 Regulates Thermogenic Capacity and Cell Fate in Brown/Beige Adipocytes. Adv. Sci. 2020, 7, 1903366. [Google Scholar] [CrossRef]
- Morigny, P.; Boucher, J.; Arner, P.; Langin, D. Lipid and glucose metabolism in white adipocytes: Pathways, dysfunction and therapeutics. Nat. Rev. Endocrinol. 2021, 17, 276–295. [Google Scholar] [CrossRef] [PubMed]
- Fernández, L.; Pannaraj, P.S.; Rautava, S.; Rodríguez, J.M. The Microbiota of the Human Mammary Ecosystem. Front. Cell Infect. Microbiol. 2020, 10, 586667. [Google Scholar] [CrossRef] [PubMed]


| Items | Content |
|---|---|
| Ingredient (%) | |
| Corn | 62.49 |
| Flour | 2.00 |
| Soybean meal | 25.20 |
| Corn protein flour | 2.00 |
| Soybean oil | 1.96 |
| Stone powder | 1.24 |
| Calcium hydrogen phosphate | 1.11 |
| Choline chloride | 1.00 |
| Premix | 3.00 |
| Nutritional level (%) | |
| Crude protein | 18.50 |
| Metabolizable energy (MJ/kg) | 12.55 |
| Ca | 0.80 |
| Available phosphorus | 0.33 |
| Digestible lysine | 0.90 |
| Iron (mg/kg) | 321.00 |
| Items | Control | HFD | HFDFe500 | p Value |
|---|---|---|---|---|
| ADG (g) | 27.52 ± 5.66 b | 35.30 ± 4.96 a | 26.63 ± 6.15 b | 0.003 |
| Liver yield (g/1000 g BW at slaughter) | 19.99 ± 3.17 b | 25.88 ± 5.45 a | 18.74 ± 1.77 b | 0.001 |
| Abdominal adipose tissue yield (g/1000 g BW at slaughter) | 23.99 ± 14.97 b | 41.07 ± 6.24 a | 29.75 ± 10.82 b | 0.008 |
| Items | Control | HFD | HFDFe500 | p Value |
|---|---|---|---|---|
| TC (mmol/L) | 3.78 ± 0.31 c | 14.17 ± 1.54 a | 9.26 ± 1.92 b | 0.001 |
| TG (mmol/L) | 1.75 ± 0.18 c | 3.87 ± 0.26 a | 2.72 ± 0.24 b | <0.001 |
| AST (U/L) | 230.59 ± 9.16 b | 305.42 ± 15.33 a | 250.32 ± 5.22 ab | 0.040 |
| ALT (U/L) | 2.51 ± 0.27 b | 7.66 ± 1.82 a | 3.39 ± 0.74 b | 0.014 |
| Phyla | Control | HFD | HFDFe500 | p Value |
|---|---|---|---|---|
| Firmicutes | 43.60 ± 2.28 b | 38.41 ± 2.54 b | 50.41 ± 1.41 a | 0.004 |
| Bacteroidota | 35.80 ± 2.47 ab | 40.85 ± 2.23 a | 31.88 ± 1.82 b | 0.035 |
| unclassified | 7.93 ± 0.30 a | 6.72 ± 1.27 ab | 3.83 ± 1.10 b | 0.028 |
| Desulfobacterota | 3.57 ± 0.63 | 5.27 ± 0.50 | 3.84 ± 0.58 | 0.112 |
| Actinobacteriota | 2.27 ± 0.53 b | 3.39 ± 0.24 b | 5.69 ± 1.00 a | 0.008 |
| Proteobacteria | 2.28 ± 0.17 | 2.73 ± 0.18 | 2.80 ± 0.26 | 0.188 |
| Verrucomicrobiota | 1.60 ± 0.28 a | 1.23 ± 0.21 a | 0.44 ± 0.03 b | 0.003 |
| Synergistota | 0.79 ± 0.30 | 0.82 ± 0.10 | 0.49 ± 0.12 | 0.434 |
| Campylobacterota | 1.12 ± 0.25 a | 0.28 ± 0.05 b | 0.50 ± 0.18 b | 0.013 |
| Deferribacterota | 1.04 ± 0.33 a | 0.29 ± 0.08 b | 0.11 ± 0.04 b | 0.011 |
| Genera | Control | HFD | HFDFe500 | p Value |
|---|---|---|---|---|
| Bacteroides | 23.89 ± 5.09 | 16.18 ± 2.40 | 26.22 ± 6.55 | 0.354 |
| Rikenellaceae_RC9_gut_group | 18.93 ± 0.84 a | 19.53 ± 1.39 a | 12.69 ± 1.59 b | 0.004 |
| Prevotellaceae_UCG-001 | 18.05 ± 4.09 | 21.30 ± 2.27 | 14.30 ± 4.91 | 0.467 |
| Faecalibacterium | 13.70 ± 1.69 | 15.03 ± 1.65 | 21.72 ± 3.85 | 0.098 |
| Desulfovibrio | 10.84 ± 1.97 | 13.03 ± 1.05 | 9.66 ± 1.40 | 0.311 |
| Olsenella | 1.79 ± 0.24 | 3.16 ± 0.41 | 4.04 ± 1.88 | 0.383 |
| Ligilactobacillus | 3.38 ± 0.43 | 3.19 ± 0.13 | 3.05 ± 0.35 | 0.779 |
| Oscillibacter | 3.52 ± 0.27 | 3.43 ± 0.27 | 2.49 ± 0.20 | 0.058 |
| Escherichia-Shigella | 1.81 ± 0.19 | 2.79 ± 0.49 | 2.92 ± 0.28 | 0.075 |
| Merdimonas | 4.08 ± 0.47 | 2.36 ± 0.36 | 2.92 ± 0.96 | 0.196 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, T.; Chen, S.; Qiu, L.; Guo, Q.; Wang, Z.; Jiang, Y.; Bai, H.; Bi, Y.; Chang, G. Effect of High Dietary Iron on Fat Deposition and Gut Microbiota in Chickens. Animals 2024, 14, 2254. https://doi.org/10.3390/ani14152254
Yang T, Chen S, Qiu L, Guo Q, Wang Z, Jiang Y, Bai H, Bi Y, Chang G. Effect of High Dietary Iron on Fat Deposition and Gut Microbiota in Chickens. Animals. 2024; 14(15):2254. https://doi.org/10.3390/ani14152254
Chicago/Turabian StyleYang, Ting, Shihao Chen, Lingling Qiu, Qixin Guo, Zhixiu Wang, Yong Jiang, Hao Bai, Yulin Bi, and Guobin Chang. 2024. "Effect of High Dietary Iron on Fat Deposition and Gut Microbiota in Chickens" Animals 14, no. 15: 2254. https://doi.org/10.3390/ani14152254
APA StyleYang, T., Chen, S., Qiu, L., Guo, Q., Wang, Z., Jiang, Y., Bai, H., Bi, Y., & Chang, G. (2024). Effect of High Dietary Iron on Fat Deposition and Gut Microbiota in Chickens. Animals, 14(15), 2254. https://doi.org/10.3390/ani14152254





