Novel Approach for Evaluating Pregnancy-Associated Glycoprotein and Inflammation Markers during the Postpartum Period in Holstein Friesian Cows
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Management
2.2. Postpartum Clinical Health Check, Sample Collection, and Farm Assessment
2.3. Postpartum Reproductive Management
2.4. Measurement of Biomarker Concentrations (PAG, SAA, and MAA)
2.5. Genotyping of the SNP of the FOXP3 Gene
2.6. Statistical Analysis
3. Results
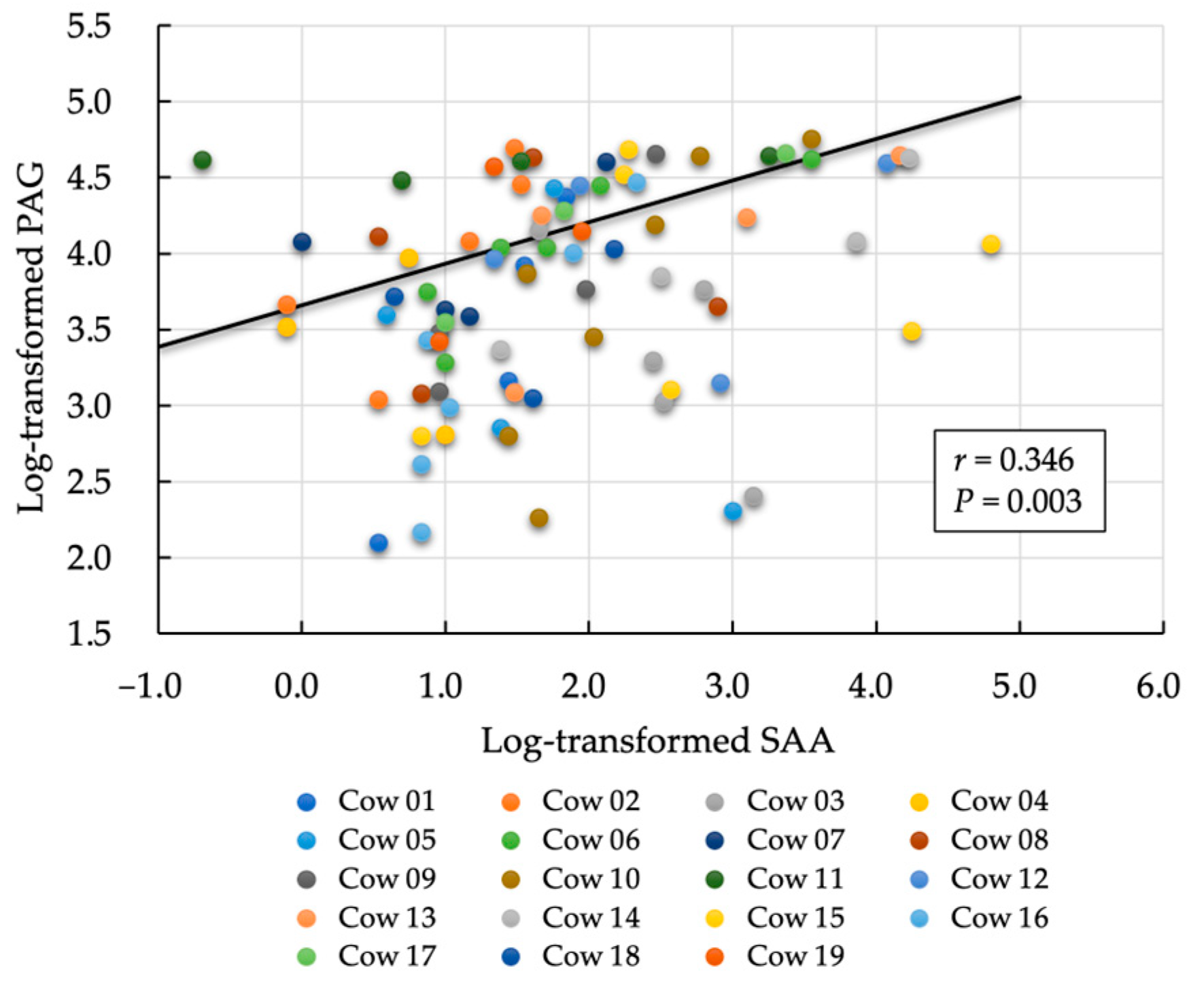
3.1. First Evaluation: Relationship between Postpartum Day and Biomarkers (PAG and SAA)
Log. PAG = 5.061 − 0.062 × days postpartum
Log. SAA = 2.550 − 0.036 × days postpartum
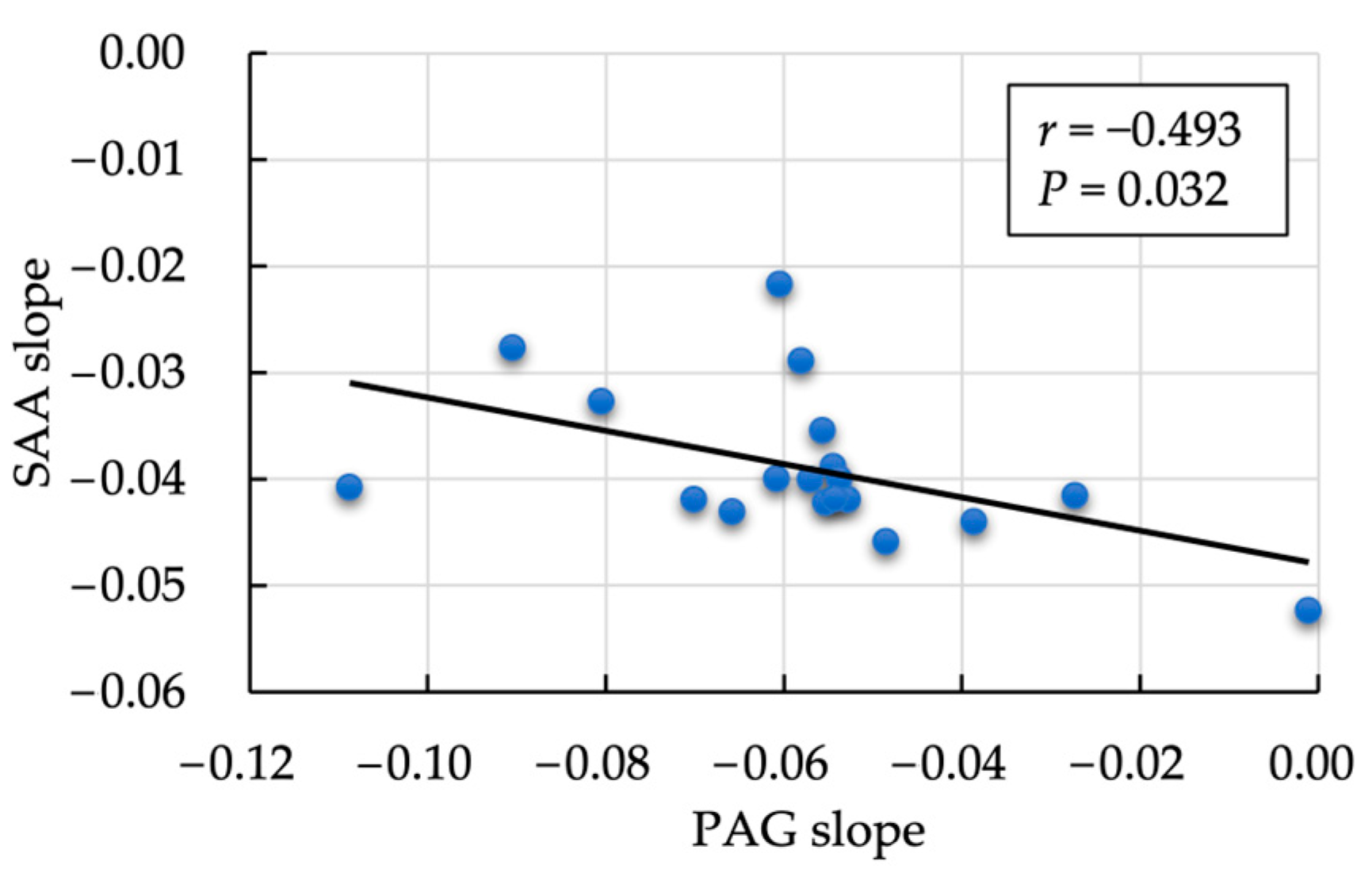
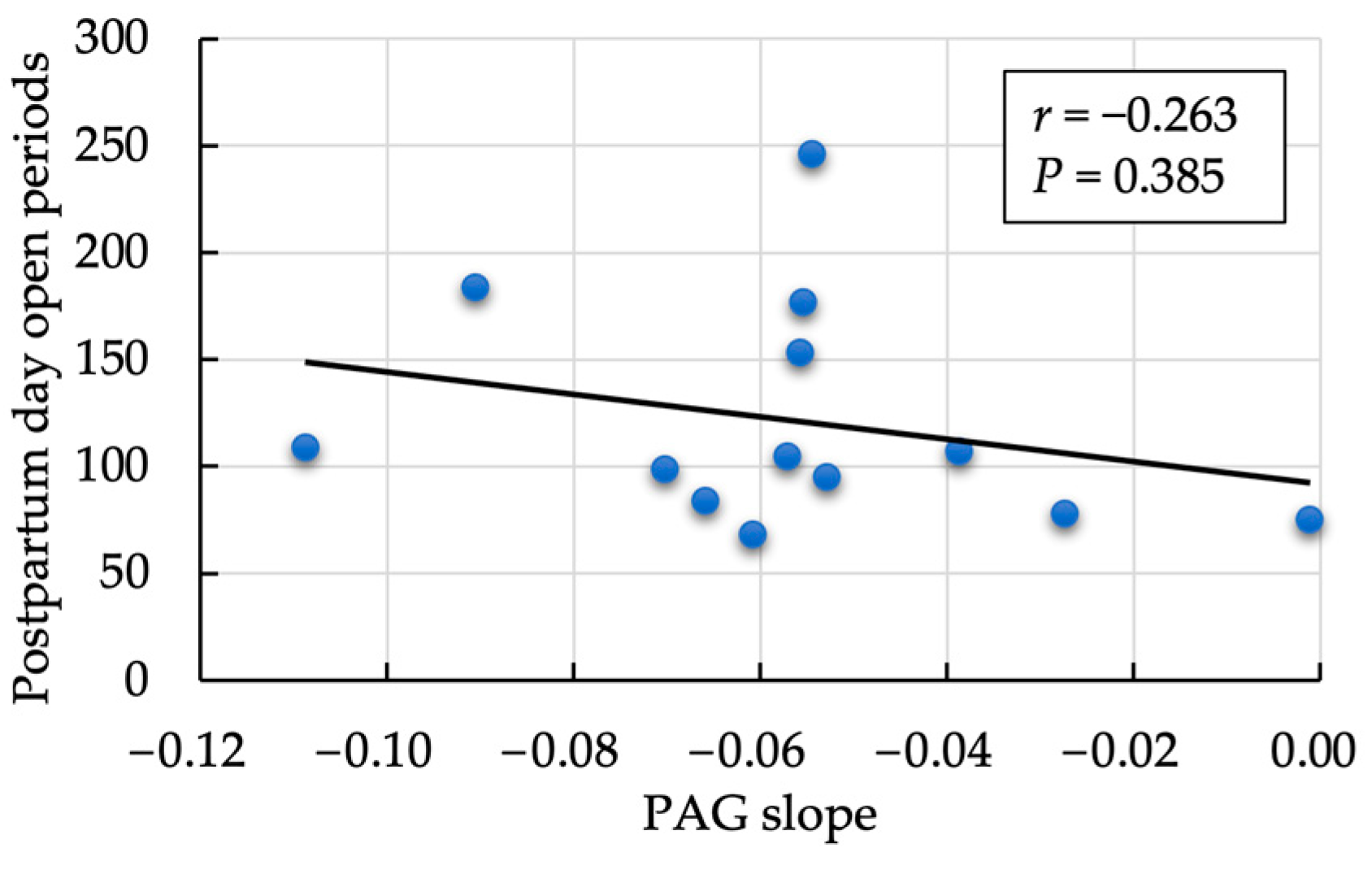
3.2. Second Evaluation: Correlation between the Postpartum Rate of Decrease in PAG and SAA
3.3. Third Evaluation: Association between Each Parameter with and without Mastitis, Follicular Cyst, and Ketosis
3.4. Fourth Evaluation: Association between FOXP3 Variants (T-Reg Genotypes) with Cyst, Mastitis, and Postpartum Days Open
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kiracofe, G.H. Uterine involution: Its role in regulating postpartum intervals. J. Anim. S. 1980, 2, 16–28. [Google Scholar]
- Raliou, M.; Dembélé, D.; Düvel, A.; Bolifraud, P.; Aubert, J.; Mary-Huard, T.; Rocha, D.; Piumi, F.; Mockly, S.; Heppelmann, M.; et al. Subclinical endometritis in dairy cattle is associated with distinct mRNA expression patterns in blood and endometrium. PLoS ONE 2019, 14, e0220244. [Google Scholar] [CrossRef] [PubMed]
- Okawa, H.; Monniaux, D.; Mizokami, C.; Fujikura, A.; Takano, T.; Sato, S.; Shinya, U.; Kawashima, C.; Yamato, O.; Fushimi, Y.; et al. Association between anti-Müllerian hormone concentration and inflammation markers in serum during the peripartum period in dairy cows. Animals 2021, 11, 1241. [Google Scholar] [CrossRef] [PubMed]
- Stephen, C.P.; Johnson, W.H.; Leblanc, S.J.; Foster, R.A.; Chenier, T.S. The impact of ecbolic therapy in the early postpartum period on uterine involution and reproductive health in dairy cows. J. Vet. Med. Sci. 2019, 81, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, S.; Kumari, S.; Rani, P.; Onteru, S.K.; Singh, D. Postpartum uterine infection and ovarian dysfunction. Indian J. Med. Res. 2018, 148, S64–S70. [Google Scholar] [PubMed]
- Heppelmann, M.; Krach, K.; Krueger, L.; Benz, P.; Herzog, K.; Piechotta, M.; Hoedemaker, M.; Bollwein, H. The effect of metritis and subclinical hypocalcemia on uterine involution in dairy cows evaluated by sonomicrometry. J. Reprod. Dev. 2015, 61, 565–569. [Google Scholar] [CrossRef] [PubMed]
- Meyerholz, M.M.; Rohmeier, L.; Eickhoff, T.; Hülsebusch, A.; Jander, S.; Linden, M.; Macias, L.; Koy, M.; Heimes, A.; Gorríz-Martín, L.; et al. Genetic selection for bovine chromosome 18 haplotypes associated with divergent somatic cell score affects postpartum reproductive and metabolic performance. J. Dairy Sci. 2019, 102, 9983–9994. [Google Scholar] [CrossRef]
- Osawa, T. Predisposing factor, diagnostic, and therapeutic aspects of persistent endometritis in postpartum cows. J. Reprod. Dev. 2021, 67, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Zhou, C.; Basang, W.; Zhu, Y.; Wang, X.; Li, C.; Chen, L.; Zhou, X. Mechanisms by which mastitis affects reproduction in dairy cow: A review. Reprod. Domest. Anim. 2021, 56, 1165–1175. [Google Scholar] [CrossRef]
- Zhang, B.; Ma, X.; Loor, J.J.; Jiang, Q.; Guo, H.; Zhang, W.; Li, M.; Lv, X.; Yin, Y.; Wen, J.; et al. Role of ORAI calcium release-activated calcium modulator 1 (ORAI1) on neutrophil extracellular trap formation in dairy cows with subclinical hypocalcemia. J. Dairy Sci. 2022, 105, 3394–3404. [Google Scholar] [CrossRef]
- Abuajamieh, M.; Kvidera, S.K.; Fernandez, M.V.; Nayeri, A.; Upah, N.C.; Nolan, E.A.; Lei, S.M.; DeFrain, J.M.; Green, G.B.; Schoenberg, K.M.; et al. Inflammatory biomarkers are associated with ketosis in periparturient Holstein cows. Res. Vet. Sci. 2016, 109, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Stassi, A.F.; Díaz, P.U.; Gasser, F.B.; Velázquez, M.M.L.; Gareis, N.C.; Salvetti, N.R.; Ortega, H.H.; Baravalle, M.E. A review on inflammation and angiogenesis as key mechanisms involved in the pathogenesis of bovine cystic ovarian disease. Theriogenology 2022, 186, 70–85. [Google Scholar] [CrossRef] [PubMed]
- Okawa, H.; Fujikura, A.; Wijayagunawardane, M.M.P.; Vos, P.L.A.M.; Taniguchi, M.; Takagi, M. Effect of diagnosis and treatment of clinical endometritis based on vaginal discharge score grading system in postpartum Holstein cows. J. Vet. Med. Sci. 2017, 79, 1545–1551. [Google Scholar] [CrossRef] [PubMed]
- Okawa, H.; Goto, A.; Wijayagunawardane, M.M.P.; Vos, P.L.A.M.; Yamato, O.; Taniguchi, M.; Takagi, M. Risk factors associated with reproductive performance in Japanese dairy cows: Vaginal discharge with flecks of pus or calving abnormality extend time to pregnancy. J. Vet. Med. Sci. 2019, 81, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Sasser, R.G.; Ruder, C.A.; Ivani, K.A.; Butler, J.E.; Hamilton, W.C. Detection of pregnancy by radioimmunoassay of a novel pregnancy-specific protein in the serum of cows and a profile of serum concentration during gestation. Biol. Reprod. 1986, 35, 936–942. [Google Scholar] [CrossRef] [PubMed]
- Mercadante, P.M.; Ribeiro, E.S.; Risco, C.; Ealy, A.D. Associations between pregnancy associated glycoproteins and pregnancy outcomes, milk yield, parity, and clinical diseases in high-producing dairy cows. J. Dairy Sci. 2016, 99, 3031–3040. [Google Scholar] [CrossRef] [PubMed]
- Hooshmandabbasi, R.; Zerbe, H.; Bauersachs, S.; de Sousa, N.M.; Boos, A.; Klisch, K. Pregnancy-associated glycoprotein in cows with retained fetal membrane. Theriogenology 2018, 105, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Wallace, R.M.; Hart, M.L.; Egen, T.E.; Schmelzle, A.; Smith, M.F.; Pohler, K.G.; Green, J.A. Bovine pregnancy associated glycoproteins can alter selected transcripts in bovine endometrial explants. Theriogenology 2019, 131, 123–132. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, N.M.; Zongo, M.; Pitala, W.; Boly, H.; Sawadogo, L.; Sanon, M.; de Figueiredo, J.R.; Gonçalves, P.B.D.; El Amiri, B.A.; Perènyi, Z.; et al. Pregnancy-associated glycoprotein concentrations during pregnancy and the postpartum period in Azawak zebu cattle. Theriogenology 2003, 59, 1131–1142. [Google Scholar] [CrossRef]
- Reese, S.T.; Geary, T.W.; Franco, G.A.; Moraes, J.G.N.; Spencer, T.E.; Pohler, K.G. Pregnancy associated glycoproteins (PAGS) and pregnancy loss in high vs sub fertility heifers. Theriogenology 2019, 135, 7–12. [Google Scholar] [CrossRef]
- Akköse, M. Comparative evaluation of two commercial pregnancy-associated glycoproteins tests for early detection of pregnancy in dairy cattle. Theriogenology 2023, 200, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Ealy, A.D.; Seekford, Z.K. Symposium review: Predicting pregnancy loss in dairy cattle. J. Dairy Sci. 2019, 102, 11798–11804. [Google Scholar] [CrossRef] [PubMed]
- Filho, R.V.O.; Franco, G.A.; Reese, S.T.; Dantas, F.G.; Fontes, P.L.P.; Cooke, R.F.; Rhinehart, J.D.; Thompson, K.W.; Pohler, K.G. Using pregnancy associated glycoproteins (PAG) for pregnancy detection at day 24 of gestation in beef cattle. Theriogenology 2020, 141, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, P.M.; Hubner, A.M.; Junior, W.M.C.; Cunha, L.L.; Garrett, E.F.; Pohler, K.G.; Dias, N.W.; Mercadante, V.R.G.; Canisso, I.F.; Lima, F.S. Characterization of pregnancy-associated glycoproteins and progesterone as a predictor of twins and conceptus loss in high-risk pregnancy Holstein cows. J. Dairy Sci. 2021, 104, 5034–5046. [Google Scholar] [CrossRef] [PubMed]
- Austin, K.J.; King, C.P.; Vierk, J.E.; Sasser, R.G.; Hansen, T.R. Pregnancy-specific protein b induces release of an alpha chemokine in bovine endometrium. Endocrinology 1999, 140, 542–545. [Google Scholar] [CrossRef] [PubMed]
- Hoeben, D.; Burvenich, C.; Massart-Leën, A.M.; Lenjou, M.; Nijs, G.; Van Bockstaele, D.V.; Beckers, J.F. In vitro effect of ketone bodies, glucocorticosteroids and bovine pregnancy-associated glycoprotein on cultures of bone marrow progenitor cells of cows and calves. Vet. Immunol. Immunopathol. 1999, 68, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Kindahl, H.; Kornmatitsuk, B.; Gustafsson, H. The cow endocrine focus before and after calving. Reprod. Domest. Anim. 2004, 39, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Haugejorden, G.; Waage, S.; Dahl, E.; Karlberg, K.; Beckers, J.F.; Ropstad, E. Pregnancy associated glycoproteins (PAG) in postpartum cows, ewes, goats, and their offspring. Theriogenology 2006, 66, 1976–1984. [Google Scholar] [CrossRef] [PubMed]
- Tefera, N.; Jeanguyot, N.; Thibier, M.; Humblot, P. Pregnancy-specific protein B (bPSPB) and progesterone monitoring of post-partum dairy cows with placental retention. J. Vet. Med. A Physiol. Pathol. Clin. Med. 2001, 48, 331–336. [Google Scholar] [CrossRef]
- Foley, C.; Chapwanya, A.; Callanan, J.J.; Whiston, R.; Miranda-CasoLuengo, R.; Lu, J.; Meijer, W.G.; Lynn, D.J.; O’ Farrelly, C.; Meade, K.G. Integrated analysis of the local and systemic changes preceding the development of post-partum cytological endometritis. BMC Genom. 2015, 16, 811. [Google Scholar]
- Sheldon, I.M.; Molinari, P.C.C.; Ormsby, T.J.R.; Bromfield, J.J. Preventing postpartum uterine disease in dairy cattle depends on avoiding, tolerating, and resisting pathogenic bacteria. Theriogenology 2020, 150, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Kononov, S.U.; Meyer, J.; Frahm, J.; Kersten, S.; Kluess, J.; Bühler, S.; Wegerich, A.; Rehage, J.; Meyer, U.; Huber, K.; et al. Dietary l-carnitine affects leukocyte count and function in dairy cows around parturition. Front. Immunol. 2022, 13, 784046. [Google Scholar] [CrossRef] [PubMed]
- Shuster, D.E.; Kehrli, M.E., Jr.; Rainard, P.; Paape, M. Complement fragment C5a and inflammatory cytokines in neutrophil recruitment during intramammary infection with Escherichia coli. Infect. Immun. 1997, 65, 3286–3292. [Google Scholar] [CrossRef] [PubMed]
- Machado, V.S.; Silva, T.H. Adaptive immunity in the postpartum uterus: Potential use of vaccines to control metritis. Theriogenology 2020, 150, 201–209. [Google Scholar] [CrossRef]
- Brodzki, P.; Kostro, K.; Brodzki, A.; Wawron, W.; Marczuk, J.; Kurek, Ł. Inflammatory cytokines and acute-phase protein concentrations in the peripheral blood and uterus of cows that developed endometritis during early postpartum. Theriogenology 2015, 84, 11–18. [Google Scholar] [CrossRef]
- Elsayed, D.H.; El-Azzazi, F.E.; Mahmoud, Y.K.; Dessouki, S.M.; Ahmed, E.A. Subclinical endometritis and postpartum ovarian resumption in respect to TNF-α, IL-8 and CRP in Egyptian buffaloes. Anim. Reprod. 2020, 17, e20190027. [Google Scholar] [CrossRef]
- Chapwanya, A.; Meade, K.G.; Doherty, M.L.; Callanan, J.J.; Mee, J.F.; O’Farrelly, C. Histopathological and molecular evaluation of Holstein-Friesian cows postpartum: Toward an improved understanding of uterine innate immunity. Theriogenology 2009, 71, 1396–1407. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, F.; Oguejiofor, C.F.; Wang, D.; Dong, S.; Yan, Z. Endometrial expression of the acute phase molecule SAA is more significant than hp in reflecting the severity of endometritis. Res. Vet. Sci. 2018, 121, 130–133. [Google Scholar] [CrossRef]
- Dadarwal, D.; Palmer, C.; Griebel, P. Mucosal immunity of the postpartum bovine genital tract. Theriogenology 2017, 104, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Workman, C.J.; Szymczak-Workman, A.L.; Collison, L.W.; Pillai, M.R.; Vignali, D.A.A. The development and function of regulatory T cells. Cell Mol. Life Sci. 2009, 66, 2603–2622. [Google Scholar] [CrossRef]
- Arishima, T.; Sasaki, S.; Isobe, T.; Ikebata, Y.; Shimbara, S.; Ikeda, S.; Kawashima, K.; Suzuki, Y.; Watanabe, M.; Sugano, S.; et al. Maternal variant in the upstream of FOXP3 gene on the X chromosome is associated with recurrent infertility in Japanese Black cattle. BMC Genet. 2017, 18, 103. [Google Scholar] [CrossRef]
- Islam, M.D.; Takagi, M.; Lee, K.W.; Chang, H.S.; Okawa, H.; Yunus, M.; Lestari, T.S.; Tacharina, M.R.; Pervin, S.; Rakib, T.M.; et al. Frequency of an X-linked maternal variant of the bovine FOXP3 gene associated with infertility in different cattle breeds: A pilot study. Animals 2022, 12, 1044. [Google Scholar] [CrossRef]
- Takagi, M.; Yamagishi, N.; Lee, I.H.; Oboshi, K.; Tsunom, M.; Wijayagunawardane, M.P.B. Reproductive management with ultrasound scanner-monitoring system for a high-yielding commercial dairy herd under stanchion management style. Asian Aust. J. Anim. Sci. 2005, 18, 949–956. [Google Scholar] [CrossRef]
- Ono, T.; Takagi, M.; Kawashima, C.; Wijayagunawardane, M.P.B.; Vos, P.L.A.M.; Taniguchi, M.; Tanihara, F.; Otoi, T. Comparative effects of different dosages of hcg on follicular development in postpartum dairy cows with cystic ovarian follicles. Front. Vet. Sci. 2018, 5, 130. [Google Scholar] [CrossRef] [PubMed]
- Thomas, F.C.; Waterston, M.; Hastie, P.; Parkin, T.; Haining, H.; Eckersall, P.D. The major acute phase proteins of bovine milk in a commercial dairy herd. BMC Vet. Res. 2015, 11, 207. [Google Scholar] [CrossRef]
- Mizukami, K.; Chang, H.-S.; Yabuki, A.; Kawamichi, T.; Kawahara, N.; Hayashi, D.; Hossain, M.A.; Rahman, M.M.; Uddin, M.M.; Yamato, O. Novel rapid genotyping assays for neuronal ceroid lipofuscinosis in Border Collie dogs and high frequency of the mutant allele in Japan. J. Vet. Diagn. Investig. 2011, 23, 1131–1139. [Google Scholar] [CrossRef] [PubMed]
- Krebs, T.; Kilic, I.; Neuenroth, L.; Wasselin, T.; Ninov, M.; Tetens, J.; Lenz, C. A multiplexed parallel reaction monitoring assay to monitor bovine pregnancy-associated glycoproteins throughout pregnancy and after gestation. PLoS ONE 2022, 23, 0271057. [Google Scholar] [CrossRef]
- Lobago, F.; Bekana, M.; Gustafsson, H.; Beckers, J.F.; Yohannes, G.; Aster, Y.; Kindahl, H. Serum profiles of pregnancy-associated glycoprotein, oestrone sulphate and progesterone during gestation and some factors influencing the profiles in Ethiopian Borana and crossbred cattle. Reprod. Domest. Anim. 2009, 44, 685–692. [Google Scholar] [CrossRef]
- Vallejo-Timaran, D.A.; Reyes, J.; Gilbert, R.O.; Lefebvre, R.C.; Palacio-Baena, L.G.; Maldonado-Estrada, J.G. Incidence, clinical patterns, and risk factors of postpartum uterine diseases in dairy cows from high-altitude tropical herds. J. Dairy Sci. 2021, 104, 9016–9026. [Google Scholar] [CrossRef] [PubMed]
- Trela, M.; Domańska, D.; Witkowska-Piłaszewicz, O. Diagnostic use of serum amyloid A in dairy cattle. Agriculture 2022, 12, 459. [Google Scholar] [CrossRef]

| Estimate | 95% CI | p-Value | AIC | |||
|---|---|---|---|---|---|---|
| PAG | Segment | 1.749 | 1.648 | 1.850 | 238.329 | |
| Postpartum day | −0.085 | −0.097 | −0.073 | <0.001 * | ||
| Log. PAG | Segment | 5.061 | 4.882 | 5.239 | 115.193 | |
| Postpartum day | −0.062 | −0.069 | −0.056 | <0.001 * | ||
| Log. SAA | Segment | 2.550 | 2.118 | 2.983 | 246.075 | |
| Postpartum day | −0.036 | −0.050 | −0.022 | <0.001 * | ||
| Follicular Cyst | p-Value | ||
|---|---|---|---|
| without | with | with vs. without | |
| No. of cows | 7 | 12 | |
| Days open period (mean ± SD) | 94.5 ± 14.3 | 140.4 ± 60.6 | 0.046 * |
| PAG slope | −0.061 ± 0.033 | −0.052 ± 0.021 | 0.524 |
| Estimate day 1 SAA | 2.81 ± 1.20 | 2.36 ± 9.22 | 0.367 |
| SAA slope | −0.040 ± 0.007 | −0.040 ± 0.005 | 0.860 |
| No. of cows with MAA more than 12 µg/mL (%) | 4 (57.1) | 8 (66.7) | 0.999 |
| FOXP3 Variant | ||||
|---|---|---|---|---|
| A/A | A/G | G/G | p-Value | |
| No. of cows | 2 | 13 | 4 | |
| Follicular cyst (%) | 0 (0) | 8 (61.5) | 4 (100) | 0.017 * |
| Mastitis (%) | 0 (0) | 8 (61.5) | 3 (75.0) | 0.135 |
| Days open period (mean ± SD) | 102.0 ± 9.9 | 119.9 ± 50.9 | 154.8 ± 74.5 | 0.239 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Priyo, T.W., Jr.; Edo, A.; Taura, Y.; Yamato, O.; Ono, T.; Taniguchi, M.; Widodo, O.S.; Islam, M.S.; Maki, S.; Takagi, M. Novel Approach for Evaluating Pregnancy-Associated Glycoprotein and Inflammation Markers during the Postpartum Period in Holstein Friesian Cows. Animals 2024, 14, 1459. https://doi.org/10.3390/ani14101459
Priyo TW Jr., Edo A, Taura Y, Yamato O, Ono T, Taniguchi M, Widodo OS, Islam MS, Maki S, Takagi M. Novel Approach for Evaluating Pregnancy-Associated Glycoprotein and Inflammation Markers during the Postpartum Period in Holstein Friesian Cows. Animals. 2024; 14(10):1459. https://doi.org/10.3390/ani14101459
Chicago/Turabian StylePriyo, Topas Wicaksono, Jr., Ayane Edo, Yasuho Taura, Osamu Yamato, Tetsushi Ono, Masayasu Taniguchi, Oky Setyo Widodo, Md Shafiqul Islam, Shinichiro Maki, and Mitsuhiro Takagi. 2024. "Novel Approach for Evaluating Pregnancy-Associated Glycoprotein and Inflammation Markers during the Postpartum Period in Holstein Friesian Cows" Animals 14, no. 10: 1459. https://doi.org/10.3390/ani14101459
APA StylePriyo, T. W., Jr., Edo, A., Taura, Y., Yamato, O., Ono, T., Taniguchi, M., Widodo, O. S., Islam, M. S., Maki, S., & Takagi, M. (2024). Novel Approach for Evaluating Pregnancy-Associated Glycoprotein and Inflammation Markers during the Postpartum Period in Holstein Friesian Cows. Animals, 14(10), 1459. https://doi.org/10.3390/ani14101459







