Simple Summary
This study aimed to investigate the function of the nuptial pad-secreted pheromone in anurans. By conducting transcriptome sequencing of the nuptial pads of the Yunnan pond frog, we obtained transcripts of protein pheromones that were highly expressed in the nuptial pads during the breeding season. These pheromones were homologous to the common frog amplexins and the plethodontid modulating factor (PMF) pheromone of urodeles. By means of recombinant protein expression, we obtained the single subtype of the pheromone and demonstrated its ability to shorten the duration of Yunnan pond frog pairing through behavioral experiments. The results of this study further confirm that urodeles and anurans share the PMF pheromone system. Additionally, this study validates the function of this pheromone in anurans for the first time, confirming that it serves similar functions as in urodeles, shortening the breeding process. Overall, this study provides a paradigm for exploring the function of pheromones in anurans.
Abstract
Chemical communication is an important mode of communication in the courtship and breeding processes of amphibians. In caudates, multiple components of sexual pheromones have been identified and functionally verified. One of these pheromone systems is plethodontid modulating factor (PMF). In anurans, the pheromone called amplexin was found in nuptial pads of ranids and was considered a member of the PMF system, yet its bio-function has not been tested. In this study, we obtained 18 amplexin transcript sequences from nuptial pads of Nidirana pleuraden (Amphibia, Ranidae) by transcriptome sequencing and found that the proteins translated by these transcripts are diversified, hydrophilic, and relatively stable. We also acquired a N. pleuraden amplexin isoform with the highest expression level in the transcriptome analysis through the prokaryotic expression system. Using two different animal behavioral experimental settings, we have tested the bio-function of the recombinant PMF protein (rPMF) in N. pleuraden’s reproduction and found that the rPMF does not attract females but shortens the duration of amplexus significantly. This is the first study to verify the function of the PMF pheromone in Anura, indicating the pervasiveness of chemical communication during breeding in amphibians.
1. Introduction
Amplexus is one of the most widespread reproductive behaviors in anurans [1], typically characterized by the male mounting the female’s back, with its forearms pressing under the female’s armpits to hold her tightly, so we figuratively call it “hugging”. This behavior is essential for coordinating egg deposition and sperm release [2,3]. During the breeding season, many male frogs or toads develop keratinized spines on their nuptial pads on the thumbs or forearms, like in Rhinella marina [4], Ptychohyla hypomykter [5] and Rana temporaria [6]. It is widely believed that nuptial pads strengthen the male’s grip on the female during amplexus [3]. Histomorphological studies have also shown that multicellular, alveolar glands with granular secretive products are presented beneath the surface of the nuptial pads, sharing characteristics with the well-known pheromone glands in salamanders [7]. This suggests that nuptial pads may synthesize sexual pheromones during the breeding season. Instead of via vomeronasal organ, pheromones produced in the mental gland of some plethodontid salamanders are transmitted directly into the female’s blood circulation system by dermal attrition [8]. Interestingly, examining post-amplexus female frogs reveals that their ventral skin is often abraded at the site where the male’s spiny nuptial pads contact [6], which suggest that a similar pheromone transmission pattern might exist in anurans.
Chemical communication has been proven widespread in the courtship and reproduction processes of amphibians [9,10]. In caudates, multiple courtship pheromones have been identified and functionally validated [9,10,11,12,13], most of which are proteins or peptides [14]. Early studies suggested that in anurans, vocal communication was the primary means of interaction [2,3,15]. However, increasing evidence indicates that chemical communication also plays a significant role in the reproduction and courtship behaviors of frogs and toads [5,16,17,18,19].
Currently, three major pheromone systems exist in amphibians: plethodontid receptivity factor (PRF) [9], plethodontid modulating factor (PMF) [20] and soderfin precursor-like factor (SPF) [21]. Among them, PMF was first found in the mental gland of male plethodontid salamanders [20], its members weigh 7 to 10 kDa, and it structurally belongs to the three-finger protein (TFP) superfamily. Animal behavioral experiments indicate that the PMF pheromone enhances female receptivity and reduces the duration of female courtship behaviors in plethodontid salamanders [20]. By 2013, Willaert et al. identified a set of pheromones in the nuptial pads of R. temporaria (Amphibia, Anura, Randidae), which they named amplexin(s). These secretive proteins showed homology with PMFs and were speculated to play a role in reducing amplexus duration. However, the biofunction of amplexins requires further verification [6].
To gain a deeper understanding of the biological function of pheromones in anurans, our study focuses on investigating the potential similarity between the secretions of the most prevalent breeding gland, the nuptial pad, and PMFs in caudates, as hypothesized by Willaert [6]. We selected another ranid species, the Yunnan pond frog (Nidirana pleuraden), which develops pronounced nuptial pads during the breeding season, as our target. We first performed a comparative transcriptome analysis of its nuptial pads and dorsal skin to obtain the tissue-specific transcript sequences. Subsequently, we screened out the transcripts that show sequence similarity to the reported PMF pheromones. Then, we selected the transcripts with the highest expression level for prokaryotic expression to obtain the recombinant PMF protein. Finally, we designed two sets of behavioral experiments to test the function of the recombinant pheromone.
2. Materials and Methods
2.1. Animals
In June 2017, three adult male N. pleuraden were collected from the Caohai National Nature Reserve in Bijie, Guizhou, China. The frogs were anesthetized using MS22 solution, and then their nuptial pads and dorsal skin were swiftly excised on ice. The excised tissues were immediately placed into RNase-free centrifuge tubes, flash-frozen in liquid nitrogen and subsequently stored at −80 °C for transcriptome sequencing.
For behavioral testing, 30 females N. pleuraden were captured in the reserve during the nights of 21 to 25 August 2022 and 22 to 26 July 2023. Two sets of ethological experiments were conducted separately in different years. On the day of the experiment, animals were collected individually and kept in covered plastic containers (15 × 10 × 6 cm3) with damp paper towels inside. After completing a set of experiments on the following night, the animals were released back into the wild. Given this sampling–experiment–release procedure, the possibility that the same individual was introduced into multiple sets of experiments cannot be excluded.
The frogs used in this study were all sexually mature. Male frogs were located by their advertisement calls, and female frogs were identified by having a snout-vent length greater than 55 mm and exhibiting abdominal swelling (indicative of gravidity). All experimental procedures involving animals were conducted in accordance with the regulations established by the Animal Care and Use Committee of the Chengdu Institute of Biology, Chinese Academy of Sciences (CIBDWLL2021014).
2.2. Transcriptome Sequencing and Analysis
Nuptial pads and dorsal skin samples from male N. pleuraden specimens were collected for transcriptome sequencing during breeding season. Each type of tissue included three replicates for a total of six samples. Total RNA was isolated using the TRIzol® reagent (Invitrogen, Carlsbad, CA, USA) followed by treatment with RNase-free DNase I (Promega, Madison, WI, USA) according to the manufacturers’ protocols. The concentration of extracted RNA was detected with Nanodrop2000 (Agilent, Santa Clara, CA, USA), and the integrity was examined with Agient2100, LabChip GX (Perkin Elmer, Hopkinton, MA, USA). Illumina RNA-seq libraries were prepared for 6 samples and sequenced on Illumina NovaSeq 6000 platform (Illumina, San Diego, CA, USA) with a PE150 strategy following the manufacturer’s instructions.
The expression level of predicted transcripts in each RNA-seq library was calculated as the fragments per kilobase of exon model per million mapped fragments (FPKMs) using the following formula: FPKM = (exon mapped fragments × 109)/(total mapped fragments × exon length). To identify the differentially expressed genes (DEGs), we compared FPKM values from nuptial pads and dorsal skins. DEGs were identified as |log2(fold change)| > 1 and BH corrected p < 0.01 by the DESeq2 package v1.38.3 [22]. The Trinotate v3.2.1 annotation tool [23] was used to search seven major databases for functional annotation, the software used in the retrieval and the set threshold (e-value); see Supplementary Table S1.
GO enrichment was performed on the genes highly expressed in the nuptial pads relative to the dorsal skin and elimination of redundancy by ReviGo v1.8.1 [24].
2.3. Protein Sequence Alignment and Amino Acid Characteristic Analysis
After annotation of the nuptial pads transcripts, we selected the sequences annotated as amplexin or PMF and screened the domains of these sequences using InterPro [25]. Sequences with high expression and complete domains in the nuptial pads were used for subsequent analysis. Multiple sequence alignment of amino acid sequences was conducted using the MAFFT software (version 7), utilizing the L-INS-I model for the alignment [26]. The R. temporaria amplexins (GenBank accession nos KC282376–KC282380) and two caudate PMFs (GenBank accession numbers AEO22663 and ABI48851) were used for multiple sequence alignment. Visualization of the alignment results was performed using GeneDoc v2.7 [27]. Amino acid sequence identity was calculated in the software BioEdit v7.0.9 [28] using the aligned sequences. The theoretical molecular weight, isoelectric point, instability index, and average hydrophobicity, among other characteristic parameters of the proteins, were calculated by submitting the sequence information to the ExPASy website and using the ProtParam tool (https://web.expasy.org/protparam/ (accessed on 6 April 2024)).
2.4. Recombinant Expression of Pheromone Protein
Recombinant PMF (rPMF) was expressed as a precursor protein with a cleavable N-terminal 6xHis affinity tag. We chose the Npamplexin05 (GenBank accession number PP583595) for expression because this isoform is the most abundantly expressed subtype in the nuptial pad of males. The bridge polymerase chain reaction technique (Overlap PCR) was used to fuse the Trx sequence, His purification tag, and enterokinase sequence at the N-terminus of the Npamplexin05. The fusion fragment was inserted into the pET32a (+) vector after double digestion with KpnI and HindIII, and the recombinant plasmid was transformed into Rosetta (DE3) cells. Single-clone colonies were inoculated into 100 mL LB (Amp: 100 μg/mL) and activated and expanded at 37 °C, 185 rpm until the culture OD600 reached about 0.6. Then, 0.1 mM IPTG was added to induce expression at 16 °C, 185 rpm for 8 h. The cells were collected by centrifugation and lysed, and the supernatant was purified using Ni-NTA affinity chromatography to obtain the Trx-His-EK-PMF fusion protein. The protein was quantified, and an appropriate amount was incubated with enterokinase at 20 °C for 16 h to cleave off the N-terminal fusion fragment. The protease digestion system was then subjected to Ni-NTA affinity chromatography to recover the target protein PMF. Purified rPMFs were taken for SDS-PAGE analysis.
2.5. Behavioral Experiments and Data Analysis
Two sets of behavioral tests were conducted, each consisting of 30 trials. A network surveillance camera equipped with an infrared module was installed above the test apparatus and operated through IPClient V1.0.7.57 software to record animal behavior. The following is a description of the two sets of tests.
The first group of tests was conducted to evaluate whether the rPMF protein is attractive to female N. pleuraden. The experimental aquarium was made in a do-it-yourself manner: a PVC rainwater guiding trough with a length of 1 m and a rectangular cross-section (18 cm wide) was sealed at both ends with matching plastic covers. Black markers were used to draw lines along the edges of the trough, dividing it into three areas: the areas extending 10 cm from the central line of the trough to both sides were designated as “waiting areas”, and the remaining areas on the left and right sides of the trough (40 × 18 cm2) were designated as ‘choice areas’ (Supplementary Figure S1). If the test individual spends a significantly longer total time in one choice area than in the other, it indicates a preference for the stimulus placed in that choice area. Before the test began, tap water that had been left to stand overnight was added to the experimental trough until the water depth reached 6 cm. Then, the test female frog was placed at the center of the waiting area and covered with a metal wire mesh box to restrict its movement. At the same time, 10 μL (ca. 5 μg) rPMF was injected into a sponge block (1 cm2), and then the sponge block was attached to one end of the experimental tank (below water level); at the other end is a sponge block injected with an equal volume of water (to avoid position effects, the end of the trough where the stimuli were placed was randomly determined in each trial), and the camera’s recording function was turned on. After a 10-min animal adaptation period, the metal wire mesh box was removed, allowing the test female to move freely in the experimental trough. Once the female left the waiting area, a 10-min timer started. After the timing ended, the test female and the stimuli were removed from the trough, the camera’s recording function was turned off, and the trough was carefully cleaned with a detergent. After cleaning, the trough was rinsed several times with standing tap water before proceeding to the next trial.
Another group of tests was conducted to evaluate the effect of the rPMF protein during the amplexus process. The experimental tank was a white PVC basin (50 × 30 × 25 cm3). Before the experiment began, tap water that had been left to stand overnight was added to the tank until the water depth reached 6 cm. Subsequently, a 1 mL syringe needle was used to gently puncture the skin under the female’s armpit, and after injecting 10 μL (ca 5 μg) rPMF protein solution, the female was quickly embraced with a model frog and then placed in the tank with the camera’s recording function turned on (Supplementary Figure S2). After the female showed struggling movements, the time was recorded and the camera was turned off. The test female and model frog were then removed from the tank, and the tank and model frog were carefully cleaned with a detergent. After cleaning, they were rinsed several times with standing tap water. The same process was repeated for another female with the difference being that the PMF protein solution was replaced with distilled water, treating the two frogs as one experiment.
Based on the activity rhythm of the N. pleuraden, the experiments were conducted from 20:00 to 02:00 each day. Behavioral experiments were conducted in the yard of a farmhouse near the Caohai Nature Reserve (26.873391° N, 104.225566° E, 2178.84 m asl), where environmental factors are similar to the natural breeding habitats of the animals. The first set of experiments commenced on 23 August, 24 August, and 25 August 2022. The second set of experiments was conducted on 24 July, 25 July, and 26 July 2023. The dosage of rPMF in all experiments was determined based on the research conducted by Houck et al. on recombinant PRF [29].
We used the player plugin built into IPClient to watch the animal behavior recorded by the camera. For the choice experiment, we started timing when the test female left the waiting area and tallied the total time the animal spent on both stimulus sides of the experimental tank (crossing the midline of the tank is used as an indicator for changing stimulus sides). For the amplexus experiment, we started timing immediately after the test female was placed in the tank and stopped timing when the female showed clear struggling movements and recorded the intermediate time.
We compared two sets of data obtained from experiments with the same design (corresponding to the time the test animals were active on different stimulus sides, n = 30). Since the data did not conform to normality, a Wilcoxon rank-sum test was used. All tests were two-tailed with a significance level (p) of 0.05. Statistical comparisons of the data were performed with R, and the bar graphs of the behavior experiment results were drawn using the ggplot2 package [30] in R.
3. Results
3.1. PMF Sequence Acquisition
Quantifying gene expression helps to describe the degree of difference between different sequencing samples. After normalizing the reads count by calculating the probability of hypothesis testing and correcting for multiple testing, we found that there are more upregulated genes (810) in the nuptial pad of N. pleuraden compared to its dorsal skin (Supplementary Table S2). The GO enrichment results of these genes (Figure 1) showed that they were enriched in protein-related GO terms such as regulation of protein maturation (G0:1903317) and regulation of protein activation cascade (GO:2000257), indicating that the nuptial pad of N. pleuraden had more vigorous protein synthesis activity than the dorsal skin. At the same time, they were also enriched in regard to keratinization (G0:0031424), which was consistent with the nuptial spines growing on the surface of the nuptial pad in the breeding season. Meanwhile, by searching the annotation information of these highly expressed genes (Supplementary Table S3) and conducting domain screening, we found 18 sequences annotated as amplexin in the nuptial pads of the N. pleuraden. The most highly expressed gene (Npampliexin05) ranks within the top 10% of upregulated genes in terms of expression levels. The sequence accession numbers are shown in Table 1.
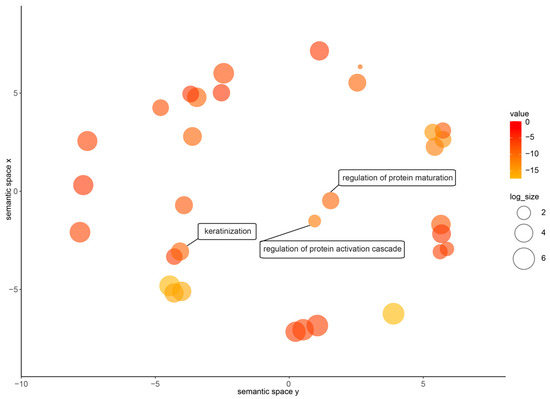
Figure 1.
Enriched GO terms of upregulated genes in nuptial pad of N. pleuraden with interested term highlighted.

Table 1.
ID, name and expression information of N. pleuraden PMF.
3.2. Amino acid Sequence Characteristics of N. pleuraden PMF
Multiple sequence alignment results (Figure 2) show that the PMF pheromone sequences of the N. pleuraden can generally be divided into a signal peptide segment of 19 to 60 amino acids and a subsequent mature protein segment of 71 to 108 amino acids, although some sequences show insertions. Comparing with the R. temporaria amplexin, the cysteine distribution of N. pleuraden PMF was consistent with that of the caudate PMF.

Figure 2.
The multiple sequences alignment result of N. pleuraden PMFs. The green background highlights the cysteine among aligned sequences.
The amino acid identity (Table 2) of N. pleuraden PMF was relatively low, about 34.9%, while that of R. temporaria was relatively high, about 86.8%, which is mainly due to the single amino acid insertions occurring in the middle of the N. pleuraden PMF sequences and large sequence-specific regions at the signal peptide region and tail.

Table 2.
Amino acid sequence identity of amphibian PMFs (%).
After removing the signal peptide segment, the molecular weight of N. pleuraden PMF pheromones ranges between 8.14 and 12.36 kDa. The isoelectric point varies widely from 4 to 8, indicating that these pheromones have subtypes that are negatively charged, uncharged, or weakly positively charged in neutral solutions. The instability index also varies significantly, ranging from 20 to 45, suggesting that some subtypes are stable while others are not. The total average hydrophilicity values are all negative, indicating that the N. pleuraden amplexins are water-soluble. Additionally, despite significant variations in amino acid sequences, the estimated half-life of these pheromones is overwhelmingly 5.5 h (Table 3), suggesting that the N. pleuraden PMFs may have long residual action.

Table 3.
Characteristic of the mature pheromone proteins in N. pleuraden.
3.3. Recombinant Expression of N. pleuraden Amplexin
According to the differential expression results of the transcriptome, we selected the sequence with the highest expression level among all PMF-related genes, Npamplexin05 (average FPKM = 6093), for PMF protein recombinant expression. SDS-PAGE results (Figure 3) showed that the undigested purified protein molecular weight was about 31 kDa, and after digestion, the purified protein molecular weight had obvious bands at about 11 kDa, which was consistent with the prediction, indicating that the recombinant protein expression experiment was successful. Finally, we harvested 1 mg of amplexin protein, which is about 11 kDa in size and 90% in purity.
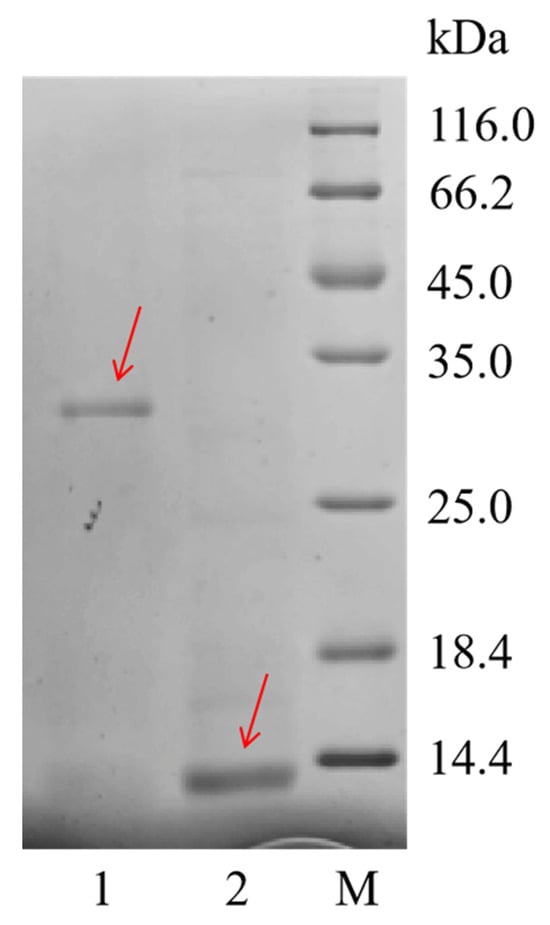
Figure 3.
SDS-PAGE image of rPMF protein. Lane M: protein molecular weight standard; Lane 1: Purified protein before digestion; Lane 2: Purified protein after digestion. Red arrows indicate bands of recombinant proteins.
3.4. Functional Verification of N. pleuraden Amplexin
In the mate choice preference experiment (Figure 4), we observed that female N. pleuraden spent a significantly longer time on the side with distilled water than on the side with the rPMF (390.2 ± 53.09 s vs. 209.7 ± 53.09 s, Z = 2.91, p < 0.05). Additionally, 22 test frogs immediately swam to the end with distilled water after adapting to the environment, suggesting that PMF has a repelling effect on females.
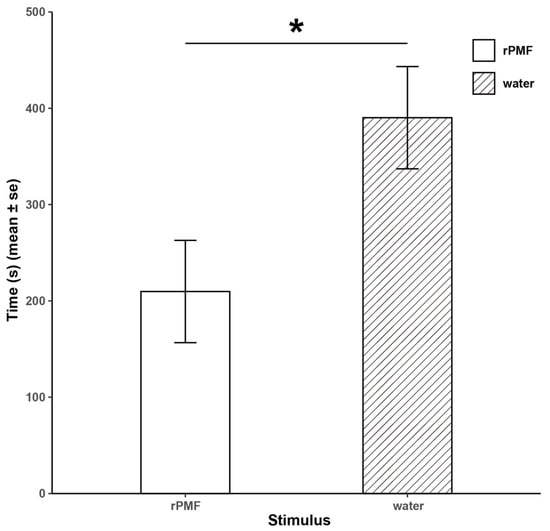
Figure 4.
Results of the mate choice preference experiment. * p < 0.05.
In the amplexus experiment (Figure 5), the time it took for the female frogs injected with PMF to start struggling was significantly shorter than for those injected with distilled water (163.6 ± 35.53 s vs. 244.4 ± 35.53 s, Z = 2.61, p < 0.05). The results of the two experiments corroborate each other, indicating that PMF plays a role in shortening the amplexus process in N. pleuraden.
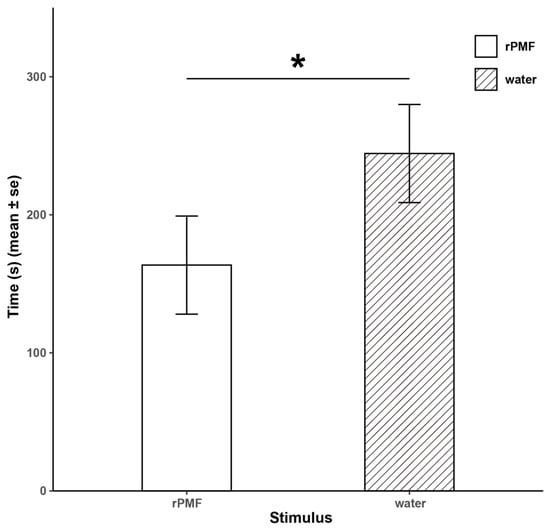
Figure 5.
Results of the amplexus experiment. * p < 0.05.
4. Discussion
The nuptial pad is the most thoroughly studied amphibian reproductive gland to date, and it is reported in both Anura and Caudata. During the breeding season, the conical protrusions of the nuptial pad’s dermis penetrate the epidermal germinative layer to form breeding spines [8,31,32]. Histological studies indicate that the nuptial pad shares a similar composition with the pheromone-producing gland in Caudata [7]. The discovery of amplexin in the nuptial pads of the R. temporaria suggests that the secretion of the nuptial pad is proteinaceous pheromones, which are homologous to the PMF in Caudata [6]. By comparing the transcriptomes of the nuptial pad and the dorsal skin of N. pleuraden during the breeding season, we found 18 genes highly expressed in the nuptial pad annotated as amplexin, consistent with findings in the R. temporaria, indicating the similarity of secretions in the nuptial pads across anurans. The results of multiple sequence alignment showed that both N. pleuraden and R. temporaria amplexins had homology and similar cysteine skeleton with PMFs in tailed amphibians. Based on sequence homology and the cysteine skeleton, we believe that amplexin should be considered as a member of the PMF pheromone system.
The PMF pheromones are reported to have multiple isoforms in Caudata, and the discovery of amplexins in R. temporaria suggests that multiple subtypes of PMF also exist in anurans. We have found 18 amplexins in the nuptial pad of the N. pleuraden, further confirming the diversification of the PMF system. Interestingly, although multiple subtypes of PMFs are present in different amphibian groups, the identity of these PMF sequences varies significantly between groups. For instance, in studies on the red-legged salamanders, Plethodon shermani, the PMFs show about 30% amino acid sequence identity [33]. We calculated the amino acid sequence identity in auran’s PMFs and found approximately 40.2% in N. pleuraden and 86.8% in R. temporaria, suggesting a high degree of diversity in PMFs among amphibians [34]. This diversity is probably the result of long-term co-evolution with multiple female receptors. Diversity in sequence and structure may help male pheromones bind to more potential female receptors or respond quickly to slight changes in the receptor sequence [35]. It is worth noting that the sequence identity of R. temporaria amplexins is much higher than that of the other amphibian species’ PMFs. The discovery process of R. temporaria amplexins was through the construction of a cDNA library of the nuptial pad and the use of primers for RACE PCR. In this process, only one individual of R. temporaria and one primer designed according to the signal peptide were used [6]. We speculate that other PMF subtypes may be ignored in this process. With the development of omics technology, the use of multi-omics in the study of pheromones in the future will be conducive to achieving efficient pheromone identification. From a physicochemical perspective, the isoelectric point and instability index of the N. pleuraden amplexins show significant variation, reflecting its lower sequence similarity. Moreover, PMF belongs to the three-finger protein superfamily, most of which are characterized by the sequence variability and structural stability maintained by disulfide bonds [36]. Multiple sequence alignments indicate that the N. pleuraden and the R. temporaria amplexins as well as the caudates’ PMF all have a stable cysteine skeleton, suggesting a potentially similar function.
PMF has been reported in all species of Plethodontidae [33,34,37,38], where its various subtypes in combination were sufficient to increase female sexual receptivity, achieving the effect of shortening the female’s tail-straddling walk time [20]. It was previously thought to have originated 42–50 mya after the divergence of the species of the family Plethodontidae and Salamandridae [39,40], but the homologous amplexins was found in the R. temporaria [6] and in the N. pleuraden, suggesting that the origin of this pheromone system should be dated back to 297–300 mya before the separate evolution of the ancestors of the caudates and anurans [41,42].
The results of a previous study show that a single subtype does not alter female behavior; a mixture of PMFs lacking the three main subtypes (PMF-G, PMF-H, PMF-I) can actually decrease female sexual receptivity and prolong courtship duration [43,44]. This not only suggests that the function of this class of pheromones may not be similar to SPF system’s gender attraction [9,45] but also indicates that the functions of the subtypes within this pheromone system are non-redundant and act in a synergistic manner, which may have promoted the increasingly complex and diverse chemical signaling in male plethodontid salamanders. Willaert et al. discovered amplexin in the nuptial pads of R. temporaria and based on its homology to PMF sequences in caudates [6] speculated that it could have a similar function; the amplexin enters the female’s circulatory system directly through abrasions caused by nuptial pad friction, thereby reducing amplexus duration and decreasing the risk of predation during mating. Our experimental results imply that PMF functions not only via the circulatory system but also through the animals’ olfactory system. The highly expressed PMF subtype in the nuptial pads of N. pleuraden does not exhibit an attractive effect on females in the mate choice experiment, similar to findings in caudates, confirming that PMF does not function as a gender attractant like SPF. Moreover, in subsequent amplexus experiments, we found that females injected with rPMF typically escaped faster than those injected with distilled water, which is consistent with the hypothesis suggested by Willaert et al. about amplexin’s function [6]. Therefore, we believe that the function of PMF in N. pleuraden is to reduce the duration of amplexus. Functionally, whether in caudates or anurans, the role of PMF pheromones is essentially consistent, indicating that this pheromone system has widespread applicability. The observation in caudates that some single subtypes do not function [43,44] did not occur in our study. We speculate the primary reason is that we chose the most highly expressed sequence in the nuptial pads for recombinant protein expression, which is the main subtype of N. pleuraden amplexin, thus exhibiting functionality in subsequent behavioral experiments. However, the function of interaction of multiple PMF subtypes in N. pleuraden remains to be further verified.
5. Conclusions
In conclusion, our study further proves that both caudates and anurans share the PMF pheromone system, and both are protein pheromones with the same cysteine skeleton structure. At the same time, the function of PMF pheromones in anurans was verified for the first time, and it was confirmed that these pheromones had a similar function in both caudates and anurans, shortening the reproductive process. In the Caudata, male salamanders deliver PMF pheromones through ‘biting’ by presenting their submandibular (mental) gland to females, while in the Anura, male frogs deliver pheromones through ‘hugging’ by pressing their nuptial pad against females.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ani14111550/s1, Figure S1: Diagram of the setup of animal preference trials; Figure S2: (a) A model frog for amplexus experiments; (b) an experiment in progress; Table S1: The databases and software used for non-redundant transcripts function annotation; Table S2: Upregulated genes in the nuptial pads of N. pleuraden and their annotation information; Table S3: GO enrichment of upregulated genes in the nuptial pads of N. pleuraden.
Author Contributions
Conceptualization, X.L., J.J., J.R. and F.X.; methodology, P.Z. and Y.G.; software, P.Z. and Y.G.; validation, P.Z., Y.G., B.W., H.Y. and S.H.; formal analysis, P.Z. and Y.G.; investigation, P.Z. and Y.G.; resources, P.Z., Y.G., B.W., H.Y. and S.H.; data curation, P.Z. and Y.G.; writing—original draft preparation, P.Z. and Y.G.; writing—review and editing, X.L., J.J., J.R. and F.X.; visualization, P.Z. and Y.G.; supervision, F.X.; project administration, F.X.; funding acquisition, Y.G., B.W., J.J. and F.X. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (No. 32170428), the Opening project of CAS key Laboratory of Mountain Ecological Restoration and Bioresource Utilization & Ecological Restoration and Biodiversity Conservation Key Laboratory of Sichuan Province, Chengdu Institute of Biology, Chinese Academy of Sciences (No. KXYSWS2205), the Second Tibetan Plateau Scientific Expedition and Research Program (STEP, 2019QZKK05010503), and China Biodiversity Observation Networks (Sino BON).
Institutional Review Board Statement
All experimental procedures involving animals were strictly conducted in accordance with the regulations established by the Animal Care and Use Committee of the Chengdu Institute of Biology, Chinese Academy of Sciences (CIBDWLL2021014).
Informed Consent Statement
Not applicable.
Data Availability Statement
All the raw sequencing data generated in this study are available in the National Center for Biotechnology Information database under the BioProject number PRJNA1098347. The data are publicly available as of the date of publication.
Acknowledgments
We are grateful to Yuanfei Wang and Yiheng Lin from the Chengdu Institute of Biology, Chinese Academy of Sciences for participating in the behavioral experiment. We thank the anonymous reviewers for the comments and recommendations for the publication of this manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Carvajal-Castro, J.D.; López-Aguirre, Y.; Ospina-L, A.M.; Santos, J.C.; Rojas, B.; Vargas-Salinas, F. Much more than a clasp: Evolutionary patterns of amplexus diversity in anurans. Biol. J. Linn. Soc. 2020, 129, 652–663. [Google Scholar] [CrossRef]
- Wells, K.D. The Ecology and Behavior of Amphibians; University of Chicago Press: Chicago, IL, USA, 2007. [Google Scholar]
- Duellman, W.E.; Trueb, L. Biology of Amphibians; McGraw-Hill: New York, NY, USA, 1986; p. 670. [Google Scholar]
- Kelehear, C.; Shine, R. Non-reproductive male cane toads (Rhinella marina) withhold sex-identifying information from their rivals. Biol. Lett. 2019, 15, 20190462. [Google Scholar] [CrossRef] [PubMed]
- Luna, M.C.; Mcdiarmid, R.W.; Faivovich, J. From erotic excrescences to pheromone shots: Structure and diversity of nuptial pads in anurans. Biol. J. Linn. Soc. 2018, 124, 403–446. [Google Scholar] [CrossRef]
- Willaert, B.; Bossuyt, F.; Janssenswillen, S.; Adriaens, D.; Baggerman, G.; Matthijs, S.; Pauwels, E.; Proost, P.; Raepsaet, A.; Schoofs, L.; et al. Frog nuptial pads secrete mating season-specific proteins related to salamander pheromones. J. Exp. Biol. 2013, 216, 4139–4143. [Google Scholar] [CrossRef] [PubMed]
- Thomas, E.O.; Tsang, L.; Licht, P. Comparative histochemistry of the sexually dimorphic skin glands of anuran amphibians. Copeia 1993, 1993, 133–143. [Google Scholar] [CrossRef]
- Houck, L.D.; Reagan, N.L. Male courtship pheromones increase female receptivity in a plethodontid salamander. Anim. Behav. 1990, 39, 729–734. [Google Scholar] [CrossRef]
- Kikuyama, S.; Toyoda, F.; Ohmiya, Y.; Matsuda, K.; Tanaka, S.; Hayashi, H. Sodefrin: A female-attracting peptide pheromone in newt cloacal glands. Science 1995, 267, 1643–1645. [Google Scholar] [CrossRef] [PubMed]
- Rollmann, S.M.; Houck, L.D.; Feldhoff, R.C. Proteinaceous pheromone affects female receptivity in a terrestrial salamander. Science 1999, 285, 1907–1909. [Google Scholar] [CrossRef] [PubMed]
- Maex, M.; Treer, D.; De Greve, H.; Proost, P.; Van Bocxlaer, I.; Bossuyt, F. Exaptation as a mechanism for functional reinforcement of an animal pheromone system. Curr. Biol. 2018, 28, 1–6. [Google Scholar] [CrossRef]
- Nakada, T.; Toyoda, F.; Matsuda, K.; Nakakura, T.; Hasunuma, I.; Yamamoto, K.; Onoue, S.; Yokosuka, M.; Kikuyama, S. Imorin: A sexual attractiveness pheromone in female red-bellied newts (Cynops pyrrhogaster). Sci. Rep. 2017, 7, 41334. [Google Scholar] [CrossRef]
- Yamamoto, K.; Kawai, Y.; Hayashi, T.; Ohe, Y.; Hayashi, H.; Toyoda, F.; Kawahara, G.; Iwata, T.; Kikuyama, S. Silefrin, a sodefrin-like pheromone in the abdominal gland of the sword-tailed newt, Cynops ensicauda. FEBS Lett. 2000, 472, 267–270. [Google Scholar] [CrossRef]
- Houck, L.D. Pheromone communication in amphibians and reptiles. Annu. Rev. Physiol. 2009, 71, 161–176. [Google Scholar] [CrossRef] [PubMed]
- Wells, K.D. The social behaviour of anuran amphibians. Anim. Behav. 1977, 25, 666–693. [Google Scholar] [CrossRef]
- Bossuyt, F.; Schulte, L.M.; Maex, M.; Janssenswillen, S.; Novikova, P.Y.; Biju, S.D.; Peer, Y.V.D.; Matthijs, S.; Roelants, K.; Martel, A.; et al. Multiple independent recruitment of sodefrin precursor-like factors in Anuran sexually dimorphic glands. Mol. Biol. Evol. 2019, 36, 1921–1930. [Google Scholar] [CrossRef] [PubMed]
- Pearl, C.A.; Cervantes, M.; Chan, M.; Ho, U.; Shoji, R.; Thomas, E.O. Evidence for a mate-attracting chemosignal in the dwarf African clawed frog Hymenochirus. Horm. Behav. 2000, 38, 67–74. [Google Scholar] [CrossRef]
- Schulte, L.M.; Martel, A.; Cruz-Elizalde, R.; Ramírez-Bautista, A.; Bossuyt, F. Love bites: Male frogs (Plectrohyla, Hylidae) use teeth scratching to deliver sodefrin precursor-like factors to females during amplexus. Front. Zool. 2021, 18, 59. [Google Scholar] [CrossRef]
- Waldman, B.; Bishop, P.J. Chemical communication in an archaic anuran amphibian. Behav. Ecol. 2004, 15, 88–93. [Google Scholar] [CrossRef]
- Houck, L.D.; Palmer, C.A.; Watts, R.A.; Arnold, S.J.; Feldhoff, P.W.; Feldhoff, R.C. A new vertebrate courtship pheromone, PMF, affects female receptivity in a terrestrial salamander. Anim. Behav. 2007, 73, 315–320. [Google Scholar] [CrossRef]
- Janssenswillen, S.; Vandebergh, W.; Treer, D.; Willaert, B.; Maex, M.; Van Bocxlaer, I.; Bossuyt, F. Origin and diversification of a salamander sex pheromone system. Mol. Biol. Evol. 2015, 32, 472–480. [Google Scholar] [CrossRef]
- Love, M.; Anders, S.; Huber, W. Differential analysis of count data–the deseq2 package. Genome Biol. 2014, 15, 10–1186. [Google Scholar] [CrossRef]
- Bryant, D.M.; Johnson, K.; DiTommaso, T.; Tickle, T.; Couger, M.B.; Payzin-Dogru, D.; Lee, T.J.; Leigh, N.D.; Kuo, T.H.; Davis, F.G.; et al. A Tissue-Mapped Axolotl de Novo Transcriptome Enables Identification of Limb Regeneration Factors. Cell Rep. 2017, 18, 762–776. [Google Scholar] [CrossRef] [PubMed]
- Supek, F.; Bošnjak, M.; Škunca, N.; Šmuc, T. REVIGO summarizes and visualizes long lists of gene ontology terms. PLoS ONE 2011, 6, e21800. [Google Scholar] [CrossRef] [PubMed]
- Paysan-Lafosse, T.; Blum, M.; Chuguransky, S.; Grego, T.; Pinto, B.L.; Salazar, G.A.; Bileschi, M.L.; Bork, P.; Bridge, A.; Colwell, L.; et al. InterPro in 2022. Nucleic Acids Res. 2023, 51, D418–D427. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed]
- KB, N. GeneDoc: Analysis and visualization of genetic variation. EMBnet News 1997, 4, 1–4. [Google Scholar] [CrossRef]
- Hall, T.; Biosciences, I.; Carlsbad, C. BioEdit: An important software for molecular biology. GERF Bull. Biosci. 2011, 2, 60–61. [Google Scholar]
- Houck, L.D.; Watts, R.A.; Arnold, S.J.; Bowen, K.E.; Kiemnec, K.M.; Godwin, H.A.; Feldhoff, P.W.; Feldhoff, R.C. A recombinant courtship pheromone affects sexual receptivity in a plethodontid salamander. Chem. Senses 2008, 33, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, L. ggplot2: Elegant Graphics for Data Analysis by WICKHAM, H. Biometrics 2011, 67, 678–679. [Google Scholar] [CrossRef]
- Forbes, M.S.; Dent, J.N.; Singhas, C.A. The developmental cytology of the nuptial pad in the red-spotted newt. Dev. Biol. 1975, 46, 56–78. [Google Scholar] [CrossRef]
- Parakkal, P.E.; Ellis, R.A. A cytochemical and electron microscopic study of the thumb pad in Rana pipiens. Exp. Cell Res. 1963, 32, 280–288. [Google Scholar] [CrossRef]
- Wilburn, D.B.; Bowen, K.E.; Gregg, R.G.; Cai, J.; Feldhoff, P.W.; Houck, L.D.; Feldhoff, R.C. Proteomic and utr analyses of a rapidly evolving hypervariable family of vertebrate pheromones. Evolution 2012, 66, 2227–2239. [Google Scholar] [CrossRef]
- Palmer, C.A.; Watts, R.A.; Hastings, A.P.; Houck, L.D.; Arnold, S.J. Rapid evolution of plethodontid modulating factor, a hypervariable salamander courtship pheromone, is driven by positive selection. J. Mol. Evol. 2010, 70, 427–440. [Google Scholar] [CrossRef] [PubMed]
- Wilburn, D.B.; Bowen, K.E.; Doty, S.; Arumugam, S.; Lane, A.N.; Feldhoff, P.W.; Feldhoff, R.C. Structural insights into the evolution of a sexy protein: Novel topology and restricted backbone flexibility in a hypervariable vertebrate pheromone. PLoS ONE 2014, 9, e96975. [Google Scholar] [CrossRef] [PubMed]
- Galat, A.; Gross, G.; Drevet, P.; Ménez, A. Conserved structural determinants in three-fingered protein domains. FEBS J. 2008, 275, 3207–3225. [Google Scholar] [CrossRef] [PubMed]
- Doty, K.A.; Wilburn, D.B.; Bowen, K.E.; Feldhoff, P.W.; Feldhoff, R.C. Co-option and evolution of non-olfactory proteinaceous pheromones in a terrestrial lungless salamander. J. Proteom. 2016, 135, 101–111. [Google Scholar]
- Wilburn, D.B.; Bowen, K.E.; Feldhoff, P.W.; Feldhoff, R.C. Proteomic analyses of courtship pheromones in the redback salamander, Plethodon cinereus. J. Chem. Ecol. 2014, 40, 928–939. [Google Scholar] [CrossRef] [PubMed]
- Palmer, C.A.; Watts, R.A.; Houck, L.D.; Picard, A.L.; Arnold, S.J. Evolutionary replacement of components in a salamander pheromone signaling complex: More evidence for phenotypic-molecular decoupling. Evolution 2007, 61, 202–215. [Google Scholar] [CrossRef] [PubMed]
- Kiemnec-Tyburczy, K.M.; Watts, R.A.; Gregg, R.G.; von Borstel, D.; Arnold, S.J. Evolutionary shifts in courtship pheromone composition revealed by EST analysis of plethodontid salamander mental glands. Gene 2009, 432, 75–81. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Suleski, M.; Hedges, S.B. TimeTree: A resource for timelines, timetrees, and divergence times. Mol. Biol. Evol. 2017, 34, 1812–1819. [Google Scholar] [CrossRef]
- Zhang, P.; Zhou, H.; Chen, Y.Q.; Liu, Y.F.; Qu, L.H. Mitogenomic perspectives on the origin and phylogeny of living amphibians. Syst. Biol. 2005, 54, 391–400. [Google Scholar] [CrossRef]
- Wilburn, D.B.; Eddy, S.L.; Chouinard, A.J.; Arnold, S.J.; Feldhoff, R.C.; Houck, L.D. Pheromone isoform composition differentially affects female behaviour in the red-legged salamander, Plethodon shermani. Anim. Behav. 2015, 100, 1–7. [Google Scholar] [CrossRef]
- Wilburn, D.B.; Arnold, S.J.; Houck, L.D.; Feldhoff, P.W.; Feldhoff, R.C. Gene duplication, co-option, structural evolution, and phenotypic tango in the courtship pheromones of plethodontid salamanders. Herpetologica 2017, 73, 206–219. [Google Scholar] [CrossRef]
- Bossuyt, F.; Maex, M.; Treer, D.; Schulte, L.M.; Van Bocxlaer, I.; Janssenswillen, S. Chemistry between salamanders: Evolution of the SPF courtship pheromone system in Salamandridae. In Chemical Signals in Vertebrates 14; Buesching, C.D., Ed.; Springer Nature Switzerland AG: Cham, Switzerland, 2019; pp. 205–220. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).