Feasibility of Nanostructured Lipid Carrier Loaded with Alpha-Mangostin and Clove Oil for Canine Periodontal Therapy
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
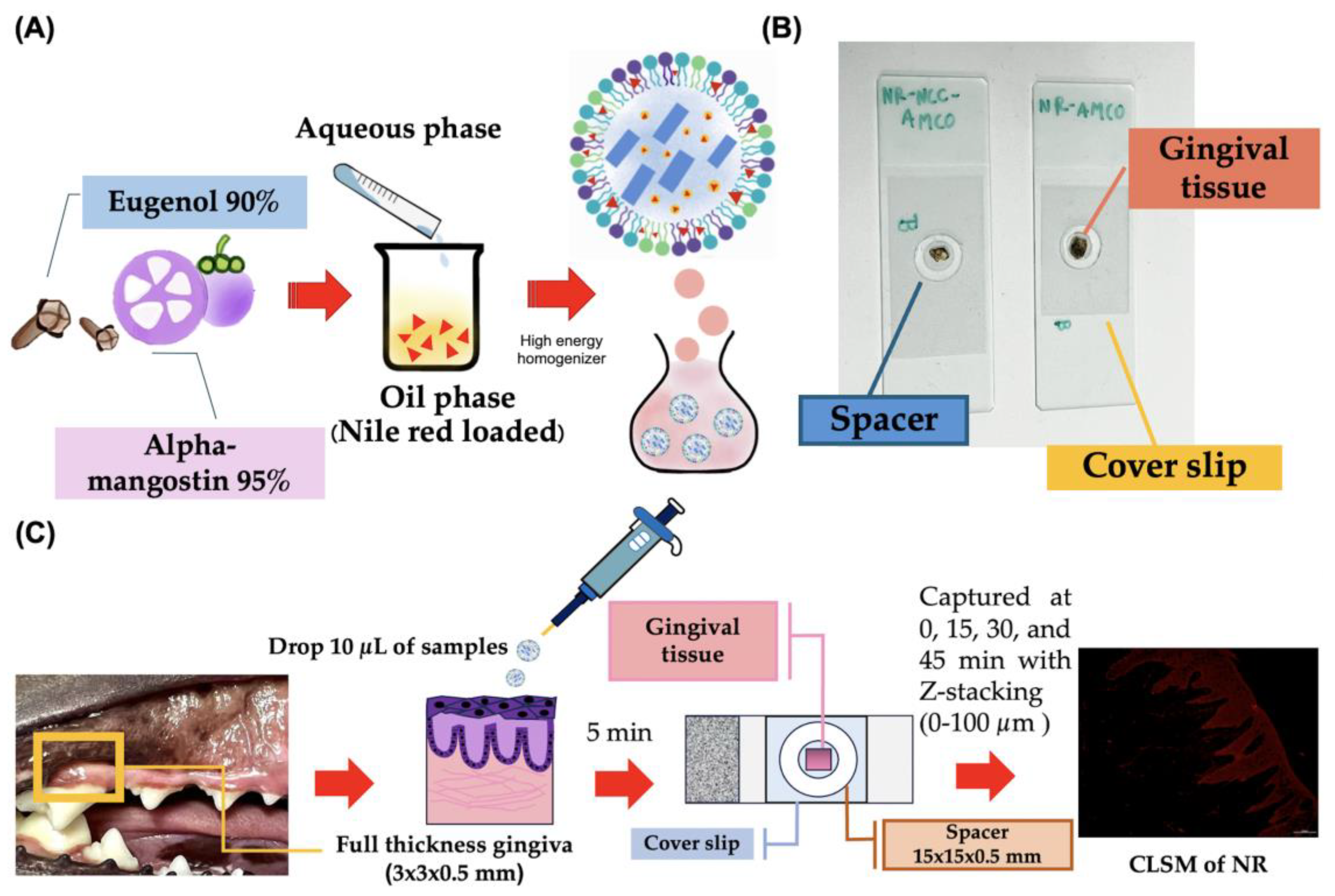
2.1. NLC-AMCO Preparation
2.1.1. The Oil Phase
2.1.2. Aqueous Phase
2.1.3. Formula Fabrication
2.2. Physicochemical Properties of NLC-AMCO
2.2.1. Nanoparticle Characteristics
2.2.2. Stability
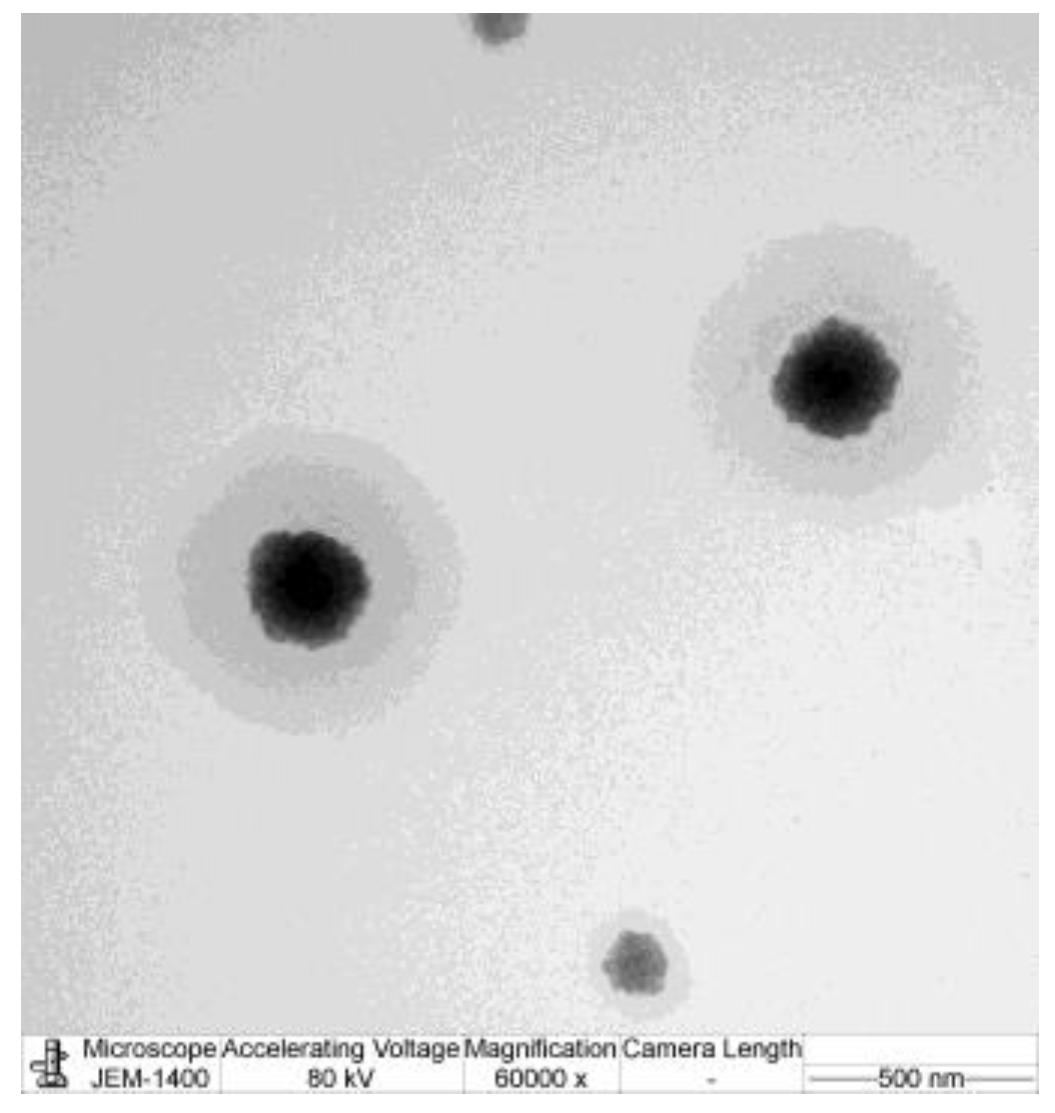
2.2.3. Transmission Electron Microscopy (TEM)
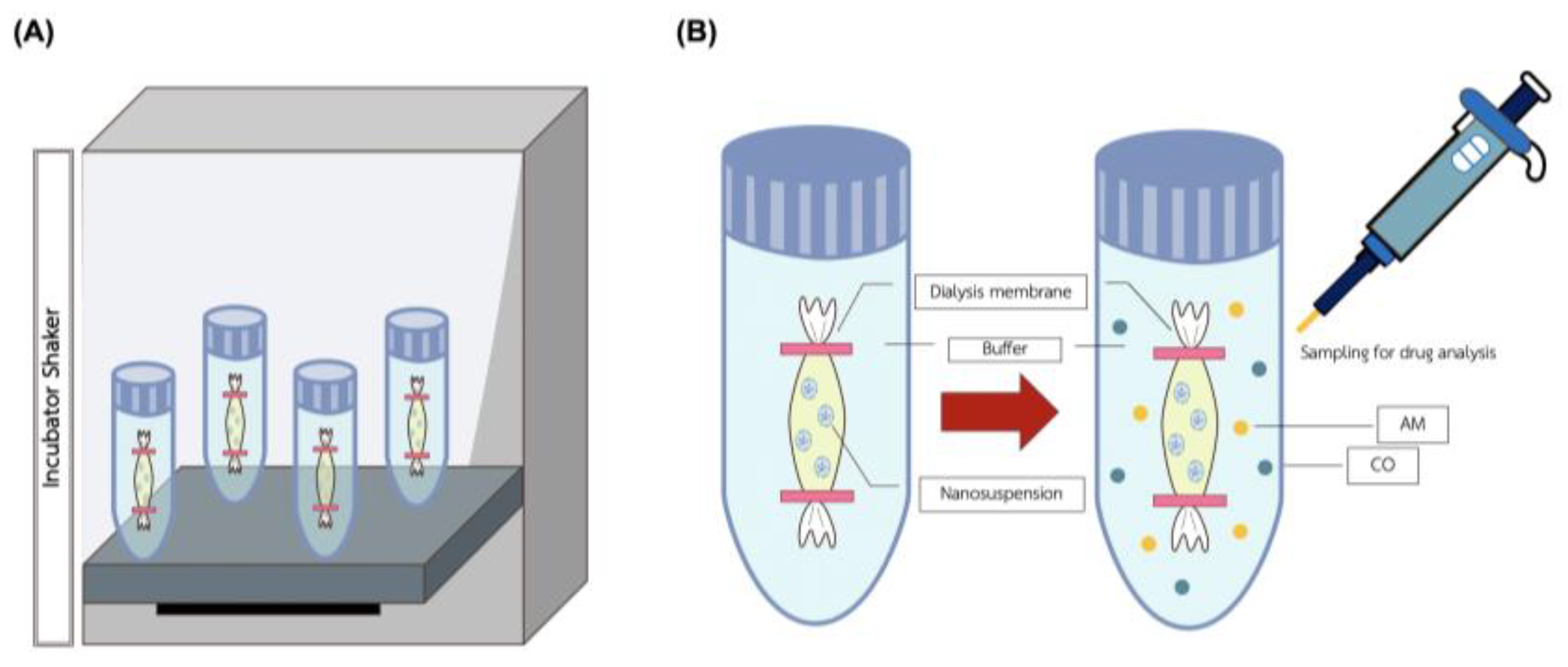
2.2.4. Kinetic Release of NLC-AMCO
2.2.5. Drug Loading and Release from NLC-AMCO
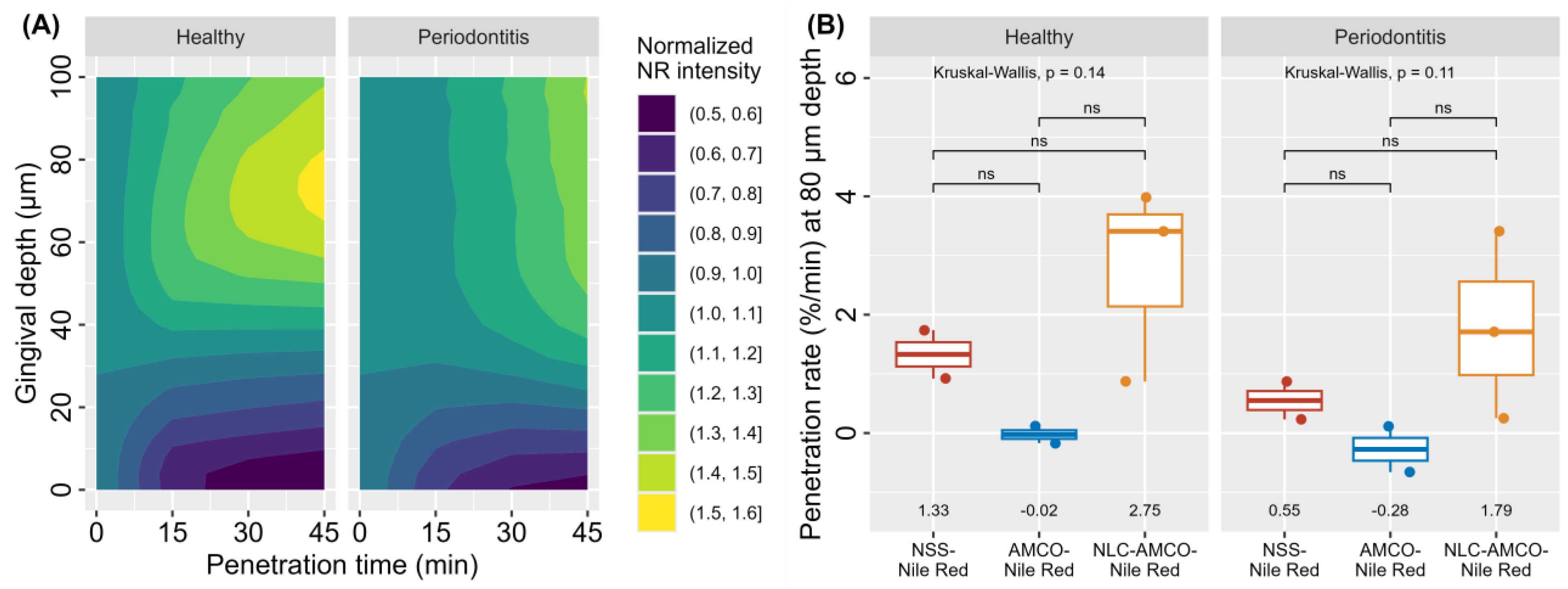
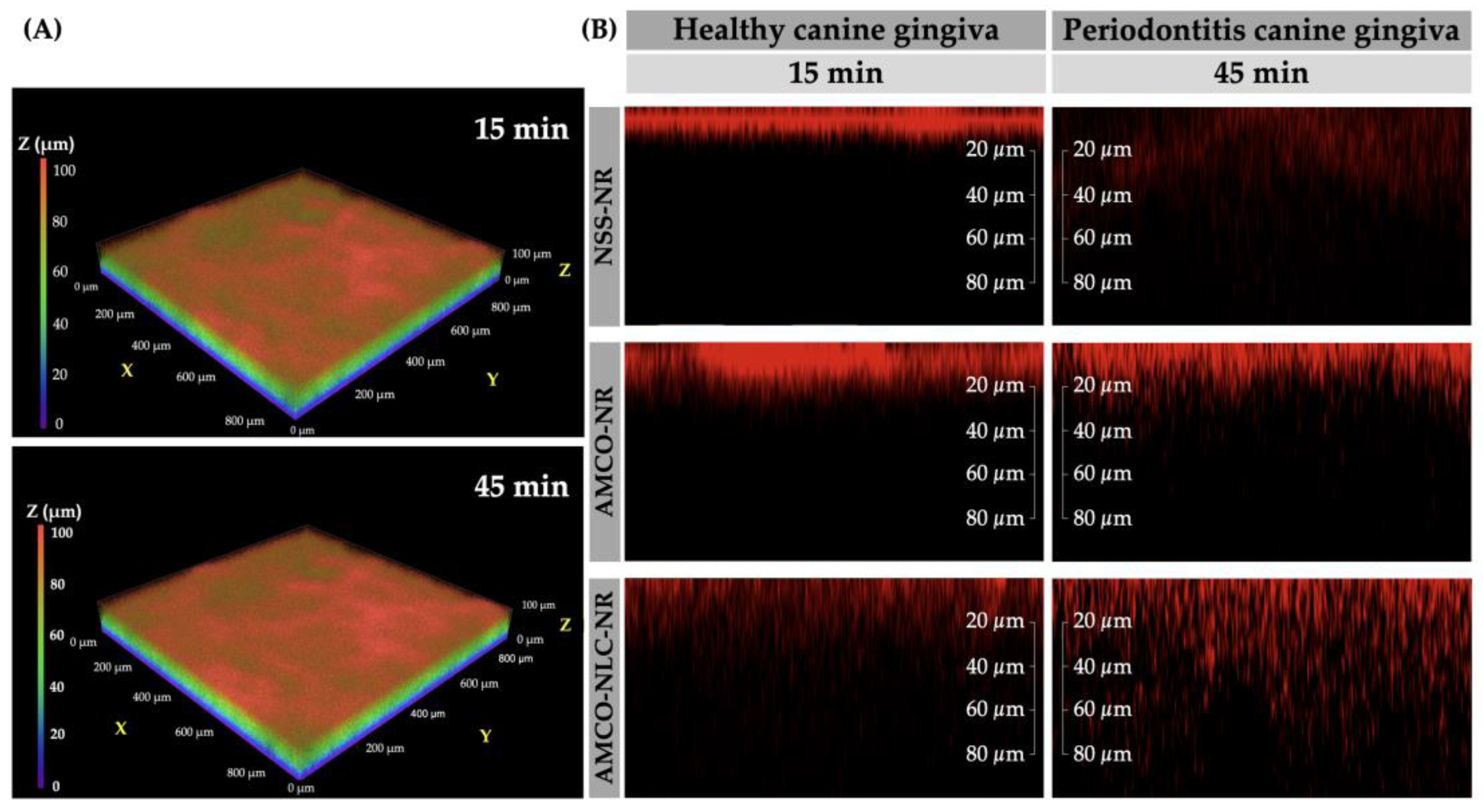
2.2.6. Realtime Ex Vivo Canine Gingiva Penetration
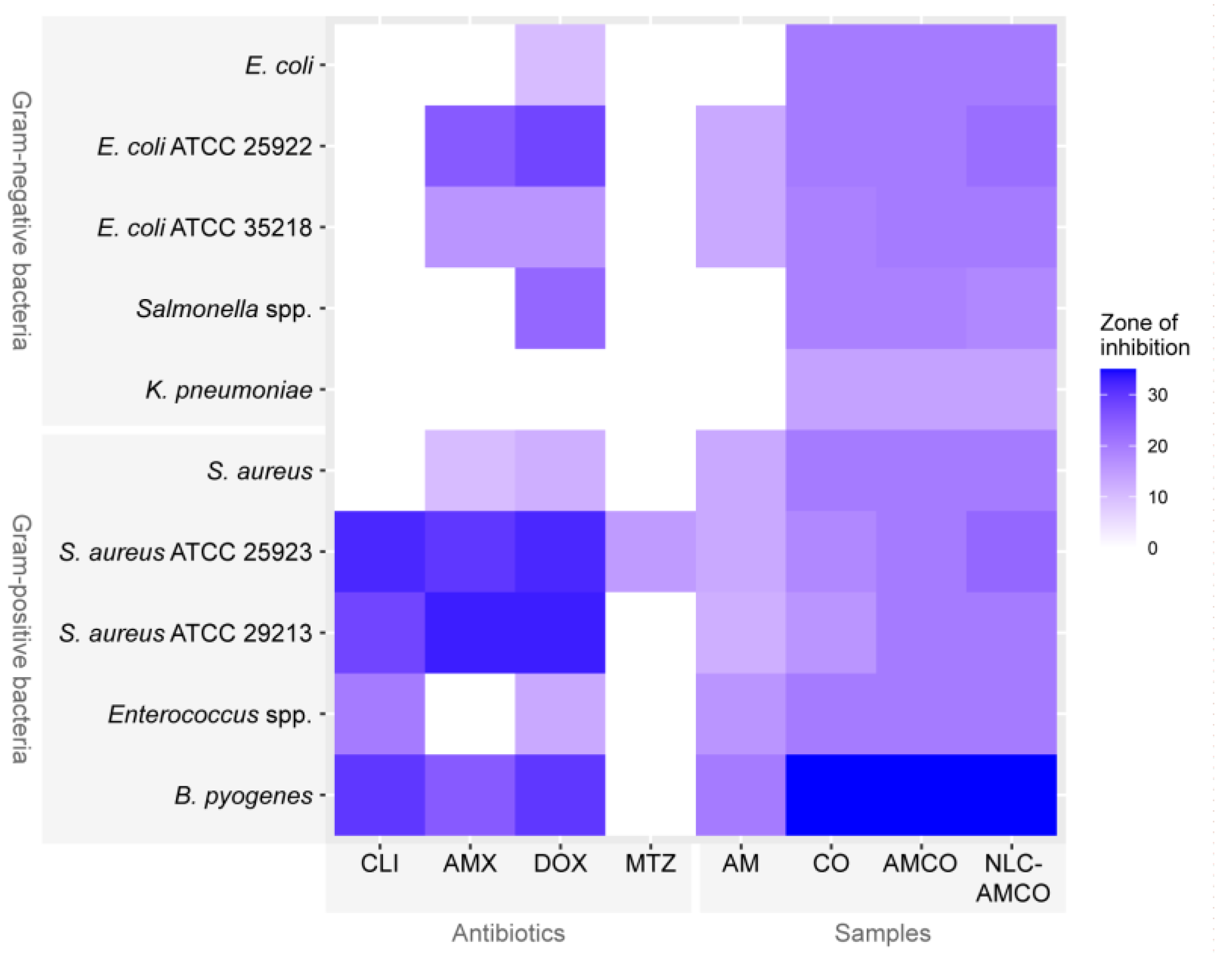
2.2.7. Antibacterial Properties
2.3. Statistical Analysis
3. Results
3.1. Physical Characteristics of NLC-AMCO
3.2. Stability of NLC-AMCO
3.3. Transmission Electron Microscopy (TEM)
3.4. Kinetic Release of NLC-AMCO
3.5. Drug Loading and Release from NLC-AMCO
3.6. Realtime Ex Vivo Canine Gingiva Penetration
3.7. Antibacterial Activity of NLC-AMCO
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McFadden, T.; Marretta, S.M. Consequences of untreated periodontal disease in dogs and cats. J. Vet. Dent. 2013, 30, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Niemiec, B.A. Periodontal disease. Top. Companion Anim. Med. 2008, 23, 72–80. [Google Scholar] [CrossRef]
- Marretta, S.M. The common and uncommon clinical presentations and treatment of periodontal disease in the dog and cat. Semin. Vet. Med. Surg. 1987, 2, 230–240. [Google Scholar]
- Enlund, K.B.; Brunius, C.; Hanson, J.; Hagman, R.; Höglund, O.V.; Gustås, P.; Pettersson, A. Dog Owners’ Perspectives on Canine Dental Health—A Questionnaire Study in Sweden. Front. Vet. Sci. 2020, 7, 298. [Google Scholar] [CrossRef] [PubMed]
- Niemiec, B. Veterinary Periodontology, 1st ed.; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Silva, N.; Abusleme, L.; Bravo, D.; Dutzan, N.; Garcia-Sesnich, J.; Vernal, R.; Hernández, M.; Gamonal, J. Host response mechanisms in periodontal diseases. J. Appl. Oral Sci. 2015, 23, 329–355. [Google Scholar] [CrossRef] [PubMed]
- Cekici, A.; Kantarci, A.; Hasturk, H.; Van Dyke, T.E. Inflammatory and immune pathways in the pathogenesis of periodontal disease. Periodontology 2000 2014, 64, 57–80. [Google Scholar] [CrossRef] [PubMed]
- Gościniak, A.; Paczkowska-Walendowska, M.; Skotnicka, A.; Ruchała, M.A.; Cielecka-Piontek, J. Can plant materials be valuable in the treatment of periodontal diseases? Practical review. Pharmaceutics 2021, 13, 2185. [Google Scholar] [CrossRef]
- Ramesh, A.; Varghese, S.S.; Doraiswamy, J.N.; Malaiappan, S. Herbs as an antioxidant arsenal for periodontal diseases. J. Intercult. Ethnopharmacol. 2016, 5, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Aljuanid, M.A.; Qaid, H.R.; Lashari, D.M.; Ridwan, R.D.; Budi, H.S.; Alkadasi, B.A.; Ramadhani, Y.; Rahmasari, R. Nano-emulsion of mangosteen rind extract in a mucoadhesive patch for periodontitis regenerative treatment: An in vivo study. J. Taibah Univ. Med. Sci. 2022, 17, 910–920. [Google Scholar] [CrossRef]
- Pulikottil, S.; Nath, S. Potential of clove of Syzygium aromaticum in development of a therapeutic agent for periodontal disease: A review. S. Afr. Dent. J. 2015, 70, 108–115. [Google Scholar]
- Tangsuksan, P.; Srichana, T.; Kettratad, M.; Nittayananta, W. Antimicrobial and Anti-inflammatory Effects of α-Mangostin Soluble Film. J. Int. Soc. Prev. Community Dent. 2022, 12, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Koh, J.J.; Qiu, S.; Zou, H.; Lakshminarayanan, R.; Li, J.; Zhou, X.; Tang, C.; Saraswathi, P.; Verma, C.; Tan, D.T.; et al. Rapid bactericidal action of Alpha-Mangostin against MRSA as an outcome of membrane targeting. Biochim. Biophys. Acta 2013, 1828, 834–844. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Xu, H.; Li, D.; Chen, J.; Yu, Z.; Deng, Q.; Li, P.; Zheng, J.; Zhang, H. Mechanisms of Rapid Bactericidal and Anti-Biofilm Alpha-Mangostin In Vitro Activity against Staphylococcus aureus. Pol. J. Microbiol. 2023, 72, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Felix, L.; Mishra, B.; Khader, R.; Ganesan, N.; Mylonakis, E. In Vitro and in Vivo Bactericidal and Antibiofilm Efficacy of Alpha Mangostin against Staphylococcus Aureus Persister Cells. Front. Cell. Infect. Microbiol. 2022, 12, 898794. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, Y.; Zhu, X.; Cao, P.; Wei, S.; Lu, Y. Antibacterial and antibiofilm activities of eugenol from essential oil of Syzygium aromaticum (L.) Merr. & L. M. Perry (clove) leaf against periodontal pathogen Porphyromonas gingivalis. Microb. Pathog. 2017, 113, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Husna, M.; Nasution, R.; Ilyas, S.; Wulandari, P.; Syahputra, A. The effect of 4% mangosteen peel extract gel on interleukin-6 (il-6) levels and clinical parameters in stage iii grade b periodontitis patients. Int. J. Appl. Dent. Sci. 2022, 8, 531–534. [Google Scholar] [CrossRef]
- Taher, Y.A.; Samud, A.M.; El-Taher, F.E.; ben-Hussin, G.; Elmezogi, J.S.; Al-Mehdawi, B.F.; Salem, H.A. Experimental evaluation of anti-inflammatory, antinociceptive and antipyretic activities of clove oil in mice. Libyan J. Med. 2015, 10, 28685. [Google Scholar] [CrossRef]
- Alqareer, A.; Alyahya, A.; Andersson, L. The effect of clove and benzocaine versus placebo as topical anesthetics. J. Dent. 2006, 34, 747–750. [Google Scholar] [CrossRef] [PubMed]
- Zanul Abidin, Z.; Mohd Salleh, N.; Himratul-Aznita, W.H.; Ahmad, S.F.; Lim, G.S.; Raja Mohd, N.; Dziaruddin, N. Antifungal effects of eugenol on Candida albicans adherence to denture polymers. PeerJ 2023, 11, e15750. [Google Scholar] [CrossRef]
- Ulanowska, M.; Olas, B. Biological Properties and Prospects for the Application of Eugenol—A Review. Int. J. Mol. Sci. 2021, 22, 3671. [Google Scholar] [CrossRef]
- Alsaad, A.; Hussien, A.A.; Gareeb, M.M. Solid lipid nanoparticles (SLN) as a novel drug delivery system: A theoretical review. Syst. Rev. Pharm. 2020, 11, 259–273. [Google Scholar]
- Chaivisuthangkura, A.; Malaikaew, Y.; Chaovanalikit, A.; Jaratrungtawee, A.; Panseeta, P.; Ratananukul, P.; Suksamrarn, S. Prenylated xanthone composition of Garcinia mangostana (mangosteen) fruit hull. Chromatographia 2009, 69, 315–318. [Google Scholar] [CrossRef]
- Sun, S.B.; Liu, P.; Shao, F.M.; Miao, Q.L. Formulation and evaluation of PLGA nanoparticles loaded capecitabine for prostate cancer. Int. J. Clin. Exp. Med. 2015, 8, 19670–19681. [Google Scholar] [PubMed]
- Nii, T.; Ishii, F. Encapsulation efficiency of water-soluble and insoluble drugs in liposomes prepared by the microencapsulation vesicle method. Int. J. Pharm. 2005, 298, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Philpotts, C.; Harding, C.; Carlile, M.; Sadler, C.; Wright, J. Ex vivo delivery and penetration of α-tocopherol acetate and linoleic acid to gingival tissue from a toothpaste formulation. Int. Dent. J. 2007, 57, 129–134. [Google Scholar] [CrossRef]
- Stracke, F.; Weiss, B.; Lehr, C.-M.; König, K.; Schaefer, U.F.; Schneider, M. Multiphoton microscopy for the investigation of dermal penetration of nanoparticle-borne drugs. J. Investig. Dermatol. 2006, 126, 2224–2233. [Google Scholar] [CrossRef] [PubMed]
- Wizenty, J.; Schumann, T.; Theil, D.; Stockmann, M.; Pratschke, J.; Tacke, F.; Aigner, F.; Wuensch, T. Recent Advances and the Potential for Clinical Use of Autofluorescence Detection of Extra-Ophthalmic Tissues. Molecules 2020, 25, 2095. [Google Scholar] [CrossRef] [PubMed]
- Hudzicki, J. Kirby-Bauer disk diffusion susceptibility test protocol. Am. Soc. Microbiol. 2009, 15, 55–63. [Google Scholar]
- Khan, S.; Sharma, A.; Jain, V. An Overview of Nanostructured Lipid Carriers and its Application in Drug Delivery through Different Routes. Adv. Pharm. Bull. 2023, 13, 446–460. [Google Scholar] [CrossRef]
- Musielak, E.; Feliczak-Guzik, A.; Nowak, I. Synthesis and Potential Applications of Lipid Nanoparticles in Medicine. Materials 2022, 15, 682. [Google Scholar] [CrossRef]
- Yostawonkul, J.; Surassmo, S.; Namdee, K.; Khongkow, M.; Boonthum, C.; Pagseesing, S.; Saengkrit, N.; Ruktanonchai, U.R.; Chatdarong, K.; Ponglowhapan, S. Nanocarrier-mediated delivery of α-mangostin for non-surgical castration of male animals. Sci. Rep. 2017, 7, 16234. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, J.P.; Neves, A.R.; Silva, A.M.; Barbosa, M.A.; Reis, M.S.; Santos, S.G. Nanostructured lipid carriers loaded with resveratrol modulate human dendritic cells. Int. J. Nanomed. 2016, 11, 3501–3516. [Google Scholar]
- Basak, S.; Singh, P.; Rajurkar, M. Multidrug Resistant and Extensively Drug Resistant Bacteria: A Study. J. Pathog. 2016, 2016, 4065603. [Google Scholar] [CrossRef] [PubMed]
- Haindongo, E.H.; Ndakolo, D.; Hedimbi, M.; Vainio, O.; Hakanen, A.; Vuopio, J. Antimicrobial resistance prevalence of Escherichia coli and Staphylococcus aureus amongst bacteremic patients in Africa: A systematic review. J. Glob. Antimicrob. Resist. 2023, 32, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Chaieb, K.; Hajlaoui, H.; Zmantar, T.; Kahla-Nakbi, A.B.; Rouabhia, M.; Mahdouani, K.; Bakhrouf, A. The chemical composition and biological activity of clove essential oil, Eugenia caryophyllata (Syzigium aromaticum L. Myrtaceae): A short review. Phytother. Res. 2007, 21, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Li, J.; Chen, Z.; Bai, X.; Yang, Z.; Wang, Z.; Yang, Y. Antibacterial activity and mechanism of clove essential oil against foodborne pathogens. LWT 2023, 173, 114249. [Google Scholar] [CrossRef]
- Dung, H.T.; Van Huy, N.; Dung, N.T.; Tung, D.T.; Yen, N.T.; Thanh, N.B.; Hien, N.T.; Hoang, T.; Lu, L.T.; Chinh, N.T. Electrospun PVA/α-mangostin nanofibers, their anti-bacterial ability and anti-oxidation performances. Vietnam J. Chem. 2022, 60, 798–808. [Google Scholar] [CrossRef]
- Junyaprasert, V.B.; Singhsa, P.; Jintapattanakit, A. Influence of chemical penetration enhancers on skin permeability of ellagic acid-loaded niosomes. Asian J. Pharm. Sci. 2013, 8, 110–117. [Google Scholar] [CrossRef]
- Rathbone, M.J.; Tucker, I.G. Mechanisms, barriers and pathways of oral mucosal drug permeation. Adv. Drug Deliv. Rev. 1993, 12, 41–60. [Google Scholar] [CrossRef]
- Niamprem, P.; Srinivas, S.P.; Tiyaboonchai, W. Penetration of Nile red-loaded nanostructured lipid carriers (NLCs) across the porcine cornea. Colloids Surf. B Biointerfaces 2019, 176, 371–378. [Google Scholar] [CrossRef]
- Greenspan, P.; Mayer, E.P.; Fowler, S.D. Nile red: A selective fluorescent stain for intracellular lipid droplets. J. Cell Biol. 1985, 100, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Sheu, H.M.; Tsai, J.C.; Lin, T.K.; Wong, T.W.; Lee, J.Y. Modified Nile red staining method for improved visualization of neutral lipid depositions in stratum corneum. J. Formos Med. Assoc. 2003, 102, 656–660. [Google Scholar] [PubMed]
- Wang, J.; Guo, X.; Li, L.; Qiu, H.; Zhang, Z.; Wang, Y.; Sun, G. Application of the Fluorescent Dye BODIPY in the Study of Lipid Dynamics of the Rice Blast Fungus Magnaporthe oryzae. Molecules 2018, 23, 1594. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Tan, C.; Shi, X.; Xu, J. Impacts of autofluorescence on fluorescence based techniques to study microglia. BMC Neurosci. 2022, 23, 21. [Google Scholar] [CrossRef] [PubMed]
- Leelapornpisid, W. Efficacy of Alpha-Mangostin for antimicrobial activity against endodontopathogenic microorganisms in a multi-species bacterial-fungal biofilm model. Arch. Oral Biol. 2022, 133, 105304. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.K.; Walls, G.; Anderson, G.; Sullivan, C.; Wong, C.A. Prolonged Bacteroides pyogenes infection in a patient with multiple lung abscesses. Respirol. Case Rep. 2024, 12, e01314. [Google Scholar] [CrossRef] [PubMed]
- Niemiec, B.A.; Gawor, J.; Tang, S.; Prem, A.; Krumbeck, J.A. The bacteriome of the oral cavity in healthy dogs and dogs with periodontal disease. Am. J. Vet. Res. 2022, 83, 50–58. [Google Scholar] [CrossRef]
- Chawnan, N.; Lampang, K.N.; Mektrirat, R.; Awaiwanont, N.; Thongkorn, K. Cultivation of bacterial pathogens and antimicrobial resistance in canine periapical tooth abscesses. Vet. Integr. Sci. 2021, 19, 513–524. [Google Scholar] [CrossRef]


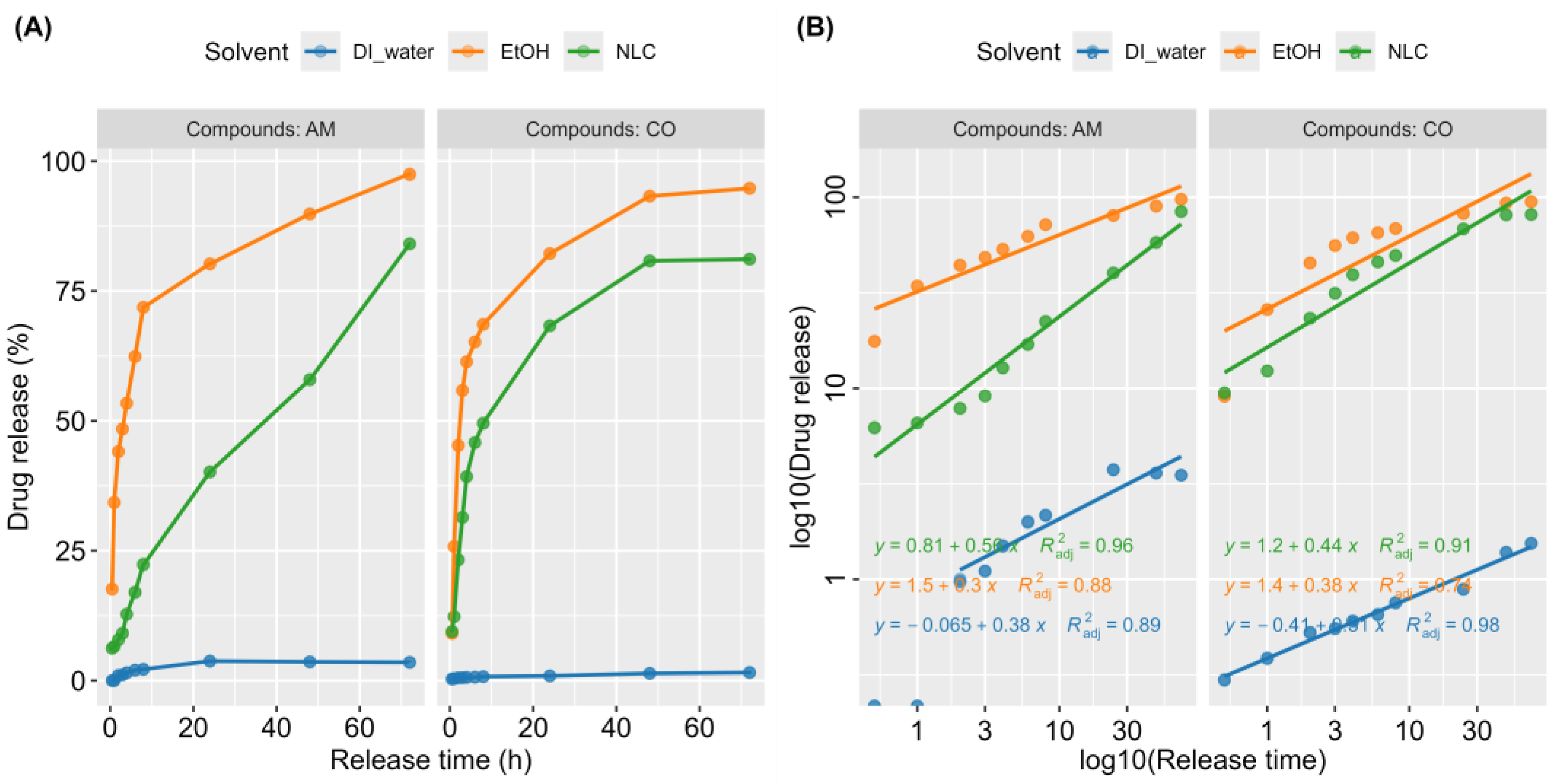
| Active Compounds | Solvent | b (Y-Intercept) | K (10b) | n (Slope) | R2 |
|---|---|---|---|---|---|
| AM | NLC | 0.81 | 6.46 | 0.56 | 0.96 |
| AM | EtOH | 1.5 | 31.62 | 0.3 | 0.88 |
| AM | DI water | −0.065 | 0.86 | 0.38 | 0.89 |
| CO | NLC | 1.2 | 15.85 | 0.44 | 0.91 |
| CO | EtOH | 1.4 | 25.12 | 0.38 | 0.74 |
| CO | DI water | −0.41 | 0.39 | 0.31 | 0.98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sawatphakdee, G.; Yostawonkul, J.; Oontawee, S.; Rodprasert, W.; Sawangmake, C.; Kornsuthisopon, C.; Yata, T.; Tabtieang, S.P.; Nowwarote, N.; Pirarat, N. Feasibility of Nanostructured Lipid Carrier Loaded with Alpha-Mangostin and Clove Oil for Canine Periodontal Therapy. Animals 2024, 14, 2084. https://doi.org/10.3390/ani14142084
Sawatphakdee G, Yostawonkul J, Oontawee S, Rodprasert W, Sawangmake C, Kornsuthisopon C, Yata T, Tabtieang SP, Nowwarote N, Pirarat N. Feasibility of Nanostructured Lipid Carrier Loaded with Alpha-Mangostin and Clove Oil for Canine Periodontal Therapy. Animals. 2024; 14(14):2084. https://doi.org/10.3390/ani14142084
Chicago/Turabian StyleSawatphakdee, Gotchagorn, Jakarwan Yostawonkul, Saranyou Oontawee, Watchareewan Rodprasert, Chenphop Sawangmake, Chatvadee Kornsuthisopon, Teerapong Yata, Sirinun Pisamai Tabtieang, Nunthawan Nowwarote, and Nopadon Pirarat. 2024. "Feasibility of Nanostructured Lipid Carrier Loaded with Alpha-Mangostin and Clove Oil for Canine Periodontal Therapy" Animals 14, no. 14: 2084. https://doi.org/10.3390/ani14142084
APA StyleSawatphakdee, G., Yostawonkul, J., Oontawee, S., Rodprasert, W., Sawangmake, C., Kornsuthisopon, C., Yata, T., Tabtieang, S. P., Nowwarote, N., & Pirarat, N. (2024). Feasibility of Nanostructured Lipid Carrier Loaded with Alpha-Mangostin and Clove Oil for Canine Periodontal Therapy. Animals, 14(14), 2084. https://doi.org/10.3390/ani14142084






