Simple Summary
Soil-transmitted worm infections, usually treated with benzimidazoles, can develop resistance due to genetic variations in a specific gene called β-tubulin isotype 1. This study aimed to create a new, quick, and accurate way to identify these genetic variations. We designed a test to spot changes in certain parts of the β-tubulin gene of Trichuris trichiura, the worm causing the infection. By using two different primers, we could distinguish between different genetic types at specific positions in the gene. We tested our method on samples from captive primates and found it to be reliable. Additionally, we explored whether the β-tubulin gene could be useful as a marker in genetic studies. Our tests worked well when we used them on samples from the field. However, we did not find any of the expected genetic variations in the worms or eggs we examined. Instead, all the samples showed the same genetic type. Despite this, our analysis of the β-tubulin gene confirmed the close relationship between T. trichiura and a related Trichuris suis species, which suggests that this gene could be valuable for understanding their evolutionary history.
Abstract
Soil-transmitted helminth (STH) infections, commonly treated with benzimidazoles, are linked to resistance through single nucleotide polymorphisms (SNPs) at position 167, 198, or 200 in the β-tubulin isotype 1 gene. The aim of this study was to establish a novel genotyping assay characterized by its rapidity and specificity. This assay was designed to detect the presence of SNPs within the partial β-tubulin gene of Trichuris trichiura. This was achieved through the biallelic discrimination at codons 167, 198, and 200 by employing the competitive binding of two allele-specific forward primers. The specificity and reliability of this assay were subsequently confirmed using Trichuris samples isolated from captive primates. Furthermore, a molecular study was conducted to substantiate the utility of the β-tubulin gene as a molecular marker. The assays showed high sensitivity and specificity when applied to field samples. Nevertheless, none of the SNPs within the β-tubulin gene were detected in any of the adult worms or eggs from the analyzed populations. All specimens consistently displayed an SS genotype. The examination of the β-tubulin gene further validated the established close relationships between the T. trichiura clade and Trichuris suis clade. This reaffirms its utility as a marker for phylogenetic analysis.
1. Introduction
Worldwide, soil-transmitted helminth (STH) infections are among the most common infections that can cause serious harm to human health. It is estimated 1.5 billion people, accounting 24% of the global population, are infected with these parasites, with a higher prevalence observed among preschool and school-age children. The main STH species are Ascaris lumbricoides (the roundworm), Trichuris trichiura (the whipworm), and Necator americanus and Ancylostoma duodenale (hookworms). These infections are transmitted through eggs found in human feces, which contaminate soil in regions with inadequate sanitation. This mainly affects impoverished and marginalized communities in tropical and subtropical areas, where the access to clean water, sanitation, and hygiene is limited [1].
The World Health Organization (WHO) has developed a strategy to control the infection of STHs. This strategy aims to regulate morbidity through the periodic treatment, known as preventive chemotherapy, of individuals at risk residing in endemic areas. The objective is to reduce and sustain low infection intensity and protect against morbidity by implementing large-scale mass drug administration programs. WHO recommends treatment with benzimidazoles (BZs) such as albendazoles and mebendazoles due to their effectiveness, affordability, and ease of administration by non-medical personnel [1]. BZs are known to exert their action by blocking the microtubule functions of parasites. This is achieved through the inhibition of β-tubulin polymerization in microtubules, leading to the subsequent inhibition of glucose uptake and transport. As a result, the parasites experience a deficiency of glycogen [2]. However, several studies suggest that the therapeutic efficacy of BZ against trichuriasis is progressively diminishing over time. This decline is believed to be partially attributed to the emergence of anthelminthic drug resistance (AR) [3,4,5], which develops because of prolonged and extensive reliance on BZ anthelmintics over an extended period of time [6]. Furthermore, the drugs are administered in single doses, and while this approach is operationally practical, it does not achieve 100% efficacy [4,7,8,9]. Therefore, this practice of administering suboptimal doses extensively over a prolonged period may contribute to the selection and development of AR. Additionally, periodic treatment has the potential to select for subpopulations of parasites that are resistant to the drugs [10,11,12]. Moreover, there are only a limited number of anthelmintic drugs that have been approved for the treatment of STH infections in humans [13,14].
Single nucleotide polymorphisms (SNPs) have been extensively employed for gene identification. Achieving allelic discrimination for a single SNP with a high degree of reliability and flexibility is of utmost importance for the precise detection of advantageous genes associated with specific SNP sites [15]. BZ resistance in T. trichiura is attributed to SNPs in the β-tubulin isotype 1 gene, specifically at codon 167, codon 200 (TTC > TAC), or codon 198 (GAG > GCG) [16,17,18,19,20]. In addition, the frequency of SNPs at codon 200 and 198 was found to increase following treatment, and it was significantly higher in individuals who exhibited a poor response to BZ compared to those who responded well [19]. To maintain the advantages of mass drug administration programs, it is crucial to have tools that can facilitate the large-scale detection of BZ resistance in human STHs. The current challenge of limited detection of phenotypic resistance may be attributed to multiple factors, including the absence of reliable and sensitive methods to monitor resistance genotypes before and after BZ treatment [21], a low frequency of resistance alleles, and the probability that BZ resistance is recessive, as in veterinary parasites [11].
To date, various platforms have been developed for genotyping individual SNPs. These include Kompetitive Allele Specific PCR (KASP) [22,23], RNase H2 enzyme-based amplification (rhAmp) [24], TaqMan [25], and semi-thermal asymmetric reverse PCR (STARP) [26]. Likewise, PCR-based methods, such as real-time PCR (RT-PCR), pyrosequencing, and genotyping assays using the SmartAmp2 method, have been developed for the detection of putative BZ resistance SNPs in human STH [17,18,27,28].
The main objectives of this study were (i) to develop a new genotyping assay, rhAmpTM SNP genotyping, for the screening of β-tubulin SNPs in T. trichiura; (ii) to assess the presence of BZ resistance-associated SNPs at positions 167, 198, and 200 within the β-tubulin gene in various populations of T. trichiura obtained from non-human primates (These SNPs are likely associated with BZ resistance in Trichuris spp.); and (iii) to conduct a molecular investigation aiming to validate the β-tubulin gene as a molecular marker applicable across different Trichuris spp. This validation was carried out to infer phylogenetic relationships between different clades of Trichuris spp. and detect the emergence of AR, thus gaining insights into its distribution among distinct clades.
2. Materials and Methods
2.1. Ethics Statement
This study did not require the approval of an ethics committee. Whipworms and eggs were isolated from stool and cecum samples from various vertebrate animal hosts. These animals were housed in zoological gardens and slaughterhouses in Spain and maintained with good animal practices.
2.2. Collection Samples
In this study, we have formulated two distinct sections. The first section is based on genotyping assays, for which we utilized T. trichiura samples, both eggs and adults, which were collected from different primate hosts. The primate host species analyzed were the Barbary macaque (Macaca sylvanus) and patas monkey (Erythrocebus patas), from Zoo Castellar (Cádiz, Spain), the vervet monkey (Chlorocebus aethiops) from Selwo Aventura (Málaga, Spain), and the Guinea baboon (Papio papio) from Parque de la Naturaleza de Cabárceno (Cantabria, Spain) (Table 1). The second section is centered on a phylogenetic analysis, also utilizing the previously mentioned samples, in addition to adult samples from suids (Sus scrofa domestica) from slaughterhouses in Seville and Huelva (Spain) and porcupine (Hystrix cristata) from Bioparc Fuengirola in Malaga, Spain (Table 1).

Table 1.
Sequences of Trichuris spp. species obtained in the present study based on β-tubulin partial gene including sample ID, host species, geographical origin, GenBank accession numbers, length, and G + C content.
Specimens were isolated from stool samples after treatment or collected from the cecum post-mortem and, consequently, washed in saline solution (0.9% w/v), and separately frozen at −20 °C until further analysis.
Sheather’s sugar solution was used for egg concentration [29], and then, they were embryonated at 32 °C for 3 to 4 weeks with potassium dichromate solution (0.2% w/v) to provide moisture to the medium and to prevent the growth of fungi and bacteria [30].
2.3. Molecular Analysis
2.3.1. DNA Extraction
Whipworm identification and morpho-biometric analysis were performed in previous studies [31,32]. According to the manufacturer’s protocol, total genomic DNA from samples (adult worms and batches of eggs) were extracted using DNeasy Blood and Tissue Kit (Qiagen, Hilden, Germany). To assess the quality of the extractions, 0.8% agarose gel electrophoresis infused with SYBRTM Safe DNA gel stained with 2% w/v Tris-Borate-EDTA (TBE) was used.
2.3.2. Genotyping by rhAmp SNP Assays
In this study, to carry out the genotyping analysis, we used a dual enzyme chemistry technology called rhAmpTM SNP genotyping [33]. This technology is based on the RNase H2-dependent polymerase chain reaction (rhPCR) and universal reporters [24]. Three SNPs from β-tubulin partial gene (codon 167, 198, and 200) were selected for the rhAmp assays. The selected SNPs with corresponding flanking sequences were submitted to the rhAmpTM Genotyping Design Tool at IDT (Integrate DNATechnologies, IDT; https://eu.idtdna.com/site/order/designtool/index/GENOTYPING_PREDESIGN (accessed on 12 February 2024)) and based on the strength of the thermodynamics, the highest ranked assays were retained. For primer design, flanking sequences shorter than 50 bp (base pairs) were extended up to 50–60 bp based on the T. trichiura reference β-tubulin sequence at the probe target sites to meet the technical requirements (Table 2). For each assay, rhAmp used two allele-specific primers and a locus-specific primer. For each SNP, synthetic gBlocksTM Gene fragments were used as known genotype controls during assays, where one represented the wild type (WT) and the other, the mutant allele (MA), and furthermore, both were mixed in an equal molar ratio to represent the heterozygous genotype (Table 3).

Table 2.
Primers designed for rhAmp SNPs Assays for each codon using rhAmpTM Genotyping Design Tool at IDT.

Table 3.
gBlocks® designed for β-tubulin partial gene of T. trichiura genotyping corresponding to the three SNPs (codon 167, 198, and 200). The SNPs are indicated in red color and in bold in the sequences.
SNP genotyping assays were performed using 0.25 μL of rhAmp SNP Assays (20X), 2.65 μL of combined rhAmp Genotyping Master Mix (2X) and rhAmp Reporter Mix (40X), 0.10 μL of nuclease-free water and 2 μL of sample DNA, and 2 μL of control template (gBlocks fragments controls) or 2 μL of nuclease-free water (for no-template control reactions). Reactions were run on the CFX Connect Real-Time PCR Detection System (Bio-Rad Laboratories, Hercules, CA, USA), and analyses were carried out using CFX Maestro Software version 2.3 (Bio-Rad, Hercules, CA, USA). The thermal conditions were 95 °C for 10 min, followed by 40 cycles at 95 °C for 10 s, 60 °C for 30 s, and 68 °C for 20 s per the published protocol (www.idtdna.com/rhAmp-SNP-protocol (accessed on 29 February 2024).
The bi-allelic specificity of the rhAmp assays was provided by two probes, one labelled with Amidite-fluorescein (FAMTM) dye and the other with Hexachloro-fluorescein (HEXTM) dye (Table 4). These different dye reporters were independently detected with excitation sources and emission filters at the respective wavelengths. A total of 91 Trichuris genomic DNA samples were quantified. Hence, each specimen was called resistant (RR), susceptible (SS), and heterozygous (RS) in relation to the melting temperature obtained.

Table 4.
Different excitation and emission spectra for fluorophores.
2.3.3. PCR and Sequencing
In the samples analyzed in the present study, the partial molecular marker gene β-tubulin was amplified by a polymerase chain reaction (PCR) using a thermal cycler (Eppendorf AG and sequenced. (Hamburg, Germany). The primers and PCR conditions were previously described by Hansen et al. [34]. Amplification reactions consisted of 5 μL (10 μM) of each primer, 5 μL of template DNA (50 ng/μL,) 25 μL of GoTaq G2 Green Master Mix, and nuclease-free water up to 50 μL. A negative (no-template DNA) control sample and a positive DNA control sample were included in each PCR reaction. PCR products were visualized on agarose gels (0.8%). Subsequently, bands were eluted and purified using the Wizard SV Gel and PCR Clean-Up System Kit (Promega, WI, USA). Once purified, PCR products were concentrated and sequenced in both directions by Stab Vida (Lisbon, Portugal).
2.3.4. Phylogenetic Studies
Accession numbers obtained in this study are available in the GenBank database (Table 1). To analyze the relationships among the different Trichuris species, additional sequences from GenBank database were included in the alignments (Supplementary Table S1).
The aligned nucleotide dataset was obtained by the MUSCLE alignment method in MEGA (Molecular Evolutionary Genetics Analysis) version 11 [35]. Moreover, the number of nucleotide differences per sequence was calculated to evaluate the identity among Trichuris β-tubulin partial sequences by Compute Pairwise Distances based on the number of differences method of MEGA11 [35].
All phylogenetic trees were inferred by two different methods, Maximum Likelihood (ML) and Bayesian Inferences (BIs). To generate the ML tree, PhyML 3.0 package [36] was used, and for the BI tree, MrBayes v3.2.6 [37] was used. To resolve the best-fit substitution model for the nucleotide dataset jModelTest [38] was employed, and the models of evolution were determined in agreement with Akaike Information Criterion [36,39]. Bootstrapping (heuristic option) of more than 1000 replications was used to examine the topology support to assess the relative reliability of the clades, and the Bayesian Posterior Probabilities (BPPs) comprised the percentage converted. Standard deviation of split frequencies was used to determine if the number of generations completed was adequate. Each dataset was run for 10 million generations, and the chain was sampled every 500 generations. In addition, trees from the first million generations were discarded based on an assessment of convergence. Empirically, during the burn, the examination of the log-likelihood values of the chain was carried out.
3. Results
3.1. SNP Genotyping Assays
rhAmpTM SNP Genotyping were completely optimized at codon 167, 198, and 200 of the β-tubulin gene in T. trichiura and were able to detect the presence or absence of the WT and MA genotypes.
Before real-time analysis, for each SNP analyzed, standard peaks were created for RR (homozygote-resistant), SS (homozygote-susceptible), and RS (heterozygote) using WT, MA, and heterozygote DNA samples obtained commercially from IDT.
For each point mutation site, the melting peaks obtained from RT-PCR were analyzed and determined as RR, SS, and RS as reported by the specific Tm. Ninety-one Trichuris samples were collected from non-human primates, and genotyping assays by RT-PCR revealed the different dots, homozygote and heterozygote, obtained in the present work (Figure 1). Positive (WT, MA, and heterozygote) and negative controls were always included, and no amplification in negative controls was observed.
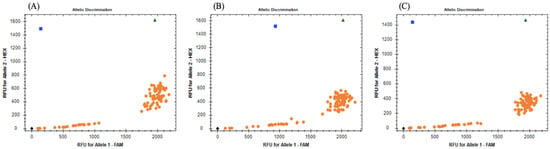
Figure 1.
Allelic discrimination plots obtained for rhAmpTM SNP genotyping assays targeting the three SNPs: (A) codon 167; (B) codon 198; (C) codon 200. Homozygous genotypes are represented by orange dots and blue squares, where the orange dots correspond to an SS and the blue squares to an RR, the green triangles represent heterozygous genotypes, and the black rhombus on the bottom left of the plot are no-template controls.
Out of the three analyzed SNPs in the partial β-tubulin gene, all specimens exhibited SS (100%). However, for codon 168, low signal intensity was observed in all twenty-one egg batch samples for both fluorophores. For codon 198, only four egg batch samples showed low signal intensity, and for codon 200, seven egg batch samples displayed low signal intensity. To ensure the accuracy of the results, all experiments were repeated twice. In concordance with these results, the resistance allele frequency (RAF) was 0.0 for each SNP studied.
3.2. Molecular Analysis
The phylogenetic tree inferred by ML and BI methods revealed three main clades (Figure 2). Clade 1 consisted of sequences from both, Trichuris sp. and T. trichiura, isolated from humans and non-human primates (100 BPP and 94% ML BV). In the BI analysis, the sequences were in polytomy, but the support for different subclades was not strong based on ML methods. Clade 2 consisted of sequences from Trichuris colobae and Trichuris suis (100 BPP and 89% ML BV), which were grouped into two distinct and well-supported subclades. Further, clade 3 consisted of sequences from Trichuris sp. isolated from H. cristata that were strongly supported (100 BPP and 85% ML BV) (Figure 2).
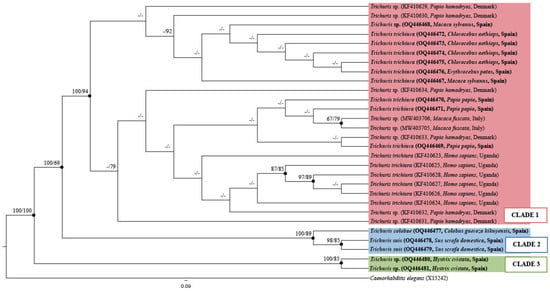
Figure 2.
Phylogenetic tree inferred based on the molecular marker β-tubulin partial gene of Trichuris species using ML method. Bayesian Posterior Probabilities (BPPs) are listed first, followed by ML bootstrap values (BVs) of clades, for clade frequencies exceeding 60%.
The results obtained were consistent with the identity analysis, where sequences clustered within the same clade displayed an inter-populational similarity percentage of over 98.7% for clade 1, ranging from 98 to 100% for clade 2, and 100% for clade 3 (Supplementary Table S2a,b). The similarity percentage between clade 1 and clade 2 ranged from 91.3 to 92.3%, between clade 1 and clade 3, 81.8–82.9%, and between clade 2 and clade 3, 82.9–83.1% (Supplementary Table S2a,b).
Phylogenetic analysis revealed a sister relationship between clade 1, which included T. trichiura and Trichuris sp. isolated from humans and non-human primates, and clade 2, which comprised T. suis and T. colobae (100 BPP and 69% ML BV). Likewise, both clades remained separate from Trichuris sp. from porcupine (Figure 2).
4. Discussion
STHs have been widely treated with mass drug administration, a highly effective approach in reducing helminth-related morbidity by limiting transmission within endemic communities. However, while this strategy brings numerous advantages to the population, it may also lead to unintended consequences, such as the gradual reduction in treatment effectiveness [40,41]. Consequently, with the expansion of drug donations, the emergence of AR becomes increasingly probable. This decline in treatment efficacy has been particularly concerning in veterinary parasites, where certain nematode species have demonstrated high levels of resistance to drugs, including BZ [11,12,42,43,44]. Moreover, in these helminths, SNPs in the β-tubulin isotype 1 gene have been associated with resistance to BZ [10,11,12].
Several tests have been proposed to identify mutations related to BZ resistance in helminths [19,45,46]. The SmartAmp2 constituted an alternative approach utilized. This method streamlines the process by implementing a single-step protocol, enabling enhanced expediency, showcasing distinct advantages over conventional PCR. Additionally, it has demonstrated efficacy in the investigation of diverse STHs identified in fecal samples. Nevertheless, utilizing this technique, the optimization of all necessary SNP detections proved unattainable, as the identification of codon 167 in T. trichiura was unsuccessful [28]. Diarra et al. [19] conducted an evaluation of the SNPs in codon 200 in A. lumbricoides using pyrosequencing, which necessitates specialized equipment. Another method employed was the RFLP-PCR, which is considered simpler and more sensitive but has certain limitations, such as cases where the DNA sequences are not recognized by commercially available restriction enzymes or possess multiple recognition sites for a single enzyme [45]. In contrast, Furtado et al. [46] employed ARMS-PCR, a technique that solely requires a conventional thermocycler, offering a straightforward and immediate result. Broccanello et al. [47] conducted a comparison of the accuracy, sensitivity, and costs of TaqMan, KASP, and rhAmpTM SNP Genotyping methods in sugar beet (Beta vulgaris L.). The sensitivity test revealed that both TaqMan and rhAmp were able to accurately determine SNP genotypes. In the case of rhAmpTM SNP Genotyping, 24 of the 33 SNPs exhibited 100% concordance with other two technologies. The genotype concordance with both technologies for the remaining nine targets exceeded 99%.
This study represents the initial endeavor to establish rhAmp assays for genotyping SNPs in the β-tubulin partial gene of Trichuris spp. samples. The primary objective was to develop a rapid and highly sensitive method capable of discriminating between different alleles in Trichuris samples, while also determining the genotyping frequencies. The developed rhAmp methods proved effective in accurately identifying the three different genotypes. Sequencing of the PCR products further confirmed the high conservation of the region flanked by the primers (PCR) and probes (rhAmp) which also contained the mutation.
None of the analyzed SNPs were found in our examined samples. Hence, the prevalence observed in our study was found to be lower compared to previously reported prevalence rates for other parasites. For instance, the prevalence of A. lumbricoides was reported to be 0.5% [46], while Haemonchus contortus showed a much higher prevalence of 74% [48]. Nonetheless, certain studies examining theses SNPs in parasites of veterinary significance, such as P. equorum and Ascaridia galli, have failed to detect these alterations, even in parasite populations subjected to frequent treatments throughout the year [49,50,51]. However, our findings are consistent with those obtained by Hansen et al. [34], wherein none of the 27 adult worms and 39 egg batched of Trichuris from human analyzed, or the 49 adult worms of Trichuris samples from baboons, exhibited mutations in any of the analyzed SNPs. This suggests that the identified SNPs may not be the primary mechanism responsible for BZ resistance in these nematode species.
The methodology proposed here offers a robust tool for screening the emergence of anthelmintic resistance mutations in populations of parasitic nematodes. Furthermore, it represents a rapid, highly sensitive, and specific technique that obviates the need for PCR amplification, a thermocycler, and electrophoresis.
Additionally, according to previous phylogenetic studies carried out by other authors, which were based on both nuclear and mitochondrial markers and focused on Trichuris species, two main clades have been identified. These clades have been previously referred to as the “T. trichiura lineage”, which parasitizes humans and non-human primates, and the “T. suis lineage”, which infects suids and other primates, such as Colobus guereza kikuyensis or Papio ursinus [31,32]. Furthermore, various authors have reported the hypothesis that a complex whipworm species may exist in primates, suggesting that different Trichuris species infect both primates and humans [32,52,53,54,55,56]. In addition, our results align with those obtained by Rivero et al. [57], where, based on nuclear and mitochondrial markers as well, the Trichuris sequences isolated from porcupines were distinct from the rest of the analyzed sequences, remaining isolated from the rest of the sequences in the obtained tree. As a result, the results obtained confirmed the sister relationship between clade 1 and clade 2 as identified in the phylogenetic tree. This finding corroborates the utility of β-tubulin as an additional marker, in addition to those previously described, for the phylogenetic analysis of the Trichuris species.
5. Conclusions
The current study provides a novel genotyping assay for assessing the prevalence of frequency of AR-associated β-tubulin SNPs at codons 167, 198, and 200 in T. trichiura. This investigation has showcased the efficacy of rhAmp methods in accurately discriminating and identifying β-tubulin SNPs within T. trichiura adults and eggs. Consequently, rhAmpTM SNP Genotyping can be considered a molecular tool that is both rapid and sensitive for the in vitro assessment of BZ susceptibility in Trichuris spp. Moreover, using pooled samples, it can be proposed as a cost-effective method, offering immediate practical advantages in the field.
Furthermore, this study provides further insights into the phylogeny of β-tubulin in Trichuris spp., reaffirming the previously established close relationship between the T. trichiura clade and T. suis clade. Thus, we affirm its usefulness as a marker for phylogenetic analysis.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ani14111545/s1, Table S1: Sequences of Trichuris spp. and outgroups species obtained from GenBank and used for phylogenetic analysis; Table S2a: Intra-specific and inter-specific similarity observed in β-tubulin partial gene sequences in Trichuris species isolated from different hosts. Values are given in percentages (%); Table S2b: Continuation of Table S2a. Intra-specific and inter-specific similarity observed in β-tubulin partial gene sequences in Trichuris species isolated from different hosts. Values are given in percentages (%).
Author Contributions
Conceptualization, R.C. and J.R.; methodology, J.R. and R.C.; validation, J.R. and R.C.; formal analysis, J.R.; investigation, J.R. and R.C.; data curation, J.R.; writing—original draft preparation, J.R.; writing—review and editing, J.R., R.C. and C.C.; supervision, C.C.; project administration, C.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors want to extend their gratitude to the zoological gardens (Zoo Castellar, Selwo Aventura, Parque Naturaleza de Cabárceno and Bioparc Fuengirola) for providing the analyzed samples.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- World Health Organization. Soil-Transmitted Infections. Available online: https://www.who.int/news-room/fact-sheets/detail/soil-transmitted-helminth-infections (accessed on 18 January 2023).
- Kern, P. Echinococcus granulosus infection: Clinical presentation, medical treatment and outcome. Langenbecks Arch. Surg. 2003, 388, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Soukhathammavong, P.A.; Sayasone, S.; Phongluxa, K.; Xayaseng, V.; Utzinger, J.; Vounatsou, P.; Hatz, C.; Akkhavong, K.; Keiser, J.; Odermatt, P. Low efficacy of single-dose albendazole and mebendazole against hookworm and effect on concomitant helminth infection in Lao PDR. PLoS Negl. Trop. Dis. 2012, 6, e1417. [Google Scholar] [CrossRef] [PubMed]
- Moser, W.; Schindler, C.; Keiser, J. Efficacy of recommended drugs against soil transmitted helminths: Systematic review and network meta-analysis. BMJ 2017, 358, j4307. [Google Scholar] [CrossRef] [PubMed]
- Moser, W.; Schindler, C.; Keiser, J. Drug Combinations Against Soil-Transmitted Helminth Infections. Adv. Parasitol. 2019, 103, 91–115. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, R.M. Drug resistance in nematodes of veterinary importance: A status report. Trends Parasitol. 2004, 20, 477–481. [Google Scholar] [CrossRef] [PubMed]
- Keiser, J.; Utzinger, J. Efficacy of current drugs against soil-transmitted helminth infections: Systematic review and meta-analysis. JAMA 2008, 299, 1937–1948. [Google Scholar] [CrossRef] [PubMed]
- Vercruysse, J.; Behnke, J.M.; Albonico, M.; Ame, S.M.; Angebault, C.; Bethony, J.M.; Engels, D.; Guillard, B.; Nguyen, T.V.; Kang, G.; et al. Assessment of the anthelmintic efficacy of albendazole in school children in seven countries where soil-transmitted helminths are endemic. PLoS Negl. Trop. Dis. 2011, 5, e948. [Google Scholar] [CrossRef] [PubMed]
- Levecke, B.; Montresor, A.; Albonico, M.; Ame, S.M.; Behnke, J.M.; Bethony, J.M.; Noumedem, C.D.; Engels, D.; Guillard, B.; Kotze, A.C.; et al. Assessment of anthelmintic efficacy of mebendazole in school children in six countries where soil-transmitted helminths are endemic. PLoS Negl. Trop. Dis. 2014, 8, e3204. [Google Scholar] [CrossRef]
- Kwa, M.S.; Veenstra, J.G.; Roos, M.H. Benzimidazole resistance in Haemonchus contortus is correlated with a conserved mutation at amino acid 200 in beta-tubulin isotype 1. Mol. Biochem. Parasitol. 1994, 63, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Prichard, R. Genetic variability following selection of Haemonchus contortus with anthelmintics. Trends Parasitol. 2001, 17, 445–453. [Google Scholar] [CrossRef]
- Ghisi, M.; Kaminsky, R.; Maser, P. Phenotyping and genotyping of Haemonchus contortus isolates reveals a new putative candidate mutation for benzimidazole resistance in nematodes. Vet. Parasitol. 2007, 144, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Keiser, J.; Utzinger, J. The drugs we have and the drugs we need against major helminth infections. Adv. Parasitol. 2010, 73, 197–230. [Google Scholar] [CrossRef] [PubMed]
- Olliaro, P.; Seiler, J.; Kuesel, A.; Horton, J.; Clark, J.N.; Don, R.; Keiser, J. Potential drug development candidates for human soil-transmitted helminthiases. PLoS Negl. Trop. Dis. 2011, 5, e1138. [Google Scholar] [CrossRef] [PubMed]
- Tehseen, M.M.; Zheng, Y.; Wyatt, N.A.; Bolton, M.D.; Yang, S.; Xu, S.S.; Li, X.; Chu, C. Development of STARP Marker Platform for Flexible SNP Genotyping in Sugarbeet. Agronomy 2023, 13, 1359. [Google Scholar] [CrossRef]
- Bennett, A.B.; Anderson, T.J.; Barker, G.C.; Michael, E.; Bundy, D.A. Sequence variation in the Trichuris trichiura beta-tubulin locus: Implications for the development of benzimidazole resistance. Int. J. Parasitol. 2002, 32, 1519–1528. [Google Scholar] [CrossRef] [PubMed]
- Diawara, A.; Drake, L.J.; Suswillo, R.R.; Kihara, J.; Bundy, D.A.; Scott, M.E.; Halpenny, C.; Stothard, J.R.; Prichard, R.K. Assays to detect beta-tubulin codon 200 polymorphism in Trichuris trichiura and Ascaris lumbricoides. PLoS Negl. Trop. Dis. 2009, 3, e397. [Google Scholar] [CrossRef] [PubMed]
- Diawara, A.; Schwenkenbecher, J.M.; Kaplan, R.M.; Prichard, R.K. Molecular and biological diagnostic tests for monitoring benzimidazole resistance in human soil-transmitted helminths. Am. J. Trop. Med. Hyg. 2013, 88, 1052–1061. [Google Scholar] [CrossRef]
- Diawara, A.; Halpenny, C.M.; Churcher, T.S.; Mwandawiro, C.; Kihara, J.; Kaplan, R.M.; Streit, T.G.; Idaghdour, Y.; Scott, M.E.; Basáñez, M.G.; et al. Association between response to albendazole treatment and β-tubulin genotype frequencies in soil-transmitted helminths. PLoS Negl. Trop. Dis. 2013, 30, 7:e2247. [Google Scholar] [CrossRef] [PubMed]
- Mendes de Oliveira, V.N.G.; Zuccherato, L.W.; Dos Santos, T.R.; Rabelo, É.M.L.; Furtado, L.F.V. Detection of Benzimidazole Resistance-Associated Single-Nucleotide Polymorphisms in the Beta-Tubulin Gene in Trichuris trichiura from Brazilian Populations. Am. J. Trop. Med. Hyg. 2022, 107, 640–648. [Google Scholar] [CrossRef]
- Vercruysse, J.; Levecke, B.; Prichard, R. Human soil-transmitted helminths: Implications of mass drug administration. Curr. Opin. Infect. Dis. 2012, 25, 703–708. [Google Scholar] [CrossRef]
- Semagn, K.; Babu, R.; Hearne, S.; Olsen, M. Single nucleotide polymorphism genotyping using Kompetitive Allele Specific PCR (KASP): Overview of the technology and its application in crop improvement. Mol. Breed. 2014, 33, 1–14. [Google Scholar] [CrossRef]
- Ertiro, B.T.; Ogugo, V.; Worku, M.; Das, B.; Olsen, M.; Labuschagne, M.; Semagn, K. Comparison of Kompetitive Allele Specific PCR (KASP) and genotyping by sequencing (GBS) for quality control analysis in maize. BMC Genom. 2015, 16, 908. [Google Scholar] [CrossRef] [PubMed]
- Dobosy, J.R.; Rose, S.D.; Beltz, K.R.; Rupp, S.M.; Powers, K.M.; Behlke, M.A.; Walder, J.A. RNase H-dependent PCR (rhPCR): Improved specificity and single nucleotide polymorphism detection using blocked cleavable primers. BMC Biotechnol. 2011, 11, 80. [Google Scholar] [CrossRef] [PubMed]
- Woodward, J. Bi-allelic SNP genotyping using the TaqMan assay. Methods Mol. Biol. 2014, 1145, 67–74. [Google Scholar] [CrossRef]
- Long, Y.M.; Chao, W.S.; Ma, G.J.; Xu, S.S.; Qi, L.L. An innovative SNP genotyping method adapting to multiple platforms and throughputs. Theor. Appl. Genet. 2017, 130, 597–607. [Google Scholar] [CrossRef] [PubMed]
- Schwenkenbecher, J.M.; Albonico, M.; Bickle, Q.; Kaplan, R.M. Characterization of beta-tubulin genes in hookworms and investigation of resistance-associated mutations using real-time PCR. Mol. Biochem. Parasitol. 2007, 156, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Rashwan, N.; Scott, M.; Prichard, R. Rapid Genotyping of β-tubulin Polymorphisms in Trichuris trichiura and Ascaris lumbricoides. PLoS Negl. Trop. Dis. 2017, 11, e0005205. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.K.; Singla, L.D. Chapter 6: Diagnostic Trends in Parasitic Diseases of Animals. In Veterinary Diagnostics; Gupta, R.P., Garg, S.R., Nehra, V., Lather, D., Eds.; Statish Serial Publishing House: Delhi, India, 2013; pp. 81–112. [Google Scholar]
- Horii, Y.; Usui, M. Experimental transmission of Trichuris ova from monkeys to man. Trans. R. Soc. Trop. Med. Hyg. 1985, 79, 423. [Google Scholar] [CrossRef] [PubMed]
- Rivero, J.; García-Sánchez, Á.M.; Zurita, A.; Cutillas, C.; Callejón, R. Trichuris trichiura isolated from Macaca sylvanus: Morphological, biometrical, and molecular study. BMC Vet. Res. 2020, 16, 445, Erratum in: BMC Vet. Res. 2021, 17, 160. [Google Scholar] [CrossRef]
- Rivero, J.; Cutillas, C.; Callejón, R. Trichuris trichiura (Linnaeus, 1771) From Human and Non-human Primates: Morphology, Biometry, Host Specificity, Molecular Characterization, and Phylogeny. Front. Vet. Sci. 2021, 7, 626120. [Google Scholar] [CrossRef]
- Beltz, K.; Tsang, D.; Wang, J.; Rose, S.; Bao, Y.; Wang, Y.; Larkin, K.; Rupp, S.; Schrepfer, D.; Datta, K.; et al. A high-performing and cost-effective SNP genotyping method using rhPCR and universal reporters. Adv. Biosci. Biotechnol. 2018, 9, 497–512. [Google Scholar] [CrossRef]
- Hansen, T.V.; Thamsborg, S.M.; Olsen, A.; Prichard, R.K.; Nejsum, P. Genetic variations in the beta-tubulin gene and the internal transcribed spacer 2 region of Trichuris species from man and baboons. Parasit. Vectors 2013, 6, 236. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Guindon, S.; Dufayard, J.F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New algorithms and methods to estimate maximum-likelihood phylogenies: Assessing the performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Huelsenbeck, J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef]
- Posada, D. jModelTest: Phylogenetic model averaging. Mol. Biol. Evol. 2008, 25, 1253–1256. [Google Scholar] [CrossRef] [PubMed]
- Posada, D.; Buckley, T.R. Model selection and model averaging in phylogenetics: Advantages of akaike information criterion and bayesian approaches over likelihood ratio tests. Syst. Biol. 2004, 53, 793–808. [Google Scholar] [CrossRef] [PubMed]
- Humphries, D.; Nguyen, S.; Boakye, D.; Wilson, M.; Cappello, M. The promise and pitfalls of mass drug administration to control intestinal helminth infections. Curr. Opin. Infect. Dis. 2012, 25, 584–589. [Google Scholar] [CrossRef] [PubMed]
- Furtado, L.F.; de Paiva Bello, A.C.P.; Rabelo, É.M.L. Benzimidazole resistance in helminths: From problem to diagnosis. Acta Trop. 2016, 162, 95–102. [Google Scholar] [CrossRef]
- Geerts, S.; Gryseels, B. Anthelmintic resistance in human helminths: A review. Trop. Med. Int. Health 2001, 6, 915–921. [Google Scholar] [CrossRef]
- van Wyk, J.A. Refugia-overlooked as perhaps the most potent factor concerning the development of anthelmintic resistance. J. Vet. Res. 2001, 68, 55–67. [Google Scholar]
- Wolstenholme, A.J.; Fairweather, I.; Prichard, R.; von Samson-Himmelstjerna, G.; Sangster, N.C. Drug resistance in veterinary helminths. Trends Parasitol. 2004, 20, 469–476. [Google Scholar] [CrossRef]
- Furtado, L.F.V.; Magalhães, J.G.S.; Rabelo, É.M.L. Standardization and application of a modified RFLP-PCR methodology for analysis of polymorphisms linked to treatment resistance in Ancylostoma braziliense. Parasit. Vectors 2018, 11, 540. [Google Scholar] [CrossRef]
- Furtado, L.F.V.; Medeiros, C.d.S.; Zuccherato, L.W.; Alves, W.P.; de Oliveira, V.N.G.M.; da Silva, V.J.; Miranda, G.S.; Fujiwara, R.T.; Rabelo, É.M.L. First identification of the benzimidazole resistance-associated F200Y SNP in the beta-tubulin gene in Ascaris lumbricoides. PLoS ONE 2019, 14, e0224108. [Google Scholar] [CrossRef] [PubMed]
- Broccanello, C.; Chiodi, C.; Funk, A.; McGrath, J.M.; Panella, L.; Stevanato, P. Comparison of three PCR-based assays for SNP genotyping in plants. Plant Methods 2018, 14, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Dixit, A.K.; Das, G.; Dixit, P.; Singh, A.P.; Kumbhakar, N.K.; Sankar, M.; Sharma, R.L. An assessment of benzimidazole resistance against caprine nematodes in Central India. Trop. Anim. Health Prod. 2017, 49, 1471–1478. [Google Scholar] [CrossRef] [PubMed]
- Tydén, E.; Engström, A.; Morrison, D.A.; Höglund, J. Sequencing of the β-tubulin genes in the ascarid nematodes Parascaris equorum and Ascaridia galli. Mol. Biochem. Parasitol. 2013, 190, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Tydén, E.; Dahlberg, J.; Karlberg, O.; Höglund, J. Deep amplicon sequencing of preselected isolates of Parascaris equorum in β-tubulin codons associated with benzimidazole resistance in other nematodes. Parasit. Vectors 2014, 7, 410. [Google Scholar] [CrossRef]
- Tarbiat, B.; Jansson, D.S.; Tydén, E.; Höglund, J. Evaluation of benzimidazole resistance status in Ascaridia galli. Parasitology 2017, 144, 1338–1345. [Google Scholar] [CrossRef]
- Liu, G.H.; Gasser, R.B.; Su, A.; Nejsum, P.; Peng, L.; Lin, R.Q.; Li, M.W.; Xu, M.J.; Zhu, X.Q. Clear genetic distinctiveness between human-and pig-derived Trichuris based on analysis of mitochondrial datasets. PLoS Negl. Trop. Dis. 2012, 6, e1539. [Google Scholar] [CrossRef]
- Cavallero, S.; De Liberato, C.; Friedrich, K.G.; Di Cave, D.; Masella, V.; D’Amelio, S.; Berrilli, F. Genetic heterogeneity and phylogeny of Trichuris spp. from captive non-human primates based on ribosomal DNA sequence data. Infect. Genet. Evol. 2015, 34, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Cavallero, S.; Nejsum, P.; Cutillas, C.; Callejón, R.; Doležalová, J.; Modrý, D.; D’Amelio, S. Insights into the molecular systematics of Trichuris infecting captive primates based on mitochondrial DNA analysis. Vet. Parasitol. 2019, 272, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Hawash, M.B.; Andersen, L.O.; Gasser, R.B.; Stensvold, C.; Nejsum, P. Mitochondrial genome analyses suggest multiple Trichuris species in humans, baboons, and pigs from different geographical regions. PLoS Negl. Trop. Dis. 2015, 9, e0004059. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Zhao, B.; Hoberg, E.P.; Li, M.; Zhou, X.; Gu, X.; Lai, W.; Peng, X.; Yang, G. Genetic characterisation and phylogenetic status of whipworms (Trichuris spp.) from captive non-human primates in China, determined by nuclear and mitochondrial sequencing. Parasit. Vectors 2018, 11, 516. [Google Scholar] [CrossRef]
- Rivero, J.; García-Sánchez, Á.M.; Callejón, R.; Cutillas, C. Characterization of Trichuris species from porcupine (Hystrix cristata) at zoological garden of Spain. Acta Trop. 2022, 228, 106276. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).