Antibiotic Resistance of Bacteria Isolated from Clinical Samples and Organs of Rescued Loggerhead Sea Turtles (Caretta caretta) in Southern Italy
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Sample Collection
2.3. Bacterial Isolation and Identification
2.4. Antibiotic Susceptibility Testing
2.5. Data Analysis
3. Results
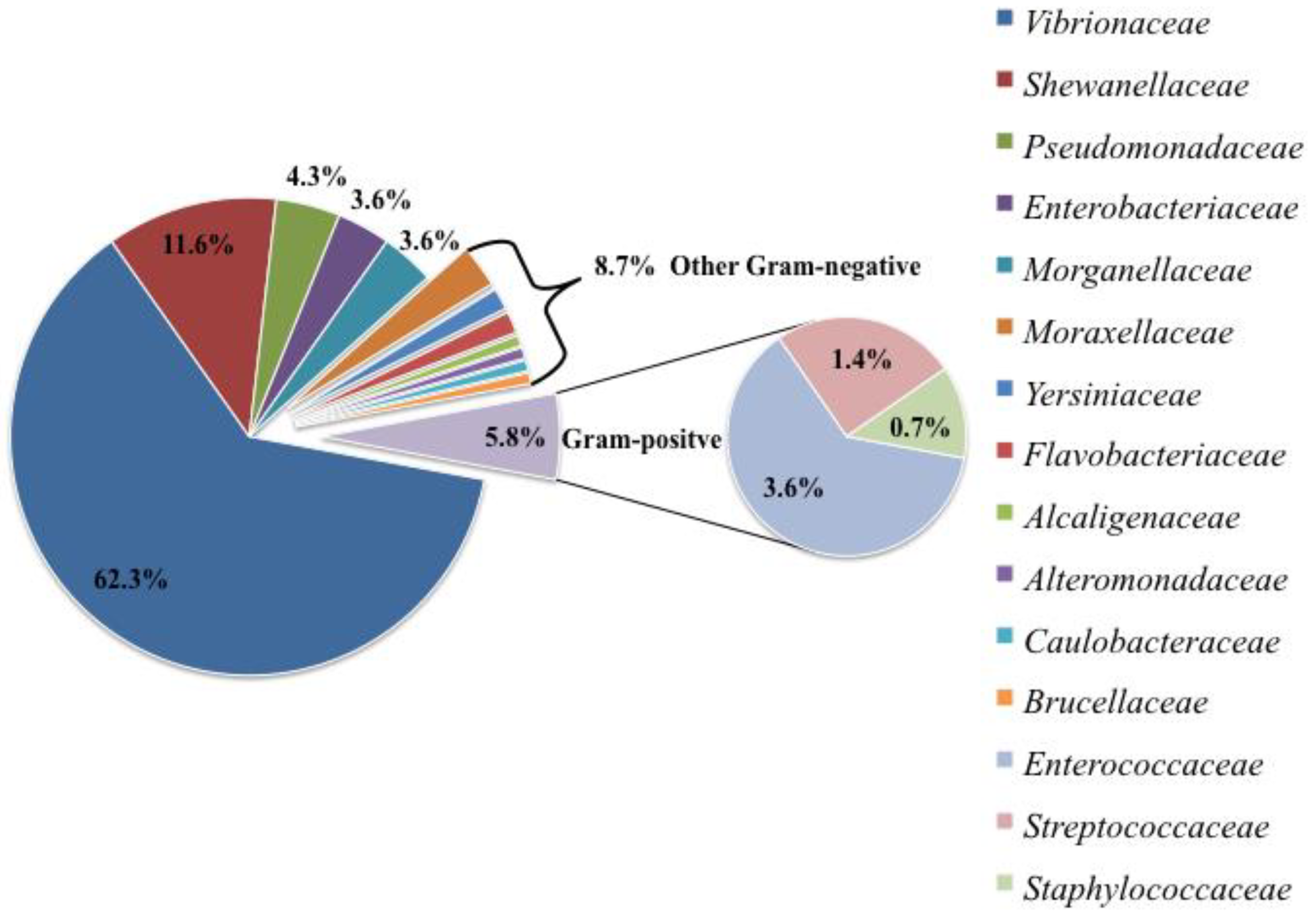
3.1. Bacterial Isolation and Identification
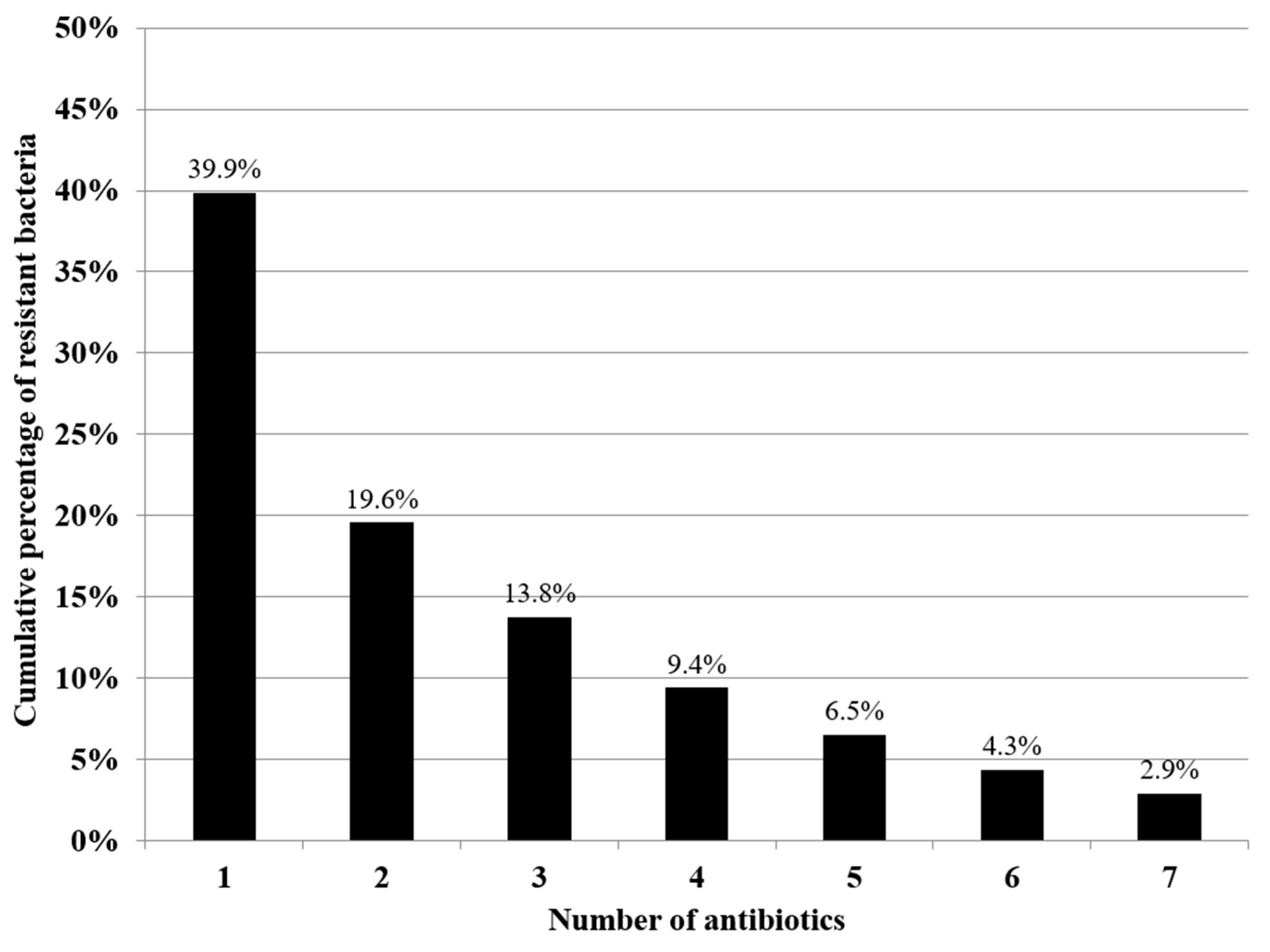
3.2. Antibiotic Susceptibility Testing
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martinez, J.L. General principles of antibiotic resistance in bacteria. Drug Discov. Today Technol. 2014, 11, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Reygaert, W.C. An overview of the antimicrobial resistance mechanisms of bacteria. AIMS Microbiol. 2018, 4, 482–501. [Google Scholar] [CrossRef] [PubMed]
- D’Costa, V.M.; King, C.E.; Kalan, L.; Morar, M.; Sung, W.W.L.; Schwarz, C.; Froese, D.; Zazula, G.; Calmels, F.; Beruyne, R.; et al. Antibiotic resistance is ancient. Nature 2011, 477, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Munita, J.M.; Arias, C.A. Mechanisms of Antibiotic Resistance. Microbiol. Spectr. 2016, 4, 481–511. [Google Scholar] [CrossRef] [PubMed]
- Dadgostar, P. Antimicrobial Resistance: Implications and Costs. Infect. Drug Resist. 2019, 12, 3903–3910. [Google Scholar] [CrossRef]
- Despotovic, M.; de Nies, L.; Bhanu Busi, S.; Wilmes, P. Reservoirs of antimicrobial resistance in the context of One Health. Curr. Opin. Microbiol. 2023, 73, 102291. [Google Scholar] [CrossRef] [PubMed]
- Friedman, N.D.; Temkin, E.; Carmeli, Y. The negative impact of antibiotic resistance. Clin. Microbiol. Infect. 2016, 22, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Report World Health Organization. Antimicrobial Resistance: An Emerging Water, Sanitation and Hygiene Issue; World Health Organization: Geneva, Switzerland, 2014.
- Bengtsson, B.; Greko, C. Antibiotic resistance—Consequences for animal health, welfare, and food production. Ups J. Med. Sci. 2014, 119, 96–102. [Google Scholar] [CrossRef]
- Arnold, K.E.; Williams, N.J.; Bennett, M. ‘Disperse abroad in the land’: The role of wildlife in the dissemination of antimicrobial resistance. Biol. Lett. 2016, 12, 20160137. [Google Scholar] [CrossRef]
- Radhouani, H.; Silva, N.; Poeta, P.; Torres, C.; Correia, S.; Igrejas, G. Potential impact of antimicrobial resistance in wildlife, environment and human health. Front. Microbiol. 2014, 5, 23. [Google Scholar] [CrossRef]
- Allen, H.K.; Donato, J.; Wang, H.H.; Cloud-Hansen, K.A.; Davies, J.; Handelsman, J. Call of the wild: Antibiotic resistance genes in natural environments. Nat. Rev. Microbiol. 2010, 8, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Vittecoq, M.; Godreuil, S.; Prugnolle, F.; Durand, P.; Brazier, L.; Renaud, N.; Arnal, A.; Aberkane, S.; Jean-Pierre, H.; Gauthier-Clerc, M.; et al. Antimicrobial resistance in wildlife. J. Appl. Ecol. 2016, 53, 519–529. [Google Scholar] [CrossRef]
- Plaza-Rodriíguez, C.; Alt, K.; Grobbel, M.; Hammerl, J.A.; Irrgang, A.; Szabo, I.; Stingl, K.; Schuh, E.; Wiehle, L.; Pfefferkorn, B.; et al. Wildlife as Sentinels of Antimicrobial Resistance in Germany? Front. Vet. Sci. 2021, 7, 627821. [Google Scholar] [CrossRef] [PubMed]
- Gambino, D.; Savoca, D.; Sucato, A.; Gargano, V.; Gentile, A.; Pantano, L.; Vicari, D.; Alduina, R. Occurrence of Antibiotic Resistance in the Mediterranean Sea. Antibiotics 2022, 11, 332. [Google Scholar] [CrossRef] [PubMed]
- Marangi, M.; Carlucci, R.; Carlino, P.; Fanizza, C.; Cirelli, G.; Maglietta, R.; Beneduce, L. Dolphins and sea turtles may host zoonotic parasites and pathogenic bacteria as indicators of anthropic pressure in the Gulf of Taranto (Northern Ionian Sea, Central-Eastern Mediterranean Sea). Vet. Res. Commun. 2022, 46, 1157–1166. [Google Scholar] [CrossRef] [PubMed]
- Tacão, M.; Correia, A.; Henriques, I. Resistance to broad-spectrum antibiotics in aquatic systems: Anthropogenic activities modulate the dissemination of blaCTX-M-like genes. Appl. Environ. Microbiol. 2012, 78, 4134–4140. [Google Scholar] [CrossRef] [PubMed]
- Hazen, E.L.; Abrahms, B.; Brodie, S.; Carroll, G.; Jacox, M.G.; Savoca, M.S.; Scales, K.L.; Sydeman, W.J.; Bograd, S.J. Marine top predators as climate and ecosystem sentinels. Front. Ecol. Environ. 2019, 17, 565–574. [Google Scholar] [CrossRef]
- Bossart, G.D. Marine mammals as sentinel species for oceans and human health. Vet. Pathol. 2011, 48, 676–690. [Google Scholar] [CrossRef]
- Frederiksen, M.; Mavor, R.A.; Wanless, S. Seabirds as environmental indicators: The advantages of combining data sets. Mar. Ecol. Prog. Ser. 2007, 352, 205–211. [Google Scholar] [CrossRef]
- Aguirre, A.A.; Lutz, P.L. Marine turtles as sentinels of ecosystem health: Is fibropapillomatosis an indicator? EcoHealth 2004, 1, 275–283. [Google Scholar] [CrossRef]
- Mariani, G.; Bellucci, F.; Cocumelli, C.; Raso, C.; Hochscheid, S.; Roncari, C.; Nerone, E.; Recchi, S.; Di Giacinto, F.; Olivieri, V.; et al. Dietary Preferences of Loggerhead Sea Turtles (Caretta caretta) in Two Mediterranean Feeding Grounds: Does Prey Selection Change with Habitat Use throughout Their Life Cycle? Animals 2023, 13, 654. [Google Scholar] [CrossRef]
- Casale, P.; Broderick, A.; Camiñas, J.; Cardona, L.; Carreras, C.; Demetropoulos, A.; Fuller, W.; Godley, B.; Hochscheid, S.; Kaska, Y.; et al. Mediterranean Sea turtles: Current knowledge and priorities for conservation and research. Endanger. Species Res. 2018, 36, 229–267. [Google Scholar] [CrossRef]
- Al-Bahry, S.N.; Al-Zadjali, M.A.; Mahmoud, I.Y.; Elshafie, A.E. Biomonitoring marine habitats in reference to antibiotic resistant bacteria and ampicillin resistance determinants from oviductal fluid of the nesting green sea turtle, Chelonia mydas. Chemosphere 2012, 87, 1308–1315. [Google Scholar] [CrossRef]
- Alduina, R.; Gambino, D.; Presentato, A.; Gentile, A.; Sucato, A.; Savoca, D.; Filippello, S.; Visconti, G.; Caracappa, G.; Vicari, D.; et al. Is “Caretta Caretta” a Carrier of Antibiotic Resistance in the Mediterranean Sea? Antibiotics 2020, 9, 116. [Google Scholar] [CrossRef]
- Blasi, M.F.; Migliore, L.; Mattei, D.; Rotini, A.; Thaller, M.C.; Alduina, R. Antibiotic Resistance of Gram-Negative Bacteria from Wild Captured Loggerhead Sea Turtles. Antibiotics 2020, 9, 162. [Google Scholar] [CrossRef]
- Trotta, A.; Marinaro, M.; Sposato, A.; Galgano, M.; Ciccarelli, S.; Paci, S.; Corrente, M. Antimicrobial Resistance in Loggerhead Sea Turtles (Caretta caretta): A Comparison between Clinical and Commensal Bacterial Isolates. Animals 2021, 11, 2435. [Google Scholar] [CrossRef]
- Ebani, V.V. Bacterial Infections in Sea Turtles. Vet. Sci. 2023, 10, 333. [Google Scholar] [CrossRef]
- Frazzon, A.P.G. Antibiotic-resistant bacteria in free-living marine species. Vet. Rec. 2016, 179, 648–649. [Google Scholar] [CrossRef]
- Mazaris, A.D.; Broder, B.; Matsinos, Y.G. An individual based model of a sea turtle population to analyze effects of age dependent mortality. Ecol. Model. 2006, 198, 174–182. [Google Scholar] [CrossRef]
- Oroís, J.; Montesdeoca, N.; Camacho, M.; Arenciba, A.; Calabuig, P. Causes of Stranding and Mortality, and Final Disposition of Loggerhead Sea Turtles (Caretta caretta) Admitted to a wildlife Rehabillitation Center in Gran Canaria Island, Spain (1998–2014): A Long-Term Retrospective Study. PLoS ONE 2016, 11, e0149398. [Google Scholar] [CrossRef]
- Ullmann, J.; Stachowitsch, M. A Critical review of the Mediterranean sea turtle rescue network: A web looking for a weaver. Nat. Conserv. 2015, 10, 45–69. [Google Scholar] [CrossRef]
- Wallace, B.P.; Heppell, S.S.; Lewison, R.L.; Kelez, S.; Crowder, L.B. Impacts of fisheries bycatch on loggerhead turtles worldwide inferred from reproductive value analyses. J. Appl. Ecol. 2008, 45, 1076–1085. [Google Scholar] [CrossRef]
- DiMatteo, A.; Canñadas, A.; Roberts, J.; Sparks, L.; Panigada, S.; Boisseau, O.; Moscrop, A.; Fortuna, C.M.; Lauriano, G.; Holcer, D.; et al. Basin-wide estimates of loggerhead turtle abundance in the Mediterranean Sea derived from line transect surveys. Front. Mar. Sci. 2022, 9, 930412. [Google Scholar] [CrossRef]
- Maffucci, F.; Corrado, R.; Palatella, L.; Borra, M.; Marullo, S.; Hochscheid, S.; Lacorata, G.; Iudicone, D. Seasonal heterogeneity of ocean warming: A mortality sink for ectotherm colonizers. Sci. Rep. 2016, 6, 23983. [Google Scholar] [CrossRef] [PubMed]
- Wyneken, J. The Anatomy of Sea Turtles; NMFS-SEFSC-470; US Department of Commerce, NOAA Technical Memorandum: Washington, DC, USA, 2001; p. 172.
- Poppi, L.; Marchiori, E. Standard Protocol for Post-Mortem Examination on Sea Turtles. Guidelines Produced within the Adriatic project “Network for the Conservation of Cetaceans and Sea Turtles in the Adriatic (NETCET). 2013. Available online: https://www.plavi-svijet.org/bw/wp-content/uploads/2017/05/NETCET_Standard-protocols-for-post-mortem-examination-of-sea-turtles (accessed on 16 July 2024).
- Cowan, S.T.; Steel, K.J. Manual for the Identification of Medical Bacteria, 3rd ed.; Barrow, G.I., Feltham, R.K.A., Eds.; Cambridge University Press: Cambridge, UK, 1965. [Google Scholar]
- Hucker, G.J.; Conn, H.J. Methods of Gram Staining; New York Agricultural Experiment Station: New York, NY, USA, 1923. [Google Scholar]
- Clinical and Laboratory Standards Institute. Methods for Antimicrobial Disk Susceptibility Testing of Bacteria Isolated from Aquatic Animals; Approved Guideline; CLSI Document VET03-A; Clinical&Laboratory Standards Institute: Wayne, PA, USA, 2006. [Google Scholar]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals, 5th ed.; CLSI Standard VET01; Clinical&Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- Alderman, D.J.; Smith, P. Development of draft protocols of standard reference methods for antimicrobial agent susceptibility testing of bacteria associated with fish diseases. Aquaculture 2001, 196, 211–243. [Google Scholar] [CrossRef]
- Smith, P.; Christofilogiannis, P. Application of Normalised Resistance Interpretation to the detection of multiple low-level resistance in strains of Vibrio anguillarum obtained from Greek fish farms. Aquaculture 2007, 272, 223–230. [Google Scholar] [CrossRef]
- Krumperman, P.H. Multiple antibiotic resistance indexing of Escherichia coli to identify high-risk sources of fecal contamination of foods. Appl. Environ. Microbiol. 1983, 46, 165–170. [Google Scholar] [CrossRef]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Arizza, V.; Vecchioni, L.; Caracappa, S.; Sciurba, G.; Berlinghieri, F.; Gentile, A.; Persichetti, M.F.; Arculeo, M.; Alduina, R. New insight into the gut microbiome in loggerhead sea turtles Caretta caretta stranded on the Mediterranean coast. PLoS ONE 2019, 14, e0220329. [Google Scholar] [CrossRef]
- Biagi, E.; D’Amico, F.; Soverini, M.; Angelini, V.; Barone, M.; Turroni, S.; Rampelli, S.; Pari, S.; Brigidi, P.; Candela, M. Faecal bacterial communities from Mediterranean loggerhead sea turtles (Caretta caretta). Environ. Microbiol. Rep. 2018, 11, 361–371. [Google Scholar] [CrossRef]
- Work, T.M.; Balazs, G.H.; Wolcott, M.; Morris, R. Bacteraemia in free-ranging Hawaiian green turtles Chelonia mydas with fibropapillomatosis. Dis. Aquat. Org. 2003, 53, 41–46. [Google Scholar] [CrossRef]
- Fichi, G.; Cardeti, A.; Cersini, C.; Mancusi, M.; Guarducci, G.; Di Guardo, G.; Terracciano, G. Bacterial and viral pathogens detected in sea turtles stranded along the coast of Tuscany, Italy. Vet. Microbiol. 2016, 185, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.; Chang, C.; Li, T. Antimicrobial-resistance profiles of gram-negative bacteria isolated from green turtles (Chelonia mydas) in Taiwan. Environ. Pollut. 2021, 277, 116870. [Google Scholar] [CrossRef] [PubMed]
- Pace, A.; Dipineto, L.; Fioretti, A.; Hochscheid, S. Loggerhead Sea turtles as sentinels in the western Mediterranean: Antibiotic resistance and environment-related modifications of Gram-negative bacteria. Mar. Pollut. Bull. 2019, 149, 110575. [Google Scholar] [CrossRef]
- Zavala-Norzagaray, A.A.; Aguirre, A.A.; Velazquez-Roman, J.; Flores-Villaseñor, H.; León-Sicairos, N.; Ley-Quiñonez, C.P.; Hernández-Díaz, L.D.J.; Canizalez-Roman, A. Isolation, characterization, and antibiotic resistance of Vibrio spp. in sea turtles from Northwestern Mexico. Front. Microbiol. 2015, 6, 635. [Google Scholar] [CrossRef] [PubMed]
- Orós, J.; Torrent, A.; Calabuig, P.; Déniz, S. Disease sand causes of mortality among sea turtles stranded in the Canary Islands, Spain (1998-2001). Dis. Aquat. Org. 2005, 63, 13–24. [Google Scholar] [CrossRef]
- Sheikh, H.I.; Alhamadin, N.I.I.; Liew, H.J.; Fadhlina, A.; Wahid, M.E.A.; Musa, N.; Jalal, K.C.A. Virulence Factors of the Zoonotic Pathogen Vibrio alginolyticus: A Review and Bibliometric Analysis. Appl. Biochem. Microbiol. 2024, 60, 514–531. [Google Scholar] [CrossRef]
- Zhang, X.H.; Austin, B. Pathogenicity of Vibrio harveyi to salmonids. J. Fish Dis. 2000, 23, 93–102. [Google Scholar] [CrossRef]
- Osorio, C.R.; Vences, A.; Matanza, X.M.; Terceti, M.S. Photobacterium damselae subsp. damselae, a generalist pathogen with unique virulence factors and high genetic diversity. J. Bacteriol. 2018, 200, e00002-18. [Google Scholar] [CrossRef]
- Velazquez-Roman, J.; Leon-Sicairos, N.; De Jesus Hernandez-Diaz, L.; Canizalez-Roman, A. Pandemic Vibrio parahaemolyticus O3:K6 on the American continent. Front. Cell Infect. Microbiol. 2014, 3, 110. [Google Scholar] [CrossRef]
- Scarano, C.; Spanu, C.; Ziino, G.; Pedonese, F.; Dalmasso, A.; Spanu, V.; Virdis, S.; De Santis, E.P.L. Antibiotic resistance of Vibrio species isolated from Sparus aurata reared in Italian mariculture. New Microbiol. 2014, 37, 329–337. [Google Scholar] [PubMed]
- You, K.G.; Bong, C.W.; Lee, C.W. Antibiotic resistance and plasmid profiling of Vibrio spp. in tropical waters of Peninsular Malaysia. Environ. Monit. Assess 2016, 188, 171. [Google Scholar] [CrossRef]
- Elmahdi, S.; DaSilva, L.V.; Parveen, S. Antibiotic resistance of Vibrio parahaemolyticus and Vibrio vulnificus in various countries: A review. Food. Microbiol. 2016, 57, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Snoussi, M.; Hajlaoui, H.; Noumi, E.; Zanetti, S.; Bakhrouf, A. Phenotypic and molecular characterization of Vibrio alginolyticus strains recovered from juveniles and older Sparus aurata reared in a Tunisian marine farm. Ann. Microbiol. 2008, 58, 141–146. [Google Scholar] [CrossRef]
- Kuschke, S.G. What lives on and in the sea turtle? A literature review of sea turtle bacterial microbiota. Anim. Microbiome 2022, 4, 52. [Google Scholar] [CrossRef]
- Fernandes, M.; Grilo, M.L.; Carneiro, C.; Cunha, E.; Tavares, L.; Patino-Martinez, J.; Oliveira, M. Antibiotic Resistance and Virulence Profiles of Gram-Negative Bacteria Isolated from Loggerhead Sea Turtles (Caretta caretta) of the Island of Maio, Cape Verde. Antibiotics 2021, 10, 771. [Google Scholar] [CrossRef] [PubMed]
- Holt, H.M.; Gahrn-Hansen, B.; Bruun, B. Shewanella algae and Shewanella putrefaciens: Clinical and Microbiological Characteristics. Clin. Microbiol. Infect. 2005, 11, 347–352. [Google Scholar] [CrossRef]
- Yousfi, K.; Bekal, S.; Usongo, V.; Touati, A. Current trends of human infections and antibiotic resistance of the genus Shewanella. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 1353–1362. [Google Scholar] [CrossRef] [PubMed]
- Santoro, M.; Hernandez, G.; Caballero, M.; Garcia, F. Potential bacterial pathogens carried by nesting leatherback turtles (Dermochelys coriacea) in Costa Rica. Chelonian Conserv. Biol. 2008, 7, 104–108. [Google Scholar] [CrossRef]
- Oliveira, M.; Serrano, I.; Santos, J.P.; Bilocq, F.; Pereira, N.; de Santos Loureiro, N.; Tavares, L.; Pirnay, J.P.; De Vos, D. Pseudomonads from Wild Free-Living Sea Turtles in Príncipe Island, Gulf of Guinea. Ecol. Indic. 2017, 81, 260–264. [Google Scholar] [CrossRef]
- Zavala-Norzagaray, A.A.; Aguirre, A.A.; Angulo-Zamudio, U.A.; Ley-Quiñonez, C.P.; Flores-Villaseñor, H.; León-Sicairos, N.; Velázquez-Román, J.; Elorriaga-Verplancken, F.R.; Zavala-Félix, K.A.; Hart, C.E.; et al. Isolation, characterization, and antimicrobial susceptibility of bacteria isolated from sea lion (Zalophus californianus) pups in northwestern Mexico. J. Wildl. Dis. 2022, 58, 500–511. [Google Scholar] [CrossRef] [PubMed]
- Al-Bahry, S.; Mahmoud, I.; Elshafie, A.; Al-Harthy, A.; Al-Ghafri, S.; Al-Amri, I.; Alkindi, A. Bacterial flora and antibiotic resistance from eggs of green turtles Chelonia mydas: An indication of polluted effluents. Mar. Pollut. Bull. 2009, 58, 720–725. [Google Scholar] [CrossRef] [PubMed]
- Al-Bahry, S.N.; Mahmoud, I.Y.; Al-Zadjali, M.; Elshafie, A.; Al-Harthy, A.; Al-Alawi, W. Antibiotic resistant bacteria as bio-indicator of polluted effluent in the green turtles, Chelonia mydas in Oman. Mar. Environ. Res. 2011, 71, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Foti, M.; Giacopello, C.; Bottari, T.; Fisichella, V.; Rinaldo, D.; Mammina, C. Antibiotic Resistance of Gram Negatives isolates from loggerhead sea turtles (Caretta caretta) in the central Mediterranean Sea. Mar. Pollut. Bull. 2009, 58, 1363–1366. [Google Scholar] [CrossRef] [PubMed]
- Ahasan, M.S.; Picard, J.; Elliott, L.; Kinobe, R.; Owens, L.; Ariel, E. Evidence of antibiotic resistance in Enterobacteriales isolated from green sea turtles, Chelonia mydas on the Great Barrier Reef. Mar. Pollut. Bull. 2017, 120, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Pace, A.; Rinaldi, L.; Ianniello, D.; Borrelli, L.; Cringoli, G.; Fioretti, A.; Hochscheid, S.; Dipineto, L. Gastrointestinal investigation of parasites and Enterobacteriaceae in loggerhead sea turtles from Italian coasts. BMC Vet. Res. 2019, 15, 370. [Google Scholar] [CrossRef] [PubMed]
- Inurria, A.; Suárez-Pérez, A.; Calabuig, P.; Orós, J. Citrobacter freundii-associated lesions in stranded loggerhead sea turtles (Caretta caretta). Vet. Pathol. 2024, 61, 140–144. [Google Scholar] [CrossRef]
- Vega-Manriquez, D.X.; Dávila-Arrellano, R.P.; Eslava-Campos, C.A.; Salazar Jiménez, E.; Negrete-Philippe, A.C.; Raigoza-Figueras, R.; Muñoz-Tenería, F.A. Identification of bacteria present in ulcerative stomatitis lesions of captive sea turtles Chelonia mydas. Vet. Res. Commun. 2018, 42, 251–254. [Google Scholar] [CrossRef] [PubMed]
- Maravic, A.; Skocibusic, M.; Cvjetan, S.; Samanic, I.; Fredotovic, Z.; Puizina, J. Prevalence and diversity of extended-spectrum-beta-lactamase-producing Enterobacteriaceae from marine beach waters. Mar. Pollut. Bull. 2015, 90, 60–67. [Google Scholar] [CrossRef]
- Gootz, T.D.; Jackson, D.B.; Sherris, J.C. Development of resistance to cephalosporins in clinical strains of Citrobacter spp. Antimicrob. Agents Chemother. 1984, 25, 591–595. [Google Scholar] [CrossRef]
- Literacka, E.; Bedenic, B.; Baraniak, A.; Fiett, J.; Tonkic, M.; Jajic-Bencic, I.; Gniadkowski, M. blaCTX-M genes in Escherichia coli strains from Croatian hospitals are located in new (blaCTX-M-3a) and widely spread (blaCTX-M-3a, blaCTX-M-15) genetic structures. Antimicrob. Agents Chemother. 2009, 53, 1630–1635. [Google Scholar] [CrossRef]
- Ojer-Usoz, E.; Gonzalez, D.; García-Jalon, I.; Vitas, A.I. High dissemination of extended-spectrum b-lactamase-producing Enterobacteriaceae in effluents from wastewater treatment plants. Water Res. 2014, 56, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Chen, H.; Yang, Q.; Chen, M.; Wang, H. High prevalence of plasmid-mediated quinolone resistance genes qnr and aac(60)-Ib-cr in clinical isolates of Enterobacteriaceae from nine teaching hospitals in China. Antimicrob. Agents Chemother. 2008, 52, 4268–4273. [Google Scholar] [CrossRef] [PubMed]
- Adeolu, M.; Alnajar, S.; Naushad, S.; Gupta, R.S. Genome-based phylogeny and taxonomy of the ‘Enterobacteriales’: Proposal for Enterobacterales ord. nov. divided into the families Enterobacteriaceae, Erwiniaceae fam. nov., Pectobacteriaceae fam. nov., Yersiniaceae fam. nov., Hafniaceae fam. nov., Morganellaceae fam. nov., and Budviciaceae fam. nov. Int. J. Syst. Evol. Microbiol. 2016, 66, 5575–5599. [Google Scholar] [CrossRef]
- Orós, J.; Camacho, M.; Calabuig, P.; Rial-Berriel, C.; Montesdeoca, N.; Déniz, S.; Luzardo, O.P. Postmortem investigations on leatherback sea turtles (Dermochelys coriacea) stranded in the Canary Islands (Spain) (1998–2017): Evidence of anthropogenic impacts. Mar. Pollut. Bull 2021, 167, 112340. [Google Scholar] [CrossRef] [PubMed]
- Cardoso-Brito, V.; Raposo, A.C.S.; Pires, T.T.; Pinna, M.H.; Oria, A.P. Conjunctival bacterial flora and antimicrobial susceptibility of captive and free-living sea turtles in brazil. Vet. Ophthalmol. 2019, 22, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Santoro, M.; Hernández, G.; Caballero, M. Aerobic bacterial flora of nesting green turtles (Chelonia mydas) from Tortuguero National Park, Costa Rica. J. Zoo. Wildl. Med. 2006, 37, 549–552. [Google Scholar] [CrossRef] [PubMed]
- Prichula, J.; Pereira, R.I.; Wachholz, G.R.; Cardoso, L.A.; Tolfo, N.C.; Santestevan, N.A.; Medeiros, A.W.; Tavares, M.; Frazzon, J.; d’Azevedo, P.A.; et al. Resistance to antimicrobial agents among enterococci isolated from fecal samples of wild marine species in the southern coast of Brazil. Mar. Pollut. Bull. 2016, 105, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Innis, C.J.; Braverman, H.; Cavin, J.M.; Ceresia, M.L.; Baden, L.R.; Kuhn, D.M.; Frasca, S., Jr.; McGowan, J.P.; Hirokawa, K.; Weber, E.S., 3rd; et al. Diagnosis and management of Enterococcus spp infections during rehabilitation of cold-stunned Kemp’s ridley turtles (Lepidochelys kempii): 50 cases (2006–2012). J. Am. Vet. Med. Assoc. 2014, 245, 315–323. [Google Scholar] [CrossRef]
- Tsai, M.; Chang, C.; Li, T. Multiple-antibiotic resistance of Enterococcus faecalis in an endangered olive ridley sea turtle (Lepidochelys olivacea): A case report. Indian J. Anim. Res. 2019, 1064, 1–6. [Google Scholar] [CrossRef]
- Kristich, C.J.; Rice, L.B.; Arias, C.A. Enterococcal Infection—Treatment and Antibiotic Resistance. In Enterococci: From Commensals to Leading Causes of Drug Resistant Infection; Gilmore, M.S., Clewell, D.B., Ike, Y., Shankar, N., Eds.; Massachusetts Eye and Ear Infirmary: Boston, MA, USA, 2014. [Google Scholar] [PubMed]
- Short, F.S.; Lôbo-Hajdu, G.; Guimarães, S.M.; Laport, M.S.; Silva, R. Antimicrobial-Resistant Bacteria from Free-Living Green Turtles (Chelonia mydas). Antibiotics 2023, 12, 1268. [Google Scholar] [CrossRef] [PubMed]
- Aung, M.S.; Urushibara, N.; Kawaguchiya, M.; Ohashi, N.; Hirose, M.; Kudo, K.; Tsukamoto, N.; Ito, M.; Kobayashi, N. Antimicrobial Resistance, Virulence Factors, and Genotypes of Enterococcus Faecalis and Enterococcus Faecium Clinical Isolates in Northern Japan: Identification of OptrA in ST480 E. Faecalis. Antibiotics 2023, 12, 108. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.R.; Townsend, F.I.; Lane, S.M.; Dyar, E.; Hohn, A.A.; Rowles, T.K.; Staggs, L.A.; Wells, R.S.; Balmer, B.C.; Schwacke, L.H. Survey of antibiotic-resistant bacteria isolated from bottlenose dolphins Tursiops truncatus in the southeastern USA. Dis. Aquat. Organ 2014, 108, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Stracy, M.; Snitser, O.; Yelin, I.; Amer, Y.; Parizade, M.; Katz, R.; Rimler, G.; Wolf, T.; Herzel, E.; Koren, G.; et al. Minimizing treatment-induced emergence of antibiotic resistance in bacterial infections. Science 2022, 375, 889–894. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, S.; Tschudin-Sutter, S.; Egli, A.; Osthoff, M. Optimizing antibiotic therapies to reduce the risk of bacterial resistance. Eur. J. Intern. Med. 2022, 99, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Kohanski, M.A.; DePristo, M.A.; Collins, J.J. Sublethal antibiotic treatment leads to multidrug resistance via radical-induced mutagenesis. Mol. Cell 2010, 37, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Beaber, J.W.; Hochhut, B.; Waldor, M.K. SOS response promotes horizontal dissemination of antibiotic resistance genes. Nature 2004, 427, 72–74. [Google Scholar] [CrossRef] [PubMed]
- López, E.; Elez, M.; Matic, I.; Blázquez, J. Antibiotic-mediated recombination: Ciprofloxacin stimulates SOS-independent recombination of divergent sequences in Escherichia coli. Mol. Microbiol. 2007, 64, 83–93. [Google Scholar] [CrossRef]
- WHO. The WHO AWaRe (Access, Watch, Reserve) Antibiotic Book; World Health Organization: Geneva, Switzerland, 2022.
- Laborda, P.; Sanz-García, F.; Ochoa-Sánchez, L.E.; Gil-Gil, T.; Hernando-Amado, S.; Martínez, J.L. Wildlife and Antibiotic Resistance. Front. Cell Infect. Microbiol. 2022, 12, 873989. [Google Scholar] [CrossRef]
- Di Renzo, L.; De Angelis, M.E.; Torresi, M.; Di Lollo, V.; Di Teodoro, G.; Averaimo, D.; Defourny, S.V.P.; Di Giacinto, F.; Profico, C.; Olivieri, V.; et al. First Report of Septicaemic Listeriosis in a Loggerhead Sea Turtle (Caretta caretta) Stranded along the Adriatic Coast: Strain Detection and Sequencing. Animals 2022, 12, 2364. [Google Scholar] [CrossRef]
- Esposito, E.; Paduano, G.; Iaccarino, D. First Detection of Listeria monocytogenes in Stranded Loggerhead Sea Turtle (Caretta caretta) along the Coast of Campania Region (Southern Italy). In Proceedings of the 7th Mediterranean Conference on Marine Turtles, Tetouan, Morocco, 18–21 October 2022. [Google Scholar]
- Pace, A.; Vicari, N.; Rigamonti, S.; Magnino, S.; Borrelli, L.; Dipineto, L.; Fioretti, A.; Hochscheid, S.; Tavares, L.; Duarte, A. Detection of Chlamydial DNA from Mediterranean Loggerhead Sea Turtles in Southern Italy. Animals 2022, 12, 715. [Google Scholar] [CrossRef] [PubMed]
- Rubini, S.; Baruffaldi, M.; Taddei, R.; D’Annunzio, G.; Scaltriti, E.; Tambassi, M.; Menozzi, I.; Bondesan, G.; Mazzariol, S.; Centelleghe, C.; et al. Loggerhead Sea Turtle as Possible Source of Transmission for Zoonotic Listeriosis in the Marine Environment. Vet. Sci. 2023, 10, 344. [Google Scholar] [CrossRef] [PubMed]
- Waltzek, T.B.; Cortes-Hinojosa, G.; Wellehan, J.F.X., Jr.; Gray, G.C. Marine mammal zoonoses: A review of disease manifestations. Zoonoses Public Health 2012, 59, 521–535. [Google Scholar] [CrossRef]
- Aguirre, A.A.; Gardner, S.C.; Marsh, J.C.; Delgado, S.G.; Limpus, C.J.; Nichols, W.J. Hazards associated with the consumption of sea turtles meat and eggs: A review for health care workers and the general public. Ecohealth 2006, 3, 141–153. [Google Scholar] [CrossRef]
- Warwick, C.; Arena, P.C.; Steedman, C. Health implications associated with exposure to farmed and wild sea turtles. J. R. Soc. Med. Sh. Rep. 2013, 4, 1–7. [Google Scholar] [CrossRef] [PubMed]
- WHO. Antimicrobial Stewardship Interventions: A Practical Guide; WHO Regional Office for Europe: Copenhagen, Denmark, 2021.
- Chuen-Im, T.; Suriyant, D.; Sawetsuwannakun, K.; Kitkumthorn, N. The Occurrence of Vibrionaceae, Staphylococcaceae, and Enterobacteriaceae in Green Turtle Chelonia mydas Rearing Seawater. J. Aquat. Anim. Health 2019, 31, 303–310. [Google Scholar] [CrossRef]
- Ahasan, M.S.; Kinobe, R.; Elliott, L.; Owens, L.; Scott, J.; Picard, J.; Huerlimann, R.; Ariel, E. Bacteriophage versus antibiotic therapy on gut bacterial communities of juvenile green turtle, Chelonia mydas. Environ. Microbiol. 2019, 21, 2871–2885. [Google Scholar] [CrossRef]
- Delli Paoli Carini, A.; Ariel, E.; Picard, J.; Elliott, L. Antibiotic Resistant Bacterial Isolates from Captive Green Turtles and In Vitro Sensitivity to Bacteriophages. Int. J. Microbiol. 2017, 2017, 5798161. [Google Scholar] [CrossRef]


| Antibiotics | Phenotypic Profile of Bacterial Strains (%) | ||
|---|---|---|---|
| Susceptible | Intermediate | Resistant | |
| CAZ | 78.1% | 4.4% | 17.5% |
| DO | 76.8% | 9.4% | 13.8% |
| ENR | 74.6% | 13.0% | 12.3% |
| UB | 75.6% | 13.3% | 11.1% |
| CN | 66.4% | 16.8% | 16.8% |
| OXY | 64.5% | 23.2% | 12.3% |
| SXT | 85.4% | 1.5% | 13.1% |
| Bacterial Family | Percentage of Resistant Strains | ||||||
|---|---|---|---|---|---|---|---|
| CAZ | DO | ENR | UB | CN | OXY | SXT | |
| Vibrionaceae | 8.1% | 7% | 5.8% | 1.2% | 10.5% | 7% | 8.2% |
| Shewanellaceae | 6.3% | 18.8% | 6.3% | 6.7% | 6.3% | 6.3% | 6.3% |
| Pseudomonadaceae | 50% | 50% | 50% | 33.3% | 33.3% | 50% | 66.7% |
| Enterobacteriaceae | 60% | 60% | 60% | 40% | 60% | 40% | 40% |
| Morganellaceae | 20% | 80% | 80% | 80% | 60% | 80% | 60% |
| Enterococcaceae | 75% | 0% | 20% | 60% | 100% | 0% | 0% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esposito, E.; Pace, A.; Affuso, A.; Oliviero, M.; Iaccarino, D.; Paduano, G.; Maffucci, F.; Fusco, G.; De Carlo, E.; Hochscheid, S.; et al. Antibiotic Resistance of Bacteria Isolated from Clinical Samples and Organs of Rescued Loggerhead Sea Turtles (Caretta caretta) in Southern Italy. Animals 2024, 14, 2103. https://doi.org/10.3390/ani14142103
Esposito E, Pace A, Affuso A, Oliviero M, Iaccarino D, Paduano G, Maffucci F, Fusco G, De Carlo E, Hochscheid S, et al. Antibiotic Resistance of Bacteria Isolated from Clinical Samples and Organs of Rescued Loggerhead Sea Turtles (Caretta caretta) in Southern Italy. Animals. 2024; 14(14):2103. https://doi.org/10.3390/ani14142103
Chicago/Turabian StyleEsposito, Emanuele, Antonino Pace, Andrea Affuso, Maria Oliviero, Doriana Iaccarino, Gianluigi Paduano, Fulvio Maffucci, Giovanna Fusco, Esterina De Carlo, Sandra Hochscheid, and et al. 2024. "Antibiotic Resistance of Bacteria Isolated from Clinical Samples and Organs of Rescued Loggerhead Sea Turtles (Caretta caretta) in Southern Italy" Animals 14, no. 14: 2103. https://doi.org/10.3390/ani14142103
APA StyleEsposito, E., Pace, A., Affuso, A., Oliviero, M., Iaccarino, D., Paduano, G., Maffucci, F., Fusco, G., De Carlo, E., Hochscheid, S., & Di Nocera, F. (2024). Antibiotic Resistance of Bacteria Isolated from Clinical Samples and Organs of Rescued Loggerhead Sea Turtles (Caretta caretta) in Southern Italy. Animals, 14(14), 2103. https://doi.org/10.3390/ani14142103





