Ningxiang Pig-Derived Parabacteroides distasonis HNAU0205 Alleviates ETEC-Induced Intestinal Apoptosis, Oxidative Damage, and Inflammation in Piglets
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
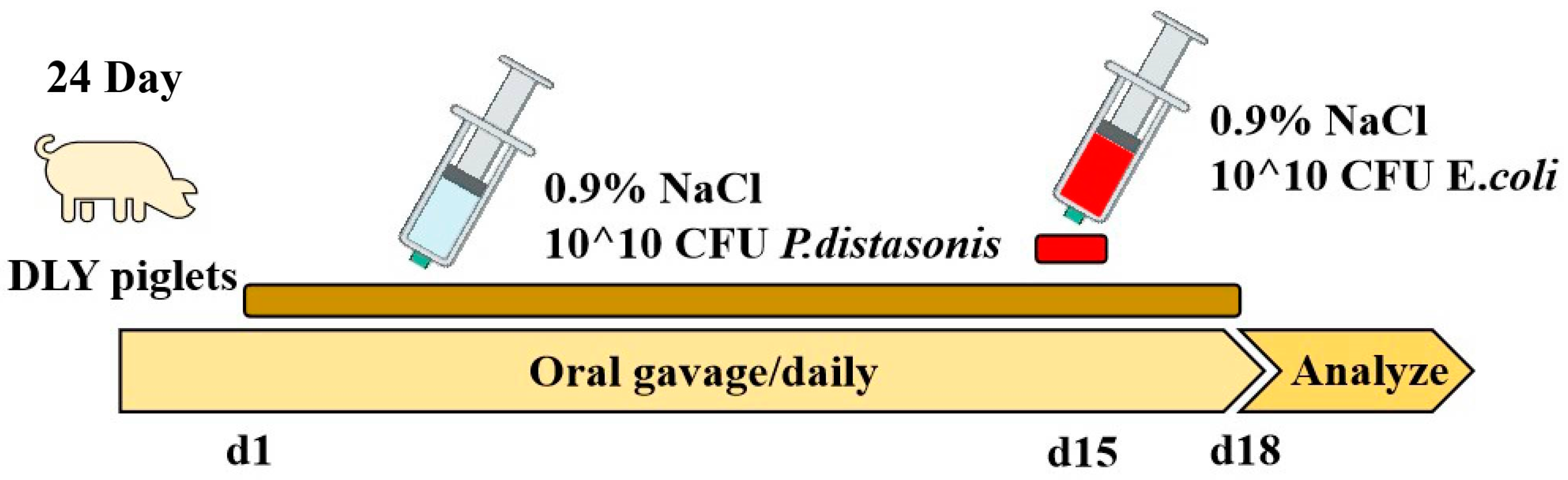
2.1. Piglet Experimental Design
2.2. Growth Performance and Relative Organ Weight
2.3. Histopathology
2.4. Real-Time Reverse Transcription PCR
2.5. Serum Inflammatory Cytokine
2.6. SCFAs
2.7. Statistical Analysis
3. Results
3.1. Growth Performance and Relative Organ Weight
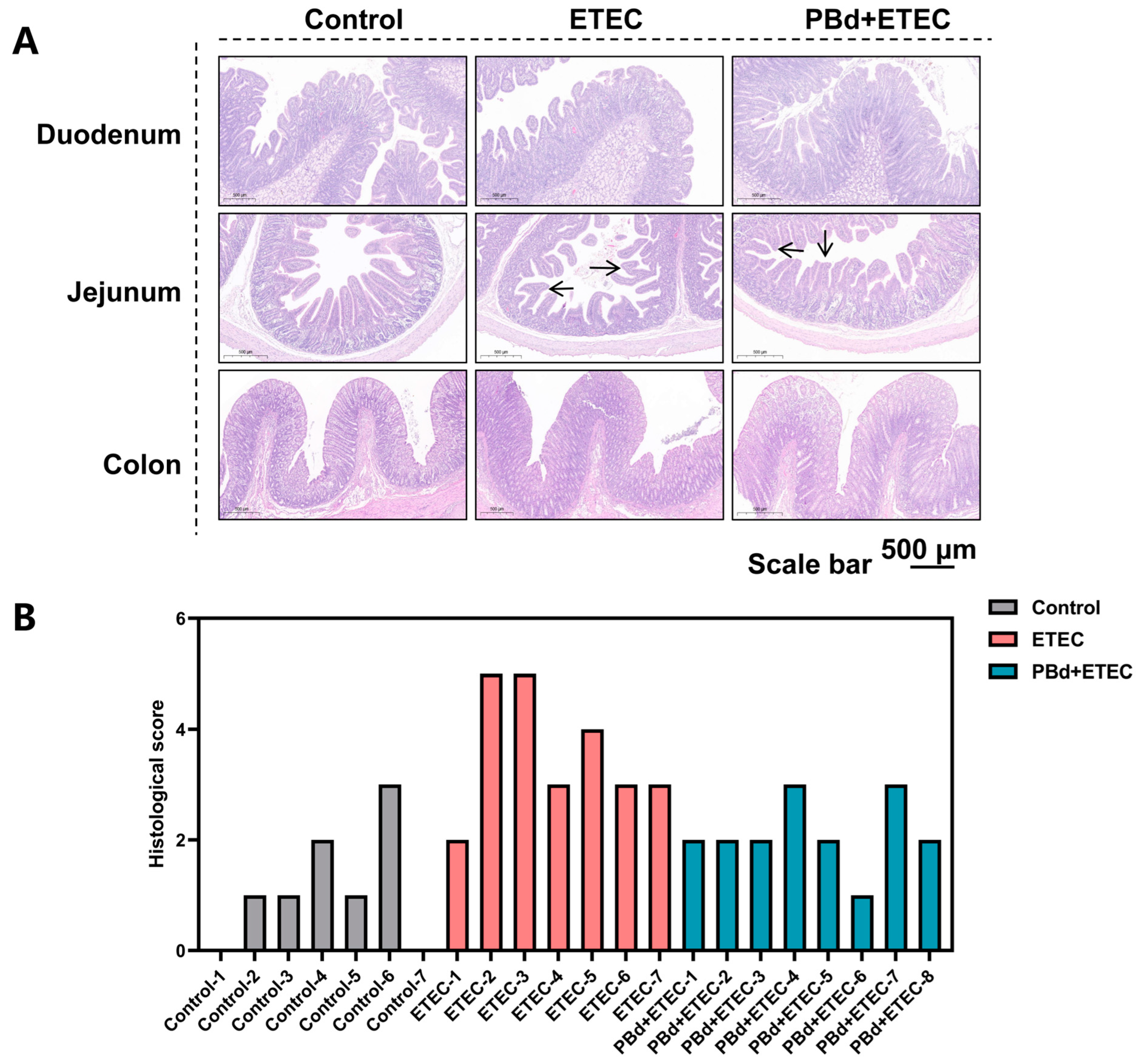
3.2. Intestinal Histomorphology
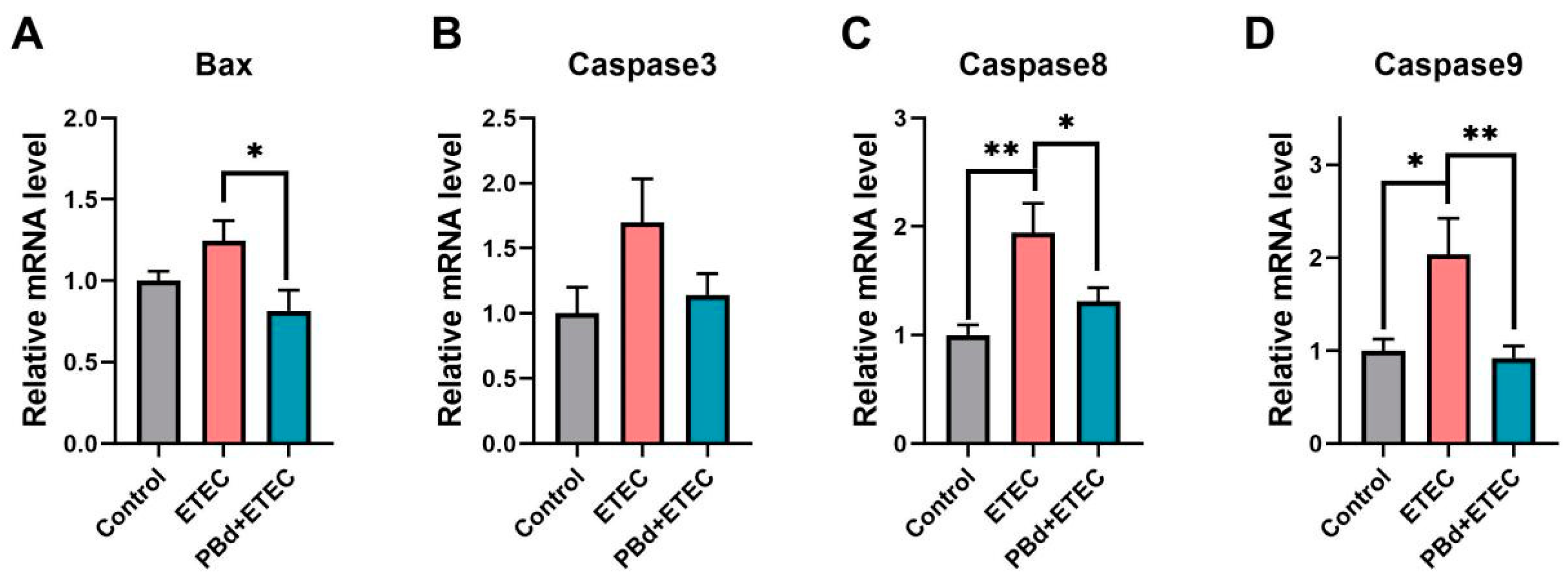
3.3. Expression of Apoptosis-Related Genes
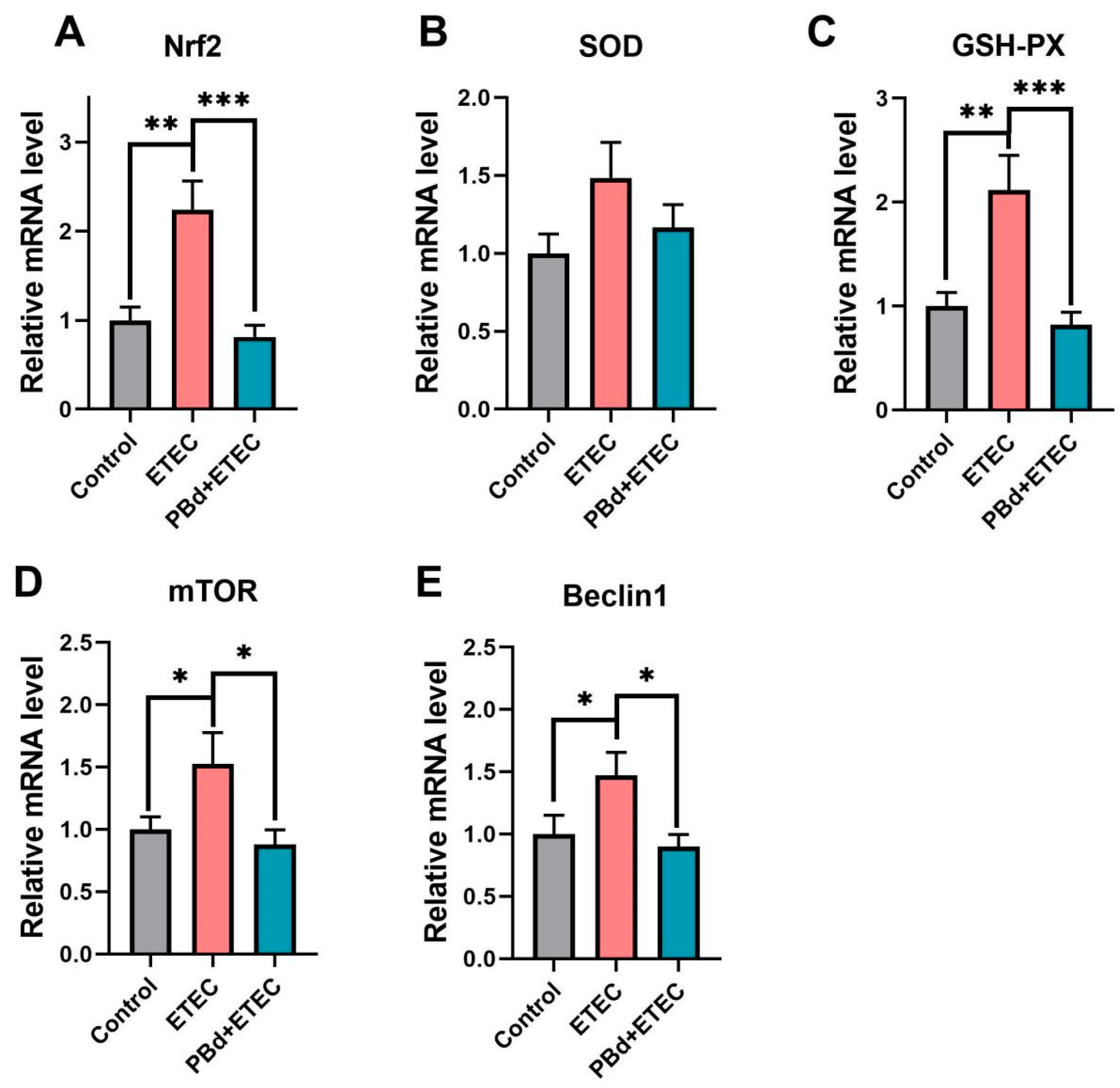
3.4. Expression of Antioxidant-Related Genes
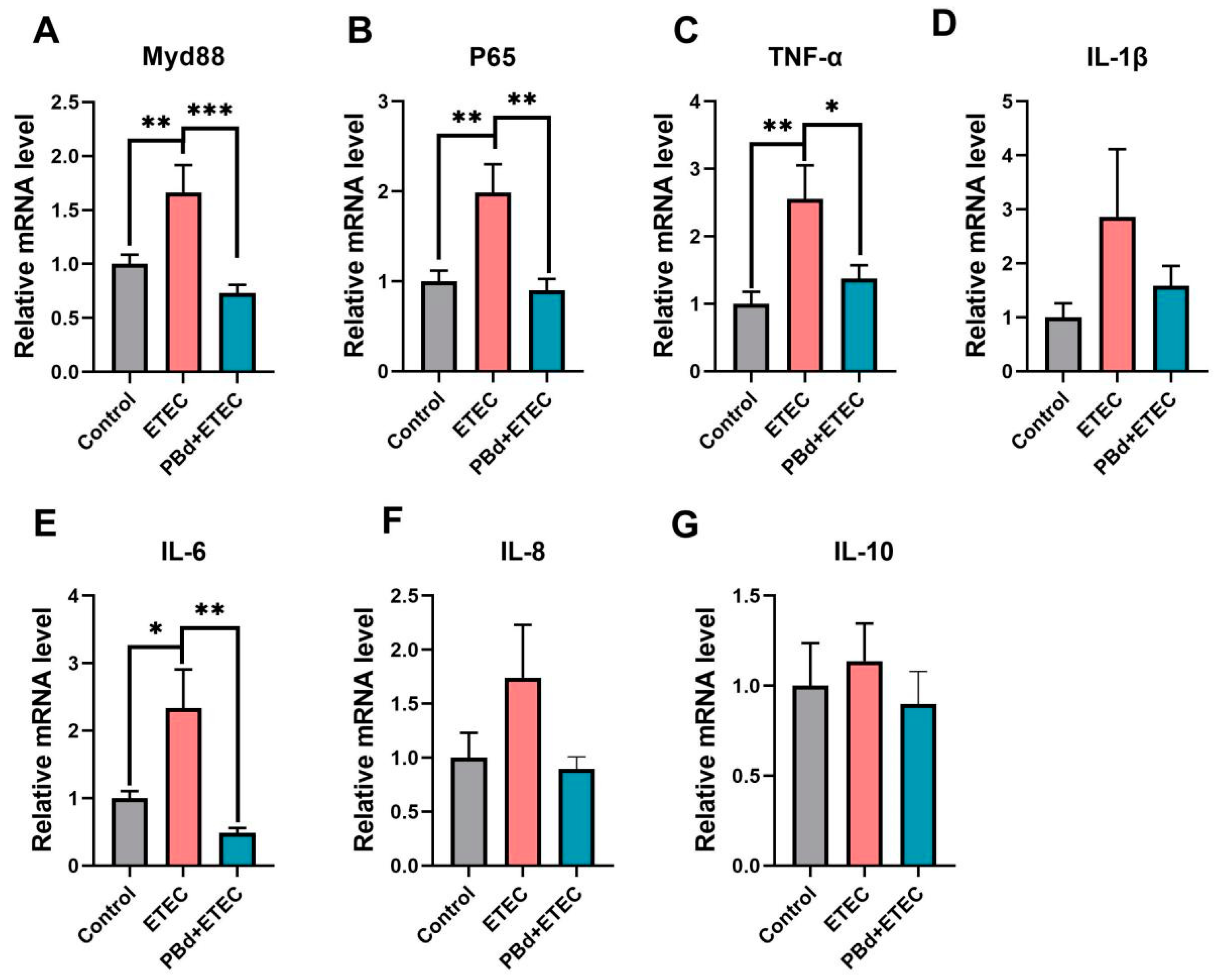
3.5. Inflammatory Biomarkers
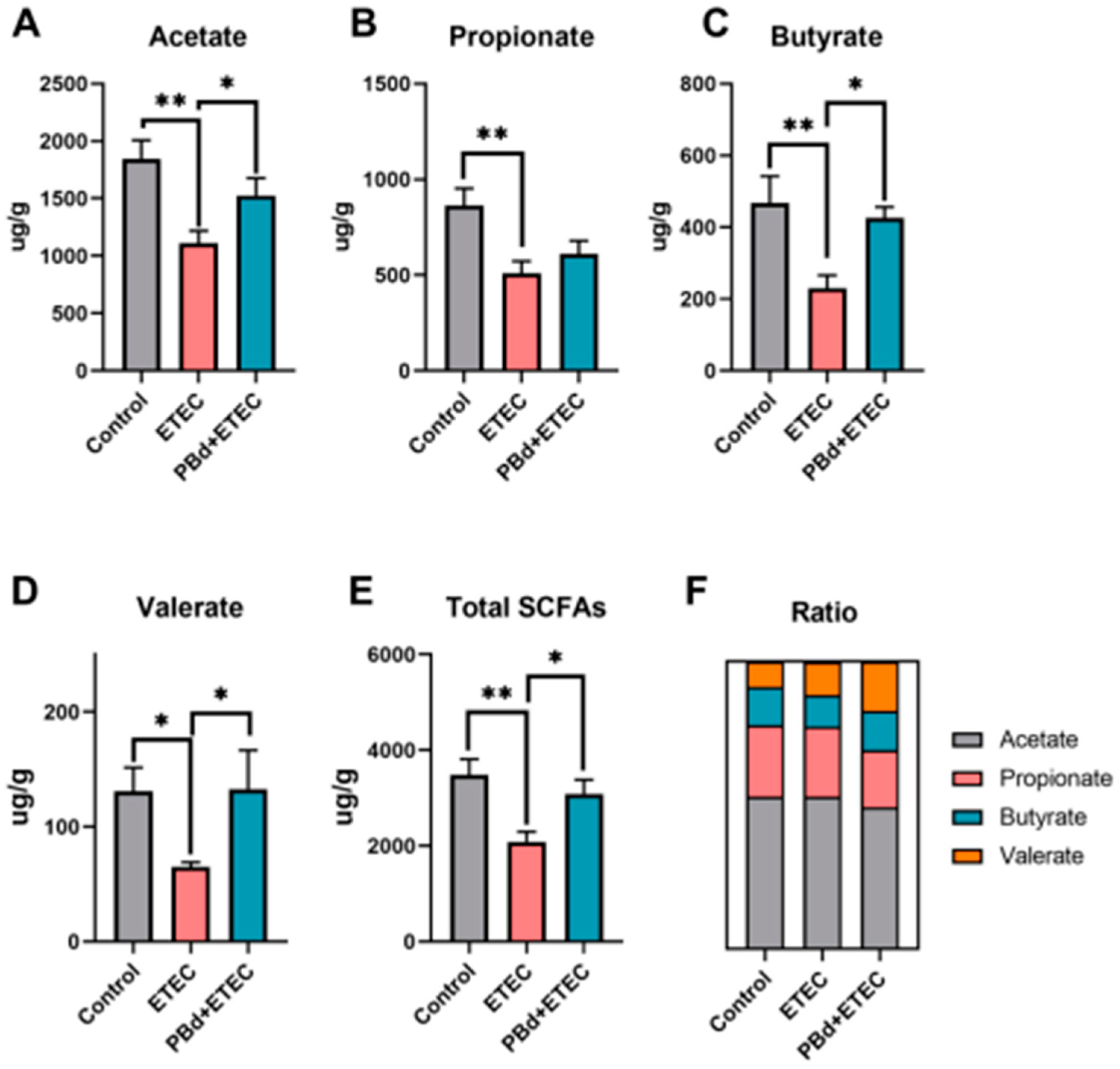
3.6. SCFAs
4. Discussion
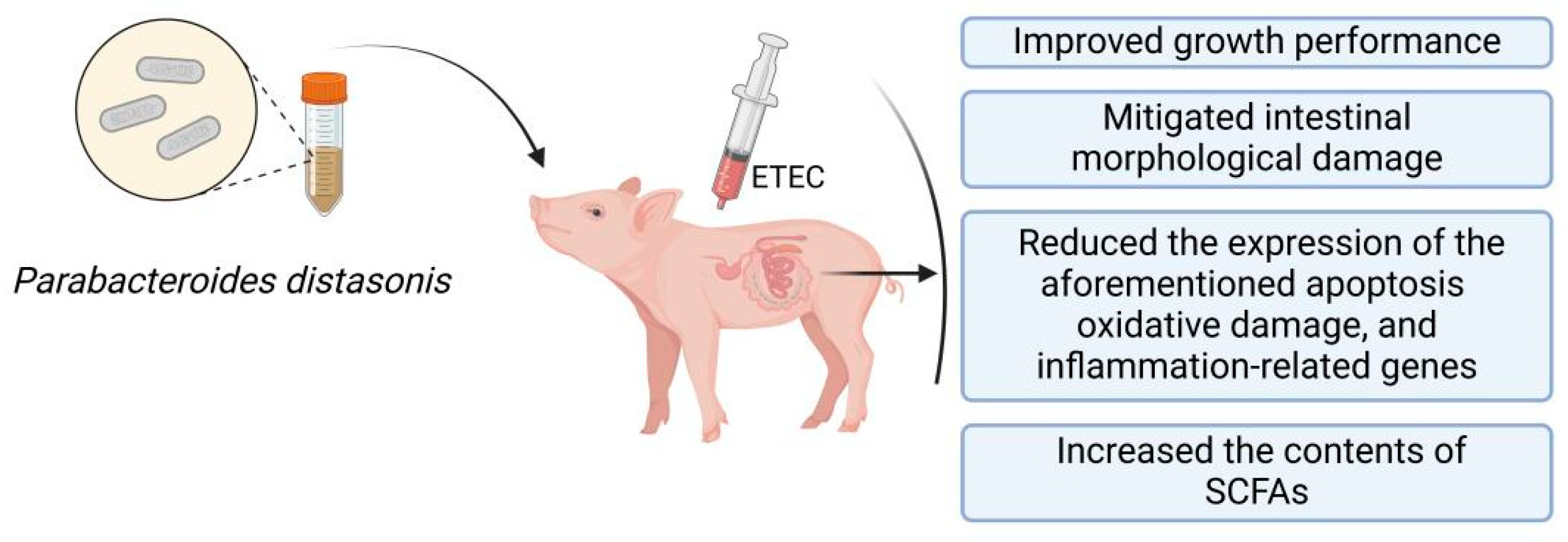
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tang, X.; Xiong, K.; Fang, R.; Li, M. Weaning Stress and Intestinal Health of Piglets: A Review. Front. Immunol. 2022, 13, 1042778. [Google Scholar] [CrossRef]
- Yin, J.; Wu, M.M.; Xiao, H.; Ren, W.K.; Duan, J.L.; Yang, G.; Li, T.J.; Yin, Y.L. Development of an Antioxidant System after Early Weaning in Piglets. J. Anim. Sci. 2014, 92, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Song, M.; Liu, Y.; Ji, P. Enterotoxigenic Escherichia coli Infection of Weaned Pigs: Intestinal Challenges and Nutritional Intervention to Enhance Disease Resistance. Front. Immunol. 2022, 13, 885253. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, M.J.; Berrios, R.; Stelzhammer, S.; Bracarense, A.P.F.R.L. Ingestion of Organic Acids and Cinnamaldehyde Improves Tissue Homeostasis of Piglets Exposed to Enterotoxic Escherichia coli (ETEC). J. Anim. Sci. 2020, 98, skaa012. [Google Scholar] [CrossRef]
- Pang, J.; Liu, Y.; Kang, L.; Ye, H.; Zang, J.; Wang, J.; Han, D. Bifidobacterium Animalis Promotes the Growth of Weaning Piglets by Improving Intestinal Development, Enhancing Antioxidant Capacity, and Modulating Gut Microbiota. Appl. Environ. Microbiol. 2022, 88, e01296-22. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Q.; Wu, X.; Pan, Y.; Wang, L.; Cui, C.; Guo, Y.; Zhu, L.; Peng, J.; Wei, H. Early-Life Intervention Using Fecal Microbiota Combined with Probiotics Promotes Gut Microbiota Maturation, Regulates Immune System Development, and Alleviates Weaning Stress in Piglets. Int. J. Mol. Sci. 2020, 21, 503. [Google Scholar] [CrossRef]
- Li, W.; Xu, B.; Wang, L.; Sun, Q.; Deng, W.; Wei, F.; Ma, H.; Fu, C.; Wang, G.; Li, S. Effects of Clostridium butyricum on Growth Performance, Gut Microbiota and Intestinal Barrier Function of Broilers. Front. Microbiol. 2021, 12, 777456. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.K.K. Parabacteroides distasonis: An Emerging Probiotic? Gut 2023, 72, 1635–1636. [Google Scholar] [CrossRef]
- Sun, H.; Guo, Y.; Wang, H.; Yin, A.; Hu, J.; Yuan, T.; Zhou, S.; Xu, W.; Wei, P.; Yin, S.; et al. Gut Commensal Parabacteroides distasonis Alleviates Inflammatory Arthritis. Gut 2023, 72, 1664–1677. [Google Scholar] [CrossRef]
- Wang, K.; Liao, M.; Zhou, N.; Bao, L.; Ma, K.; Zheng, Z.; Wang, Y.; Liu, C.; Wang, W.; Wang, J.; et al. Parabacteroides distasonis Alleviates Obesity and Metabolic Dysfunctions via Production of Succinate and Secondary Bile Acids. Cell Rep. 2019, 26, 222–235.e5. [Google Scholar] [CrossRef]
- Kuang, J.; Wang, J.; Li, Y.; Li, M.; Zhao, M.; Ge, K.; Zheng, D.; Cheung, K.C.P.; Liao, B.; Wang, S.; et al. Hyodeoxycholic Acid Alleviates Non-Alcoholic Fatty Liver Disease through Modulating the Gut-Liver Axis. Cell Metab. 2023, 35, 1752–1766.e8. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Wong, C.C.; Jia, Z.; Liu, W.; Liu, C.; Ji, F.; Pan, Y.; Wang, F.; Wang, G.; Zhao, L.; et al. Parabacteroides distasonis Uses Dietary Inulin to Suppress NASH via Its Metabolite Pentadecanoic Acid. Nat. Microbiol. 2023, 8, 1534–1548. [Google Scholar] [CrossRef] [PubMed]
- Ezeji, J.C.; Sarikonda, D.K.; Hopperton, A.; Erkkila, H.L.; Cohen, D.E.; Martinez, S.P.; Cominelli, F.; Kuwahara, T.; Dichosa, A.E.K.; Good, C.E.; et al. Parabacteroides distasonis: Intriguing Aerotolerant Gut Anaerobe with Emerging Antimicrobial Resistance and Pathogenic and Probiotic Roles in Human Health. Gut Microbes 2021, 13, 1922241. [Google Scholar] [CrossRef]
- Ma, M.; Fu, T.; Wang, Y.; Zhang, A.; Gao, P.; Shang, Q.; Yu, G. Polysaccharide from Edible Alga Enteromorpha clathrata Improves Ulcerative Colitis in Association with Increased Abundance of Parabacteroides spp. in the Gut Microbiota of Dextran Sulfate Sodium-Fed Mice. Mar. Drugs 2022, 20, 764. [Google Scholar] [CrossRef] [PubMed]
- Gaifem, J.; Mendes-Frias, A.; Wolter, M.; Steimle, A.; Garzón, M.J.; Ubeda, C.; Nobre, C.; González, A.; Pinho, S.S.; Cunha, C.; et al. Akkermansia muciniphila and Parabacteroides distasonis Synergistically Protect from Colitis by Promoting ILC3 in the Gut. mBio 2024, 15, e00078-24. [Google Scholar] [CrossRef]
- Cuffaro, B.; Assohoun, A.L.W.; Boutillier, D.; Súkeníková, L.; Desramaut, J.; Boudebbouze, S.; Salomé-Desnoulez, S.; Hrdý, J.; Waligora-Dupriet, A.J.; Maguin, E.; et al. In Vitro Characterization of Gut Microbiota-Derived Commensal Strains: Selection of Parabacteroides distasonis Strains Alleviating TNBS-Induced Colitis in Mice. Cells 2020, 9, 2104. [Google Scholar] [CrossRef]
- Zhang, L.; Yao, X.; Ma, M.; Ding, Y.; Zhang, H.; He, X.; Song, Z. Protective Effect of l -Theanine against DSS-Induced Colitis by Regulating the Lipid Metabolism and Reducing Inflammation via the NF-ΚB Signaling Pathway. J. Agric. Food Chem. 2021, 69, 14192–14203. [Google Scholar] [CrossRef]
- Feng, J.; Wang, L.; Chen, Y.; Xiong, Y.; Wu, Q.; Jiang, Z.; Yi, H. Effects of Niacin on Intestinal Immunity, Microbial Community and Intestinal Barrier in Weaned Piglets during Starvation. Int. Immunopharmacol. 2021, 95, 107584. [Google Scholar] [CrossRef]
- Li, H.; Ma, L.; Li, Z.; Yin, J.; Tan, B.; Chen, J.; Jiang, Q.; Ma, X. Evolution of the Gut Microbiota and Its Fermentation Characteristics of Ningxiang Pigs at the Young Stage. Animals 2021, 11, 638. [Google Scholar] [CrossRef]
- Canibe, N.; Højberg, O.; Kongsted, H.; Vodolazska, D.; Lauridsen, C.; Nielsen, T.S.; Schönherz, A.A. Review on Preventive Measures to Reduce Post-Weaning Diarrhoea in Piglets. Animals 2022, 12, 2585. [Google Scholar] [CrossRef]
- Middelkoop, A.; Costermans, N.; Kemp, B.; Bolhuis, J.E. Feed Intake of the Sow and Playful Creep Feeding of Piglets Influence Piglet Behaviour and Performance before and after Weaning. Sci. Rep. 2019, 9, 16140. [Google Scholar] [CrossRef] [PubMed]
- Blavi, L.; Solà-Oriol, D.; Llonch, P.; López-Vergé, S.; Martín-Orúe, S.M.; Pérez, J.F. Management and Feeding Strategies in Early Life to Increase Piglet Performance and Welfare around Weaning: A Review. Animals 2021, 11, 302. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Yan, W.; Ma, Y.; Fang, J. The Impact of Probiotics on Gut Health via Alternation of Immune Status of Monogastric Animals. Anim. Nutr. 2021, 7, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.F.; Nyachoti, M. Using Probiotics to Improve Swine Gut Health and Nutrient Utilization. Anim. Nutr. 2017, 3, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Han, X.; Tang, S.; Xiao, W.; Tan, Z.; Zhou, C.; Wang, M.; Kang, J. Magnolol and Honokiol Regulate the Calcium-Activated Potassium Channels Signaling Pathway in Enterotoxigenic Escherichia coli-Induced Diarrhea Mice. Eur. J. Pharmacol. 2015, 755, 66–73. [Google Scholar] [CrossRef]
- Xu, J.; Jia, Z.; Xiao, S.; Long, C.; Wang, L. Effects of Enterotoxigenic Escherichia coli Challenge on Jejunal Morphology and Microbial Community Profiles in Weaned Crossbred Piglets. Microorganisms 2023, 11, 2646. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Yin, J.; Chen, S.; Duan, J.; Liu, G.; Li, T.; Li, N.; Peng, Y.; Tan, B.; Yin, Y. Proteome Analysis for the Global Proteins in the Jejunum Tissues of Enterotoxigenic Escherichia coli-Infected Piglets. Sci. Rep. 2016, 6, 25640. [Google Scholar] [CrossRef]
- Gāliņa, D.; Ansonska, L.; Valdovska, A. Effect of Probiotics and Herbal Products on Intestinal Histomorphological and Immunological Development in Piglets. Vet. Med. Int. 2020, 2020, 3461768. [Google Scholar] [CrossRef] [PubMed]
- Helal, F.; El-Badawi, A.; El-Naggar, S.; Shourrap, M.; Aboelazab, O.; Abu Hafsa, S. Probiotics Role of Saccharomyces cerevisiae and Bacillus subtilis in Improving the Health Status of Rabbits’ Gastrointestinal Tract. Bull. Natl. Res. Cent. 2021, 45, 66. [Google Scholar] [CrossRef]
- Maklad, E.H.; Gabr, A.A.; Ragab, M.A.; El-hady, M.A.A.A.; Hegazya, B.K.; Mark, C. Effect of Probiotic Supplementation on Nutrients Digestibility and Intestinal Histomorphology of Growing Rabbits. J. Anim. Poult. Prod. 2023, 14, 91–97. [Google Scholar] [CrossRef]
- Pi, D.; Liu, Y.; Shi, H.; Li, S.; Odle, J.; Lin, X.; Zhu, H.; Chen, F.; Hou, Y.; Leng, W. Dietary Supplementation of Aspartate Enhances Intestinal Integrity and Energy Status in Weanling Piglets after Lipopolysaccharide Challenge. J. Nutr. Biochem. 2014, 25, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Shi, Y.; Zhang, Y.; Zhang, M.; Zhang, L.; Ma, Z.; Zhao, D.; Wang, L.; Yu, H.; Hou, Y.; et al. Lactobacillus rhamnosus LB1 Alleviates Enterotoxigenic Escherichia coli-Induced Adverse Effects in Piglets by Improving Host Immune Response and Anti-Oxidation Stress and Restoring Intestinal Integrity. Front. Cell. Infect. Microbiol. 2021, 11, 724401. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Yu, M. Role of Goblet Cells in Intestinal Barrier and Mucosal Immunity. J. Inflamm. Res. 2021, 14, 3171–3183. [Google Scholar] [CrossRef] [PubMed]
- Qian, S.; Wei, Z.; Yang, W.; Huang, J.; Yang, Y.; Wang, J. The Role of BCL-2 Family Proteins in Regulating Apoptosis and Cancer Therapy. Front. Oncol. 2022, 12, 985363. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, G.; Samal, D.; Khandayataray, P.; Murthy, M.K. A Review on Caspases: Key Regulators of Biological Activities and Apoptosis. Mol. Neurobiol. 2023, 60, 5805–5837, ISBN 1203502303. [Google Scholar] [CrossRef] [PubMed]
- Kaloni, D.; Diepstraten, S.T.; Strasser, A.; Kelly, G.L. BCL-2 Protein Family: Attractive Targets for Cancer Therapy. Apoptosis 2023, 28, 20–38. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Bin, P.; Liu, S.; Chen, S.; Yin, J.; Liu, G.; Tang, Z.; Ren, W. Enterotoxigenic Escherichia coli Infection Promotes Apoptosis in Piglets. Microb. Pathog. 2018, 125, 290–294. [Google Scholar] [CrossRef] [PubMed]
- Ching, J.C.Y.; Jones, N.L.; Ceponis, P.J.M.; Karmali, M.A.; Sherman, P.M. Escherichia coli Shiga-like Toxins Induce Apoptosis and Cleavage of Poly(ADP-Ribose) Polymerase via in Vitro Activation of Caspases. Infect. Immun. 2002, 70, 4669–4677. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Xing, M.; Gu, X. Research Progress on Oxidative Stress and Its Nutritional Regulation Strategies in Pigs. Animals 2021, 11, 1384. [Google Scholar] [CrossRef]
- Novais, A.K.; Deschêne, K.; Martel-Kennes, Y.; Roy, C.; Laforest, J.P.; Lessard, M.; Matte, J.J.; Lapointe, J. Weaning Differentially Affects Mitochondrial Function, Oxidative Stress, Inflammation and Apoptosis in Normal and Low Birth Weight Piglets. PLoS ONE 2021, 16, e0247188. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Z.; Fernandes, J.M.O.; Gao, Y.; Yin, P.; Liu, Y.; Tian, L.; Xie, S.; Niu, J. Beneficial Effects on Growth, Haematic Indicators, Immune Status, Antioxidant Function and Gut Health in Juvenile Nile Tilapia (Oreochromis niloticus) by Dietary Administration of a Multi-Strain Probiotic. Aquac. Nutr. 2020, 26, 1369–1382. [Google Scholar] [CrossRef]
- Yu, Y.; Li, Q.; Zeng, X.; Xu, Y.; Jin, K.; Liu, J.; Cao, G. Effects of Probiotics on the Growth Performance, Antioxidant Functions, Immune Responses, and Caecal Microbiota of Broilers Challenged by Lipopolysaccharide. Front. Vet. Sci. 2022, 9, 846649. [Google Scholar] [CrossRef]
- Lei, X.G.; Zhu, J.H.; Cheng, W.H.; Bao, Y.; Ho, Y.S.; Reddi, A.R.; Holmgren, A.; Arnér, E.S.J. Paradoxical Roles of Antioxidant Enzymes: Basic Mechanisms and Health Implications. Physiol. Rev. 2015, 96, 307–364. [Google Scholar] [CrossRef] [PubMed]
- Kahroba, H.; Shirmohamadi, M.; Hejazi, M.S.; Samadi, N. The Role of Nrf2 Signaling in Cancer Stem Cells: From Stemness and Self-Renewal to Tumorigenesis and Chemoresistance. Life Sci. 2019, 239, 116986. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, X.; Zhang, J. Bridges between Mitochondrial Oxidative Stress, ER Stress and MTOR Signaling in Pancreatic β Cells. Cell. Signal. 2016, 28, 1099–1104. [Google Scholar] [CrossRef] [PubMed]
- Kang, R.; Zeh, H.J.; Lotze, M.T.; Tang, D. The Beclin 1 Network Regulates Autophagy and Apoptosis. Cell Death Differ. 2011, 18, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.; Zhang, H.; Guo, Q.; Duan, Y.; Chen, S.; Han, M.; Li, F.; Jin, M.; Wang, Y. Engineered Bacillus Subtilis Alleviates Intestinal Oxidative Injury through Nrf2-Keap1 Pathway in Enterotoxigenic Escherichia coli (ETEC) K88-Infected Piglet. J. Zhejiang Univ. Sci. B 2023, 24, 496–509. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-ΚB Signaling in Inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Sato, S.; Hemmi, H.; Hoshino, K.; Kaisho, T.; Sanjo, H.; Takeuchi, O.; Sugiyama, M.; Okabe, M.; Takeda, K.; et al. Role of Adaptor TRIF in the MyD88-Independent Toll-like Receptor Signaling Pathway. Science 2003, 301, 640–643. [Google Scholar] [CrossRef]
- Li, H.; Liu, X.; Shang, Z.; Qiao, J. Clostridium butyricum Helps to Alleviate Inflammation in Weaned Piglets Challenged with Enterotoxigenic Escherichia coli K88. Front. Vet. Sci. 2021, 8, 683863. [Google Scholar] [CrossRef]
- Li, H.; Zhang, L.; Chen, L.; Zhu, Q.; Wang, W.; Qiao, J. Lactobacillus acidophilus Alleviates the Inflammatory Response to Enterotoxigenic Escherichia coli K88 via Inhibition of the NF-ΚB and P38 Mitogen-Activated Protein Kinase Signaling Pathways in Piglets. BMC Microbiol. 2016, 16, 273. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhang, S.; Li, S.; Zhang, Q.; Cai, Y.; Li, P.; Li, H.; Shen, B.; Liao, Q.; Hong, Y.; et al. Indoleacrylic Acid Produced by Parabacteroides distasonis Alleviates Type 2 Diabetes via Activation of AhR to Repair Intestinal Barrier. BMC Biol. 2023, 21, 90. [Google Scholar] [CrossRef] [PubMed]
- Koh, G.Y.; Kane, A.V.; Wu, X.; Crott, J.W. Parabacteroides distasonis Attenuates Tumorigenesis, Modulates Inflammatory Markers and Promotes Intestinal Barrier Integrity in Azoxymethane-Treated A/J Mice. Carcinogenesis 2021, 41, 909–917. [Google Scholar] [CrossRef] [PubMed]
- Fernández, J.; Redondo-Blanco, S.; Gutiérrez-del-Río, I.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Colon Microbiota Fermentation of Dietary Prebiotics towards Short-Chain Fatty Acids and Their Roles as Anti-Inflammatory and Antitumour Agents: A Review. J. Funct. Foods 2016, 25, 511–522. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, H.; Chen, T.; Shi, L.; Wang, D.; Tang, D. Regulatory Role of Short-Chain Fatty Acids in Inflammatory Bowel Disease. Cell Commun. Signal. 2022, 20, 64. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Pan, Q.; Xin, F.Z.; Zhang, R.N.; He, C.X.; Chen, G.Y.; Liu, C.; Chen, Y.W.; Fan, J.G. Sodium Butyrate Attenuates High-Fat Diet-Induced Steatohepatitis in Mice by Improving Gut Microbiota and Gastrointestinal Barrier. World J. Gastroenterol. 2017, 23, 60–75. [Google Scholar] [CrossRef]
- Gao, G.; Zhou, J.; Wang, H.; Ding, Y.; Zhou, J.; Chong, P.H.; Zhu, L.; Ke, L.; Wang, X.; Rao, P.; et al. Effects of Valerate on Intestinal Barrier Function in Cultured Caco-2 Epithelial Cell Monolayers. Mol. Biol. Rep. 2022, 49, 1817–1825. [Google Scholar] [CrossRef]
| Items | Control | ETEC | PBd + ETEC |
|---|---|---|---|
| IBW, kg | 6.22 ± 0.11 | 6.40 ± 0.09 | 6.30 ± 0.12 |
| FBW, kg | 11.70 ± 0.52 a | 9.87 ± 0.51 b | 11.43 ± 0.23 a |
| ADG, kg/d | 0.68 ± 0.031 a | 0.58 ± 0.03 b | 0.67 ± 0.013 a |
| ADFI, kg/d | 0.43 ± 0.027 a | 0.34 ± 0.02 b | 0.42 ± 0.02 ab |
| F/G, kg/kg | 0.64 ± 0.05 | 0.60 ± 0.05 | 0.63 ± 0.04 |
| Anus temperature, °C | 39.36 ± 0.54 b | 40.56 ± 0.22 a | 39.80 ± 0.10 ab |
| Liver, g/kg | 2.58 ± 0.08 | 2.81 ± 0.14 | 2.66 ± 0.10 |
| Spleen, g/kg | 0.19 ± 0.01 b | 0.27 ± 0.02 a | 0.23 ± 0.02 ab |
| Items | Control | ETEC | PBd + ETEC |
|---|---|---|---|
| Duodenum | |||
| Villus height, µm | 371.49 ± 90.54 a | 240.26 ± 51.49 b | 394.63 ± 30.25 a |
| Crypt depth, µm | 307.79 ± 61.00 b | 389.90 ± 66.28 a | 289.11 ± 49.10 b |
| V/C | 1.26 ± 0.43 a | 0.63 ± 0.19 b | 1.40 ± 0.29 a |
| Jejunum | |||
| Villus height, µm | 531.15 ± 64.84 a | 204.25 ± 56.75 b | 477.49 ± 73.06 a |
| Crypt depth, µm | 195.53 ± 36.67 | 241.98 ± 64.55 | 224.41 ± 21.99 |
| V/C | 2.77 ± 0.42 a | 0.86 ± 0.21 c | 2.13 ± 0.27 b |
| Histological score | 0.86 ± 0.26 c | 3.57 ± 0.43 a | 1.87 ± 0.30 b |
| Colon | |||
| Crypt depth, µm | 464.06 ± 27.62 | 579.28 ± 133.37 | 515.48 ± 150.61 |
| Items | Control | ETEC | PBd + ETEC |
|---|---|---|---|
| TNF-α, pg/mL | 208.05 ± 89.34 | 476.97 ± 135.02 | 225.46 ± 41.37 |
| IL-1β, pg/mL | 479.44 ± 91.27 | 730.56 ± 149.28 | 434.22 ± 84.70 |
| IL-4, pg/mL | 293.38 ± 96.70 | 1191.60 ± 514.37 | 454.41 ± 94.09 |
| IL-6, pg/mL | 157.50 ± 44.16 b | 653.31 ± 215.21 a | 201.53 ± 60.29 b |
| IL-8, pg/mL | 35.15 ± 3.84 | 49.72 ± 9.78 | 55.85 ± 15.05 |
| IL-10, pg/mL | 837.04 ± 243.38 b | 1864.81 ± 447.15 a | 1180.25 ± 212.88 ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Z.; Zhang, L.; Li, H.; Li, J.; Zhang, Z.; Tan, B.; Wang, J. Ningxiang Pig-Derived Parabacteroides distasonis HNAU0205 Alleviates ETEC-Induced Intestinal Apoptosis, Oxidative Damage, and Inflammation in Piglets. Animals 2024, 14, 2156. https://doi.org/10.3390/ani14152156
Wu Z, Zhang L, Li H, Li J, Zhang Z, Tan B, Wang J. Ningxiang Pig-Derived Parabacteroides distasonis HNAU0205 Alleviates ETEC-Induced Intestinal Apoptosis, Oxidative Damage, and Inflammation in Piglets. Animals. 2024; 14(15):2156. https://doi.org/10.3390/ani14152156
Chicago/Turabian StyleWu, Zichen, Longlin Zhang, Hongkun Li, Junyao Li, Zihao Zhang, Bie Tan, and Jing Wang. 2024. "Ningxiang Pig-Derived Parabacteroides distasonis HNAU0205 Alleviates ETEC-Induced Intestinal Apoptosis, Oxidative Damage, and Inflammation in Piglets" Animals 14, no. 15: 2156. https://doi.org/10.3390/ani14152156
APA StyleWu, Z., Zhang, L., Li, H., Li, J., Zhang, Z., Tan, B., & Wang, J. (2024). Ningxiang Pig-Derived Parabacteroides distasonis HNAU0205 Alleviates ETEC-Induced Intestinal Apoptosis, Oxidative Damage, and Inflammation in Piglets. Animals, 14(15), 2156. https://doi.org/10.3390/ani14152156







