Calming Hungarian Grey Cattle in Headlocks Using Processed Nasal Vocalization of a Mother Cow
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals and Housing
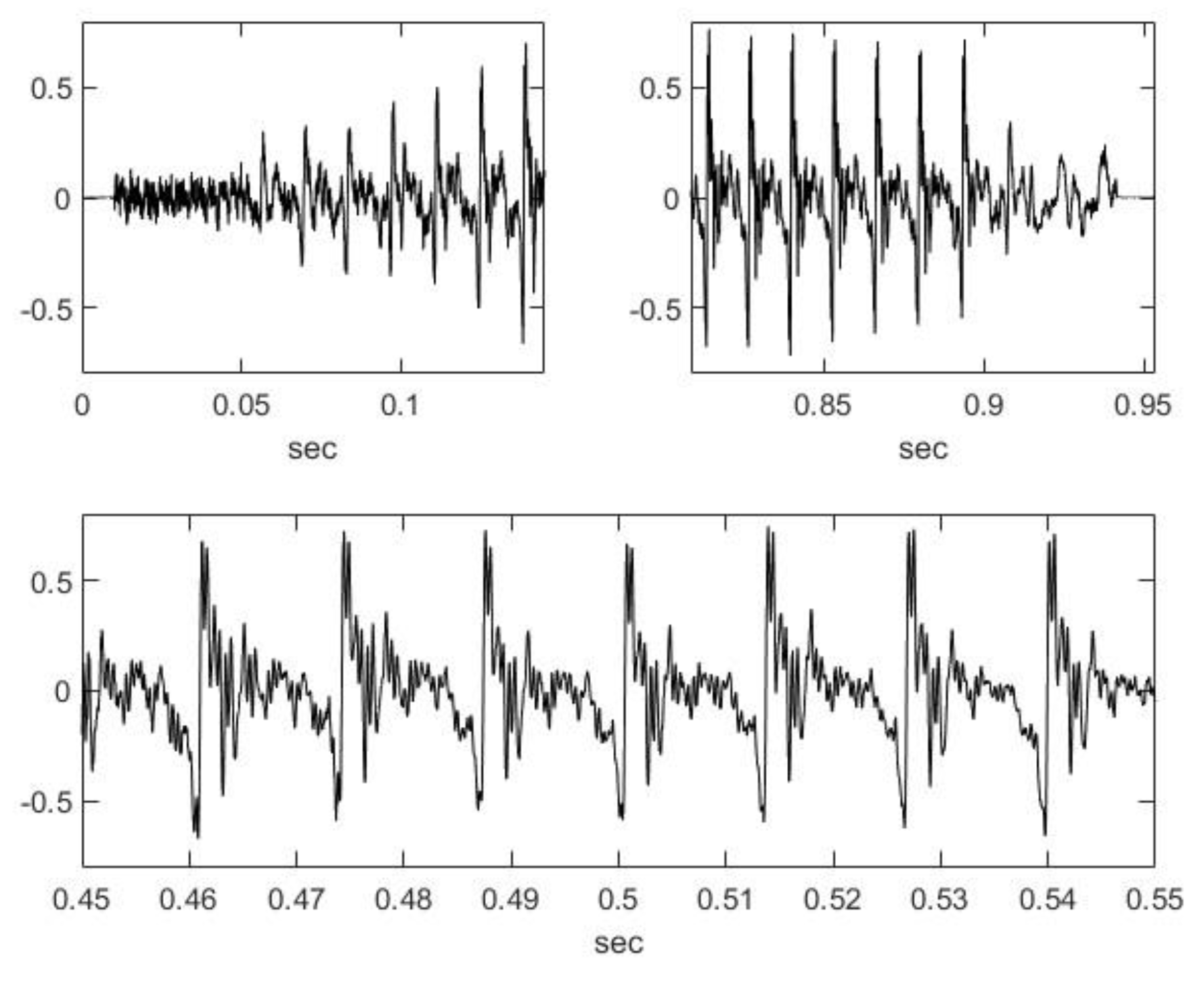
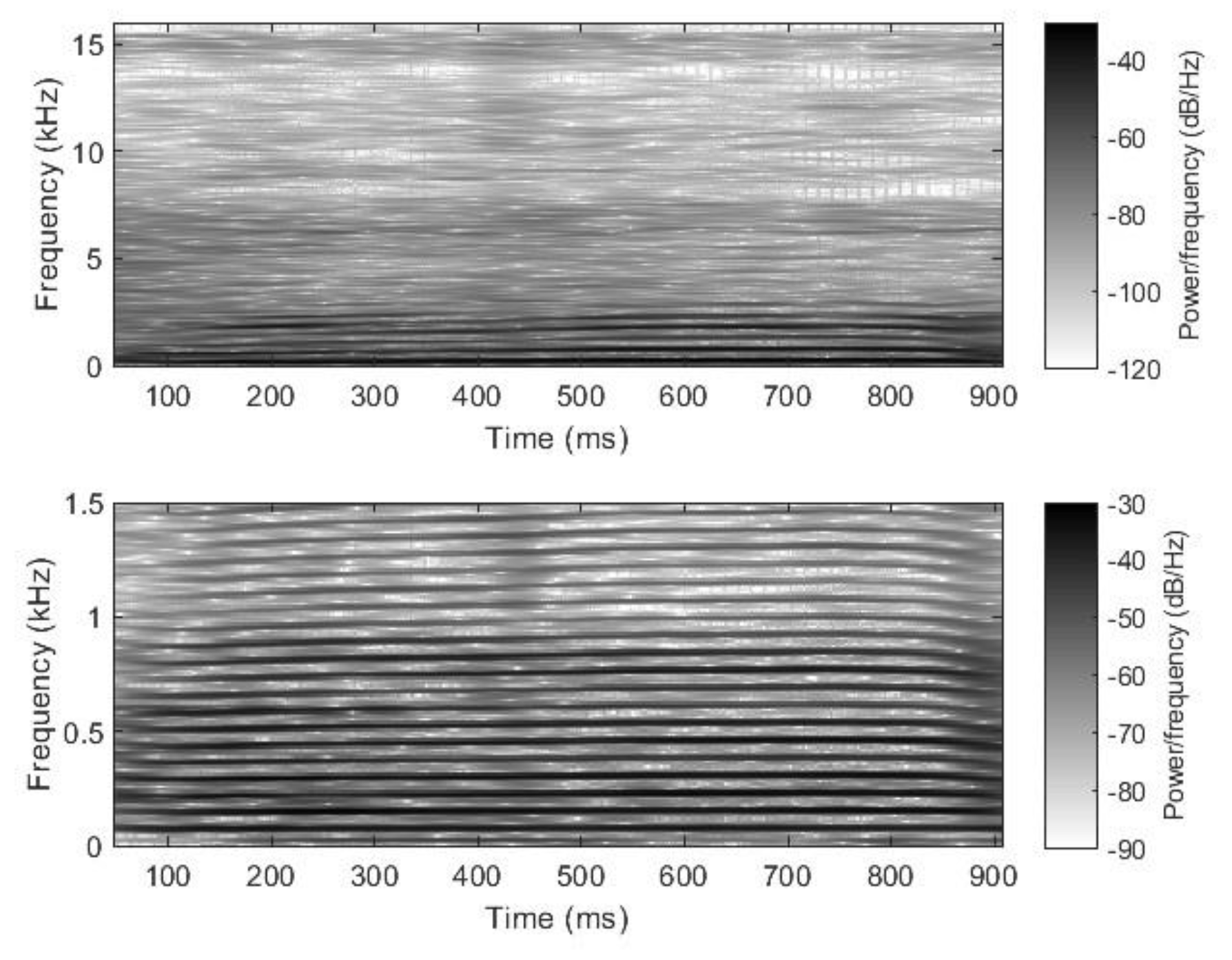
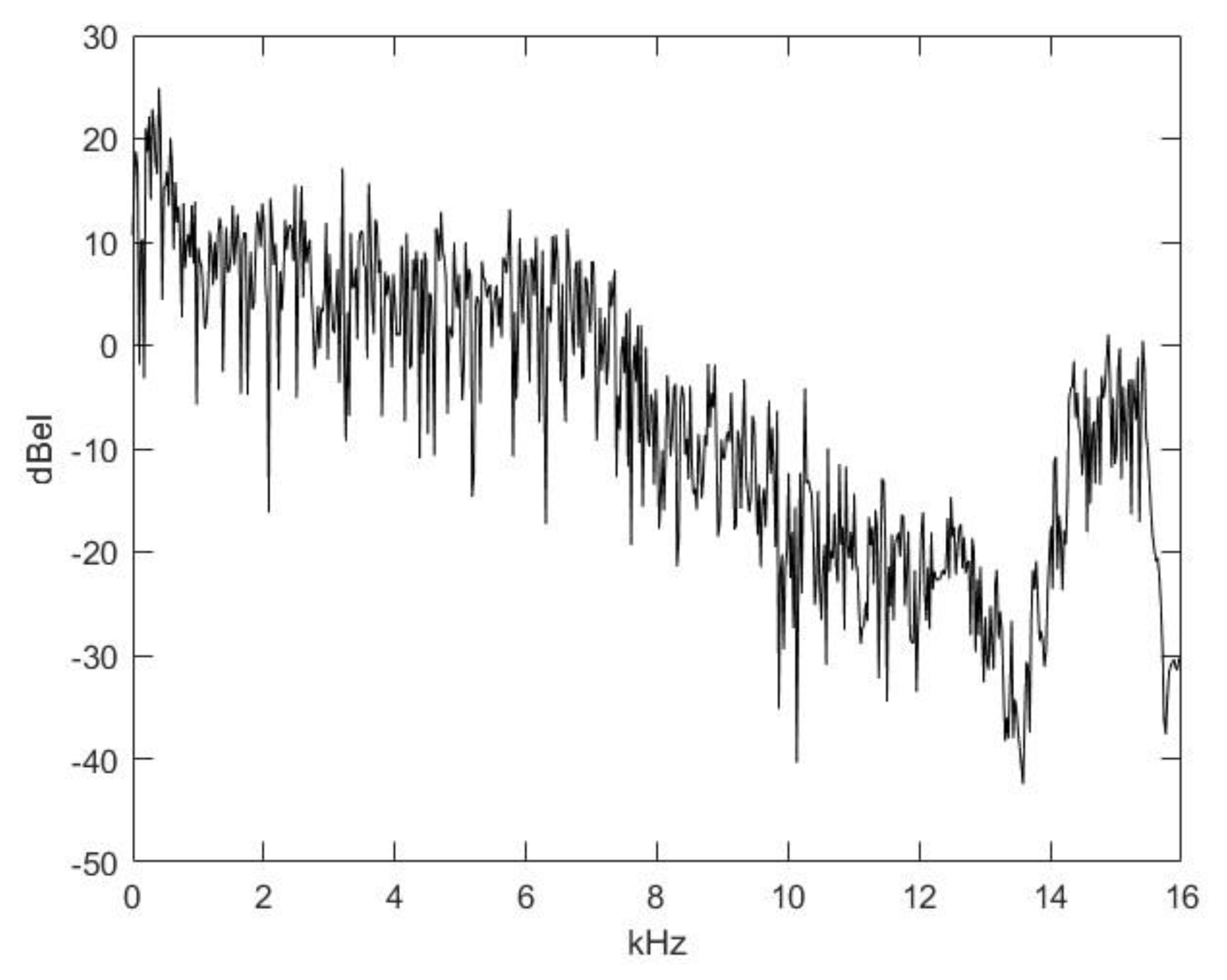
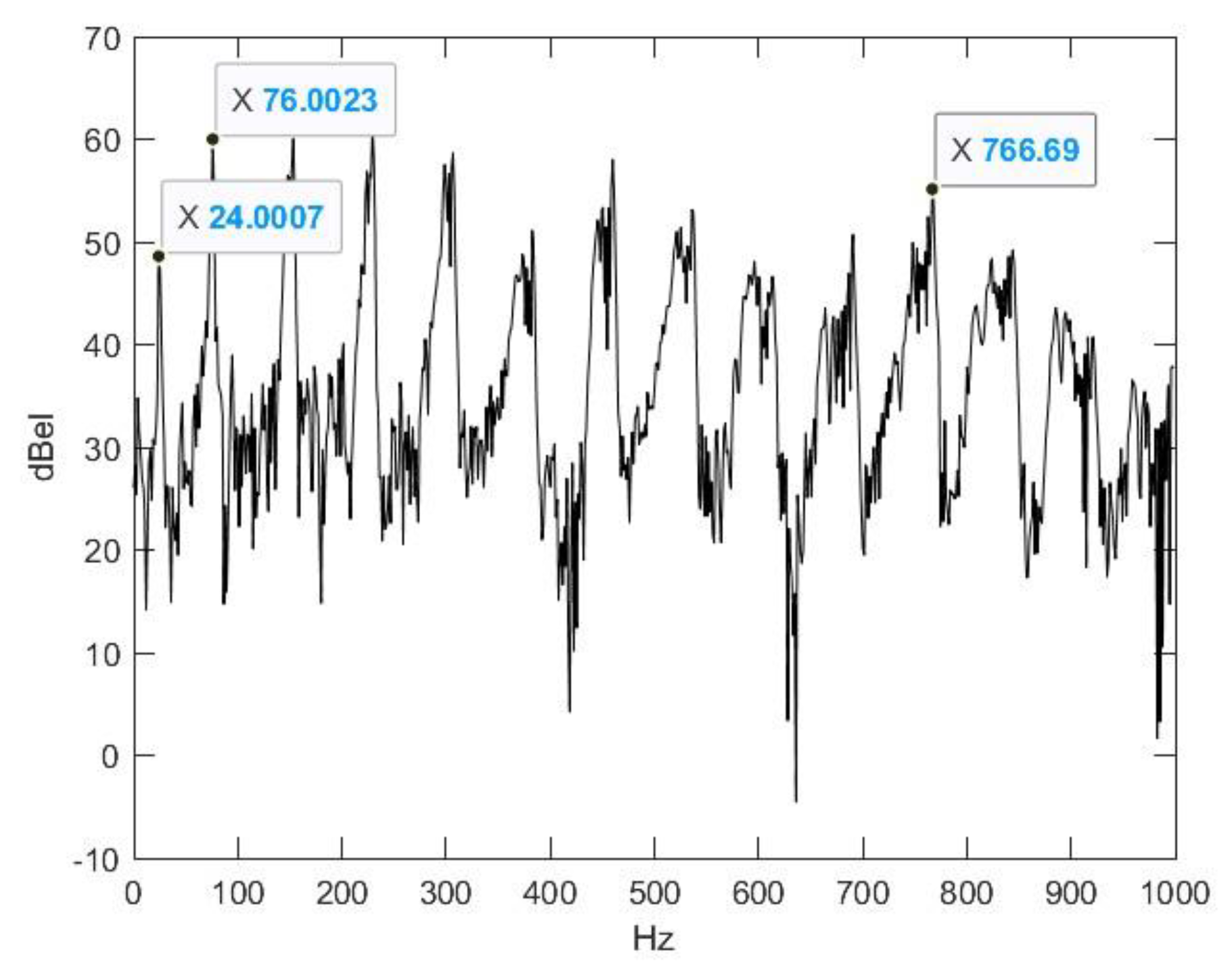
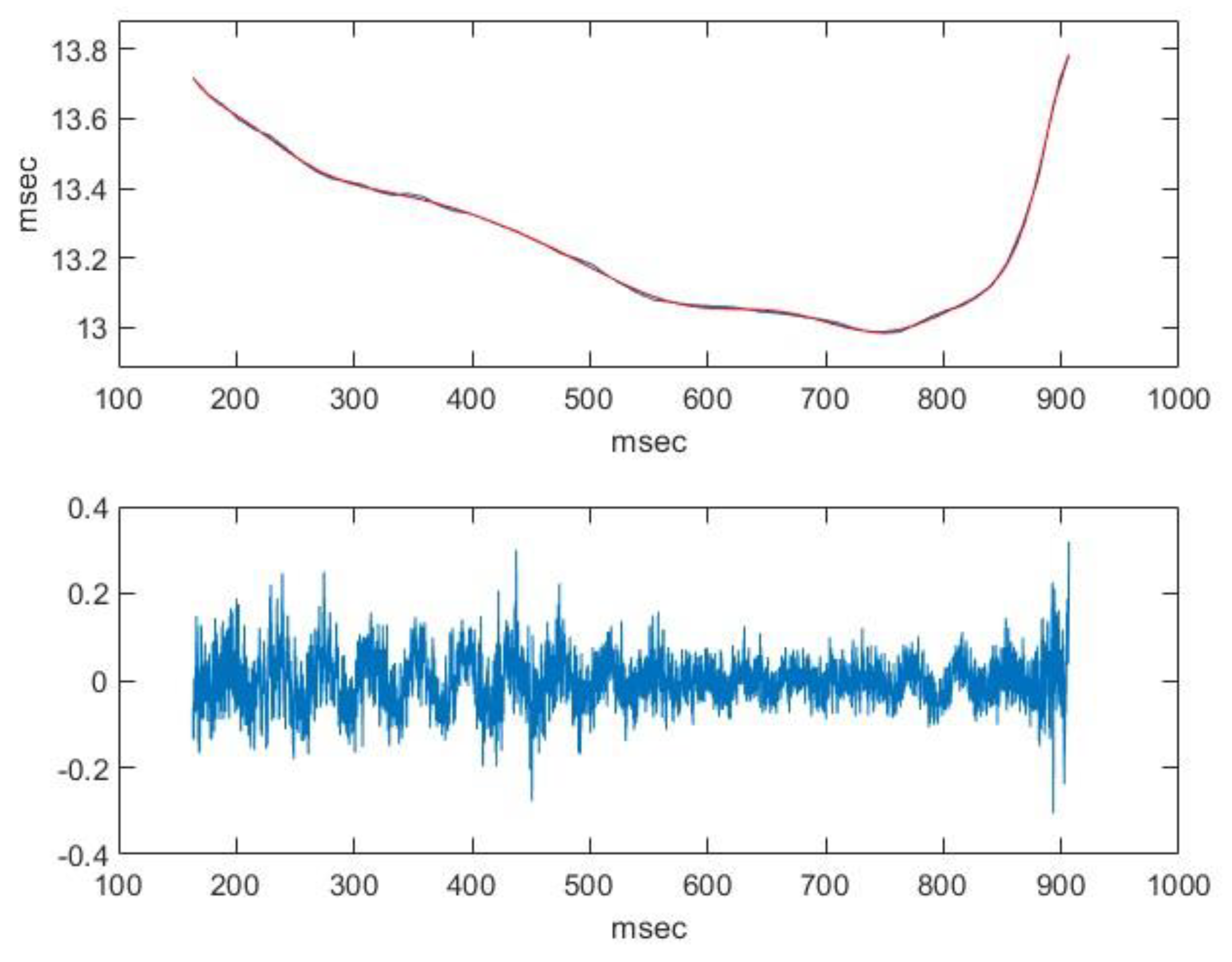
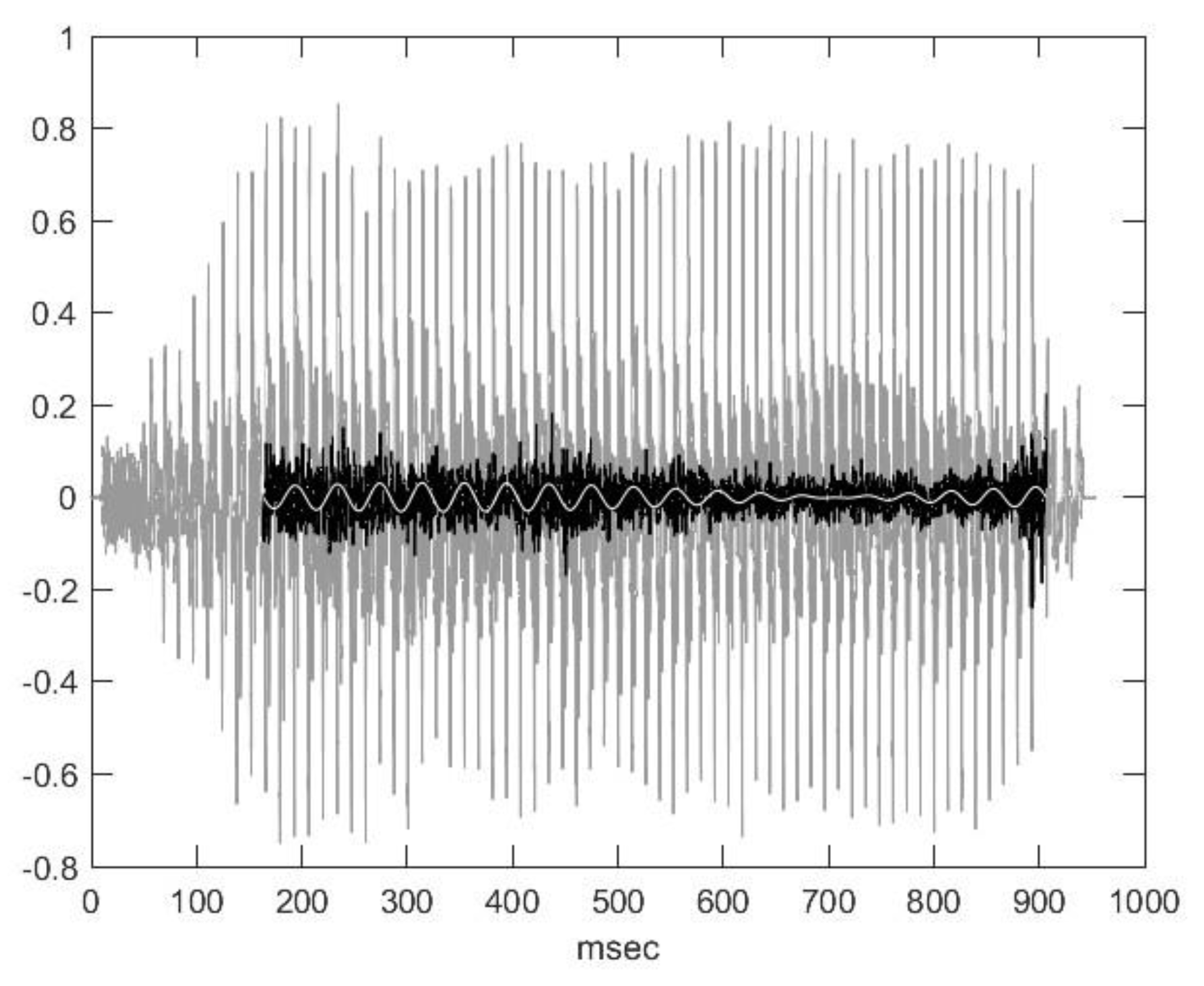
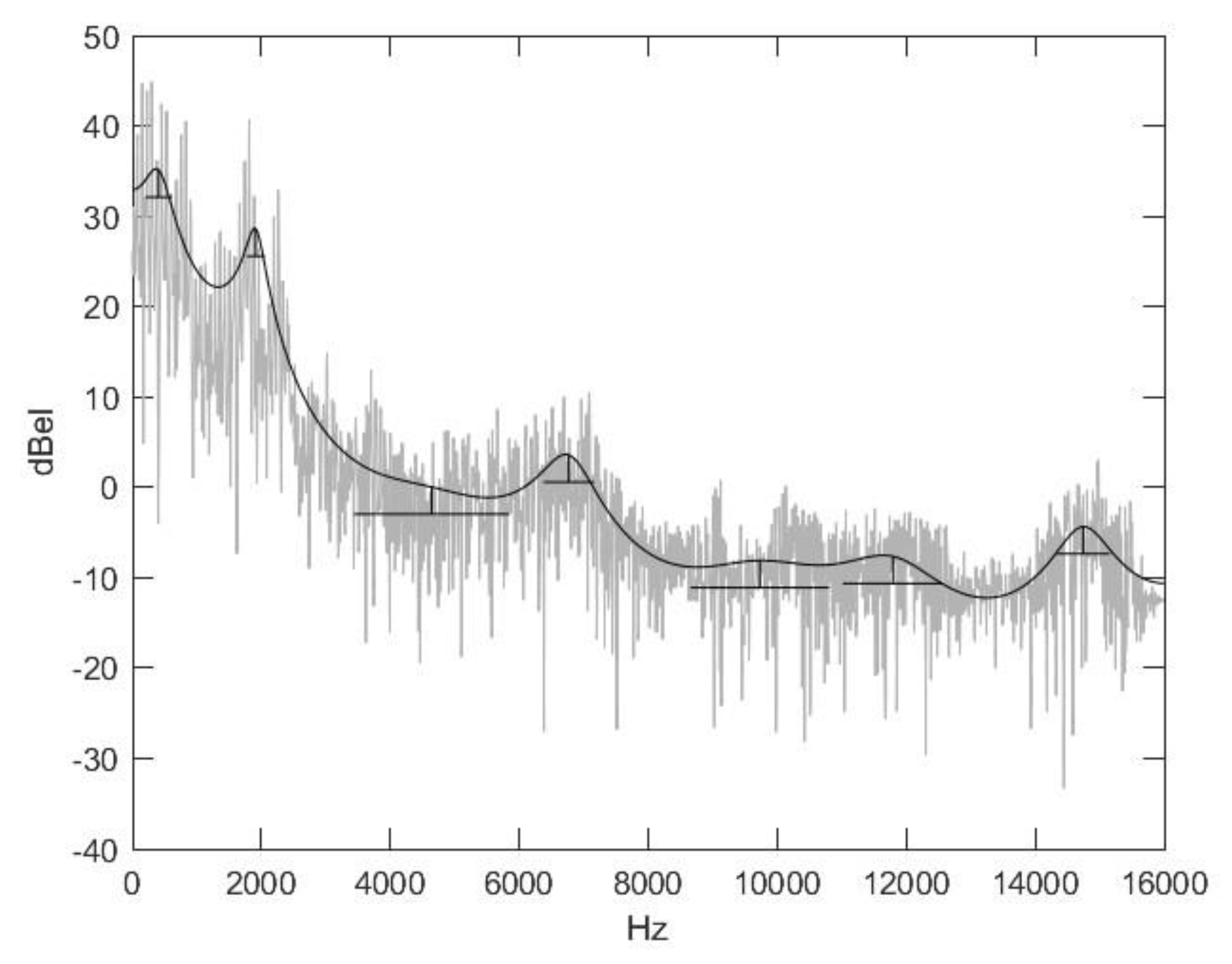
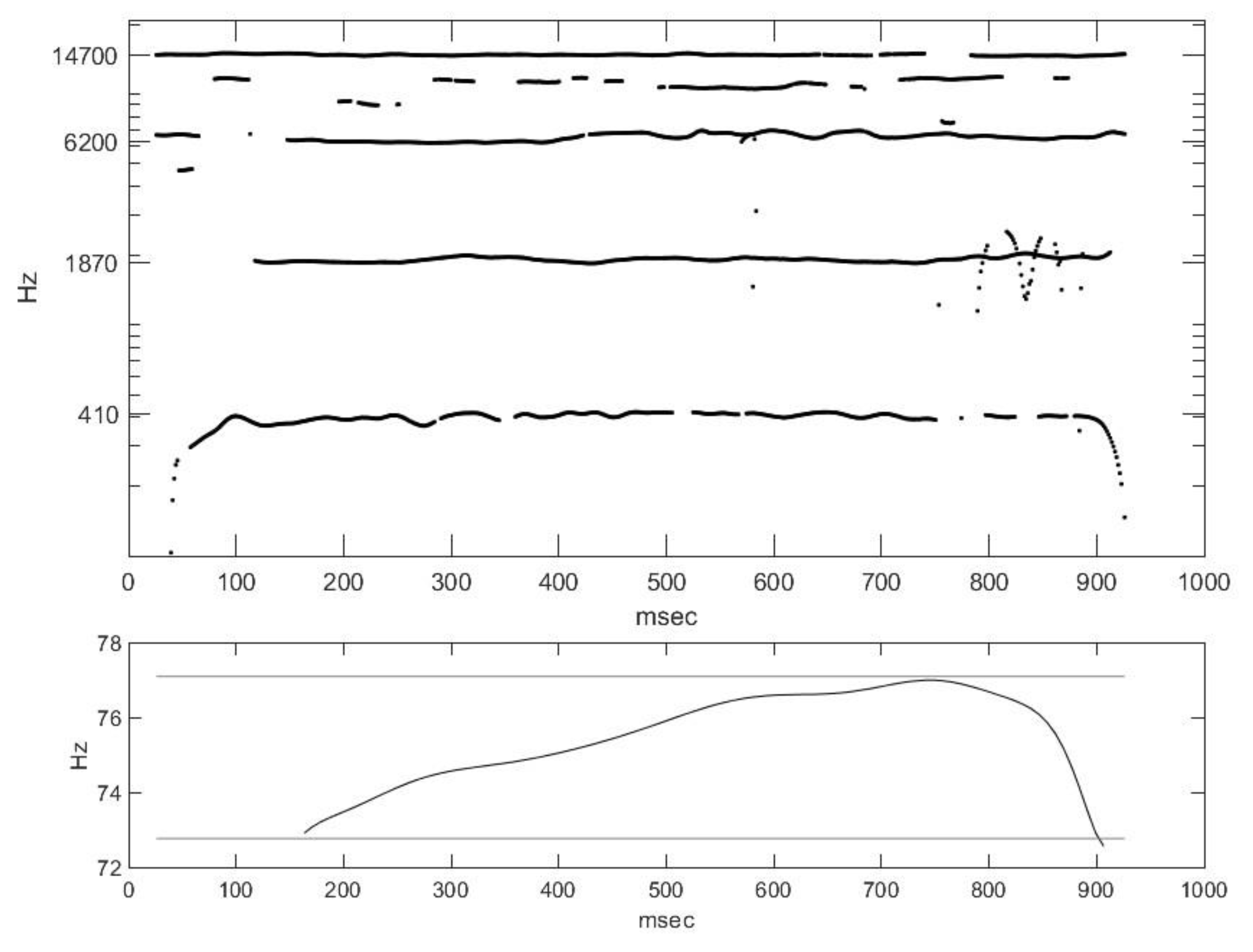
2.2. Nasal Sound Recording and Analysis
2.3. Experimental Procedure
2.4. HRV Analyses
2.5. Temperament Assessment
2.6. Meteorological Effects
2.7. Statistical Analysis
3. Results and Discussion
3.1. Analysis of the Used Sound
3.2. Heart Rate Variability and Flight Speed
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Węglarz, A. Meat quality defined based on pH and colour depending on cattle category and slaughter season. Czech J. Anim. Sci. 2010, 55, 548–556. [Google Scholar] [CrossRef]
- Mueller, R.; Schrader, L. Behavioural consistency during social separation and personality in dairy cows. Behaviour 2005, 142, 1289–1306. [Google Scholar]
- Færevik, G.; Jensen, M.B.; Bøe, K.E. Dairy calves social preferences and the significance of a companion animal during separation from the group. Appl. Anim. Behav. Sci. 2006, 99, 205–221. [Google Scholar] [CrossRef]
- Frid, A.; Dill, L. Human-caused disturbance stimuli as a form of predation risk. Conserv. Ecol. 2002, 6, 11. [Google Scholar] [CrossRef]
- Leiner, L.; Fendt, M. Behavioural fear and heart rate responses of horses after exposure to novel objects: Effects of habituation. Appl. Anim. Behav. Sci. 2011, 131, 104–109. [Google Scholar] [CrossRef]
- Christensen, J.W.; Rundgren, M. Predator odour per se does not frighten domestic horses. Appl. Anim. Behav. Sci. 2008, 112, 136–145. [Google Scholar] [CrossRef]
- Ahmadinejad, M.; Hassani, A.; Kharazian, F. The responses of horses to predator stimuli. Int. J. Vet. Res. 2010, 4, 5–9. [Google Scholar]
- Vetters, M.D.D.; Engle, T.E.; Ahola, J.K.; Gardin, T. Comparison of flight speed and exit score as measurements of temperament in beef cattle. Am. Soc. Anim. Sci. 2013, 91, 374–381. [Google Scholar]
- Petherick, J.C.; Holroyd, R.G.; Doogan, V.J.; Venus, B.K. Productivity, carcass and meat quality of lot-fed Bos indicus cross steers grouped according to temperament. Aust. J. Exp. Agric. 2002, 42, 389–398. [Google Scholar] [CrossRef]
- Richardson, E.C.; Herd, R.M.; Archer, J.A.; Arthur, P.F. Metabolic differences in Angus steers divergently selected for residual feed intake. Aust. J. Exp. Agric. 2004, 44, 441–452. [Google Scholar] [CrossRef]
- Nkrumah, D.; Crews, H.; Basarab, J.A.; Price, M.A.; Okine, E.K.; Wang, Z.; Li, C.; Moore, S.S. Genetic and phenotypic relationships of feeding behavior and temperament with performance, feed efficiency, ultrasound, and carcass merit of beef cattle. J. Anim. Sci. 2007, 85, 2382–2390. [Google Scholar] [CrossRef] [PubMed]
- Voisinet, B.D.; Grandin, T.; O’Connor, S.F.; Tatum, J.D.; Deesing, M.J. Bos indicus cross feedlot cattle with excitable temperaments have tougher meat and a higher incidence of borderline dark cutters. Meat Sci. 1997, 46, 367–377. [Google Scholar] [CrossRef] [PubMed]
- King, D.A.; Schuehle Pfieffer, C.E.; Randel, R.D.; Welsh, T.H., Jr.; Oliphint, R.A.; Baird, B.E.; Curley, K.O., Jr.; Vann, R.C.; Hale, D.S.; Savell, J.W. Influence of animal temperament and stress responsiveness on the carcass quality and beef tenderness of feedlot cattle. Meat Sci. 2006, 74, 546–556. [Google Scholar] [CrossRef] [PubMed]
- Vann, R.C. Relationships between carcass quality and temperament in beef cattle. In Proceedings of the 38rd Annual Research Symposium on Beef Improvement Federation, Choctaw, MA, USA, 18–21 April 2006; pp. 69–72. [Google Scholar]
- Fell, L.R.; Colditz, I.G.; Walker, K.H.; Watson, D.L. Associations between temperament, performance and immune function in cattle entering a commercial feedlot. Aust. J. Exp. Agric. 1999, 39, 795–802. [Google Scholar] [CrossRef]
- Cafe, L.M.; Robinson, D.L.; Ferguson, D.M.; Mcintyre, B.L.; Geesink, G.H.; Greenwood, P.L. Cattle temperament: Persistence of assessments and associations with productivity, efficiency, carcass and meat quality traits. J. Anim. Sci. 1999, 89, 1452–1465. [Google Scholar] [CrossRef]
- Turner, S.P.; Navajas, E.A.; Hyslop, J.J.; Ross, D.W.; Richardson, R.I.; Prieto, N.; Bell, M. Associations between response to handling and growth and meat quality in frequently handled Bos taurus beef cattle. J. Anim. Sci. 2011, 89, 4239–4248. [Google Scholar] [CrossRef]
- Francisco, C.L.; Resende, F.D.; Benatti, J.M.; Castilhos, A.M.; Cooke, R.F.; Jorge, A.M. Impacts of temperament on Nellore cattle: Physiological responses, feedlot performance, and carcass characteristics. J. Anim. Sci. 2015, 93, 5419–5429. [Google Scholar] [CrossRef]
- Trenkle, A.; Topel, D.G. Relationship of some endocrine measurements to growth and carcass composition of cattle. J. Anim. Sci. 1978, 46, 1604–1609. [Google Scholar] [CrossRef]
- Grandin, T. Behavioral agitation during handling of cattle is persistent over time. Appl. Anim. Behav. Sci. 1993, 36, 1. [Google Scholar] [CrossRef]
- Burdick, N.C.; Carroll, J.A.; Hulbert, L.E.; Dailey, J.W.; Ballou, M.A.; Randel, R.D.; Willard, S.T.; Vann, R.C.; Welsh, T.H. Temperament influences endotoxin-induced changes in rectal temperature, sickness behavior, and plasma epinephrine concentrations in bulls. Innate Immun. 2010, 17, 355–364. [Google Scholar] [CrossRef]
- Curley, K.O., Jr.; Paschal, J.C.; Welsh, T.H., Jr.; Randel, R.D. Technical note: Exit velocity as a measure of cattle temperament is repeatable and associated with serum concentration of cortisol in Brahman bulls. J. Anim. Sci. 2006, 84, 3100–3103. [Google Scholar] [CrossRef] [PubMed]
- Vann, R.C.; Burdick, N.C.; Lyons, J.G.; Welsh, T.H.; Randel, R.D. Temperament Influences Stress Hormone and IgG Concentrations in Angus cross Calves; Animal and Dairy Sciences Annual Report; Mississipi State University: Starkville, MS, USA, 2010; pp. 36–39. [Google Scholar]
- Scanga, J.A.; Belk, K.E.; Tatum, J.D.; Grandin, T.; Smith, G. Factors contributing to the incidence of dark cutting beef. J. Anim. Sci. 1998, 76, 2040–2047. [Google Scholar] [CrossRef] [PubMed]
- Grandin, T. Behavioral principles of livestock handling. Prof. Anim. Sci. 1989, 5, 1–11. [Google Scholar] [CrossRef]
- Burrow, H.M. Measurements of temperament and their relationships with performance traits of beef cattle. Anim. Breed. Abstr. 1997, 65, 477–495. [Google Scholar]
- Voisinet, B.D.; Grandin, T.; Tatum, J.D.; O’Connor, S.F.; Struthers, J.J. Feedlot cattle with calm temperaments have higher average daily gains than cattle with excitable temperaments. J. Anim. Sci. 1997, 75, 892–896. [Google Scholar] [CrossRef]
- Plasse, D.; Warnick, A.C.; Koger, M. Reproductive behavior of Bos Indicus females in a subtropical environment IV. Length of estrous cycle, duration of estrus, time of ovulation, fertilization and embryo survival in grade Brahman heifers. J. Anim. Sci. 1970, 30, 63–72. [Google Scholar] [CrossRef]
- Cooke, R.F.; Bohnert, D.W.; Meneghetti, M.; Losi, T.C.; Vasconcelos, J.L.M. Effects of temperament on pregnancy rates to fixed-timed AI in Bos Indicus beef cows. Livest. Sci. 2011, 142, 108–113. [Google Scholar] [CrossRef]
- Burdick, N.C.; Randel, R.D.; Carroll, J.A.; Welsh, T.H., Jr. Interactions between temperament, stress, and immune function in cattle. Int. J. Zool. 2011, 2011, 373197. [Google Scholar] [CrossRef]
- Réale, D.; Reader, S.M.; Sol, D.; McDougall, P.T.; Dingemanse, N.J. Integrating animal temperament within ecology and evolution. Biol. Rev. Camb. Philos. Soc. 2007, 82, 291–318. [Google Scholar] [CrossRef]
- Burrow, H.M.; Seifert, G.W.; Corbet, N.J. A new technique for measuring temperament in cattle. Proc. Aust. Soc. Anim. Prod. 1988, 17, 154–157. [Google Scholar]
- Rocha, L.M. Validation of Stress Indicators for the Assessment of Animal Welfare and Prediction of Pork Meat Quality Variation at Commercial Level. Doctoral Thesis, Laval University, Quebec City, QC, Canada, 2016. [Google Scholar]
- Warriss, P. The growth and body composition of animals. Meat Sci. Introd. Text 2009, 9–25. [Google Scholar] [CrossRef]
- Burrow, H.M.; Dillon, R.D. Relationships between temperament and growth in a feedlot and commercial carcass traits of Bos indicus crossbreds. Aust. J. Exp. Agric. 1997, 37, 407–411. [Google Scholar] [CrossRef]
- Müller, R.; von Keyserlingk, M.A.G. Consistency of flight speed and its correlation to productivity and to personality in Bos taurus beef cattle. Appl. Anim. Behav. Sci. 2006, 99, 193–204. [Google Scholar] [CrossRef]
- Sant’Anna, A.C.; Paranhos da Costa, M.J.R.; Baldi, F.; Rueda, P.M.; Albuquerque, L.G. Genetic associations between flight speed and growth traits in Nellore cattle. J. Anim. Sci. 2012, 90, 3427–3432. [Google Scholar] [CrossRef]
- Burrow, H.M.; Corbet, N.J. Genetic and environmental factors affecting temperament of zebu and zebu-derived beef cattle grazed at pasture in the tropics. Aust. J. Agric. Res. 2000, 51, 155–162. [Google Scholar] [CrossRef]
- Graf, G.C.; Petersen, W.E. Changes in respiration and heart rates, body temperatures, plasma lactic acid levels and plasma creatinine levels caused by stress in dairy cattle. J. Dairy Sci. 1953, 36, 1036–1048. [Google Scholar] [CrossRef]
- Bianca, W. The relation between respiratory rate and heart rate in the calf subjected to severe heat stress. J. Agric. Sci. 1958, 51, 321–324. [Google Scholar] [CrossRef]
- Thomas, J.W.; Moore, L.A. Variations in heart rate of dairy cows. J. Dairy Sci. 1951, 34, 321–328. [Google Scholar] [CrossRef]
- Kovács, L.; Tőzsér, J.; Szenci, O.; Póti, P.; Kézér, F.L.; Ruff, F.; Gábriel-Tőzsér, G.; Hoffmann, D.; Bakonyi, M.; Jurkovich, V. Cardiac responses to palpation per rectum in lactating and nonlactating dairy cows. J. Dairy Sci. 2014, 97, 6955–6963 . [Google Scholar] [CrossRef]
- Malik, M.; Bigger, J.T.; Camm, A.J.; Kleiger, R.E.; Malliani, A.; Moss, A.J.; Schwartz, P.J. Heart rate variability: Standards of measurement, physiological interpretation, and clinical use. Eur. Heart J. 1996, 17, 354–381. [Google Scholar] [CrossRef]
- Mohr, E.; Langbein, J.; Nürnberg, G. Heart rate variability: A noninvasive approach to measure stress in calves and cows. Physiol.Behav. 2002, 75, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Nasim, K.; Jahan, H.A.; Syed, A.S. Heart Rate Variability—A Review. J. Basic Appl. Sci. 2011, 7, 71–77. [Google Scholar]
- Hsu, C.H.; Tsai, M.Y.; Huang, G.S.; Lin, T.C.; Chen, K.P.; Ho, S.T.; Shyu, L.Y.; Li, C.Y. Poincaré plot indexes of heart rate variability detect dynamic autonomic modulation during general anesthesia induction. Acta Anaesthesiol. Taiwanica 2012, 50, 12e18. [Google Scholar] [CrossRef] [PubMed]
- Katsikadelis, M.; Pilianidis, T.; Mantzouranis, N.; Fatouros, I.; Agelousis, N. Heart rate variability of young TableTennis players with the use of the Multiball training. Biol. Exerc. 2014, 10, 25–35. [Google Scholar] [CrossRef]
- Hernández-Cruz, G.; Quezada-Chacon, J.T.; González-Fimbres, R.A.; Flores-Miranda, F.J.; Naranjo-Orellana, J.; Rangel-Colmenero, B.R. Effect of consecutive matches on heart rate variability in elite volleyballplayers. Rev. Psicol. Deport. 2017, 26, 9–14. [Google Scholar]
- Laborde, S.; Mosley, E.; Mertgen, A. A unifying conceptual framework of factors associated to cardiac vagal control. Heliyon 2018, 4, e01002. [Google Scholar] [CrossRef]
- Ghatak, S.K.; Aditya, S. Poincare parameters and principal component analysis of Heart rate variability of subjects with health disorder. arXiv 2018, arXiv:1802.10289. [Google Scholar]
- Stewart, M.; Stookey, J.M.; Stafford, K.J.; Tucker, C.B.; Rogers, A.R.; Dowling, S.K.; Verkerk, G.A.; Schaefer, A.L.; Webster, J.R. Effects of local anesthetic and a nonsteroidal antiinflammatory drug on pain responses of dairy calves to hot-iron dehorning. J. Dairy Sci. 2009, 92, 1512–1519. [Google Scholar] [CrossRef]
- Konold, T.; Bone, G.E.; Simmons, M.M. Time and frequency domain analysis of heart rate variability in cattle affected by bovine spongiform encephalopathy. BMC Res. Notes 2011, 4, 259. [Google Scholar] [CrossRef]
- Kovács, L.; Nagy, K.; Szenci, O.; Tőzsér, J. Heart rate variability during milking in dairy cows [In Hungarian]. Magy. Állatorvosok Lapja 2012, 134, 653–661. [Google Scholar]
- Trenk, L.; Kuhl, J.; Aurich, J.; Aurich, C.; Nagel, C. Heart rate and heart rate variability in pregnant dairy cows and their fetuses determined by fetomaternal electrocardiography. Theriogenology 2015, 84, 1405–1410. [Google Scholar] [CrossRef] [PubMed]
- Stewart, M.; Stafford, K.J.; Dowling, S.K.; Schaefer, A.L.; Webster, J.R. Eye temperature and heart rate variability of calves disbudded with or without local anaesthetic. Physiol. Behav. 2008, 93, 789–797. [Google Scholar] [CrossRef] [PubMed]
- Mialon, M.M.; Deiss, V.; Andanson, S.; Anglard, F.; Doreau, M.; Veissier, I. An assessment of the impact of rumenocentesis on pain and stress in cattle and the effect of local anaesthesia. Vet. J. 2012, 194, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.G.; Cheon, E.J.; Bai, D.S.; Lee, Y.H.; Koo, B.H. Stress and Heart Rate Variability: A Meta-Analysis and Review of the Literature. Psychiatry Investig. 2018, 15, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Andrade, O.; Orihuela, A.; Solano, J.; Galina, C. Some effects of repeated handling and the use of a mask on stress responses in zebu cattle during restraint. Appl. Anim. Behav.Sci. 2001, 71, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Boissy, A.; Manteuffel, G.; Jensen, M.B.; Moe, R.O.; Spruijt, B.; Keeling, L.J.; Winckler, C.; Forkman, B.; Dimitrov, I.; Langbein, J.; et al. Assessment of positive emotions in animals to improve their welfare. Physiol. Behav. 2007, 92, 375–397. [Google Scholar] [CrossRef] [PubMed]
- Yeates, J.W.; Main, D.C.J. Assessment of positive welfare: A review. Vet. J. 2008, 175, 293–300. [Google Scholar] [CrossRef]
- Mellor, D.J.; Beausoleil, N.J.; Littlewood, K.E.; McLean, A.N.; McGreevy, P.D.; Jones, B.; Wilkins, C. The 2020 five domains model: Including human–animal interactions in assessments of animal welfare. Animals 2020, 10, 1870. [Google Scholar] [CrossRef]
- Rault, J.L.; Waiblinger, S.; Boivin, X.; Hemsworth, P. The power of a positive human–animal relationship for animal welfare. Front. Vet. Sci. 2020, 7, 590867. [Google Scholar] [CrossRef]
- Lange, A.; Waiblinger, S.; van Hasselt, R.; Mundry, R.; Futschik, A.; Lürzel, S. Effects of restraint on heifers during gentle human-animal interactions. Appl. Anim. Behav. Sci. 2021, 243, 105445. [Google Scholar] [CrossRef]
- Siebert, K.; Langbein, J.; Schön, P.C.; Tuchscherer, A.; Puppe, B. Degree of social isolation affects behavioural and vocal response patterns in dwarf goats (Capra hircus). Appl. Anim. Behav. Sci. 2011, 131, 53–62. [Google Scholar] [CrossRef]
- Padilla de la Torre, M.; Briefer, E.F.; Reader, T.; McElligott, A.G. Acoustic analysis of cattle (Bos taurus) mother–offspring contact calls from a source–filter theory perspective. Appl. Anim. Behav. Sci. 2015, 163, 58–68. [Google Scholar] [CrossRef]
- Waiblinger, S.; Menke, C.; Korff, J.; Bucher, A. Previous handling and gentle interactions affect behaviour and heart rate of dairy cows during a veterinary procedure. Appl. Anim. Behav. Sci. 2004, 85, 31–42. [Google Scholar] [CrossRef]
- Kiley, M. The vocalizations of ungulates, their causation and function. Z. Tierpsychol. 1972, 31, 171–222. [Google Scholar] [CrossRef] [PubMed]
- Green, A.C.; Johnston, I.N.; Clark, C.E.F. Invited review: The evolution of cattle bioacoustics and application for advanced dairy systems. Animal 2018, 12, 1250–1259. [Google Scholar] [CrossRef]
- Biabani, M.; Farrell, M.; Zoghi, M.; Egan, G.; Jaberzadeh, S. Crossover design in transcranial direct current stimulation studies on motor learning: Potential pitfalls and difficulties in interpretation of findings. Rev.Neurosci. 2018, 29, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Janzekovic, M.; Stajnko, D.; Brus, M.; Vindis, P. Polar sport tester for cattle heart rate measurements. In Advanced Knowledge Application in Practice; IntechOpen Limited: London, UK, 2010. [Google Scholar] [CrossRef]
- Jonckheer-Sheehy, V.S.; Vinke, C.M.; Ortolani, A. Validation of a Polar® human heart rate monitor for measuring heart rate and heart rate variability in adult dogs under stationary conditions. J. Vet.Behav. Clin. Appl.Res. 2012, 7, 205–212. [Google Scholar] [CrossRef]
- Kovács, L.; Kézér, F.L.; Jurkovich, V.; Kulcsár-Huszenicza, M.; Tőzsér, J. Heart rate variability as an indicator of chronic stress caused by lameness in dairy cows. PLoS ONE 2015, 10, e0134792. [Google Scholar] [CrossRef]
- Després, G.; Veissier, I.; Boissy, A. Effect of autonomic blockers on heart period variability in calves: Evaluation of the sympatho-vagal balance. Physiol. Res. 2002, 51, 347–353. [Google Scholar]
- Hagen, K.; Langbein, J.; Schmied, C.; Lexer, D.; Waiblinger, S. Heart rate variability in dairy cows—Influences of breed and milking system. Physiol. Behav. 2005, 85, 195–204. [Google Scholar] [CrossRef]
- Schmied, C.; Waiblinger, S.; Scharl, T.; Leisch, F.; Boivin, X. Stroking of different body regions by a human: Effects on behaviour and heart rate of dairy cows. Appl. Anim. Behav. Sci. 2008, 109, 25–38. [Google Scholar] [CrossRef]
- Minero, M.; Canali, E.; Ferrante, V.; Carenzi, C. Measurement and time domain analysis of heart rate variability in dairy cattle. Vet. Rec. 2001, 149, 772–774. [Google Scholar] [CrossRef] [PubMed]
- Gygax, L.; Neuffer, I.; Kaufmann, C.; Hauser, R.; Wechsler, B. Restlessness behaviour, heart rate and heart-rate variability of dairy cows milked in two types of automatic milking systems and auto-tandem milking parlours. Appl. Anim. Behav. Sci. 2008, 109, 167–179. [Google Scholar] [CrossRef]
- Benti, A. Practical Manual on Veterinary Clinical Diagnostic Approach. J. Vet. Sci. Technol. 2016, 7, 1–10. [Google Scholar]
- Coombes, S.V.; Gardner, G.E.; Pethick, D.W.; McGilchrist, P. The impact of beef cattle temperament assessed using flight speed on muscle glycogen, muscle lactate and plasma lactate concentrations at slaughter. Meat Sci. 2014, 98, 815–821. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, Y.; Ishii, Y. Recognition of two psychological conditions of a single cow by her voice. Comput. Electron. Agric. 2008, 62, 67–72. [Google Scholar] [CrossRef]
- Melillo, P.; Bracale, M.; Pecchia, L. Nonlinear Heart Rate Variability features for real-life stress detection. Case study: Students under stress due to university examination. BioMed. Eng. Online 2011, 10, 96. [Google Scholar] [CrossRef]
- Kovalčik, K.; Kovalčik, M. Learning ability and memory testing in cattle of different ages. Appl. Anim. Behav. Sci. 1986, 15, 27–29. [Google Scholar]
- Piovezan, U.; Cyrillo, J.N.S.G.; Costa, M.J.R.P. Breed and selection line differences in the temperament of beef cattle. Acta Sci. Anim. Sci. 2013, 35, 207–213. [Google Scholar] [CrossRef]
- Bravi, A.; Longtin, A.; Seely, A.J.E. Review and classification of variability analysis techniques with clinical applications. Biomed. Eng. Online 2011, 10, 90. [Google Scholar] [CrossRef]
- Szabó, C.; Vizesi, Z.; Vincze, A. Heart Rate and Heart Rate Variability of Amateur Show Jumping Horses Competing on Different Levels. Animals 2021, 11, 693. [Google Scholar] [CrossRef]
- von Borell, E.; Langbein, J.; Després, G.; Hansen, S.; Leterrier, C.; Marchant, J.; Marchant-Forde, R.; Minero, M.; Mohr, E.; Prunier, A.; et al. Heart rate variability as a measure of autonomic regulation of cardiac activity for assessing stress and welfare in farm animals—A review. Physiol. Behav. 2007, 92, 293–316. [Google Scholar] [CrossRef] [PubMed]
- Watts, J.M.; Stookey, J.M. The propensity of cattle to vocalise during handling and isolation is affected by phenotype. Appl. Anim. Behav. Sci. 2001, 74, 81–95. [Google Scholar] [CrossRef]
- Hall, S.J.G.; Vince, M.A.; Walser, E.S.; Garson, P.J. Vocalisations of the Chillingham cattle. Behaviour 1988, 104, 78–104. [Google Scholar]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2022, 18, e3000410. [Google Scholar] [CrossRef]


| Trait | Maternal Sound | Day of Test | Sequence | p | |||||
|---|---|---|---|---|---|---|---|---|---|
| No | Yes | 1st | 2nd | YN | NY | Sound | Round | Seq. | |
| HR (1/min) | 34.4 | 25.8 | 30.0 | 31.1 | 35.0 | 23.9 | 0.1544 | 0.7667 | 0.0852 |
| HR max (1/min) | 121.6 | 115.4 | 126.4 | 110.2 | 118.4 | 118.5 | 0.4824 | 0.1415 | 0.9833 |
| SD1 (ms) | 16.9 | 17.1 | 19.1 | 14.9 | 14.5 | 20.2 | 0.9131 | 0.2220 | 0.1057 |
| SD2 (ms) | 16.9 | 17.7 | 19.3 | 15.2 | 15.2 | 19.9 | 0.9484 | 0.2312 | 0.1647 |
| SD2/SD1 | 0.97 | 1.07 | 1.03 | 1.02 | 1.04 | 1.01 | 0.0110 | 0.8051 | 0.5139 |
| RMSSD (ms) | 23.5 | 23.6 | 26.5 | 20.5 | 20.1 | 28.1 | 0.8955 | 0.2200 | 0.0979 |
| pNN50 (%) | 9.20 | 9.45 | 9.48 | 9.08 | 9.01 | 9.57 | 0.1997 | 0.1650 | 0.0396 |
| VLF (%) | 12.6 | 12.9 | 13.5 | 12.0 | 13.5 | 11.7 | 0.9993 | 0.5727 | 0.5058 |
| HF (%) | 18.9 | 19.3 | 17.4 | 21.0 | 19.0 | 19.2 | 0.9083 | 0.1820 | 0.9794 |
| LF (%) | 57.2 | 57.1 | 59.9 | 54.4 | 53.2 | 62.3 | 0.8428 | 0.1365 | 0.0176 |
| LF/HF | 3.27 | 2.98 | 3.54 | 2.74 | 2.77 | 3.59 | 0.7319 | 0.1856 | 0.1480 |
| Flight speed (s/m) | 0.92 | 1.08 | 1.07 | 0.94 | 1.07 | 0.90 | 0.0409 | 0.1557 | 0.0199 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lenner, Á.; Papp, Z.L.; Szabó, C.; Komlósi, I. Calming Hungarian Grey Cattle in Headlocks Using Processed Nasal Vocalization of a Mother Cow. Animals 2024, 14, 135. https://doi.org/10.3390/ani14010135
Lenner Á, Papp ZL, Szabó C, Komlósi I. Calming Hungarian Grey Cattle in Headlocks Using Processed Nasal Vocalization of a Mother Cow. Animals. 2024; 14(1):135. https://doi.org/10.3390/ani14010135
Chicago/Turabian StyleLenner, Ádám, Zoltán Lajos Papp, Csaba Szabó, and István Komlósi. 2024. "Calming Hungarian Grey Cattle in Headlocks Using Processed Nasal Vocalization of a Mother Cow" Animals 14, no. 1: 135. https://doi.org/10.3390/ani14010135
APA StyleLenner, Á., Papp, Z. L., Szabó, C., & Komlósi, I. (2024). Calming Hungarian Grey Cattle in Headlocks Using Processed Nasal Vocalization of a Mother Cow. Animals, 14(1), 135. https://doi.org/10.3390/ani14010135






