Platelet-Rich Plasma Proteome of Mares Susceptible to Persistent-Breeding-Induced Endometritis Differs from Resistant Mares
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Husbandry
2.2. Platelet-Rich Preparation
2.3. Extraction of PRP Proteins
2.4. In-Gel Tryptic Digestion of Samples
2.5. Mass Spectrometry
2.6. Acquisition of Proteomics Data
2.7. Statistical Analysis
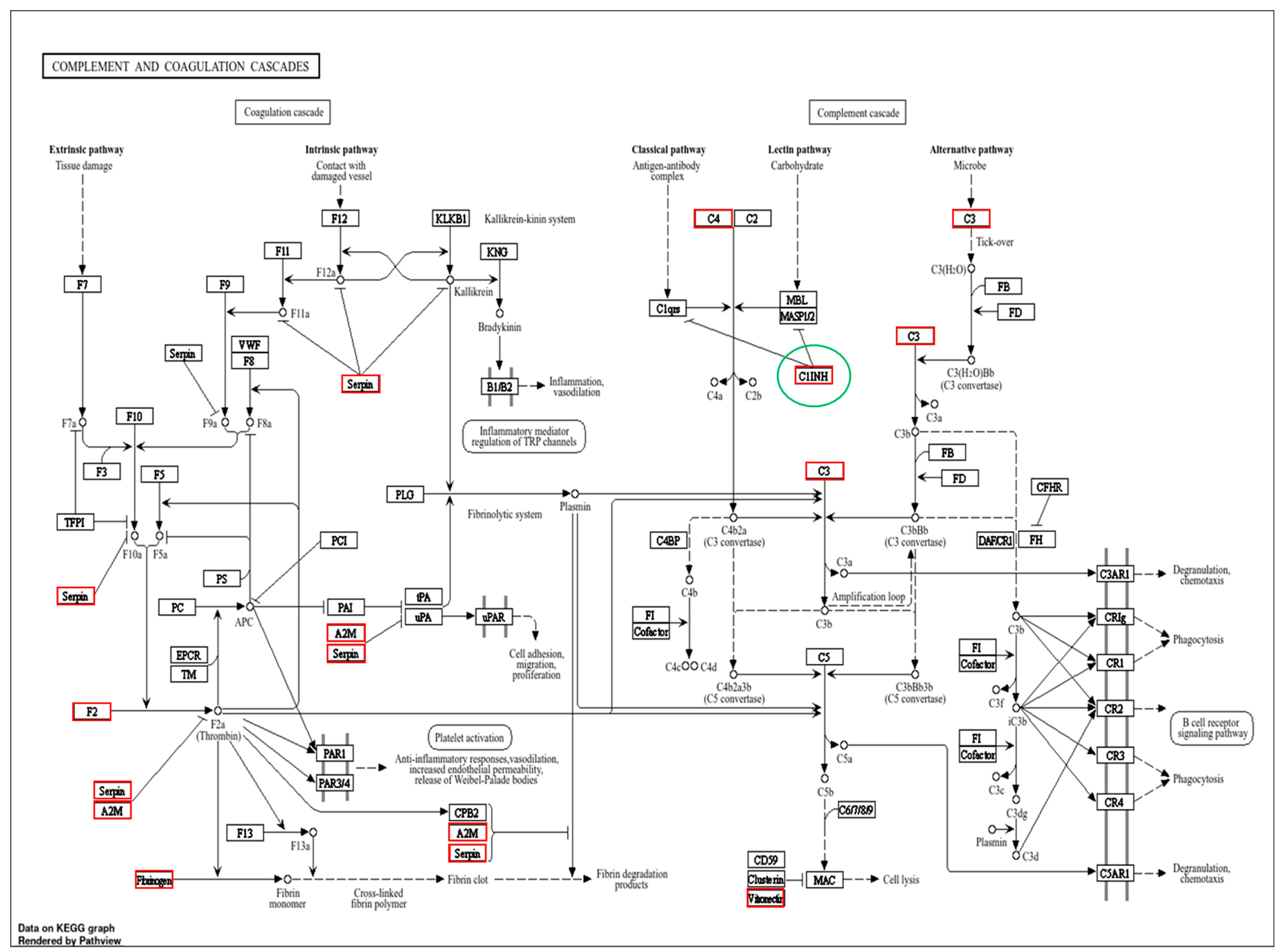
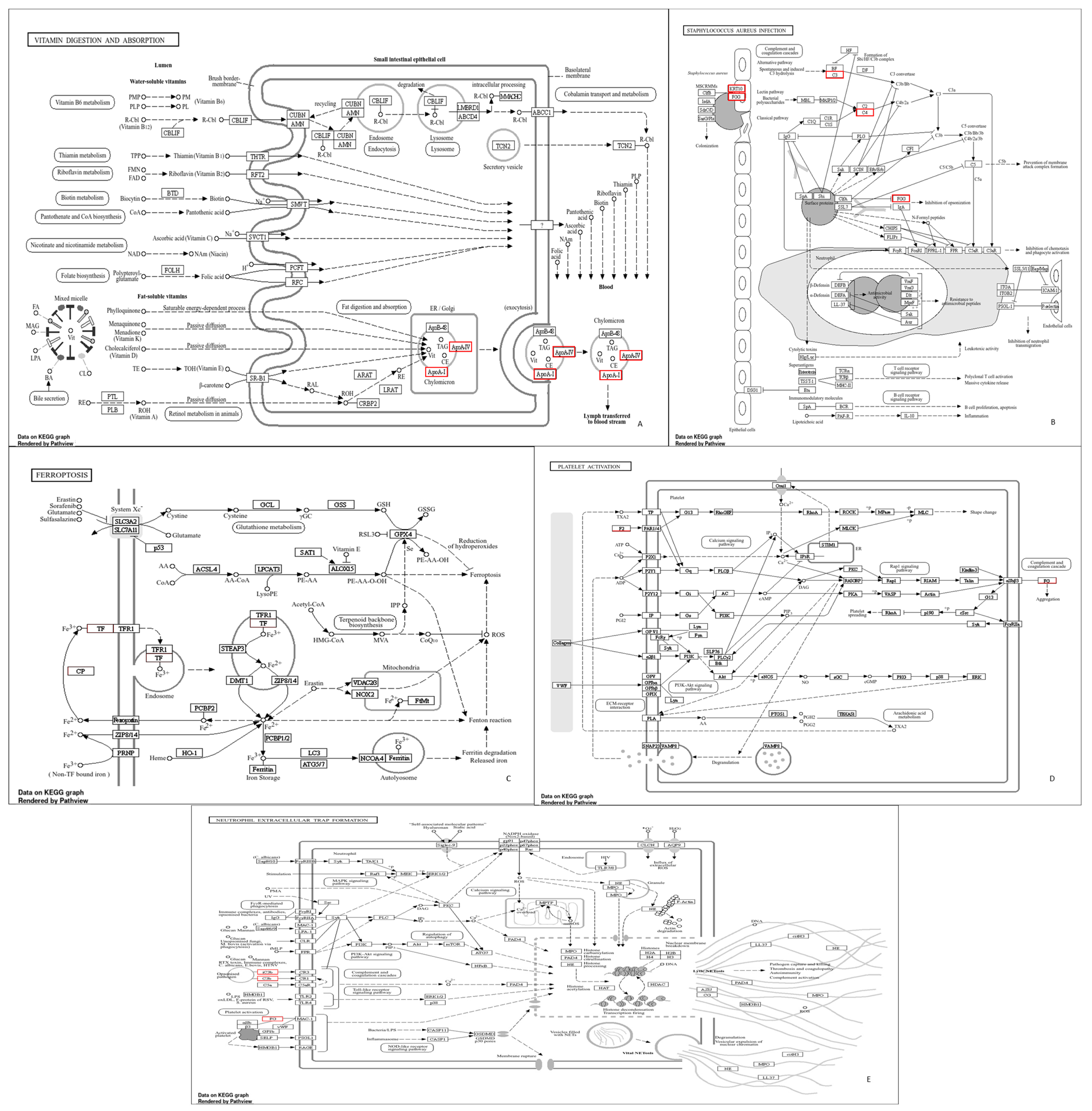
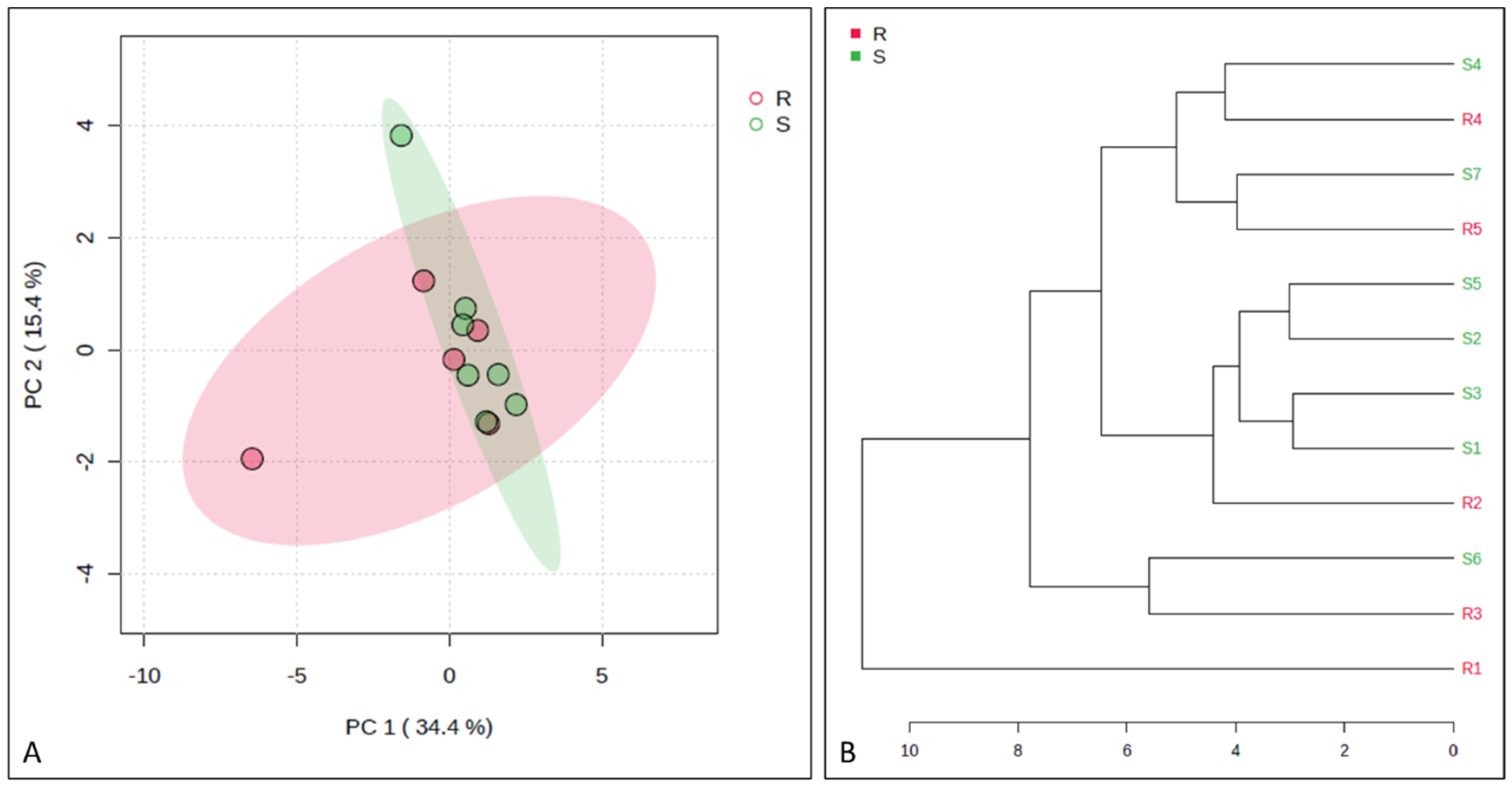
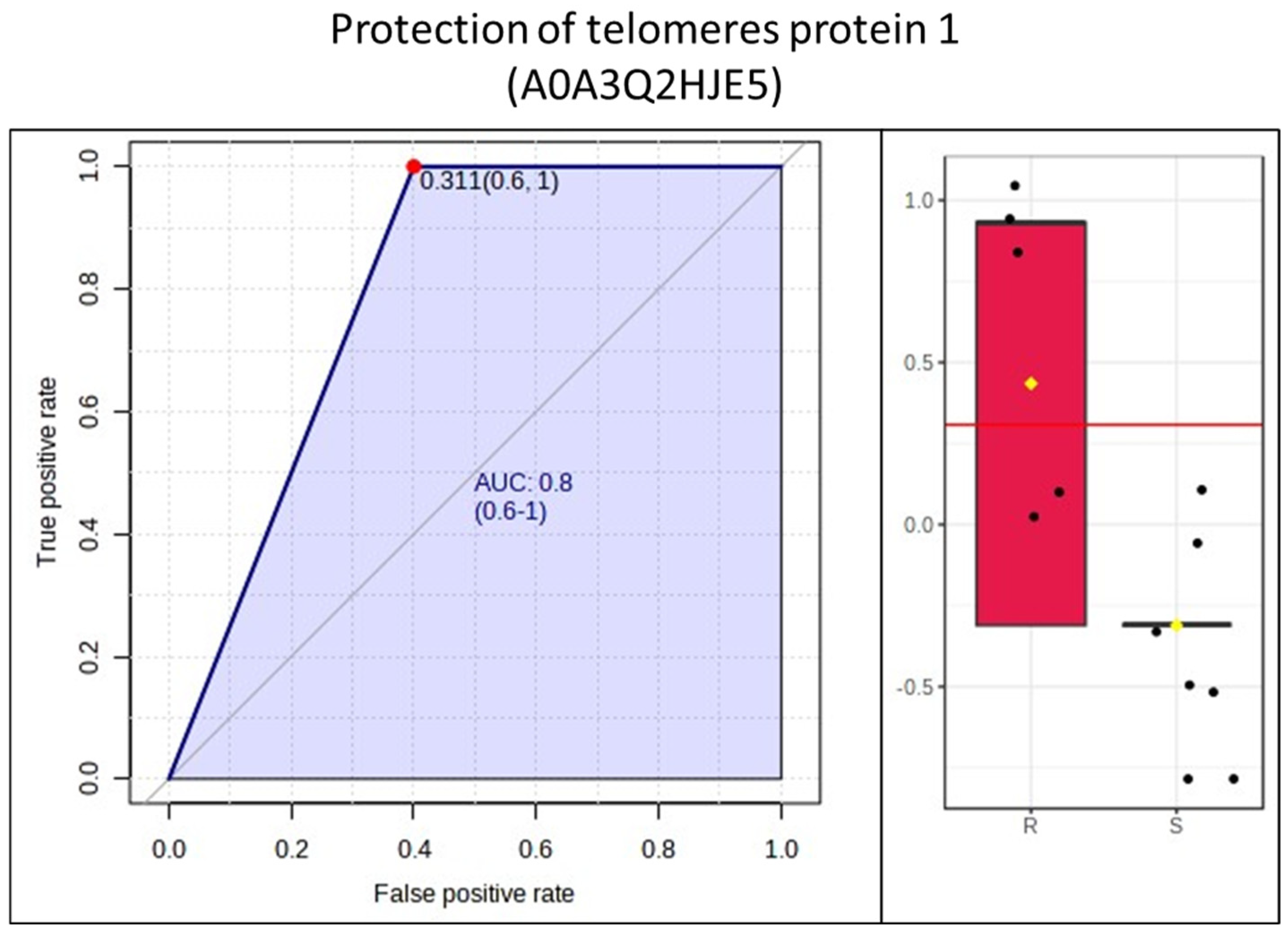
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Troedsson, M.; Liu, I.; Crabo, B. Sperm transport and survival in the mare. Theriogenology 1998, 49, 905–915. [Google Scholar] [CrossRef] [PubMed]
- Troedsson, M.H.T. Therapeutic considerations for mating-induced endometritis. Pferdeheilkunde 1997, 13, 516–520. [Google Scholar] [CrossRef]
- Troedsson, M. Uterine clearance and resistance to persistent endometritis in the mare. Theriogenology 1999, 52, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Traub-Dargatz, J.L.; Salman, M.D.; Voss, J.L. Medical problems of adult horses, as ranked by equine practitioners. J. Am. Vet. Med. Assoc. 1991, 198, 1745–1747. [Google Scholar] [CrossRef] [PubMed]
- Watson, E.D. Post-breeding endometritis in the mare. Anim. Reprod. Sci. 2000, 60–61, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Leblanc, M.M. Persistent mating-induced endometritis. In Current Therapy in Equine Medicine, 5th ed.; Science, E., Ed.; Saunders: St. Louis, MO, USA, 2003; pp. 234–237. [Google Scholar]
- Leblanc, M.; Neuwirth, L.; Jones, L.; Cage, C.; Mauragis, D. Differences in uterine position of reproductively normal mares and those with delayed uterine clearance detected by scintigraphy. Theriogenology 1998, 50, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Fumuso, E.; Giguère, S.; Wade, J.; Rogan, D.; Videla-Dorna, I.; Bowden, R.A. Endometrial IL-1β, IL-6 and TNF-α, mRNA expression in mares resistant or susceptible to post-breeding endometritis: Effects of estrous cycle, artificial insemination, and immunomodulation. Vet. Immunol. Immunopathol. 2003, 96, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Kaplanski, G.; Marin, V.; Montero-Julian, F.; Mantovani, A.; Farnarier, C. IL-6: A regulator of the transition from neutrophil to monocyte recruitment during inflammation. Trends Immunol. 2003, 24, 25–29. [Google Scholar] [CrossRef]
- Fumuso, E.A.; Aguilar, J.; Giguère, S.; Rivulgo, M.; Wade, J.; Rogan, D. Immune parameters in mares resistant and susceptible to persistent post-breeding endometritis: Effects of immunomodulation. Vet. Immunol. Immunopathol. 2007, 118, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Christoffersen, M.; Woodward, E.; Bojesen, A.; Petersen, M.; Squires, E.; Lehn-Jensen, H.; Troedsson, M. Effect of immunomodulatory therapy on the endometrial inflammatory response to induced infectious endometritis in susceptible mares. Theriogenology 2012, 78, 991–1004. [Google Scholar] [CrossRef] [PubMed]
- Canisso, I.F.; Stewart, J.; da Silva, M.A.C. Endometritis: Managing persistent post-breeding endometritis. Vet. Clin. N. Am. Equine Pr. 2016, 32, 465–480. [Google Scholar] [CrossRef] [PubMed]
- Canisso, I.F.; Segabinazzi, L.G.; Fedorka, C.E. Persistent breeding-induced endometritis in mares—A multifaceted challenge: From clinical aspects to immunopathogenesis and pathobiology. Int. J. Mol. Sci. 2020, 21, 1432. [Google Scholar] [CrossRef] [PubMed]
- Ferris, R.A.; McCue, P.M.; Borlee, G.I.; Loncar, K.D.; Hennet, M.L.; Borlee, B.R. In vitro efficacy of nonantibiotic treatments on biofilm disruption of gram-negative pathogens and an in vivo model of infectious endometritis utilizing isolates from the equine uterus. J. Clin. Microbiol. 2016, 54, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Scoggin, C.F. Endometritis: Nontraditional Therapies. Vet. Clin. N. Am. Equine Pr. 2016, 32, 499–511. [Google Scholar] [CrossRef]
- Metcalf, E. The effect of Platelet-Rich Plasma (PRP) on intraluminal fluid and pregnancy rates in mares susceptible to Persistent Mating-Induced Endometritis (PMIE). J. Equine Vet. Sci. 2014, 34, 128. [Google Scholar] [CrossRef]
- Reghini, M.F.S.; Ramires Neto, C.; Segabinazzi, L.G.; Castro Chaves, M.M.B.; Dell’Aqua, C.D.P.F.; Bussiere, M.C.C.; Dell’Aqua, J.A.; Papa, F.O.; Alvarenga, M.A. Inflammatory response in chronic degenerative endometritis mares treated with platelet-rich plasma. Theriogenology 2016, 86, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Friso, A.d.M. Resposta Inflamatória Uterina de Éguas com Endometrite Persistente Pós-Cobertura Tratadas com Firocoxib. 2016. Available online: https://repositorio.unesp.br/items/d8a3c27b-1842-4573-9222-56984a0e3275 (accessed on 1 June 2024).
- Segabinazzi, L.G.; Friso, A.M.; Correal, S.B.; Crespilho, A.M.; Dell’Aqua, J.A.; Miró, J.; Papa, F.O.; Alvarenga, M.A. Uterine clinical findings, fertility rate, leucocyte migration, and COX-2 protein levels in the endometrial tissue of susceptible mares treated with platelet-rich plasma before and after AI. Theriogenology 2017, 104, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Pedraza, C.E.; Nikolcheva, L.G.; Kaartinen, M.T.; Barralet, J.E.; McKee, M.D. Osteopontin functions as an opsonin and facilitates phagocytosis by macrophages of hydroxyapatite-coated microspheres: Implications for bone wound healing. Bone 2008, 43, 708–716. [Google Scholar] [CrossRef] [PubMed]
- Segabinazzi, L.G.T.M.; Podico, G.; Rosser, M.F.; Nanjappa, S.G.; Alvarenga, M.A.; Canisso, I.F. Three manual noncommercial methods to prepare equine platelet-rich plasma. Animals 2021, 11, 1478. [Google Scholar] [CrossRef] [PubMed]
- Shevchenko, A.; Tomas, H.; Havlis, J.; Olsen, J.V.; Mann, M. In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat. Protoc. 2006, 1, 2856–2860. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Zhou, G.; Ewald, J.; Chang, L.; Hacariz, O.; Basu, N.; Xia, J. Using MetaboAnalyst 5.0 for LC–HRMS spectra processing, multi-omics integration and covariate adjustment of global metabolomics data. Nat. Protoc. 2022, 17, 1735–1761. [Google Scholar] [CrossRef] [PubMed]
- Vincendeau, P.; Bouteille, B. Immunology and immunopathology of African trypanosomiasis. An. Acad. Bras. Cienc. 2006, 78, 645–665. [Google Scholar] [CrossRef] [PubMed]
- Amara, U.; Rittirsch, D.; Flierl, M.; Bruckner, U.; Klos, A.; Gebhard, F.; Lambris, J.D.; Huber-Lang, M. Interaction between the coagulation and complement system. Adv. Exp. Med. Biol. 2008, 632, 68–76. [Google Scholar]
- Ali, R.A.; Wuescher, L.M.; Worth, R.G. Platelets: Essential components of the immune system. Curr. Trends Immunol. 2015, 16, 65–78. [Google Scholar] [PubMed]
- Roy, N.; Ohtani, K.; Hidaka, Y.; Amano, Y.; Matsuda, Y.; Mori, K.; Hwang, I.; Inoue, N.; Wakamiya, N. Three pentraxins C-reactive protein, serum amyloid p component and pentraxin 3 mediate complement activation using Collectin CL-P1. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Hurler, L.; Toonen, E.J.M.; Kajdácsi, E.; van Bree, B.; Brandwijk, R.J.M.G.E.; de Bruin, W.; Lyons, P.A.; Bergamaschi, L.; Sinkovits, G.; Cervenak, L.; et al. Distinction of early complement classical and lectin pathway activation via quantification of C1s/C1-INH and MASP-1/C1-INH complexes using novel ELISAs. Front. Immunol. 2022, 13, 1039765. [Google Scholar] [CrossRef] [PubMed]
- Zeerleder, S. C1-Inhibitor: More Than a Serine Protease Inhibitor. Semin. Thromb. Hemost. 2011, 37, 362–374. [Google Scholar] [CrossRef] [PubMed]
- Wolska, A.; Dunbar, R.L.; Freeman, L.A.; Ueda, M.; Amar, M.J.; Sviridov, D.O.; Remaley, A.T. Apolipoprotein C-II: New findings related to genetics, biochemistry, and role in triglyceride metabolism. Atherosclerosis 2017, 267, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.D.; Tontonoz, P. Liver X receptors at the intersection of lipid metabolism and atherogenesis. Atherosclerosis 2015, 242, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Fessler, M.B. The challenges and promise of targeting the liver X receptors for treatment of inflammatory disease. Pharmacol. Ther. 2017, 181, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Green, D.S.; Young, H.A.; Valencia, J.C. Current prospects of type II interferon γ signaling and autoimmunity. J. Biol. Chem. 2017, 292, 13925–13933. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Rane, G.; Dai, X.; Shanmugam, M.K.; Arfuso, F.; Samy, R.P.; Lai, M.K.P.; Kappei, D.; Kumar, A.P.; Sethi, G. Ageing and the telomere connection: An intimate relationship with inflammation. Ageing Res. Rev. 2016, 25, 55–69. [Google Scholar] [CrossRef] [PubMed]
- Sanders, J.L.; Newman, A.B. Telomere Length in Epidemiology: A Biomarker of Aging, Age-Related Disease, Both, or Neither? Epidemiol. Rev. 2013, 35, 112–131. [Google Scholar] [CrossRef] [PubMed]
- O’Donovan, A.; Pantell, M.S.; Puterman, E.; Dhabhar, F.S.; Blackburn, E.H.; Yaffe, K.; Cawthon, R.M.; Opresko, P.L.; Hsueh, W.-C.; Satterfield, S.; et al. Cumulative Inflammatory Load Is Associated with Short Leukocyte Telomere Length in the Health, Aging and Body Composition Study. PLoS ONE 2011, 6, e19687. [Google Scholar] [CrossRef] [PubMed]
- Aubert, G.; Lansdorp, P.M.; Forrester, S.J.; Booz, G.W.; Sigmund, C.D.; Coffman, T.M.; Kawai, T.; Rizzo, V.; Scalia, R.; Eguchi, S.; et al. Telomeres and Aging. Physiol. Rev. 2008, 88, 557–579. [Google Scholar] [CrossRef] [PubMed]
- Freund, A.; Orjalo, A.V.; Desprez, P.-Y.; Campisi, J. Inflammatory networks during cellular senescence: Causes and consequences. Trends Mol. Med. 2010, 16, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Rodier, F.; Campisi, J. Four faces of cellular senescence. J. Cell Biol. 2011, 192, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Andrews, N.P.; Fujii, H.G.J. Weyand Telomeres and immunological diseases of aging. Gerontology 2010, 56, 390–403. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, P.C.; Hemerly, A.S.; Villarroel, R.; Van Montagu, M.; Inzé, D. The Arabidopsis functional homolog of the p34cdc2 protein kinase. Plant Cell 1991, 3, 531–540. [Google Scholar] [CrossRef]
- Barnum, S.R. C4a: An Anaphylatoxin in Name Only. J. Innate Immun. 2015, 7, 333–339. [Google Scholar] [CrossRef]
- Pasupuleti, M.; Walse, B.; Nordahl, E.A.; Mörgelin, M.; Malmsten, M.; Schmidtchen, A. Preservation of antimicrobial properties of complement peptide C3a, from invertebrates to humans. J. Biol. Chem. 2007, 282, 2520–2528. [Google Scholar] [CrossRef] [PubMed]
- Abebe, E.C.; Muche, Z.T.; Mariam, A.B.; Ayele, T.M.; Agidew, M.M.; Azezew, M.T.; Zewde, E.A.; Dejenie, T.A.; Mengstie, M.A. The structure, biosynthesis, and biological roles of fetuin-A: A review. Front. Cell Dev. Biol. 2022, 10, 945287. [Google Scholar] [CrossRef]
- Bokisch, V.A.; Müller-Eberhard, H.J.; Cochrane, C.G. Isolatin of a fragment (C3a) of the third component of human complement containing anaphylatoxin and chemo-tactic activity and description of an anaphylatoxin inactivator of human serum. J. Exp. Med. 1969, 129, 1109–1130. [Google Scholar] [CrossRef] [PubMed]
- Nordahl, E.A.; Rydengård, V.; Nyberg, P.; Nitsche, D.P.; Mörgelin, M.; Malmsten, M.; Björck, L.; Schmidtchen, A. Activation of the complement system generates antibacterial peptides. Proc. Natl. Acad. Sci. USA 2004, 101, 16879–16884. [Google Scholar] [CrossRef] [PubMed]
- Troedsson, M.H.; Liu, I.K.; Thurmond, M. Immunoglobulin (IgG and IgA) and complement (C3) concentrations in uterine secretion following an intrauterine challenge of Streptococcus Zooepidemicus in mares susceptible to versus resistant to chronic uterine infection1. Biol. Reprod. 1993, 49, 502–506. [Google Scholar] [CrossRef] [PubMed]
- Asbury, A.; Halliwell, R.; Foster, G.; Longino, S. Immunoglobulins in uterine secretions of mares with differing resistance to endometritis. Theriogenology 1980, 14, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Segabinazzi, L.G.T.M.; Canisso, I.F.; Podico, G.; Cunha, L.L.; Novello, G.; Rosser, M.F.; Loux, S.C.; Lima, F.S.; Alvarenga, M.A. Intrauterine Blood Plasma Platelet-Therapy Mitigates Persistent Breeding-Induced Endometritis, Reduces Uterine Infections, and Improves Embryo Recovery in Mares. Antibiotics 2021, 10, 490. [Google Scholar] [CrossRef] [PubMed]



| Whole Blood | PRP | |||||
|---|---|---|---|---|---|---|
| Platelets (×103) | WBCs (×103) | RBCs (×106) | Platelets (×103) | WBCs (×103) | RBCs (×106) | |
| Resistant (n = 5) | 163 ± 32.2 | 7.4 ± 1.8 | 931 ± 436 | 561 ± 152 | 12.6 ± 4.45 | 3.40 ± 4.92 |
| Susceptible (n = 7) | 168 ± 32.6 | 9.3 ± 1.9 | 889 ± 94 | 768 ± 395 | 17.7 ± 17.9 | 4 ± 3.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Novello, G.; Souza, F.F.; Canisso, I.F. Platelet-Rich Plasma Proteome of Mares Susceptible to Persistent-Breeding-Induced Endometritis Differs from Resistant Mares. Animals 2024, 14, 2100. https://doi.org/10.3390/ani14142100
Novello G, Souza FF, Canisso IF. Platelet-Rich Plasma Proteome of Mares Susceptible to Persistent-Breeding-Induced Endometritis Differs from Resistant Mares. Animals. 2024; 14(14):2100. https://doi.org/10.3390/ani14142100
Chicago/Turabian StyleNovello, Guilherme, Fabiana F. Souza, and Igor F. Canisso. 2024. "Platelet-Rich Plasma Proteome of Mares Susceptible to Persistent-Breeding-Induced Endometritis Differs from Resistant Mares" Animals 14, no. 14: 2100. https://doi.org/10.3390/ani14142100
APA StyleNovello, G., Souza, F. F., & Canisso, I. F. (2024). Platelet-Rich Plasma Proteome of Mares Susceptible to Persistent-Breeding-Induced Endometritis Differs from Resistant Mares. Animals, 14(14), 2100. https://doi.org/10.3390/ani14142100





