Untargeted Metabolomic Analysis Reveals Plasma Differences between Mares with Endometritis and Healthy Ones
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Selection and Grouping
2.2. Plasma Sample Collection and Processing
2.3. Metabolite Extraction
2.4. LC-MS/MS Analysis
2.5. Data Analysis
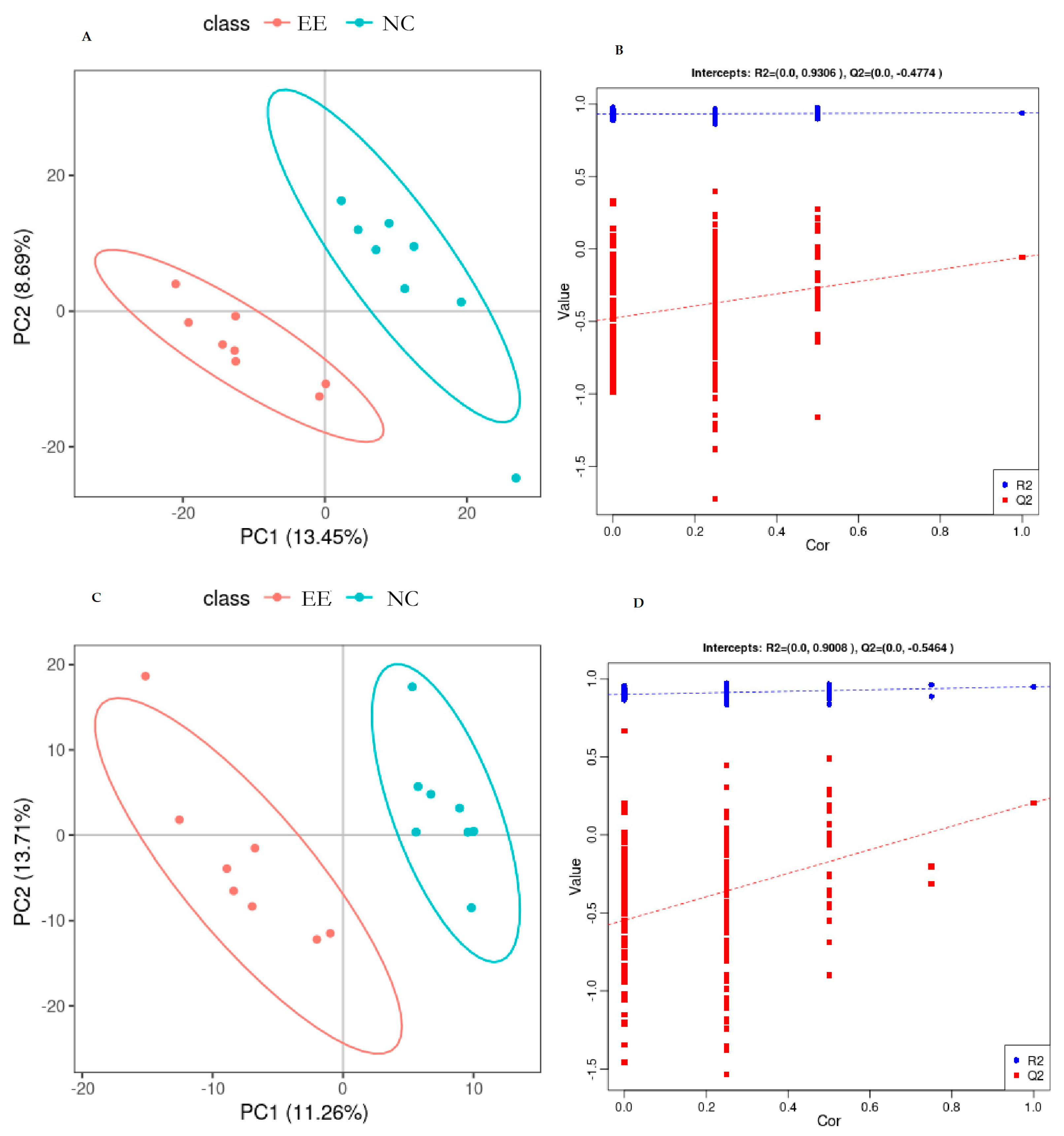
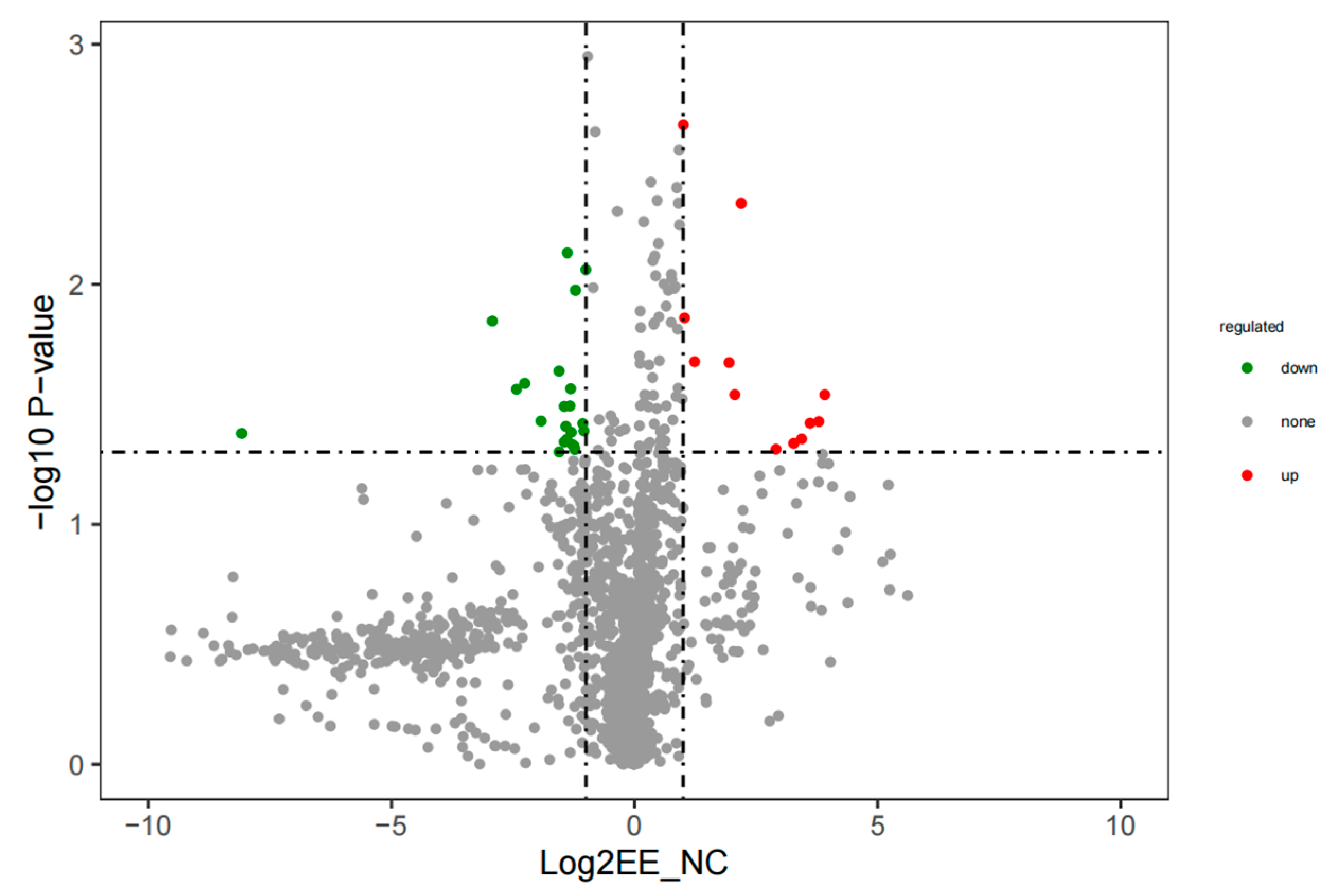
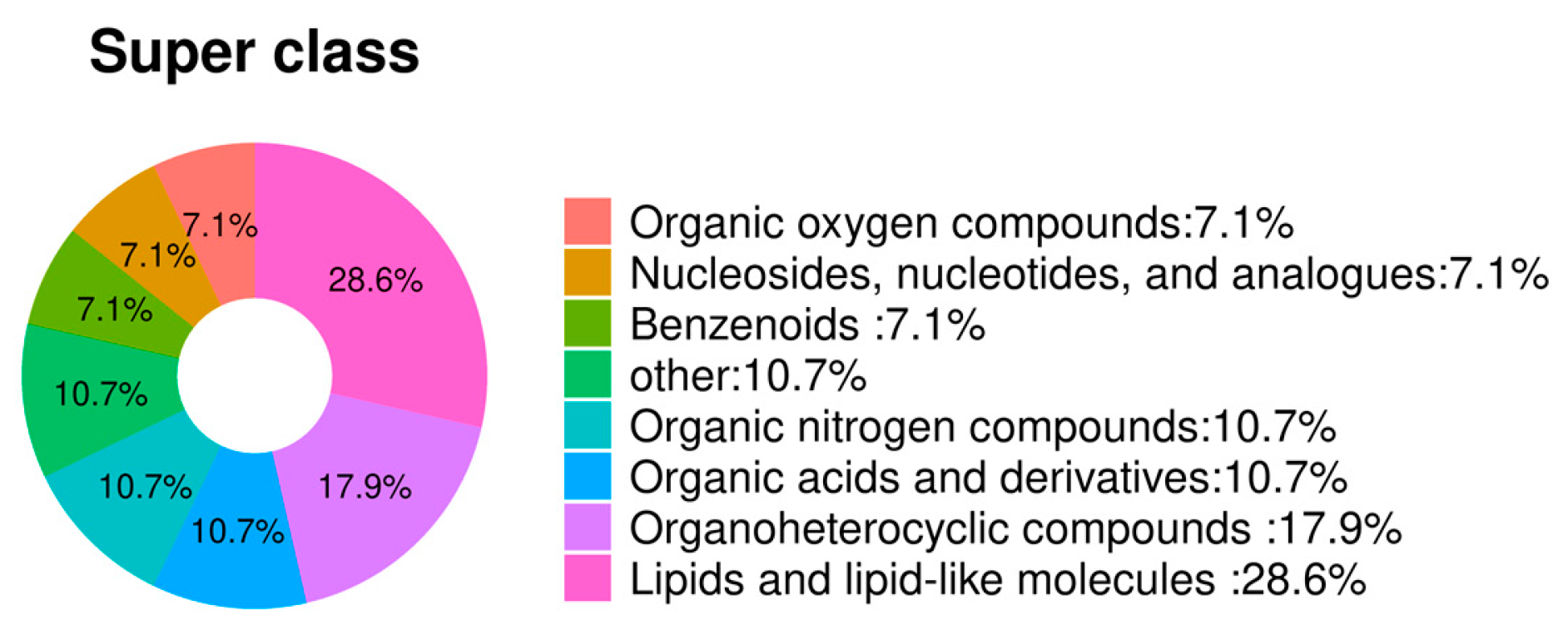
3. Results
3.1. Metabolomic Analysis of Mare Plasma
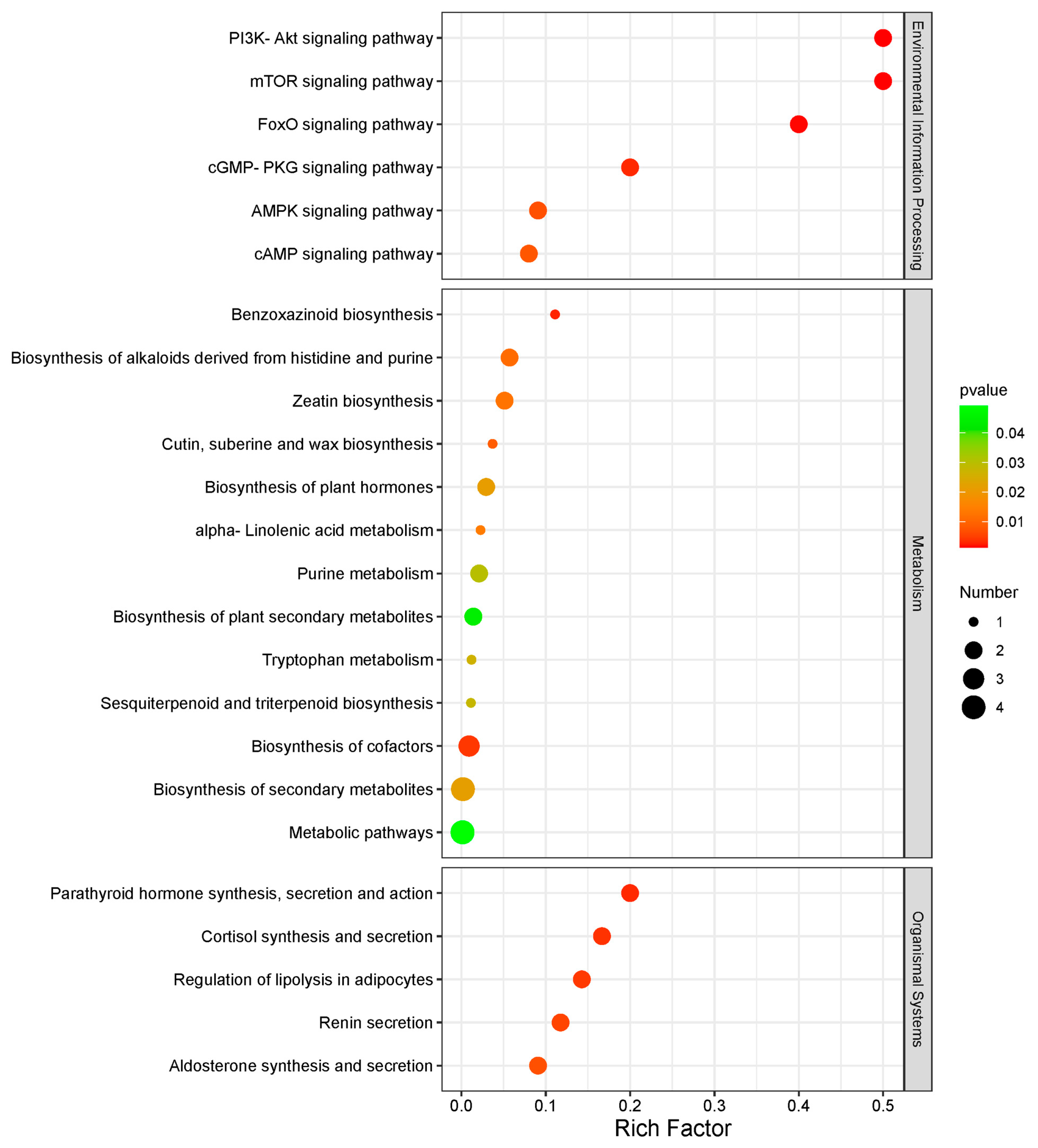
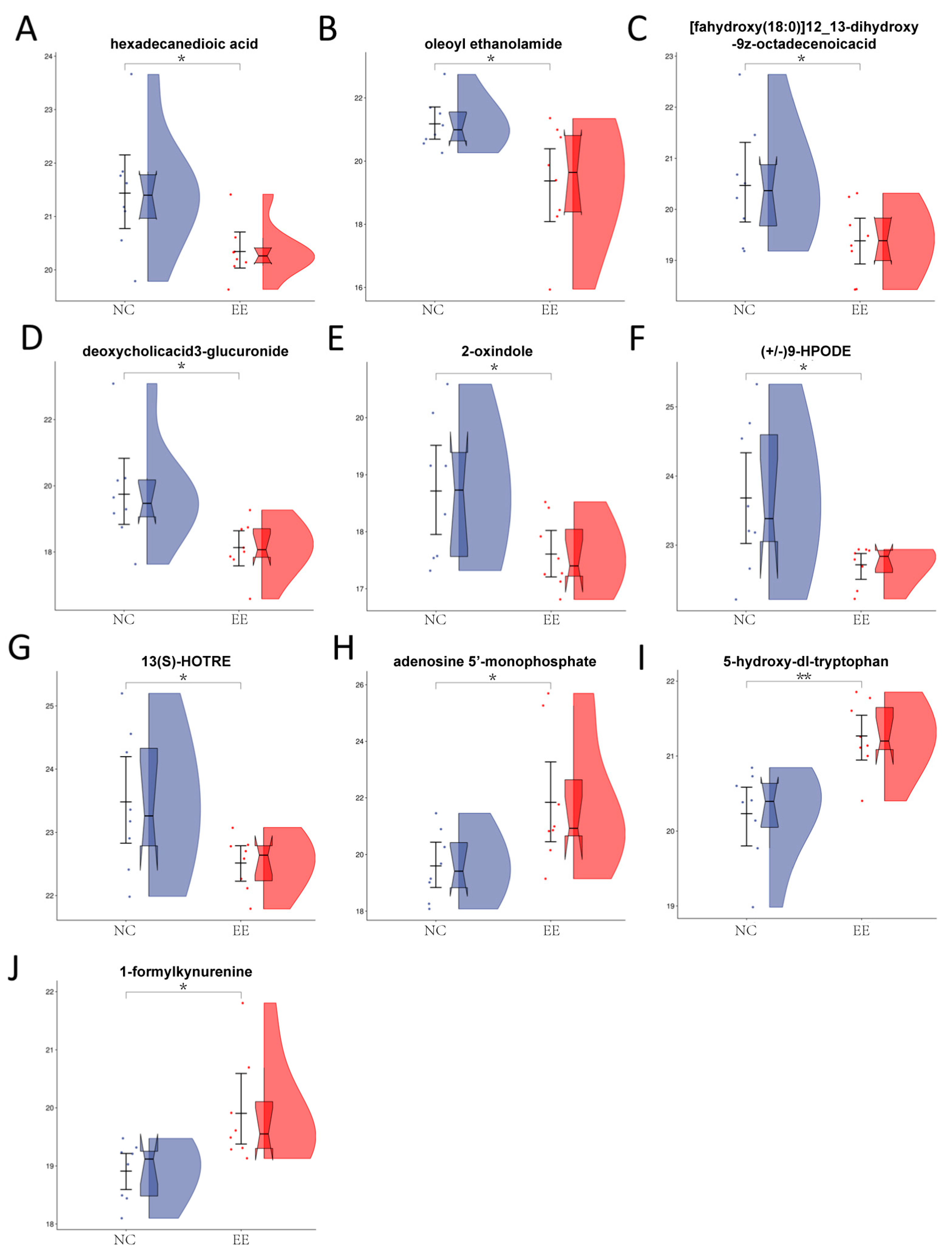
3.2. Identification and Comparison of Differentially Abundant Metabolites
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morris, L.H.; McCue, P.M.; Aurich, C. Equine Endometritis: A Review of Challenges and New Approaches. Reproduction 2020, 160, R95–R110. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhao, Y.; Gao, Y.; Zhu, Y.; Holyoak, G.R.; Zeng, S. Treatments for Endometritis in Mares Caused by Streptococcus Equi Subspecies Zooepidemicus: A Structured Literature Review. J. Equine Vet. Sci. 2021, 102, 103430. [Google Scholar] [CrossRef]
- LeBlanc, M.; Causey, R. Clinical and Subclinical Endometritis in the Mare: Both Threats to Fertility. Reprod. Domest. Anim. 2009, 44, 10–22. [Google Scholar] [CrossRef]
- Köhne, M.; Tönissen, A.; Unruh, C.; Pruß, D.; Sieme, H. Occurrence of Intrauterine Purulent Concrements in a Maiden Mare—A Case Report. J. Equine Vet. Sci. 2020, 95, 103278. [Google Scholar] [CrossRef]
- LeBlanc, M.M. Advances in the Diagnosis and Treatment of Chronic Infectious and Post–Mating-Induced Endometritis in the Mare. Reprod. Domest. Anim. 2010, 45, 21–27. [Google Scholar] [CrossRef]
- Buczkowska, J.; Kozdrowski, R.; Sikora, M.; Dzięcioł, M.; Matusz, A. Non-Traditional Treatments for Endometritis in Mares. Bulg. J. Vet. Med. 2015, 18, 285–293. [Google Scholar] [CrossRef]
- Liu, I.K.M.; Troedsson, M.H.T. The Diagnosis and Treatment of Endometritis in the Mare: Yesterday and Today. Theriogenology 2008, 70, 415–420. [Google Scholar] [CrossRef]
- Buczkowska, J.; Kozdrowski, R.; Nowak, M.; Raś, A.; Staroniewicz, Z.; Siemieniuch, M.J. Comparison of the Biopsy and Cytobrush Techniques for Diagnosis of Subclinical Endometritis in Mares. Reprod. Biol. Endocrinol. 2014, 12, 27. [Google Scholar] [CrossRef] [PubMed]
- Dunn, W.B.; Broadhurst, D.I.; Atherton, H.J.; Goodacre, R.; Griffin, J.L. Systems Level Studies of Mammalian Metabolomes: The Roles of Mass Spectrometry and Nuclear Magnetic Resonance Spectroscopy. Chem. Soc. Rev. 2011, 40, 387–426. [Google Scholar] [CrossRef]
- Fiehn, O. Metabolomics—The Link between Genotypes and Phenotypes. Plant Mol. Biol. 2002, 48, 155–171. [Google Scholar] [CrossRef]
- Zhang, A.; Sun, H.; Wang, X. Serum Metabolomics as a Novel Diagnostic Approach for Disease: A Systematic Review. Anal. Bioanal. Chem. 2012, 404, 1239–1245. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Lee, D.; Yu, J.; Lin, Y.; Lin, T. Recent Advances in LC-MS-based Metabolomics for Clinical Biomarker Discovery. Mass. Spectrom. Rev. 2023, 42, 2349–2378. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, T.; Gan, Z.; Li, H.; Li, Y.; Zhang, Y.; Zhao, X. Metabolomic Analysis of Untargeted Bovine Uterine Secretions in Dairy Cows with Endometritis Using Ultra-Performance Liquid Chromatography/Quadrupole Time-of-Flight Mass Spectrometry. Res. Vet. Sci. 2021, 139, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Dubuc, J.; Duffield, T.F.; Leslie, K.E.; Walton, J.S.; LeBlanc, S.J. Risk Factors for Postpartum Uterine Diseases in Dairy Cows. J. Dairy. Sci. 2010, 93, 5764–5771. [Google Scholar] [CrossRef] [PubMed]
- Carson, M.E. The Association of Selected Metabolites in Peripartum Dairy Cattle with Health and Production. Ph.D Thesis, University of Guelph, Guelph, ON, Canada, 2008. [Google Scholar]
- Huzzey, J.M.; Duffield, T.F.; LeBlanc, S.J.; Veira, D.M.; Weary, D.M.; von Keyserlingk, M.A.G. Short Communication: Haptoglobin as an Early Indicator of Metritis. J. Dairy Sci. 2009, 92, 621–625. [Google Scholar] [CrossRef] [PubMed]
- Pascottini, O.B.; LeBlanc, S.J. Metabolic Markers for Purulent Vaginal Discharge and Subclinical Endometritis in Dairy Cows. Theriogenology 2020, 155, 43–48. [Google Scholar] [CrossRef]
- Zhang, T.; Watson, D.G. High Performance Liquid Chromatographic Approaches to Mass Spectrometry Based Metabolomics. Curr. Metab. 2013, 1, 58–83. [Google Scholar]
- Sorgdrager, F.J.; Naudé, P.J.; Kema, I.P.; Nollen, E.A.; De Deyn, P.P. Tryptophan Metabolism in Inflammaging: From Biomarker to Therapeutic Target. Immunomodulatory Roles Tryptophan Metab. Inflamm. Cancer 2020, 15, 19. [Google Scholar] [CrossRef]
- Seo, S.K.; Kwon, B. Immune Regulation through Tryptophan Metabolism. Exp. Mol. Med. 2023, 55, 1371–1379. [Google Scholar] [CrossRef]
- Su, H.; Liu, R.; Chang, M.; Huang, J.; Jin, Q.; Wang, X. Effect of Dietary Alpha-Linolenic Acid on Blood Inflammatory Markers: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Eur. J. Nutr. 2018, 57, 877–891. [Google Scholar] [CrossRef]
- Cavina, M.; Battino, M.; Gaddi, A.V.; Savo, M.T.; Visioli, F. Supplementation with Alpha-Linolenic Acid and Inflammation: A Feasibility Trial. Int. J. Food Sci. Nutr. 2021, 72, 386–390. [Google Scholar] [CrossRef] [PubMed]
- Masoodi, M.; Kuda, O.; Rossmeisl, M.; Flachs, P.; Kopecky, J. Lipid Signaling in Adipose Tissue: Connecting Inflammation & Metabolism. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2015, 1851, 503–518. [Google Scholar]
- Juge-Aubry, C.E.; Henrichot, E.; Meier, C.A. Adipose Tissue: A Regulator of Inflammation. Best Pract. Res. Clin. Endocrinol. Metab. 2005, 19, 547–566. [Google Scholar] [CrossRef] [PubMed]
- Linden, J.; Koch-Nolte, F.; Dahl, G. Purine Release, Metabolism, and Signaling in the Inflammatory Response. Annu. Rev. Immunol. 2019, 37, 325–347. [Google Scholar] [CrossRef] [PubMed]
- Ohradanova-Repic, A.; Machacek, C.; Charvet, C.; Lager, F.; Le Roux, D.; Platzer, R.; Leksa, V.; Mitulovic, G.; Burkard, T.R.; Zlabinger, G.J. Extracellular Purine Metabolism Is the Switchboard of Immunosuppressive Macrophages and a Novel Target to Treat Diseases with Macrophage Imbalances. Front. Immunol. 2018, 9, 852. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, P.; Saha, B. Metabolic Regulation of Infection and Inflammation. Cytokine 2018, 112, 1–11. [Google Scholar] [CrossRef]
- Yang, Q.; Liu, R.; Yu, Q.; Bi, Y.; Liu, G. Metabolic Regulation of Inflammasomes in Inflammation. Immunology 2019, 157, 95–109. [Google Scholar] [CrossRef] [PubMed]
- Yeager, M.P.; Pioli, P.A.; Guyre, P.M. Cortisol Exerts Bi-Phasic Regulation of Inflammation in Humans. Dose-Response 2011, 9, 332–347. [Google Scholar] [CrossRef] [PubMed]
- Vukelic, S.; Stojadinovic, O.; Pastar, I.; Rabach, M.; Krzyzanowska, A.; Lebrun, E.; Davis, S.C.; Resnik, S.; Brem, H.; Tomic-Canic, M. Cortisol Synthesis in Epidermis Is Induced by IL-1 and Tissue Injury. J. Biol. Chem. 2011, 286, 10265–10275. [Google Scholar] [CrossRef]
- Cantero-Navarro, E.; Fernandez-Fernandez, B.; Ramos, A.M.; Rayego-Mateos, S.; Rodrigues-Diez, R.R.; Sánchez-Niño, M.D.; Sanz, A.B.; Ruiz-Ortega, M.; Ortiz, A. Renin-Angiotensin System and Inflammation Update. Mol. Cell. Endocrinol. 2021, 529, 111254. [Google Scholar] [CrossRef]
- Crowley, S.D.; Rudemiller, N.P. Immunologic Effects of the Renin-Angiotensin System. J. Am. Soc. Nephrol. JASN 2017, 28, 1350–1361. [Google Scholar] [CrossRef] [PubMed]
- Badawy, A.A.-B. Tryptophan Metabolism: A Versatile Area Providing Multiple Targets for Pharmacological Intervention. Egypt. J. Basic Clin. Pharmacol. 2019, 9, 10–32527. [Google Scholar] [CrossRef] [PubMed]
- Belladonna, M.L.; Puccetti, P.; Orabona, C.; Fallarino, F.; Vacca, C.; Volpi, C.; Gizzi, S.; Pallotta, M.T.; Fioretti, M.C.; Grohmann, U. Immunosuppression via Tryptophan Catabolism: The Role of Kynurenine Pathway Enzymes. Transplantation 2007, 84, S17–S20. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Cao, K.; Liu, K.; Xue, Y.; Roberts, A.I.; Li, F.; Han, Y.; Rabson, A.B.; Wang, Y.; Shi, Y. Kynurenic Acid, an IDO Metabolite, Controls TSG-6-Mediated Immunosuppression of Human Mesenchymal Stem Cells. Cell Death Differ. 2017, 25, 1209–1223. [Google Scholar] [CrossRef] [PubMed]
- Puccetti, P.; Grohmann, U. IDO and Regulatory T Cells: A Role for Reverse Signalling and Non-Canonical NF-κB Activation. Nat. Rev. Immunol. 2007, 7, 817–823. [Google Scholar] [CrossRef] [PubMed]
- Cribbs, A.P.; Williams, R.O. Role of the Kynurenine Pathway in Immune-Mediated Inflammation. In Targeting the Broadly Pathogenic Kynurenine Pathway; Mittal, S., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 93–107. [Google Scholar]
- Ferris, R.A.; McCue, P.M.; Borlee, G.I.; Loncar, K.D.; Hennet, M.L.; Borlee, B.R. In Vitro Efficacy of Nonantibiotic Treatments on Biofilm Disruption of Gram-Negative Pathogens and an In Vivo Model of Infectious Endometritis Utilizing Isolates from the Equine Uterus. J. Clin. Microbiol. 2016, 54, 631–639. [Google Scholar] [CrossRef]
- Genestet, C.; Le Gouellec, A.; Chaker, H.; Polack, B.; Guery, B.; Toussaint, B.; Stasia, M.J. Scavenging of Reactive Oxygen Species by Tryptophan Metabolites Helps Pseudomonas Aeruginosa Escape Neutrophil Killing. Free. Radic. Biol. Med. 2014, 73, 400–410. [Google Scholar] [CrossRef]
- Herr, N.; Bode, C.; Duerschmied, D. The Effects of Serotonin in Immune Cells. Front. Cardiovasc. Med. 2017, 4, 48. [Google Scholar] [CrossRef]
- Cloëz-Tayarani, I.; Petit-Bertron, A.-F.; Venters, H.D.; Cavaillon, J.-M. Differential Effect of Serotonin on Cytokine Production in Lipopolysaccharide-Stimulated Human Peripheral Blood Mononuclear Cells: Involvement of 5-hydroxytryptamine2A Receptors. Int. Immunol. 2003, 15, 233–240. [Google Scholar] [CrossRef]
- Ullmer, C.; Schmuck, K.; Kalkman, H.O.; Lübbert, H. Expression of Serotonin Receptor mRNAs in Blood Vessels. FEBS Lett. 1995, 370, 215–221. [Google Scholar] [CrossRef]
- Katz, M.F.; Farber, H.W.; Dodds-Stitt, Z.; Cruikshank, W.W.; Bear, D.J. Serotonin-Stimulated Aortic Endothelial Cells Secrete a Novel T Lymphocyte Chemotactic and Growth Factor. J. Leucoc. Biol. 1994, 55, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Wang, Z.; Wang, B.; Hou, B.; Cheng, J.; Bai, T.; Zhang, Y.; Wang, W.; Yan, L.; Zhang, J. Expanding the Application of Tryptophan: Industrial Biomanufacturing of Tryptophan Derivatives. Front. Microbiol. 2023, 14, 1099098. [Google Scholar] [CrossRef] [PubMed]
- Khetmalis, Y.M.; Shivani, M.; Murugesan, S.; Sekhar, K.V.G.C. Oxindole and Its Derivatives: A Review on Recent Progress in Biological Activities. Biomed. Pharmacother. Biomed. Pharmacother. 2021, 141, 111842. [Google Scholar] [CrossRef]
- Lee, J.-H.; Wood, T.K.; Lee, J. Roles of Indole as an Interspecies and Interkingdom Signaling Molecule. Trends Microbiol. 2015, 23, 707–718. [Google Scholar] [CrossRef] [PubMed]
- Bunders, C.A.; Minvielle, M.J.; Worthington, R.J.; Ortiz, M.; Cavanagh, J.; Melander, C. Intercepting Bacterial Indole Signaling with Flustramine Derivatives. J. Am. Chem. Soc. 2011, 133, 20160–20163. [Google Scholar] [CrossRef] [PubMed]
- Bunders, C.; Cavanagh, J.; Melander, C. Flustramine Inspired Synthesis and Biological Evaluation of Pyrroloindoline Triazole Amides as Novel Inhibitors of Bacterial Biofilms. Org. Biomol. Chem. 2011, 9, 5476–5481. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Attila, C.; Cirillo, S.L.G.; Cirillo, J.D.; Wood, T.K. Indole and 7-hydroxyindole Diminish Pseudomonas aeruginosa Virulence. Microb. Biotechnol. 2009, 2, 75–90. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Kim, Y.-G.; Cho, M.H.; Kim, J.-A.; Lee, J. 7-Fluoroindole as an Antivirulence Compound against Pseudomonas aeruginosa. FEMS Microbiol. Lett. 2012, 329, 36–44. [Google Scholar] [CrossRef]
- Frei, R.; Breitbach, A.S.; Blackwell, H.E. 2-Aminobenzimidazole Derivatives Strongly Inhibit and Disperse Pseudomonas aeruginosa Biofilms. Angew. Chem. 2012, 21, 5316–5319. [Google Scholar] [CrossRef]
- Lee, J.; Cho, M.H.; Lee, J. 3-Indolylacetonitrile Decreases Escherichia Coli O157:H7 Biofilm Formation and Pseudomonas aeruginosa Virulence. Environ. Microbiol. 2011, 13, 62–73. [Google Scholar] [CrossRef]
- Pandey, R.; Swamy, K.V.; Khetmalas, M.B. Indole: A Novel Signaling Molecule and Its Applications. Indian J. Biotechnol. 2013, 12, 297–310. [Google Scholar]
- Tashiro, Y.; Toyofuku, M.; Nakajima-Kambe, T.; Uchiyama, H.; Nomura, N. Bicyclic Compounds Repress Membrane Vesicle Production and Pseudomonas Quinolone Signal Synthesis in Pseudomonas Aeruginosa. FEMS Microbiol. Lett. 2010, 304, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Chu, W.; Zere, T.R.; Weber, M.M.; Wood, T.K.; Whiteley, M.; Hidalgo-Romano, B.; Valenzuela, E.; McLean, R.J.C. Indole Production Promotes Escherichia Coli Mixed-Culture Growth with Pseudomonas aeruginosa by Inhibiting Quorum Signaling. Appl. Environ. Microbiol. 2012, 78, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Biswas, N.N.; Kutty, S.K.; Barraud, N.; Iskander, G.M.; Griffith, R.; Rice, S.A.; Willcox, M.; Black, D.S.; Kumar, N. Indole-Based Novel Small Molecules for the Modulation of Bacterial Signalling Pathways. Org. Biomol. Chem. 2015, 13, 925–937. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.H.; Molla, M.N.; Cantor, C.R.; Collins, J.J. Bacterial Charity Work Leads to Population-Wide Resistance. Nature 2010, 467, 82–85. [Google Scholar] [CrossRef] [PubMed]
- Stark, A.H.; Crawford, M.A.; Reifen, R. Update on Alpha-Linolenic Acid. Nutr. Rev. 2008, 66, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, C.M.; Roche, H.M. Conjugated Linoleic Acid and Inflammatory Cell Signalling. Prostaglandins Leukot. Essent. Fat. Acids (PLEFA) 2010, 82, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Choque, B.; Catheline, D.; Rioux, V.; Legrand, P. Linoleic Acid: Between Doubts and Certainties. Biochimie 2014, 96, 14–21. [Google Scholar] [CrossRef]
- Nagy, L.; Tontonoz, P.; Alvarez, J.G.; Chen, H.; Evans, R.M. Oxidized LDL Regulates Macrophage Gene Expression through Ligand Activation of PPARγ. Cell 1998, 93, 229–240. [Google Scholar] [CrossRef]
- Ku, G.; Thomas, C.E.; Akeson, A.L.; Jackson, R.L. Induction of Interleukin 1 Beta Expression from Human Peripheral Blood Monocyte-Derived Macrophages by 9-Hydroxyoctadecadienoic Acid. J. Biol. Chem. 1992, 267, 14183–14188. [Google Scholar] [CrossRef]
- Tontonoz, P.; Nagy, L.; Alvarez, J.G.; Thomazy, V.A.; Evans, R.M. PPARγ Promotes Monocyte/Macrophage Differentiation and Uptake of Oxidized LDL. Cell 1998, 93, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.S.; Hossain, M.S. Eicosanoids Signals in SARS-CoV-2 Infection: A Foe or Friend. Mol. Biotechnol. 2023. online ahead of print. [Google Scholar] [CrossRef]
- Cambiaggi, L.; Chakravarty, A.; Noureddine, N.; Hersberger, M. The Role of α-Linolenic Acid and Its Oxylipins in Human Cardiovascular Diseases. Int. J. Mol. Sci. 2023, 24, 6110. [Google Scholar] [CrossRef]
- Kumar, N.; Gupta, G.; Anilkumar, K.; Fatima, N.; Karnati, R.; Reddy, G.V.; Giri, P.V.; Reddanna, P. 15-Lipoxygenase Metabolites of α-Linolenic Acid,[13-(S)-HPOTrE and 13-(S)-HOTrE], Mediate Anti-Inflammatory Effects by Inactivating NLRP3 Inflammasome. Sci. Rep. 2016, 6, 31649. [Google Scholar] [CrossRef]
- Yang, L.; Guo, H.; Li, Y.; Meng, X.; Yan, L.; Zhang, D.; Wu, S.; Zhou, H.; Peng, L.; Xie, Q.; et al. Oleoylethanolamide Exerts Anti-Inflammatory Effects on LPS-Induced THP-1 Cells by Enhancing PPARα Signaling and Inhibiting the NF-κB and ERK1/2/AP-1/STAT3 Pathways. Sci. Rep. 2016, 6, 34611. [Google Scholar] [CrossRef]
- Tao, Z.; Cheng, M.; Wang, S.-C.; Lv, W.; Hu, H.-Q.; Li, C.-F.; Cao, B.-Z. JAK2/STAT3 Pathway Mediating Inflammatory Responses in Heatstroke-Induced Rats. Int. J. Clin. Exp. Pathol. 2015, 8, 6732–6739. [Google Scholar] [PubMed]
- Leiria, L.O.; Tseng, Y.-H. Lipidomics of Brown and White Adipose Tissue: Implications for Energy Metabolism. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2020, 1865, 158788. [Google Scholar] [CrossRef] [PubMed]
- Hildreth, K.; Kodani, S.D.; Hammock, B.D.; Zhao, L. Cytochrome P450-Derived Linoleic Acid Metabolites EpOMEs and DiHOMEs: A Review of Recent Studies. J. Nutr. Biochem. 2020, 86, 108484. [Google Scholar] [CrossRef]
- Totani, Y.; Saito, Y.; Ishizaki, T.; Sasaki, F.; Ameshima, S.; Miyamori, I. Leukotoxin and Its Diol Induce Neutrophil Chemotaxis through Signal Transduction Different from That of fMLP. Eur. Respir. J. 2000, 15, 75–79. [Google Scholar] [CrossRef]
- Ishizaki, T.; Ozawa, T.; Voelkel, N.F. Leukotoxins and the Lung. Pulm. Pharmacol. Ther. 1999, 12, 145–155. [Google Scholar] [CrossRef]
- Thompson, D.A.; Hammock, B.D. Dihydroxyoctadecamonoenoate Esters Inhibit the Neutrophil Respiratory Burst. J. Biosci. 2007, 32, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Levan, S.R.; Stamnes, K.A.; Lin, D.L.; Panzer, A.R.; Fukui, E.; McCauley, K.; Fujimura, K.E.; McKean, M.; Ownby, D.R.; Zoratti, E.M. Elevated Faecal 12, 13-diHOME Concentration in Neonates at High Risk for Asthma Is Produced by Gut Bacteria and Impedes Immune Tolerance. Nat. Microbiol. 2019, 4, 1851–1861. [Google Scholar] [CrossRef] [PubMed]
- Ke, R.; Xu, Q.; Li, C.; Luo, L.; Huang, D. Mechanisms of AMPK in the Maintenance of ATP Balance during Energy Metabolism. Cell Biol. Int. 2018, 42, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.C.; Hardie, D.G. AMPK: Sensing Glucose as Well as Cellular Energy Status. Cell Metab. 2017, 27, 299–313. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Gao, Y.; Mai, Z.; Li, Y.; Wang, J.; Zhao, X.; Zhang, Y. Untargeted Metabolomic Analysis Reveals Plasma Differences between Mares with Endometritis and Healthy Ones. Animals 2024, 14, 1933. https://doi.org/10.3390/ani14131933
Zhang X, Gao Y, Mai Z, Li Y, Wang J, Zhao X, Zhang Y. Untargeted Metabolomic Analysis Reveals Plasma Differences between Mares with Endometritis and Healthy Ones. Animals. 2024; 14(13):1933. https://doi.org/10.3390/ani14131933
Chicago/Turabian StyleZhang, Xijun, Yujin Gao, Zhanhai Mai, Yina Li, Jiamian Wang, Xingxu Zhao, and Yong Zhang. 2024. "Untargeted Metabolomic Analysis Reveals Plasma Differences between Mares with Endometritis and Healthy Ones" Animals 14, no. 13: 1933. https://doi.org/10.3390/ani14131933
APA StyleZhang, X., Gao, Y., Mai, Z., Li, Y., Wang, J., Zhao, X., & Zhang, Y. (2024). Untargeted Metabolomic Analysis Reveals Plasma Differences between Mares with Endometritis and Healthy Ones. Animals, 14(13), 1933. https://doi.org/10.3390/ani14131933





