Morpho-Molecular Features and Phylogenetic Relationships of Metorchis butoridi Oschmarin, 1963 (Trematoda: Opisthorchiidae) from East Asia
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Preparation of Whole Mounts
2.2. DNA Extraction, Amplification, and Sequencing
2.3. Analysis of Genetic Data
3. Results
3.1. Taxonomic Summary
3.2. Morphological Description
3.3. Genetic Data
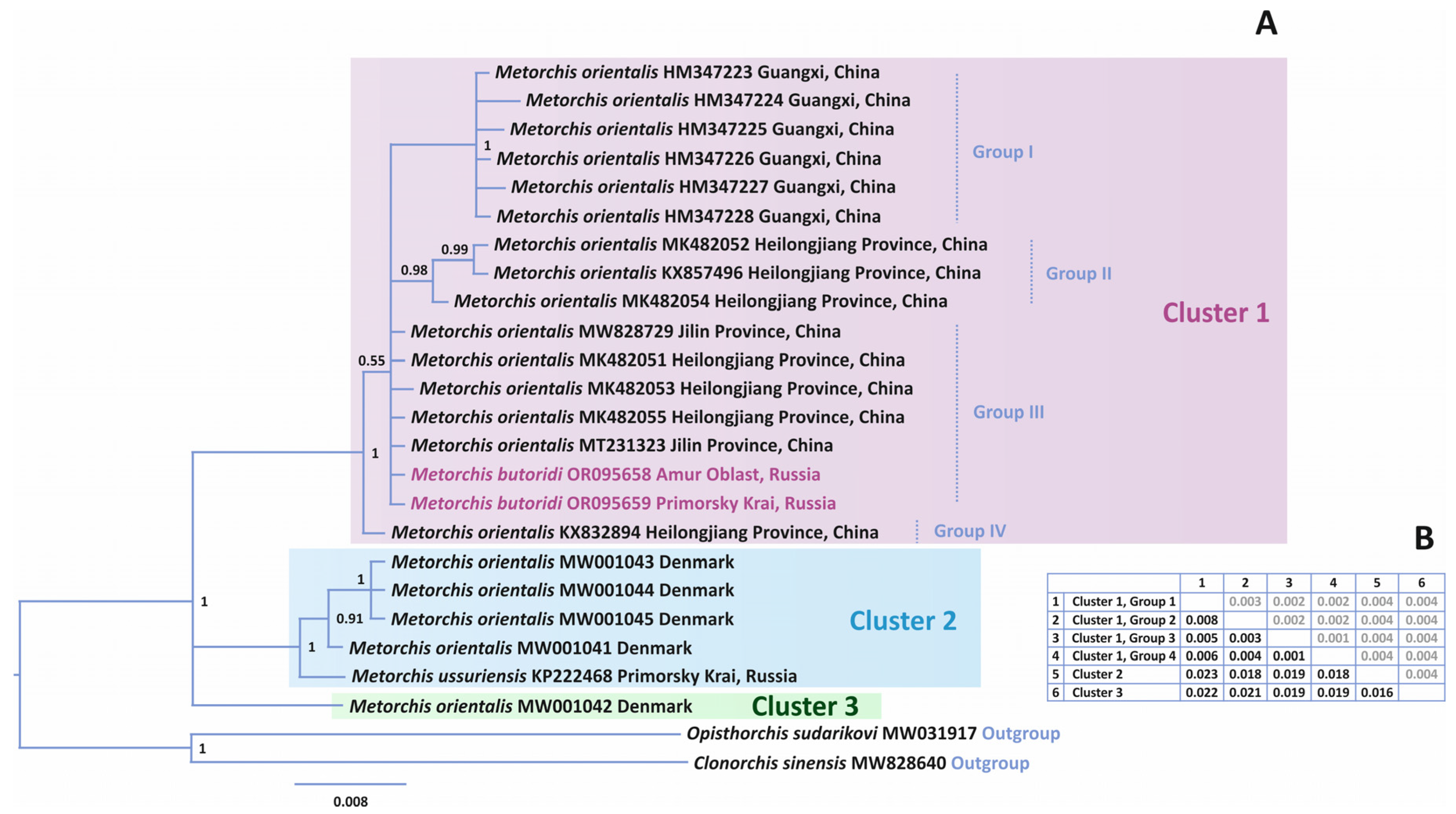
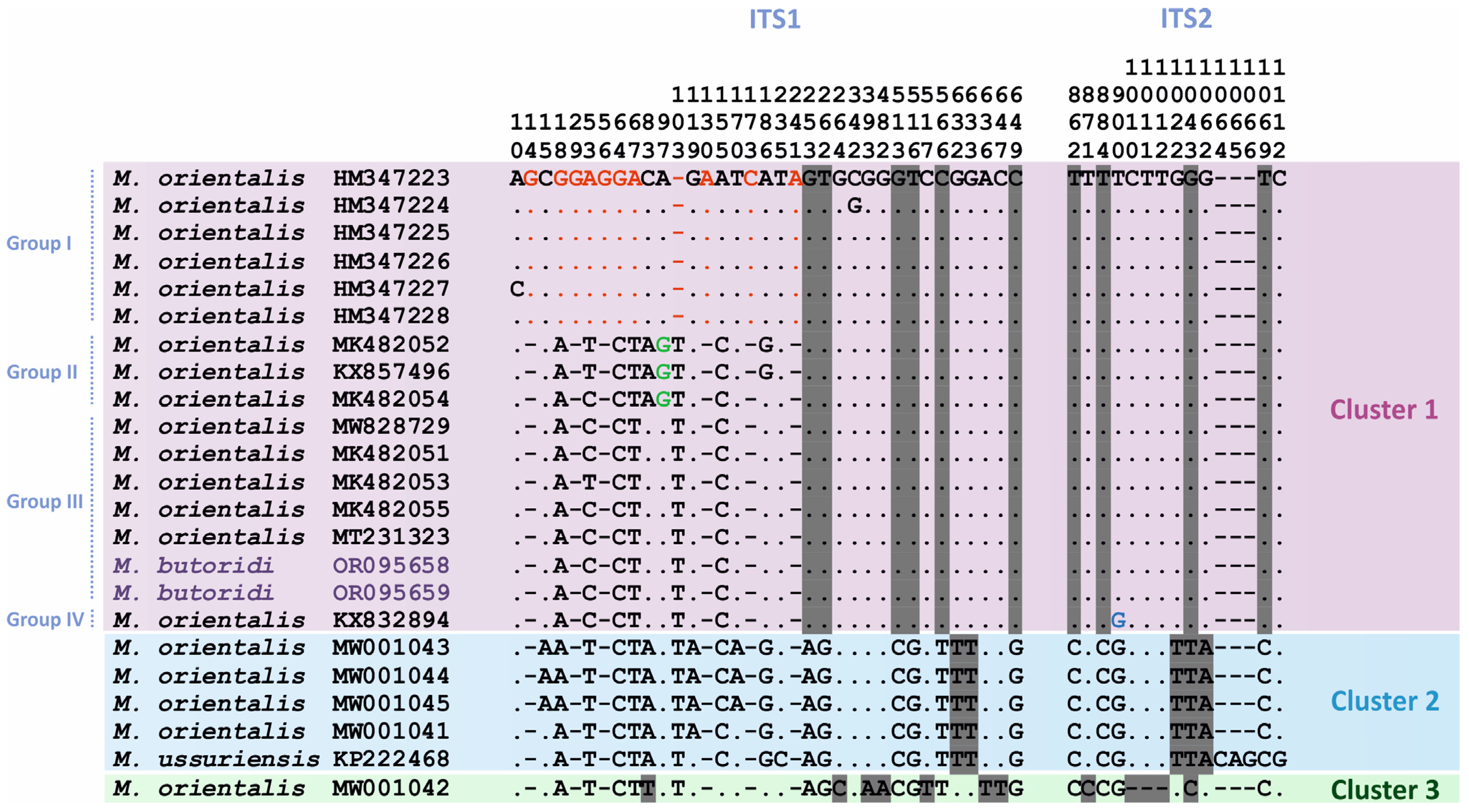
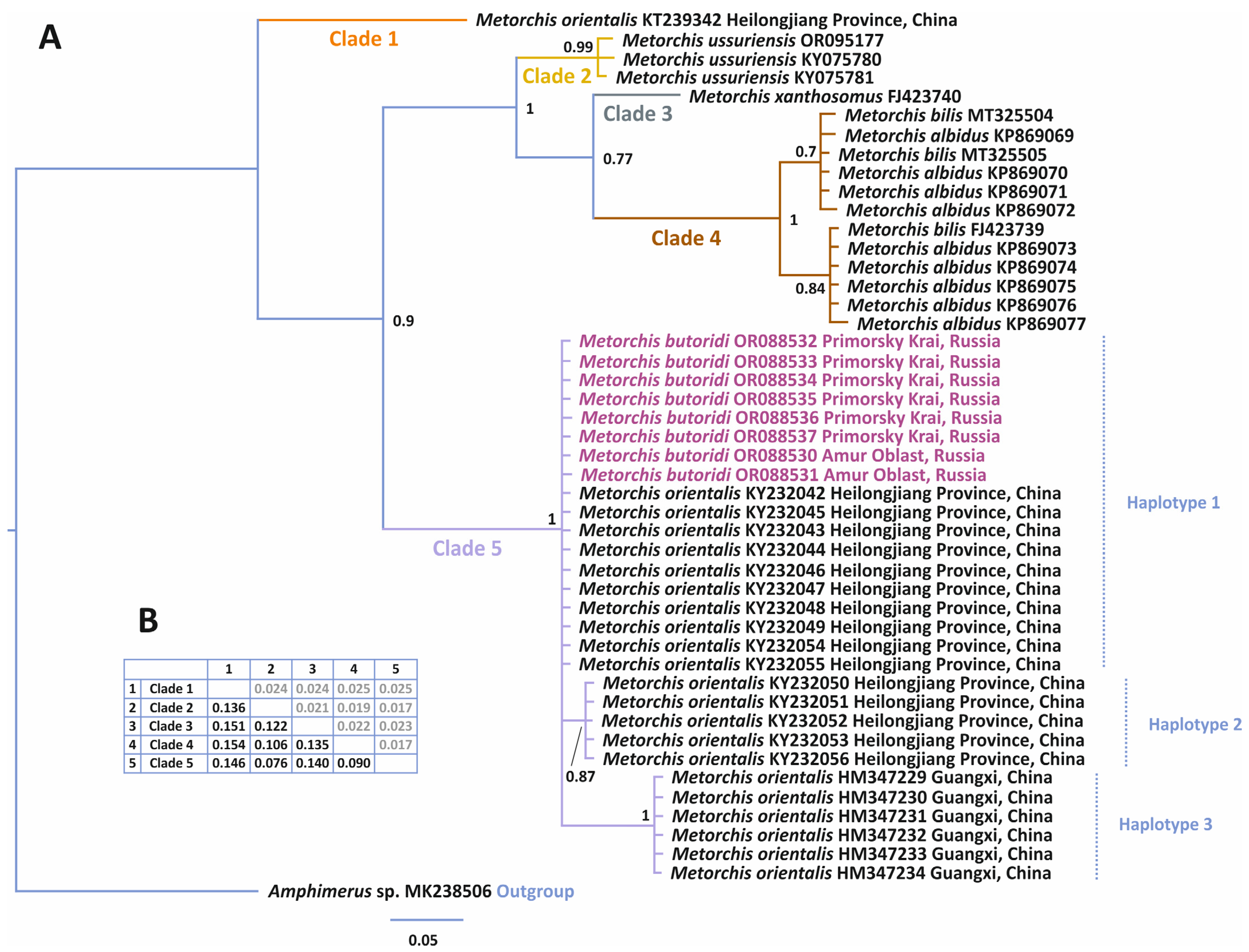
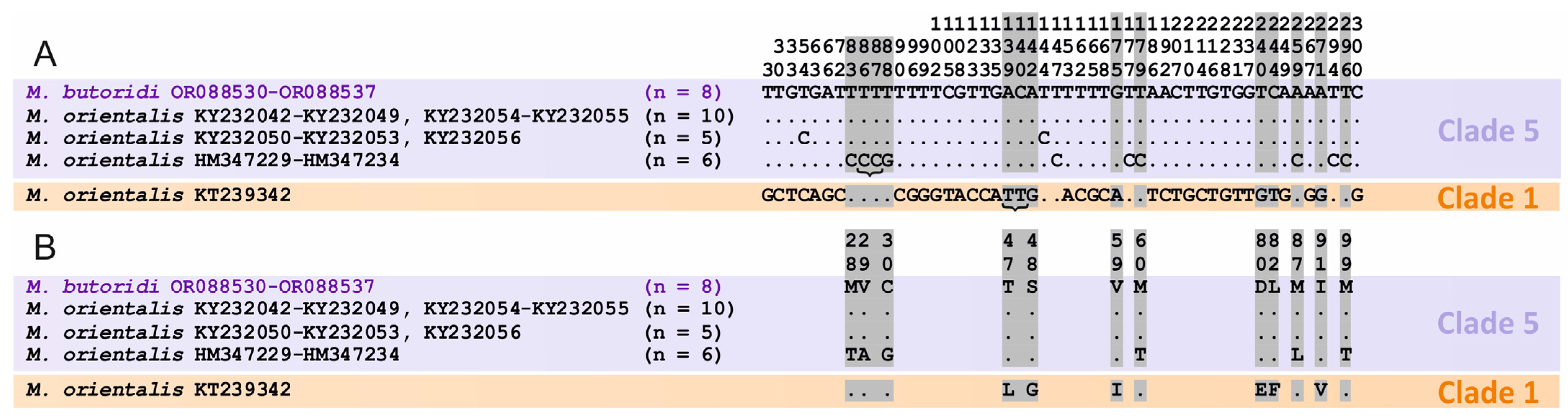
3.3.1. Intraspecific Variation
3.3.2. Phylogenetic Relationships
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sitko, J.; Bizos, J.; Sherrard-Smith, E.; Stanton, D.W.; Komorová, P.; Heneberg, P. Integrative taxonomy of European parasitic flatworms of the genus Metorchis Looss, 1899, (Trematoda, Opisthorchiidae). Parasitol. Int. 2016, 65, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Labony, S.S.; Alim, M.A.; Hasan, M.M.; Hossain, M.S.; Islam, A.; Alam, M.Z.; Tsuji, N. Anisuzzaman, Fish-borne trematode infections in wild fishes in Bangladesh. Pathog. Glob. Health 2020, 114, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Scholz, T. Family Opisthorchiidae Looss, 1899. In Keys to the Trematoda; Bray, R.A., Gibson, D.I., Jones, A., Eds.; CAB International and Natural History Museum: London, UK, 2008; Volume 3, pp. 9–49. [Google Scholar]
- Skrjabin, K.I.; Petrov, A.M. Superfamily Opisthorchoidea Faust, 1929. Osnovy trematodologii; Nauka: Moscow, Russia, 1950. (In Russian) [Google Scholar]
- Belogurov, O.I.; Leonov, V.A. Two new species of trematodes occurring in Anas acuta in Kamtchtka. Trudy Gelmintolog. Lab. 1963, 13, 212–215. (In Russian) [Google Scholar]
- Oschmarin, P.G. Helminths of Mammals and Birds of Primorskiy Kray; Izdatelstvo Akademii Nauk SSSR: Moscow, Russia, 1963. (In Russian) [Google Scholar]
- Chen, P.; Tang, Z. Notes on the morphology of Metorchis orientalis Tanabe, 1921, and M. taiwanensis Morishita, 1929. Acta. Vet. Zootech. Sin. 1981, 12, 53–61. [Google Scholar]
- Ha, N.V. One new trematode species Metorchis kimbangensis sp. nov. (Trematoda, Opisthorchiidae) parasiting in the catfish (Parasilurus asotus) from Vietnam. Tap Chi Sinh HOC 2005, 27, 21–24. [Google Scholar]
- Besprozvannykh, V.V.; Tatonova, Y.V.; Shumenko, P.G. Life cycle, morphology of developmental stages of Metorchis ussuriensis sp. nov. (Trematoda: Opisthorchiidae), and phylogenetic relationships with other opisthorchiids. J. Zool. Syst. Evol. Res. 2019, 57, 24–40. [Google Scholar] [CrossRef]
- Yang, G.; Lai, W. Studies on the life cycle of Metorchis taiwanensis. Chin. J. Vet. Sci. 1997, 17, 475–478. [Google Scholar]
- Ai, L.; Chen, S.H.; Zhang, Y.N.; Zhou, X.N.; Li, H.; Chen, M.X.; Guo, J.; Cai, Y.C.; Zhu, X.Q.; Chen, J.X. Sequences of internal transcribed spacers and two mitochondrial genes effective genetic markers for Metorchis orientalis. J. Anim. Vet. Adv. 2010, 9, 2371–2376. [Google Scholar] [CrossRef]
- Na, L.; Gao, J.-F.; Liu, G.-H.; Fu, X.; Su, X.; Yue, D.-M.; Gao, Y.; Zhang, Y.; Wang, C.-R. The complete mitochondrial genome of Metorchis orientalis (Trematoda: Opisthorchiidae): Comparison with other closely related species and phylogenetic implications. Infect. Gen. Evol. 2016, 39, 45–50. [Google Scholar] [CrossRef]
- Qiu, J.-H.; Zhang, Y.; Zhang, X.-X.; Gao, Y.; Li, Q.; Chang, Q.-C.; Wang, C.-R. Metacercaria infection status of fishborne zoonotic trematodes, except for Clonorchis sinensis in fish from the Heilongjiang Province, China. Foodborne Pathog. Dis. 2017, 14, 440–446. [Google Scholar] [CrossRef]
- Qiu, Y.-Y.; Gao, Y.; Li, Y.; Ma, X.-X.; Lv, Q.-B.; Hu, Y.; Qiu, H.-Y.; Chang, Q.-C.; Wang, C.-R. Comparative analyses of complete ribosomal DNA sequences of Clonorchis sinensis and Metorchis orientalis: IGS sequences may provide a novel genetic marker for intraspecific variation. Infect. Gen. Evol. 2020, 78, 104125. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, X.; Sun, Q.; Gong, P.; Zhang, N.; Zhang, X.; Wang, X.; Li, G.; Li, J. First case report of Metorchis orientalis from Black Swan. Int. J. Parasitol. Parasites Wildl. 2020, 13, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Truett, G.E.; Heeger, P.; Mynatt, R.L.; Walker, J.A.; Warman, M.L. Preparation of PCR-quality mouse genomic DNA with hot sodium hydroxide and tris (Hot SHOT). Biotechniques 2000, 29, 52–54. [Google Scholar] [CrossRef] [PubMed]
- Tatonova, Y.V.; Besprozvannykh, V.V.; Katugina, L.O.; Solodovnik, D.A.; Nguyen, H.M. Morphological and molecular data for highly pathogenic avian parasite Erschoviorchis anuiensis sp. n. and phylogenetic relationships within the Opisthorchiidae (Trematoda). Parasitol. Int. 2020, 75, 102055. [Google Scholar] [CrossRef] [PubMed]
- Tkach, V.V.; Timothy, D.; Littlewood, J.; Olson, P.D.; Kinsella, J.M.; Swiderski, Z. Molecular phylogenetic analysis of the Microphalloidea Ward, 1901 (Trematoda: Digenea). Syst. Parasitol. 2003, 56, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Morgan, J.A.T.; Blair, D. Nuclear rDNA ITS sequence variation in the trematode genus Echinostoma: An aid to establishing relationships within the 37-collar-spine group. Parasitol. 1995, 111, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Chelomina, G.N.; Tatonova, Y.V.; Nguyen, H.M.; Ngo, H.D. Genetic diversity of the Chinese liver fluke Clonorchis sinensis from Russia and Vietnam. Int. J. Parasitol. 2014, 44, 795–810. [Google Scholar] [CrossRef]
- Katokhin, A.V.; Shekhovtsov, S.V.; Konkow, S.; Yurlova, N.I.; Serbina, E.A.; Vodianitskai, S.N.; Fedorov, K.P.; Loktev, V.B.; Muratov, I.V.; Ohyama, F.; et al. Assessment of the genetic distinctions of Opisthorchis felineus from O. viverrini and Clonorchis sinensis by ITS2 and CO1 sequences. Dokl. Biochem. 2008, 421, 214–217. [Google Scholar] [CrossRef]
- Waeschenbach, A.; Cox, C.J.; Littlewood, D.T.J.; Porter, J.S.; Taylor, P.D. First molecular estimate of cyclostome bryozoan phylogeny confirms extensive homoplasy among skeletal characters used in traditional taxonomy. Mol. Phylogenet. Evol. 2009, 52, 241–251. [Google Scholar] [CrossRef]
- Tatonova, Y.V.; Chelomina, G.N.; Besprozvannykh, V.V. Genetic diversity of nuclear ITS1-5.8S-ITS2 rDNA sequence in Clonorchis sinensis Cobbold, 1875 (Trematoda: Opisthorchidae) from the Russian Far East. Parasitol. Int. 2012, 61, 664–674. [Google Scholar] [CrossRef]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef] [PubMed]
- Librado, P.; Rozas, J. DnaSP v5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics 2009, 25, 1451–1452. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Huelsenbeck, J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef] [PubMed]
- Sonin, M.D. Keys to the Identification of Trematodes of Fish-Eating Birds of the Palaearctic (Opisthorchids, Renicolids, Strigeids); Nauka: Moscow, Russia, 1986. (In Russian) [Google Scholar]
- Sohn, W.M.; Chai, J.Y.; Lee, S.H. Growth and development of Metorchis orientalis in chicks and its adult morphology. Kisaengchunghak Chapchi 1992, 30, 237–243. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Gong, P.; Yu, Y.; Zhang, N.; Ren, Y.; Ma, Y.; Zhao, Z.; Zhang, X.; Li, X.; et al. Prevalence of fish-borne zoonotic trematode infection in Jilin Province, China. Int. J. Parasitol. Parasites Wildl. 2022, 18, 52–60. [Google Scholar] [CrossRef]
- Duan, Y.; Al-Jubury, A.; Kania, P.W.; Buchmann, K. Trematode diversity reflecting the community structure of Danish freshwater systems: Molecular clues. Parasit. Vectors 2021, 14, 1–15. [Google Scholar] [CrossRef]
- Filimonova, L.V. Some aspects of the study of morphological variability of maritas Metorchis bilis (Braun, 1970) (Trematoda: Opisthorchiidae). Fauna Morphol. Taxon. Parasites Immun. Parasitosis 1995, 40, 95–104. (In Russian) [Google Scholar]
- Filimonova, L.V. Influence of some environmental factors on the morphology of marita Metorchis xantosomus (Creplin, 1846). Tr. Instituta Parazitol. RAN 1997, 41, 183–192. (In Russian) [Google Scholar]
- Ishii, N.; Matsuoka, F. Studies on bird trematodes. V. Intermediate host and few species of bird trematodes. Jpn. J. Exp. Med. 1935, 13, 751–756. [Google Scholar]
- Hsu, H.F.; Chow, C.Y. Studies on helminths of fowls. Some trematodes of fowls in Tsingkinpu, Kiangsu, China. Chi. Med. J. 1938, 2, 441–442. [Google Scholar]
- Nekrasov, A.V.; Pronin, N.M.; Sanzhieva, S.D.; Timoshenko, T.M. Diversity of the helminth fauna of the herring gull (Larus argentatus) of Lake Baikal: Features of spatial distribution and infestation. Parazitologiya. 1999, 33, 426–436. (In Russian) [Google Scholar]
- Dorzhiev, T.Z.; Badmaeva, E.N.; Dugarov, Z.N. Ecological and faunistic analysis of helminthes of water-marsh birds of Baikal Siberia: 3. Gulls Laridae. Nat. Inn. Asia 2020, 1, 66–78. (In Russian) [Google Scholar]
- Olson, P.D.; Tkach, V.V. Advances and trends in the molecular systematics of the parasitic Platyhelminthes. Adv. Parasitol. 2005, 60, 165–243. [Google Scholar] [CrossRef] [PubMed]
- Thaenkham, U.; Blair, D.; Nawa, Y.; Waikagul, J. Families Opisthorchiidae and Heterophyidae: Are they distinct? Parasitol. Int. 2012, 61, 90–93. [Google Scholar] [CrossRef] [PubMed]
- Cutmore, S.C.; Miller, T.L.; Curran, S.S.; Bennett, M.B.; Cribb, T.H. Phylogenetic relationships of the Gorgoderidae (Platyhelminthes: Trematoda), including the proposal of a new subfamily (Degeneriinae n. subfam.). Parasitol. Res. 2013, 112, 3063–3074. [Google Scholar] [CrossRef] [PubMed]
- Nakao, M.; Ishikawa, T.; Hibino, Y.; Ohari, Y.; Taniguchi, R.; Takeyama, T.; Nakamura, S.; Kakino, W.; Ikadai, H.; Sasaki, M. Resolution of cryptic species complexes within the genus Metagonimus (Trematoda: Heterophyidae) in Japan, with descriptions of four new species. Parasitol. Int. 2022, 90, 102605. [Google Scholar] [CrossRef]
- Kang, S.; Sultana, T.; Loktev, V.B.; Wongratanacheewin, S.; Sohn, W.M.; Eom, K.S.; Park, J.K. Molecular identification and phylogenetic analysis of nuclear rDNA sequences among three opisthorchid liver fluke species (Opisthorchiidae, Trematoda). Parasitol. Int. 2008, 57, 191–197. [Google Scholar] [CrossRef]
- Xiao, J.-Y.; Gao, J.-F.; Cai, L.-S.; Dai, Y.; Yang, C.-J.; Luo, L.; Agatsuma, T.; Wang, C.-R. Genetic variation among Clonorchis sinensis isolates from different hosts and geographical locations revealed by sequence analysis of mitochondrial and ribosomal DNA regions. Mitochondrial DNA 2013, 24, 559–564. [Google Scholar] [CrossRef]
- Thaenkham, U.; Nuamtanong, S.; Vonghachack, Y.; Yoonuan, T.; Sanguankiat, S.; Dekumyoy, P.; Prommasack, B.; Kobayashi, J.; Waikagul, J. Discovery of Opisthorchis lobatus (Trematoda: Opisthorchiidae): A new record of small liver flukes in the Greater Mekong Sub-region. J. Parasitol. 2011, 97, 1152–1158. [Google Scholar] [CrossRef]



| Gene | 28S | ITS1-5.8S-ITS2 | cox1 |
|---|---|---|---|
| PCR | forward dig12 (5′-AAG-CAT-ATC-ACT-AAG-CGG-3′) and reverse 1500R (5′-GCT-ATC-CTG-AGG-GAA-ACT-TCG-3′) [18] | forward BD1 (5′-GTC-GTA-ACA-AGG-TTT-CCG-TA-3′) [19] and reverse 28S4R (5′-TAT-TTA-GCC-TTG-GAT-GGA-GTT-TAC-C-3′) [9] | forward 5cF22 (5′-TAG-ACT-ATC-TGT-CTT-CAA-AAC-A-3′) [20] and reverse CO1-Rv (5′-AAC-AAA-TCA-TGATGC-AAA-AGG-TA-3′) [21]; forward MBCOIF* (5′-GTG-TCT-GCG-GTA-TGG-CTC-CT-3′) and reverse MBCOIR* (5′-AGC-ACC-CAG-TCC-ACA-CAA-GG-3′) |
| Sequencing (internal) | 900F (5′-CCG-TCT-TGA-AAC-ACG-GAC-CAA-G-3′), ECD2 (5′-CTT-GGT-CCG-TGT-TTC-AAG-ACG-GG-3′) [18]; 1200R (5′-GCA-TAG-TTC-ACC-ATC-TTTCGG-3′) [22]; 28S4R | F1i27* (5′-TGC-CTA-CCC-GTC-TGA-TGC-TCT-C-3′); R1i15 (5′-GTC-GTA-ACA-AGG-TTT-CCG-TA-3′) [23]; F2i03* (5′-GAC-TGC-CTA-GAT-GAG-GGG-GTG-G-3′); R2i18* (5′-GAC-AAA-GGA-CCA-ACA-ACG-GAG-C-3′) | MOR11.1* (5′-GAG-TTC-CAG-CAA-GCA-TAA-AC-3′) |
| M. butoridi Our Date n = 5 | M. butoridi [6] | M. orientalis [4] | M. orientalis [7] | M. orientalis [29] | M. orientalis [15] | M. ussuriensis [9] | M. elegans [5] | M. taiwanensis [7] | M. taiwanensis [10] | |
|---|---|---|---|---|---|---|---|---|---|---|
| Body length | 1.83–3.00 | 4.10–4.70 | 2.36–4.65 | 3.00–6.81 | 3.43 | 4.98–9.10 | 2.42–3.93 | 3.20–4.50 | 3.49–5.81 | 2.50–3.50 |
| Body width | 0.35–0.60 | 0.65–0.70 | 0.53–1.23 | 0.61–1.64 | 0.83 | 0.61–1.35 | 0.34–0.79 | 0.46–0.54 | 0.42–1.45 | 0.40–0.55 |
| Length/width ratio, % | 1:4.06–6.32 | – | – | 1:2.99–4.49 | – | – | 1:4.06–8.50 | – | 1:4.26–11.2 | – |
| Forebody length | 0.63–1.08 | – | – | 0.66–1.81 | – | – | 0.83–1.42 | – | 0.83–1.68 | – |
| Body/forebody length ratio, % | 1:2.70–3.56 | – | – | – | – | – | 1:2.30–3.20 | – | – | – |
| Oral sucker length | 0.13–0.19 | 0.22–0.30 | 0.18–0.29 | 0.27–0.38 | 0.28 | 0.33–0.43 | 0.16–0.19 | 0.25–0.25 | 0.16–0.29 | 0.18–023 |
| Oral sucker width | 0.22–0.25 | 0.30–0.34 | 0.18–0.29 | 0.30–0.38 | 0.29 | 0.31–0.37 | 0.17–0.19 | 0.25–0.32 | 0.20–0.28 | 0.20–0.23 |
| Ventral sucker length | 0.16–0.19 | 0.25–0.34 | 0.12–0.30 | 0.23–0.33 | 0.27 | 0.30–0.40 | 0.14–0.18 | 0.21–0.27 | 0.20–0.28 | 0.15–0.18 |
| Ventral sucker width | 0.16–0.20 | 0.25–0.34 | 0.12–0.30 | 0.22–0.33 | 0.27 | 0.27–0.39 | 0.14–0.17 | 0.19–0.25 | 0.20–0.27 | 0.15–0.18 |
| Ventral/oral sucker length ratio, % | 1:1.00–1.39 | – | – | – | – | – | 1:0.82–0.98 | – | – | – |
| Ventral/oral sucker width ratio, % | 1:0.70–0.86 | – | – | – | – | – | 1:0.78–0.94 | – | – | – |
| Pharynx length | 0.05–0.07 | 0.08–0.10 | 0.04–0.06 | 0.07–0.08 | 0.07 | – | 0.05–0.07 | 0.30 | 0.07–0.10 | – |
| Pharynx width | 0.05–0.07 | 0.08–0.09 | 0.04–0.06 | 0.07–0.08 | 0.07 | – | 0.05–0.07 | 0.26 | 0.05–0.08 | – |
| Esophagus length | 0 | 0 | – | 0.08–0.18 | 0.07 | – | 0.03–0.10 | – | 0.03–0.12 | – |
| Ovary length | 0.10–0.17 | 0.18–0.20 | 0.26–0.28 | 0.12–0.33 | 0.14 | 0.35–0.53 | 0.12–0.24 | 0.12–0.16 | 0.18–0.32 | 0.15–0.18 |
| Ovary width | 0.11–0.20 | 0.18–0.23 | 0.33–0.36 | 0.17–0.40 | 0.21 | 0.25–0.35 | 0.12–0.27 | 0.10–0.11 | 0.15–0.33 | 0.15–0.18 |
| Anterior testis length | 0.16–0.38 | 0.35 | 0.44–0.54 | 0.25–0.83 | 0.30 | 0.83–1.17 | 0.24–0.44 | 0.22–0.37 | 0.33–0.83 | 0.25–0.35 |
| Anterior testis width | 0.23–0.54 | 0.26 | 0.73–0.90 | 0.32–0.98 | 0.42 | 0.51–0.89 | 0.27–0.56 | 0.20–0.31 | 0.33–0.61 | 0.30–0.38 |
| Posterior testis length | 0.13–0.41 | 0.36–0.44 | 0.44–0.54 | 0.25–0.90 | 0.36 | 0.88–1.22 | 0.29–0.58 | 0.28–0.36 | 0.33–0.83 | 0.30–0.35 |
| Posterior testis width | 0.25–0.55 | 0.34 | 0.73–0.90 | 0.32–1.08 | 0.43 | 0.60–1.08 | 0.30–0.67 | 0.22–0.38 | 0.40–0.65 | 0.33–0.43 |
| Anterior end of vitellarium | 0.31–0.72 | – | – | – | – | – | 0.43–0.89 | 0.60 | – | – |
| Egg length | 0.015–0.030 | 0.025–0.027 | 0.029–0.032 | 0.029–0.032 | – | 0.025–0.029 | 0.027–0.031 | 0.024–0.028 | 0.026–0.030 | 0.023–0.028 |
| Egg width | 0.010–0.018 | 0.014–0.015 | 0.015–0.017 | 0.014–0.017 | – | 0.013–0.015 | 0.015–0.019 | 0.012–0.014 | 0.136–0.017 | 0.014–0.016 |
| Opisthorchiidae | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Metorchis orientalis MK482051 | 0.002 | 0.002 | 0.003 | 0.003 | 0.004 | 0.004 | 0.004 | 0.007 | 0.006 | 0.007 | 0.007 | 0.007 | 0.013 | 0.008 | |
| 2 | Metorchis ussuriensis KY075777 | 0.006 | 0.002 | 0.003 | 0.003 | 0.004 | 0.004 | 0.004 | 0.007 | 0.006 | 0.007 | 0.007 | 0.007 | 0.013 | 0.008 | |
| 3 | Metorchis butoridi OR095627-OR095634 | 0.003 | 0.005 | 0.003 | 0.003 | 0.004 | 0.004 | 0.004 | 0.006 | 0.006 | 0.007 | 0.007 | 0.007 | 0.013 | 0.008 | |
| 4 | Clonorchis sinensis JF823989 | 0.009 | 0.009 | 0.008 | 0.002 | 0.004 | 0.004 | 0.003 | 0.006 | 0.006 | 0.006 | 0.007 | 0.007 | 0.013 | 0.008 | |
| 5 | Opisthorchis felineus MF099790 | 0.011 | 0.011 | 0.010 | 0.006 | 0.003 | 0.004 | 0.003 | 0.006 | 0.006 | 0.006 | 0.007 | 0.007 | 0.013 | 0.008 | |
| 6 | Opisthorchis viverrini HM004188 | 0.019 | 0.019 | 0.018 | 0.015 | 0.015 | 0.004 | 0.004 | 0.007 | 0.006 | 0.007 | 0.007 | 0.007 | 0.013 | 0.008 | |
| 7 | Opisthorchis noverca KC295443 | 0.020 | 0.020 | 0.018 | 0.016 | 0.017 | 0.017 | 0.005 | 0.007 | 0.006 | 0.007 | 0.007 | 0.007 | 0.013 | 0.008 | |
| 8 | Erschoviorchis anuiensis MK877245 | 0.015 | 0.015 | 0.014 | 0.014 | 0.014 | 0.022 | 0.025 | 0.006 | 0.006 | 0.006 | 0.007 | 0.007 | 0.013 | 0.008 | |
| 9 | Amphimerus ovalis AY116876 | 0.046 | 0.048 | 0.045 | 0.045 | 0.045 | 0.052 | 0.052 | 0.042 | 0.007 | 0.007 | 0.008 | 0.007 | 0.013 | 0.008 | |
| 10 | Apophallus zalophi MG806918 | 0.043 | 0.045 | 0.042 | 0.042 | 0.042 | 0.044 | 0.050 | 0.041 | 0.056 | 0.005 | 0.007 | 0.007 | 0.012 | 0.008 | |
| 11 | Liliatrema skrjabini MT303944 | 0.048 | 0.049 | 0.047 | 0.046 | 0.048 | 0.050 | 0.052 | 0.047 | 0.059 | 0.028 | 0.007 | 0.006 | 0.013 | 0.008 | |
| 12 | Cryptocotyle lata MH025622 | 0.059 | 0.061 | 0.058 | 0.058 | 0.060 | 0.065 | 0.062 | 0.060 | 0.067 | 0.059 | 0.054 | 0.004 | 0.013 | 0.008 | |
| 13 | Cryptocotyle lingua AY222228 | 0.058 | 0.060 | 0.057 | 0.057 | 0.059 | 0.062 | 0.063 | 0.055 | 0.064 | 0.053 | 0.047 | 0.021 | 0.012 | 0.008 | |
| 14 | Pachytrema calculus AF151942 | 0.209 | 0.214 | 0.212 | 0.209 | 0.209 | 0.207 | 0.215 | 0.212 | 0.219 | 0.212 | 0.216 | 0.203 | 0.210 | 0.012 | |
| Heterophyidae (outgroup) | ||||||||||||||||
| 15 | Metagonimus suifunensis KX387456 | 0.065 | 0.063 | 0.064 | 0.060 | 0.064 | 0.065 | 0.070 | 0.065 | 0.074 | 0.065 | 0.063 | 0.069 | 0.066 | 0.198 | |
| Opisthorchiidae | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Metorchis butoridi OR088532 | 0.001 | 0.000 | 0.002 | 0.002 | 0.002 | 0.002 | 0.003 | 0.010 | 0.010 | 0.010 | 0.010 | 0.011 | 0.011 | 0.011 | 0.011 | 0.012 | 0.013 | |
| 2 | Metorchis butoridi OR088533 | 0.002 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.003 | 0.010 | 0.010 | 0.011 | 0.010 | 0.011 | 0.011 | 0.011 | 0.011 | 0.012 | 0.013 | |
| 3 | Metorchis butoridi OR088534 | 0.000 | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.003 | 0.010 | 0.010 | 0.010 | 0.010 | 0.011 | 0.011 | 0.011 | 0.011 | 0.012 | 0.013 | |
| 4 | Metorchis butoridi OR088535 | 0.004 | 0.002 | 0.004 | 0.001 | 0.002 | 0.002 | 0.003 | 0.010 | 0.010 | 0.011 | 0.010 | 0.011 | 0.011 | 0.011 | 0.011 | 0.012 | 0.013 | |
| 5 | Metorchis butoridi OR088536 | 0.004 | 0.002 | 0.004 | 0.002 | 0.002 | 0.002 | 0.003 | 0.010 | 0.010 | 0.011 | 0.010 | 0.011 | 0.011 | 0.011 | 0.011 | 0.012 | 0.013 | |
| 6 | Metorchis butoridi OR088537 | 0.004 | 0.002 | 0.004 | 0.004 | 0.004 | 0.002 | 0.003 | 0.010 | 0.010 | 0.011 | 0.010 | 0.011 | 0.011 | 0.011 | 0.011 | 0.012 | 0.013 | |
| 7 | Metorchis butoridi OR088530 | 0.003 | 0.001 | 0.003 | 0.003 | 0.003 | 0.003 | 0.003 | 0.010 | 0.010 | 0.011 | 0.010 | 0.011 | 0.011 | 0.011 | 0.011 | 0.012 | 0.013 | |
| 8 | Metorchis butoridi OR088531 | 0.013 | 0.012 | 0.013 | 0.012 | 0.010 | 0.013 | 0.012 | 0.010 | 0.010 | 0.011 | 0.011 | 0.011 | 0.010 | 0.011 | 0.011 | 0.013 | 0.013 | |
| 9 | Metorchis ussuriensis OR095177 | 0.135 | 0.134 | 0.135 | 0.132 | 0.134 | 0.132 | 0.134 | 0.137 | 0.010 | 0.011 | 0.010 | 0.011 | 0.010 | 0.012 | 0.011 | 0.012 | 0.014 | |
| 10 | Metorchis orientalis KT239342 | 0.145 | 0.145 | 0.145 | 0.145 | 0.145 | 0.147 | 0.146 | 0.153 | 0.147 | 0.009 | 0.011 | 0.011 | 0.011 | 0.011 | 0.012 | 0.012 | 0.013 | |
| 11 | Opisthorchis viverrini JF739555 | 0.151 | 0.151 | 0.151 | 0.151 | 0.151 | 0.153 | 0.152 | 0.158 | 0.157 | 0.101 | 0.010 | 0.011 | 0.011 | 0.011 | 0.011 | 0.013 | 0.013 | |
| 12 | Clonorchis sinensis MT607652 | 0.161 | 0.159 | 0.161 | 0.159 | 0.159 | 0.161 | 0.159 | 0.166 | 0.148 | 0.158 | 0.155 | 0.010 | 0.010 | 0.011 | 0.012 | 0.013 | 0.014 | |
| 13 | Opisthorchis felineus EU921260 | 0.159 | 0.157 | 0.159 | 0.155 | 0.155 | 0.159 | 0.157 | 0.157 | 0.152 | 0.156 | 0.166 | 0.151 | 0.010 | 0.011 | 0.012 | 0.013 | 0.013 | |
| 14 | Opisthorchis sudarikovi MK033132 | 0.143 | 0.141 | 0.143 | 0.140 | 0.140 | 0.143 | 0.141 | 0.145 | 0.149 | 0.143 | 0.140 | 0.145 | 0.136 | 0.011 | 0.012 | 0.012 | 0.014 | |
| 15 | Erschoviorchis anuiensis OR084097 | 0.161 | 0.160 | 0.161 | 0.159 | 0.160 | 0.162 | 0.161 | 0.164 | 0.178 | 0.164 | 0.166 | 0.158 | 0.165 | 0.159 | 0.011 | 0.013 | 0.013 | |
| 16 | Amphimerus sp. MK238506 | 0.178 | 0.179 | 0.178 | 0.178 | 0.179 | 0.181 | 0.180 | 0.183 | 0.192 | 0.199 | 0.183 | 0.199 | 0.193 | 0.191 | 0.191 | 0.011 | 0.013 | |
| 17 | Cryptocotyle lingua EU876333 | 0.222 | 0.224 | 0.222 | 0.222 | 0.223 | 0.224 | 0.225 | 0.227 | 0.231 | 0.231 | 0.224 | 0.229 | 0.235 | 0.225 | 0.239 | 0.192 | 0.013 | |
| Heterophyidae (outgroup) | |||||||||||||||||||
| 18 | Metagonimus suifunensis MK736830 | 0.243 | 0.243 | 0.243 | 0.244 | 0.244 | 0.245 | 0.242 | 0.247 | 0.258 | 0.251 | 0.244 | 0.251 | 0.249 | 0.244 | 0.248 | 0.232 | 0.240 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solodovnik, D.A.; Tatonova, Y.V.; Besprozvannykh, V.V. Morpho-Molecular Features and Phylogenetic Relationships of Metorchis butoridi Oschmarin, 1963 (Trematoda: Opisthorchiidae) from East Asia. Animals 2024, 14, 124. https://doi.org/10.3390/ani14010124
Solodovnik DA, Tatonova YV, Besprozvannykh VV. Morpho-Molecular Features and Phylogenetic Relationships of Metorchis butoridi Oschmarin, 1963 (Trematoda: Opisthorchiidae) from East Asia. Animals. 2024; 14(1):124. https://doi.org/10.3390/ani14010124
Chicago/Turabian StyleSolodovnik, Daria Andreevna, Yulia Viktorovna Tatonova, and Vladimir Vladimirovich Besprozvannykh. 2024. "Morpho-Molecular Features and Phylogenetic Relationships of Metorchis butoridi Oschmarin, 1963 (Trematoda: Opisthorchiidae) from East Asia" Animals 14, no. 1: 124. https://doi.org/10.3390/ani14010124
APA StyleSolodovnik, D. A., Tatonova, Y. V., & Besprozvannykh, V. V. (2024). Morpho-Molecular Features and Phylogenetic Relationships of Metorchis butoridi Oschmarin, 1963 (Trematoda: Opisthorchiidae) from East Asia. Animals, 14(1), 124. https://doi.org/10.3390/ani14010124





