Genome-Wide Association Study for Somatic Skeletal Traits in Duroc × (Landrace × Yorkshire) Pigs
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Animals and Phenotype
2.3. Genotyping and Quality Control
2.4. Population Structure Analysis
2.5. Genome-Wide Association Analyses
2.6. Estimation of Genetic Parameters and the Explained Phenotypic Variance
2.7. Candidate Genes and Functional Annotation
3. Results
3.1. Phenotype Statistics and Heritability Estimation
3.2. Population Structure Analysis
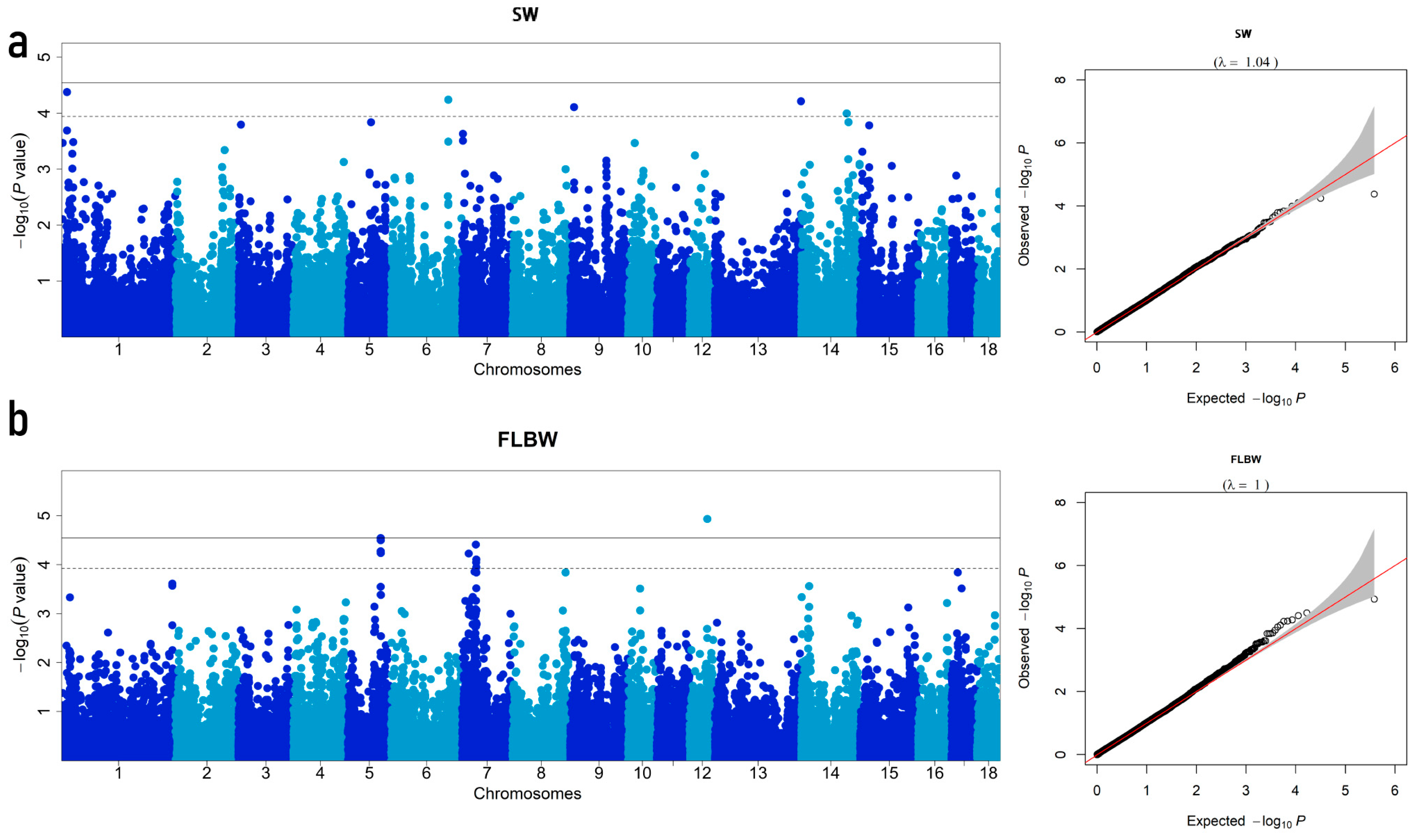
3.3. Summary of GWAS Results
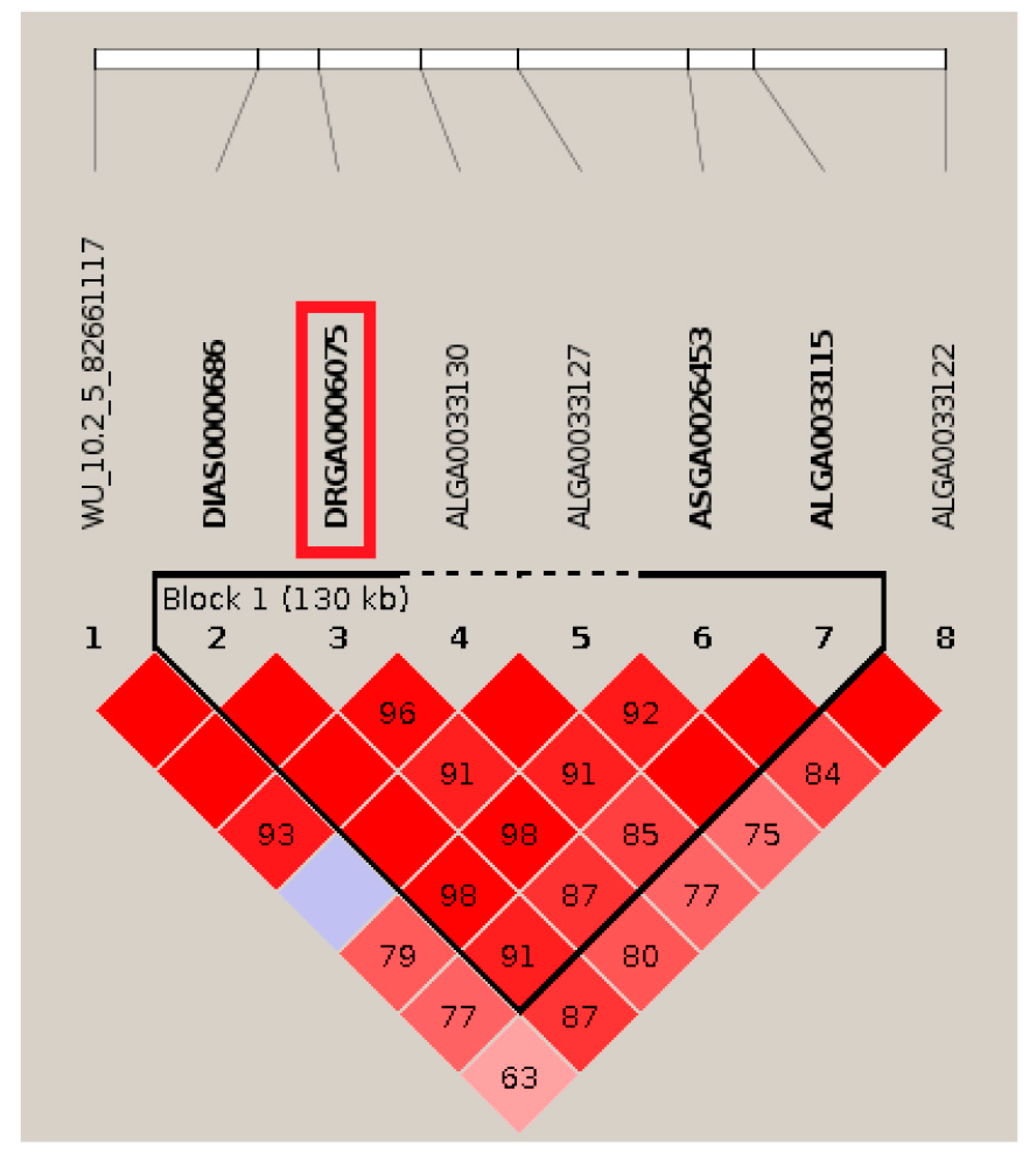
3.4. Candidate Genes and Function Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, H.; Shen, L.-Y.; Xu, Z.-C.; Kramer, L.M.; Yu, J.-Q.; Zhang, X.-Y.; Na, W.; Yang, L.-L.; Cao, Z.-P.; Luan, P. Haplotype-based genome-wide association studies for carcass and growth traits in chicken. Poult. Sci. 2020, 99, 2349–2361. [Google Scholar] [CrossRef] [PubMed]
- Terpstra, L.; Knol, D.L.; Van Coeverden, S.C.; Delemarre-van de Waal, H.A. Bone metabolism markers predict increase in bone mass, height and sitting height during puberty depending on the vdr fok1 genotype. Clin. Endocrinol. 2006, 64, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhuang, Z.; Yang, M.; Ding, R.; Quan, J.; Zhou, S.; Gu, T.; Xu, Z.; Zheng, E.; Cai, G.; et al. Genome-wide detection of genetic loci and candidate genes for body conformation traits in duroc × landrace × yorkshire crossbred pigs. Front. Genet. 2021, 12, 664343. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Hou, L.; Zhang, X.; Huang, M.; Mao, H.; Chen, H.; Ma, J.; Chen, C.; Ai, H.; Ren, J.; et al. A meta analysis of genome-wide association studies for limb bone lengths in four pig populations. BMC Genet. 2015, 16, 95. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, C.; Duan, D.; Xue, Y.; Han, X.; Wang, K.; Qiao, R.; Li, X.L.; Li, X.J. An association study on imputed whole-genome resequencing from high-throughput sequencing data for body traits in crossbred pigs. Anim. Genet. 2022, 53, 212–219. [Google Scholar] [CrossRef]
- Lan, Q.; Deng, Q.; Qi, S.; Zhang, Y.; Li, Z.; Yin, S.; Li, Y.; Tan, H.; Wu, M.; Yin, Y.; et al. Genome-wide association analysis identified variants associated with body measurement and reproduction traits in shaziling pigs. Genes 2023, 14, 522. [Google Scholar] [CrossRef]
- Browning, S.R.; Browning, B.L. Rapid and accurate haplotype phasing and missing-data inference for whole-genome association studies by use of localized haplotype clustering. Am. J. Hum. Genet. 2007, 81, 1084–1097. [Google Scholar] [CrossRef]
- Wu, X.; Fang, M.; Liu, L.; Wang, S.; Liu, J.; Ding, X.; Zhang, S.; Zhang, Q.; Zhang, Y.; Qiao, L. Genome wide association studies for body conformation traits in the chinese holstein cattle population. BMC Genom. 2013, 14, 897. [Google Scholar] [CrossRef]
- Consortium, T.W.T.C.C. Genome-wide association study of 14,000 cases of seven common diseases and 3000 shared controls. Nature 2007, 447, 661–678. [Google Scholar]
- Bakoev, S.; Getmantseva, L.; Bakoev, F.; Kolosova, M.; Gabova, V.; Kolosov, A.; Kostyunina, O. Survey of snps associated with total number born and total number born alive in pig. Genes 2020, 11, 491. [Google Scholar] [CrossRef]
- Wang, X.; Wang, L.; Shi, L.; Zhang, P.; Li, Y.; Li, M.; Tian, J.; Wang, L.; Zhao, F. Gwas of reproductive traits in large white pigs on chip and imputed whole-genome sequencing data. Int. J. Mol. Sci. 2022, 23, 13338. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, J.; Gong, H.; Cui, L.; Zhang, W.; Ma, J.; Chen, C.; Ai, H.; Xiao, S.; Huang, L.; et al. Genetic correlation of fatty acid composition with growth, carcass, fat deposition and meat quality traits based on gwas data in six pig populations. Meat Sci. 2019, 150, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.L.; Park, C.A.; Reecy, J.M. Bringing the animal qtldb and corrdb into the future: Meeting new challenges and providing updated services. Nucleic Acids Res. 2022, 50, 956–961. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.H.; Ma, M.Y.; Xiong, Y.Z. Genetic location of body composition traits in pigs. Yi Chuan = Hereditas 2004, 26, 163–166. [Google Scholar] [PubMed]
- Zhang, J.H.; Xiong, Y.Z.; Zuo, B.; Lei, M.G.; Jiang, S.W.; Li, F.E.; Zheng, R.; Li, J.L.; Xu, D.Q. Quantitative trait loci for carcass traits on pig chromosomes 4, 6, 7, 8 and 13. J. Appl. Genet. 2007, 48, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Wang, M.; Tang, Z.; Du, X.; Feng, S.; Ma, G.; Ye, D.; Cheng, H.; Wang, H.; Liu, X. Genome-wide association study of bone mineral density trait among three pig breeds. Anim. Int. J. Anim. Biosci. 2020, 14, 2443–2451. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.; Guo, Y.; Yang, G.; Yang, B.; Ren, J.; Liu, S.; Ai, H.; Ma, J.; Brenig, B.; Huang, L. A genome-wide scan for quantitative trait loci affecting limb bone lengths and areal bone mineral density of the distal femur in a white duroc x erhualian f2 population. BMC Genet. 2008, 9, 63. [Google Scholar] [CrossRef]
- Lim, S.W.; Hwang, D.; Kim, S.; Kim, J.M. Relationship between porcine carcass grades and estimated traits based on conventional and non-destructive inspection methods. J. Anim. Sci. Technol. 2022, 64, 155–165. [Google Scholar] [CrossRef]
- Ding, R.; Quan, J.; Yang, M.; Wang, X.; Zheng, E.; Yang, H.; Fu, D.; Yang, Y.; Yang, L.; Li, Z.; et al. Genome-wide association analysis reveals genetic loci and candidate genes for feeding behavior and eating efficiency in duroc boars. PLoS ONE 2017, 12, e0183244. [Google Scholar] [CrossRef]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.; Bender, D.; Maller, J.; Sklar, P.; De Bakker, P.I.; Daly, M.J. Plink: A tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef]
- Yang, J.; Lee, S.H.; Goddard, M.E.; Visscher, P.M. Gcta: A tool for genome-wide complex trait analysis. Am. J. Hum. Genet. 2011, 88, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Veerkamp, R.F.; Bouwman, A.C.; Schrooten, C.; Calus, M.P. Genomic prediction using preselected DNA variants from a gwas with whole-genome sequence data in holstein–friesian cattle. Genet. Sel. Evol. 2016, 48, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Stephens, M. Genome-wide efficient mixed-model analysis for association studies. Nat. Genet. 2012, 44, 821–824. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Cui, J.; Chazaro, I.; Cupples, L.A.; Demissie, S. Power and type i error rate of false discovery rate approaches in genome-wide association studies. BMC Genet. 2005, 6 (Suppl. S1), S134. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Zhang, Z.; Yang, B.; Guo, Y.; Ai, H.; Long, Y.; Su, Y.; Cui, L.; Zhou, L.; Wang, X.; et al. Identification of loci affecting teat number by genome-wide association studies on three pig populations. Asian-Australas. J. Anim. Sci. 2017, 30, 1–7. [Google Scholar]
- Barrett, J.C.; Fry, B.; Maller, J.; Daly, M.J. Haploview: Analysis and visualization of ld and haplotype maps. Bioinformatics 2005, 21, 263–265. [Google Scholar] [CrossRef]
- Lee, S.H.; Yang, J.; Goddard, M.E.; Visscher, P.M.; Wray, N.R. Estimation of pleiotropy between complex diseases using single-nucleotide polymorphism-derived genomic relationships and restricted maximum likelihood. Bioinformatics 2012, 28, 2540–2542. [Google Scholar] [CrossRef]
- Yang, J.; Benyamin, B.; McEvoy, B.P.; Gordon, S.; Henders, A.K.; Nyholt, D.R.; Madden, P.A.; Heath, A.C.; Martin, N.G.; Montgomery, G.W.; et al. Common snps explain a large proportion of the heritability for human height. Nat. Genet. 2010, 42, 565–569. [Google Scholar] [CrossRef]
- Ding, R.; Yang, M.; Wang, X.; Quan, J.; Zhuang, Z.; Zhou, S.; Li, S.; Xu, Z.; Zheng, E.; Cai, G. Genetic architecture of feeding behavior and feed efficiency in a duroc pig population. Front. Genet. 2018, 9, 220. [Google Scholar] [CrossRef]
- Yang, J.; Ferreira, T.; Morris, A.P.; Medland, S.E.; Madden, P.A.; Heath, A.C.; Martin, N.G.; Montgomery, G.W.; Weedon, M.N.; Loos, R.J. Conditional and joint multiple-snp analysis of gwas summary statistics identifies additional variants influencing complex traits. Nat. Genet. 2012, 44, 369–375. [Google Scholar] [CrossRef]
- Bartoš, P.; Dolan, A.; Smutný, L.; Šístková, M.; Celjak, I.; Šoch, M.; Havelka, Z. Effects of phytogenic feed additives on growth performance and on ammonia and greenhouse gases emissions in growing-finishing pigs. Anim. Feed Sci. Technol. 2016, 212, 143–148. [Google Scholar] [CrossRef]
- Warburton, C.L.; Engle, B.N.; Ross, E.M.; Costilla, R.; Moore, S.S.; Corbet, N.J.; Allen, J.M.; Laing, A.R.; Fordyce, G.; Lyons, R.E. Use of whole-genome sequence data and novel genomic selection strategies to improve selection for age at puberty in tropically-adapted beef heifers. Genet. Sel. Evol. 2020, 52, 1–13. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, Y.H.; Wang, S.; Zhang, Y.; Huang, T.; Cai, Y.D. Prediction and analysis of essential genes using the enrichments of gene ontology and kegg pathways. PLoS ONE 2017, 12, e0184129. [Google Scholar] [CrossRef]
- Xing, K.; Zhu, F.; Zhai, L.; Chen, S.; Tan, Z.; Sun, Y.; Hou, Z.; Wang, C. Identification of genes for controlling swine adipose deposition by integrating transcriptome, whole-genome resequencing, and quantitative trait loci data. Sci. Rep. 2016, 6, 23219. [Google Scholar] [CrossRef]
- Xie, C.; Mao, X.; Huang, J.; Ding, Y.; Wu, J.; Dong, S.; Kong, L.; Gao, G.; Li, C.-Y.; Wei, L. Kobas 2.0: A web server for annotation and identification of enriched pathways and diseases. Nucleic Acids Res. 2011, 39, W316–W322. [Google Scholar] [CrossRef]
- Bu, D.; Luo, H.; Huo, P.; Wang, Z.; Zhang, S.; He, Z.; Wu, Y.; Zhao, L.; Liu, J.; Guo, J.; et al. Kobas-i: Intelligent prioritization and exploratory visualization of biological functions for gene enrichment analysis. Nucleic Acids Res. 2021, 49, W317–W325. [Google Scholar] [CrossRef]
- Rivals, I.; Personnaz, L.; Taing, L.; Potier, M.C. Enrichment or depletion of a go category within a class of genes: Which test? Bioinformatics 2007, 23, 401–407. [Google Scholar] [CrossRef]
- Chen, Z.; Boehnke, M.; Wen, X.; Mukherjee, B. Revisiting the genome-wide significance threshold for common variant gwas. G3 2021, 11, jkaa056. [Google Scholar] [CrossRef]
- Armstrong, R.A. When to use the bonferroni correction. Ophthalmic Physiol. Opt. 2014, 34, 502–508. [Google Scholar] [CrossRef]
- Lu, S.; Zhao, L.-J.; Chen, X.-D.; Papasian, C.J.; Wu, K.-H.; Tan, L.-J.; Wang, Z.-E.; Pei, Y.-F.; Tian, Q.; Deng, H.-W. Bivariate genome-wide association analyses identified genetic pleiotropic effects for bone mineral density and alcohol drinking in caucasians. J. Bone Miner. Metab. 2017, 35, 649–658. [Google Scholar] [CrossRef]
- Yerges, L.M.; Klei, L.; Cauley, J.A.; Roeder, K.; Kammerer, C.M.; Ensrud, K.E.; Nestlerode, C.S.; Lewis, C.; Lang, T.F.; Barrett-Connor, E. Candidate gene analysis of femoral neck trabecular and cortical volumetric bone mineral density in older men. J. Bone Miner. Res. 2010, 25, 330–338. [Google Scholar] [CrossRef]
- Sugimoto, M.; Watanabe, T.; Sugimoto, Y. The molecular effects of a polymorphism in the 5′ utr of solute carrier family 44, member 5 that is associated with birth weight in holsteins. PLoS ONE 2012, 7, e41267. [Google Scholar] [CrossRef][Green Version]
- Klinck, R.; Laberge, G.; Bisson, M.; McManus, S.; Michou, L.; Brown, J.P.; Roux, S. Alternative splicing in osteoclasts and paget’s disease of bone. BMC Med. Genet. 2014, 15, 98. [Google Scholar] [CrossRef][Green Version]
- Baggio, L.L.; Drucker, D.J. Glucagon-like peptide-1 receptor co-agonists for treating metabolic disease. Mol. Metab. 2021, 46, 101090. [Google Scholar] [CrossRef]
- Zeng, Z.; Fei, L.; Yang, J.; Zuo, J.; Huang, Z.; Li, H. Mir-27a-3p targets glp1r to regulate differentiation, autophagy, and release of inflammatory factors in pre-osteoblasts via the ampk signaling pathway. Front. Genet. 2021, 12, 783352. [Google Scholar] [CrossRef]
- Courtland, J.L.; Bradshaw, T.W.; Waitt, G.; Soderblom, E.J.; Ho, T.; Rajab, A.; Vancini, R.; Kim, I.H.; Soderling, S.H. Genetic disruption of washc4 drives endo-lysosomal dysfunction and cognitive-movement impairments in mice and humans. eLife 2021, 10, e61590. [Google Scholar] [CrossRef]
- Du, X.; Cai, L.; Xie, J.; Zhou, X. The role of tgf-beta3 in cartilage development and osteoarthritis. Bone Res. 2023, 11, 2. [Google Scholar] [CrossRef]
- Meng, Q.; Wang, K.; Liu, X.; Zhou, H.; Xu, L.; Wang, Z.; Fang, M. Identification of growth trait related genes in a yorkshire purebred pig population by genome-wide association studies. Asian-Australas. J. Anim. Sci. 2017, 30, 462. [Google Scholar] [CrossRef]
- Zhuang, Z.; Ding, R.; Peng, L.; Wu, J.; Ye, Y.; Zhou, S.; Wang, X.; Quan, J.; Zheng, E.; Cai, G.; et al. Genome-wide association analyses identify known and novel loci for teat number in duroc pigs using single-locus and multi-locus models. BMC Genom. 2020, 21, 344. [Google Scholar] [CrossRef]
- Zeng, H.; Ge, J.; Xu, W.; Ma, H.; Chen, L.; Xia, M.; Pan, B.; Lin, H.; Wang, S.; Gao, X. Twelve loci associated with bone density in middle-aged and elderly chinese: The shanghai changfeng study. J. Clin. Endocrinol. Metab. 2023, 108, 295–305. [Google Scholar] [CrossRef]
- Vijayan, V.; Khandelwal, M.; Manglani, K.; Gupta, S.; Surolia, A. Methionine down-regulates tlr 4/myd 88/nf-κ b signalling in osteoclast precursors to reduce bone loss during osteoporosis. Br. J. Pharmacol. 2014, 171, 107–121. [Google Scholar] [CrossRef] [PubMed]
- Kocijan, R.; Harre, U.; Schett, G. Acpa and bone loss in rheumatoid arthritis. Curr. Rheumatol. Rep. 2013, 15, 366. [Google Scholar] [CrossRef] [PubMed]
- Lemann, J., Jr.; Pleuss, J.A.; Gray, R.W. Potassium causes calcium retention in healthy adults. J. Nutr. 1993, 123, 1623–1626. [Google Scholar] [CrossRef] [PubMed]
- Ha, J.; Kim, S.A.; Lim, K.; Shin, S. The association of potassium intake with bone mineral density and the prevalence of osteoporosis among older korean adults. Nutr. Res. Pract. 2020, 14, 55–61. [Google Scholar] [CrossRef]
- Jin, Z.; Kho, J.; Dawson, B.; Jiang, M.M.; Chen, Y.; Ali, S.; Burrage, L.C.; Grover, M.; Palmer, D.J.; Turner, D.L.; et al. Nitric oxide modulates bone anabolism through regulation of osteoblast glycolysis and differentiation. J. Clin. Investig. 2021, 131, e138935. [Google Scholar] [CrossRef]

| Trait | N | Mean (±SD) | Min/Kg | Max/Kg | C.V./% 1 | h2 (±SE) |
|---|---|---|---|---|---|---|
| SW | 507 | 0.63 ± 0.08 | 0.39 | 0.87 | 12.71 | 0.470 ± 0.077 |
| FLBW | 506 | 0.97 ± 0.11 | 0.67 | 1.35 | 11.79 | 0.672 ± 0.073 |
| HLBW | 542 | 1.72 ± 0.19 | 1.1 | 2.32 | 11.10 | 0.629 ± 0.071 |
| SBW | 430 | 1.45 ± 0.23 | 0.85 | 2.46 | 15.75 | 0.279 ± 0.088 |
| SW | FLBW | HLBW | SBW | |
|---|---|---|---|---|
| SW | 1 | 0.41 | 0.40 | 0.13 |
| FLBW | 0.77 + 0.11 | 1 | 0.78 | 0.21 |
| HLBW | 0.52 + 0.15 | 0.86 + 0.06 | 1 | 0.26 |
| SBW | 0.86 + 0.34 | 0.50 + 0.31 | 0.33 + 0.30 | 1 |
| Trait | SSC 1 | SNP ID 2 | Position (bp) 3 | MAF 4 | P-Value | PVE/% 5 | Candidate Gene | Distance (bp) 6 |
|---|---|---|---|---|---|---|---|---|
| SW | 1 | MARC0065727 | 12,629,436 | 0.474 | 4.20 × 10−5 | 3.92 | OPRM1 | within |
| 6 | ASGA0029572 | 137,682,936 | 0.26 | 5.74 × 10−5 | 3.72 | SLC44A5 | within | |
| 9 | ALGA0108358 | 9,320,080 | 0.218 | 7.80 × 10−5 | 2.55 | NEU3 | within | |
| 13 | WU_10.2_13_217373583 | 207,442,473 | 0.32 | 6.12 × 10−5 | 1.52 | UBE2G2 | −2059 | |
| 14 | ASGA0091963 | 109,404,872 | 0.452 | 0.000101038 | 0.55 | CRTAC1 | 14,180 | |
| FLBW | 5 | DIAS0000686 | 79,444,610 | 0.302 | 5.31 × 10−5 | 5.79 | WASHC4 | within |
| 5 | DRGA0006075 | 79,461,126 | 0.298 | 2.85 × 10−5 | 6.40 | WASHC4 | within | |
| 5 | ASGA0026453 | 79,557,821 | 0.256 | 3.18 × 10−5 | 4.09 | NOPCHAP1 | within | |
| 5 | ALGA0033115 | 79,575,118 | 0.277 | 5.81 × 10−5 | 2.99 | NOPCHAP1 | within | |
| 7 | H3GA0020119 | 16,266,396 | 0.328 | 5.93 × 10−5 | 1.45 | CDKAL1 | within | |
| 7 | MARC0074546 | 33,359,087 | 0.166 | 3.89 × 10−5 | 1.47 | ZFAND3 | −19,127 | |
| 7 | ALGA0040524 | 34,151,758 | 0.209 | 9.01 × 10−5 | 1.69 | ENSSSCG00000027778 | within | |
| 7 | H3GA0020988 | 34,502,131 | 0.267 | 0.000113219 | 1.10 | GLP1R | within | |
| 7 | ALGA0040570 | 34,538,678 | 0.267 | 7.84 × 10−5 | 1.61 | GLP1R | 4049 | |
| 12 | WU_10.2_12_44505563 | 42,721,240 | 0.303 | 1.17 × 10−5 | 1.54 | RHOT1 | within | |
| HLBW | 7 | WU_10.2_7_105204658 | 99,159,898 | 0.26 | 9.05 × 10−5 | 1.04 | TGFB3 | within |
| 8 | WU_10.2_8_144898378 | 135,537,530 | 0.363 | 0.000115895 | 2.40 | SCD5 | within | |
| 11 | H3GA0032120 | 62,244,494 | 0.28 | 9.89 × 10−5 | 1.34 | GPC5 | 98,256 | |
| 17 | ALGA0093437 | 15,401,211 | 0.302 | 0.000111254 | 4.48 | ENSSSCG00000043546 | 69,056 | |
| 17 | WU_10.2_17_17981232 | 16,253,154 | 0.315 | 0.000104166 | 7.44 | ENSSSCG00000025527 | 224,053 | |
| 17 | DRGA0016598 | 17,258,153 | 0.269 | 1.01 × 10−5 | 3.50 | PLCB1 | 487,159 | |
| SBW | 1 | INRA0007356 | 257,807,729 | 0.312 | 3.16 × 10−5 | 4.27 | TLR4 | −236,881 |
| 12 | MARC0086052 | 10,012,238 | 0.365 | 7.02 × 10−5 | 3.19 | KCNJ2 | −337,171 | |
| 12 | WU_10.2_12_11424336 | 11,240,882 | 0.247 | 1.44 × 10−6 | 5.59 | ABCA6 | within | |
| 12 | WU_10.2_12_11794440 | 11,296,596 | 0.265 | 6.30 × 10−5 | 5.07 | ABCA9 | within | |
| 15 | ALGA0084013 | 14,781,096 | 0.108 | 9.16 × 10−5 | 2.48 | ENSSSCG00000042807 | 94,194 |
| Term | Database | ID | Gene Names | Corrected P-Value |
|---|---|---|---|---|
| Rheumatoid arthritis | KEGG PATHWAY | ssc05323 | TGFB3, TLR4 | 0.032405 |
| Inward rectifier potassium channel activity | Gene Ontology | GO:0005242 | KCNJ2 | 0.003538 |
| Negative regulation of nitric oxide biosynthetic process | Gene Ontology | GO:0045019 | OPRM1 | 0.033125 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, X.; Zhou, S.; Liu, Z.; Ruan, D.; Wu, J.; Quan, J.; Zheng, E.; Yang, J.; Cai, G.; Wu, Z.; et al. Genome-Wide Association Study for Somatic Skeletal Traits in Duroc × (Landrace × Yorkshire) Pigs. Animals 2024, 14, 37. https://doi.org/10.3390/ani14010037
Gao X, Zhou S, Liu Z, Ruan D, Wu J, Quan J, Zheng E, Yang J, Cai G, Wu Z, et al. Genome-Wide Association Study for Somatic Skeletal Traits in Duroc × (Landrace × Yorkshire) Pigs. Animals. 2024; 14(1):37. https://doi.org/10.3390/ani14010037
Chicago/Turabian StyleGao, Xin, Shenping Zhou, Zhihong Liu, Donglin Ruan, Jie Wu, Jianping Quan, Enqin Zheng, Jie Yang, Gengyuan Cai, Zhenfang Wu, and et al. 2024. "Genome-Wide Association Study for Somatic Skeletal Traits in Duroc × (Landrace × Yorkshire) Pigs" Animals 14, no. 1: 37. https://doi.org/10.3390/ani14010037
APA StyleGao, X., Zhou, S., Liu, Z., Ruan, D., Wu, J., Quan, J., Zheng, E., Yang, J., Cai, G., Wu, Z., & Yang, M. (2024). Genome-Wide Association Study for Somatic Skeletal Traits in Duroc × (Landrace × Yorkshire) Pigs. Animals, 14(1), 37. https://doi.org/10.3390/ani14010037






