The Elevational Gradient of Bird Beta Diversity in the Meili Snow Mountains, Yunnan Province, China
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
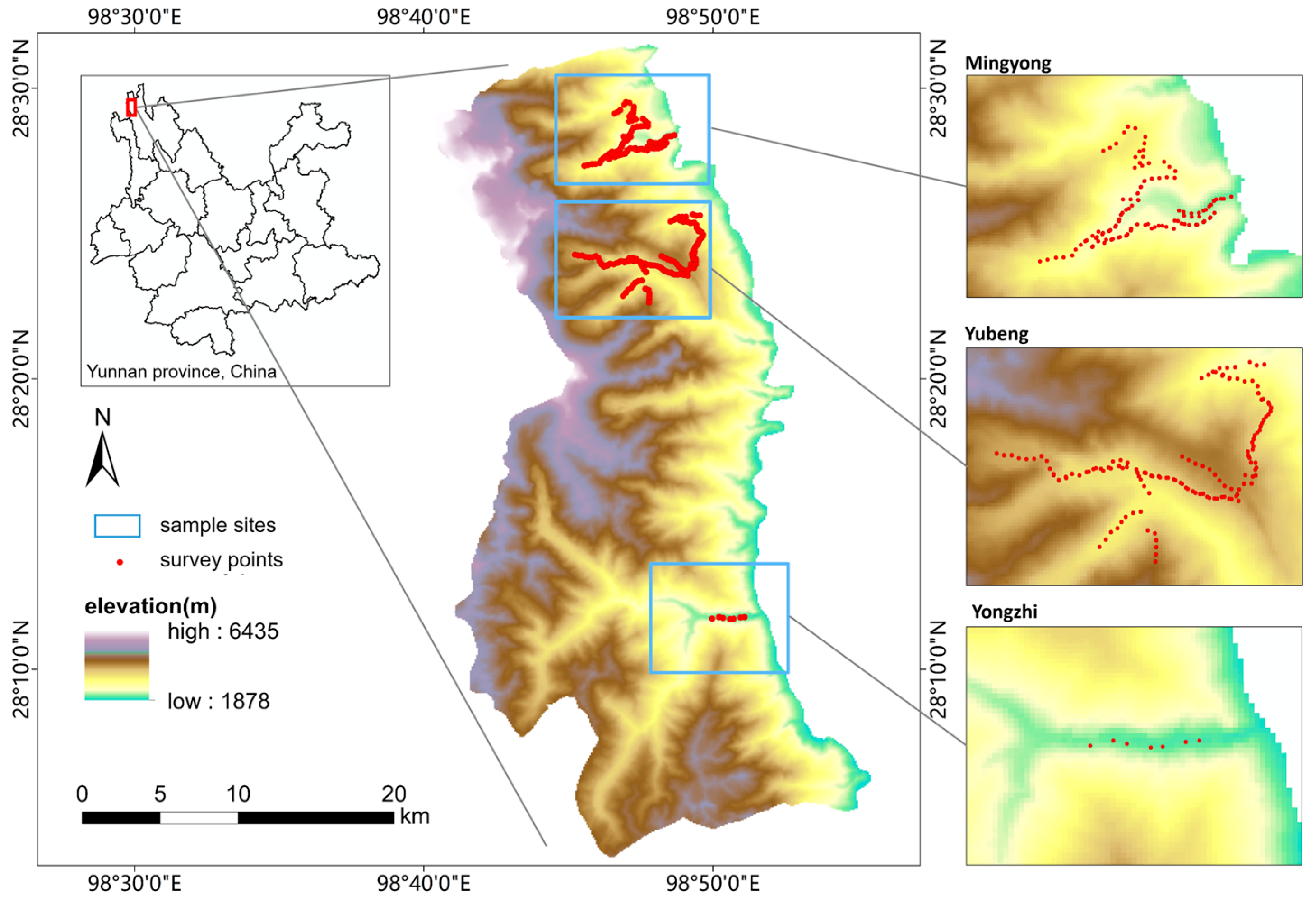
2.1. Study Area
2.2. Data Collection
2.2.1. Field Sampling and Bird Surveys
2.2.2. Phylogenetic Data
2.2.3. Functional Trait Data
2.2.4. Environmental Data
2.3. Measuring Observed and Standardized Beta Diversity in Regard to Multi-Facet Beta Diversity
2.4. Statistical Analyses
3. Results
3.1. Comparison between Turnover, Nestedness-Resultant Components, and Total Dissimilarities across Multiple Dimensions
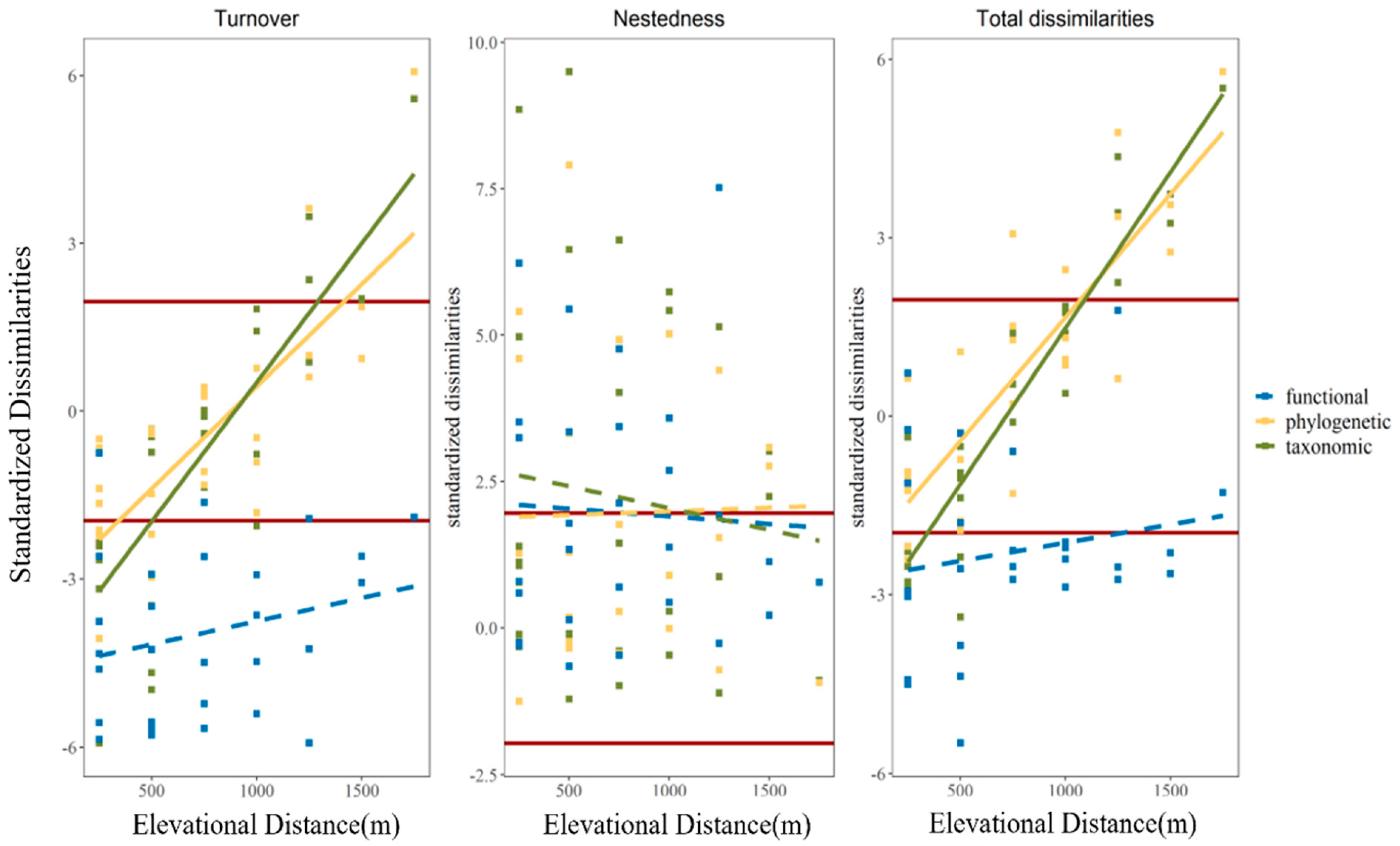
3.2. The Variation in Observed Beta Diversity along Elevational Distance
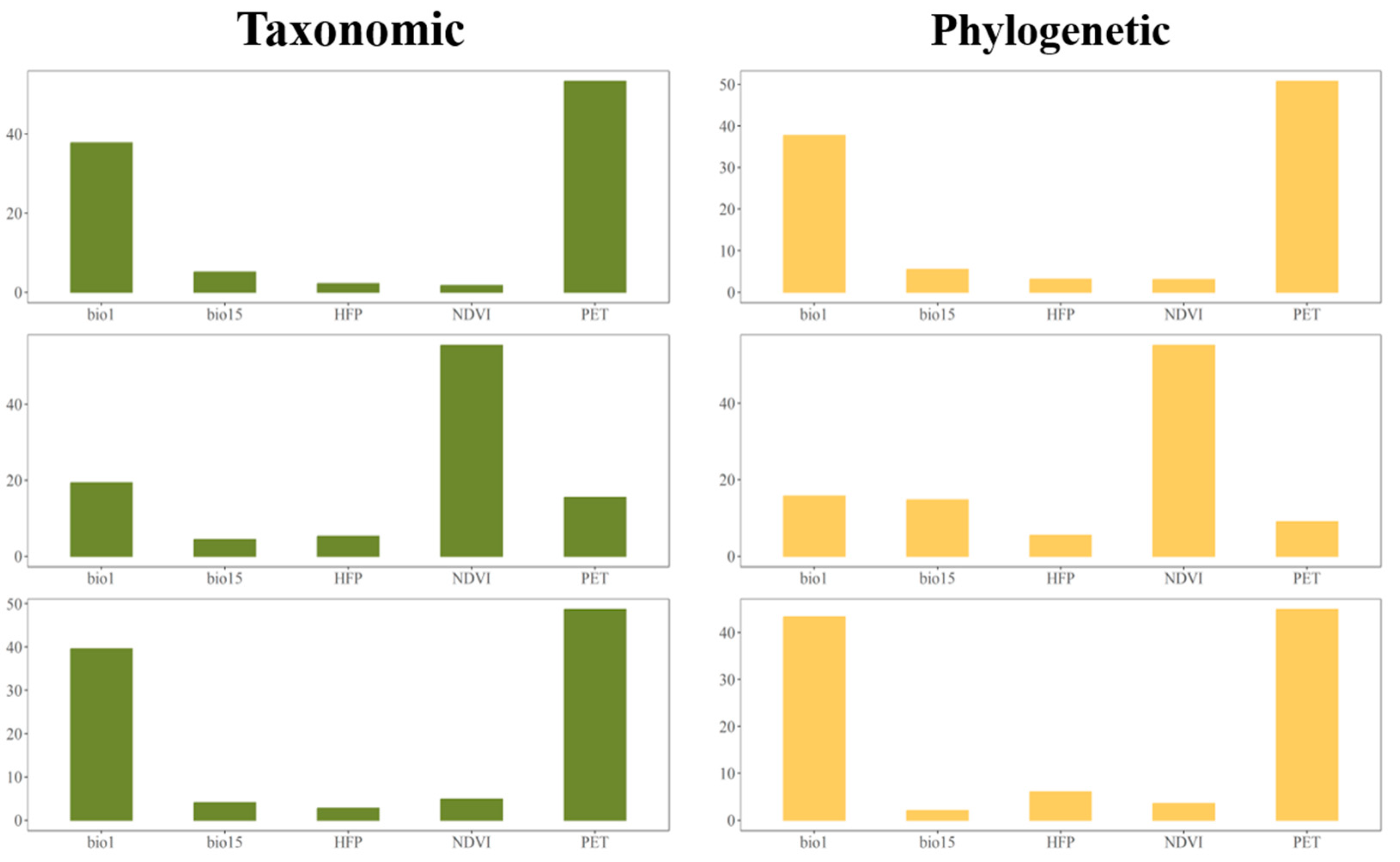
3.3. Environmental Factors
3.4. Standardized Pairwise Beta Diversity among Elevation Gradient
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zellweger, F.; Roth, T.; Bugmann, H.; Bollmann, K. Beta diversity of plants, birds and butterflies is closely associated with climate and habitat structure. Glob. Ecol. Biogeogr. 2017, 26, 898–906. [Google Scholar] [CrossRef]
- Sinha, A.; Menzies, R.K.; Chatterjee, N.; Rao, M.; Naniwadekar, R. Drivers of taxonomic, phylogenetic, and functional beta diversity of himalayan riverine birds. Front. Ecol. Evol. 2022, 10, 31. [Google Scholar] [CrossRef]
- Weinstein, B.G.; Tinoco, B.; Parra, J.L.; Brown, L.M.; McGuire, J.A.; Stiles, F.G.; Graham, C.H. Taxonomic, phylogenetic, and trait beta diversity in South American hummingbirds. Am. Nat. 2014, 184, 211–224. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, R.H. Evolution and measurement of species diversity. Taxon 1972, 21, 213–251. [Google Scholar] [CrossRef]
- Hawkins, B.A.; Felizola Diniz-Filho, J.A. Beyond Rapoport’s rule: Evaluating range size patterns of New World birds in a two-dimensional framework. Glob. Ecol. Biogeogr. 2006, 15, 461–469. [Google Scholar] [CrossRef]
- Lennon, J.J.; Koleff, P.; Greenwood, J.; Gaston, K.J. The geographical structure of British bird distributions: Diversity, spatial turnover and scale. J. Anim. Ecol. 2001, 70, 966–979. [Google Scholar] [CrossRef]
- Baselga, A. Partitioning the turnover and nestedness components of beta diversity. Glob. Ecol. Biogeogr. 2010, 19, 134–143. [Google Scholar] [CrossRef]
- Coetzee, B.W.; Robertson, M.P.; Erasmus, B.F.; Van Rensburg, B.J.; Thuiller, W. Ensemble models predict Important Bird Areas in southern Africa will become less effective for conserving endemic birds under climate change. Glob. Ecol. Biogeogr. 2009, 18, 701–710. [Google Scholar] [CrossRef]
- Wu, F.; Yang, X.J.; Yang, J.X. Additive diversity partitioning as a guide to regional montane reserve design in Asia: An example from Yunnan Province, China. Divers. Distrib. 2010, 16, 1022–1033. [Google Scholar] [CrossRef]
- Alanärä, A.; Burns, M.D.; Metcalfe, N.B. Intraspecific resource partitioning in brown trout: The temporal distribution of foraging is determined by social rank. J. Anim. Ecol. 2001, 70, 980–986. [Google Scholar] [CrossRef]
- Whittaker, R.H.; Niering, W.A. Vegetation of the Santa Catalina Mountains, Arizona. V. Biomass, production, and diversity along the elevation gradient. Ecology 1975, 56, 771–790. [Google Scholar] [CrossRef]
- Harrison, S.; Ross, S.J.; Lawton, J.H. Beta diversity on geographic gradients in Britain. J. Anim. Ecol. 1992, 61, 151–158. [Google Scholar] [CrossRef]
- Soininen, J.; McDonald, R.; Hillebrand, H. The distance decay of similarity in ecological communities. Ecography 2007, 30, 3–12. [Google Scholar] [CrossRef]
- Legendre, P. Interpreting the replacement and richness difference components of beta diversity. Glob. Ecol. Biogeogr. 2014, 23, 1324–1334. [Google Scholar] [CrossRef]
- Du, Y.; Fan, L.; Xu, Z.; Wen, Z.; Cai, T.; Feijo, A.; Hu, J.; Lei, F.; Yang, Q.; Qiao, H. A multi-faceted comparative perspective on elevational beta-diversity: The patterns and their causes. Proc. R Soc. B 2021, 288, 20210343. [Google Scholar] [CrossRef]
- Koleff, P.; Gaston, K.J.; Lennon, J.J. Measuring beta diversity for presence–absence data. J. Anim. Ecol. 2003, 72, 367–382. [Google Scholar] [CrossRef]
- Baselga, A.; Jiménez-Valverde, A.; Niccolini, G. A multiple-site similarity measure independent of richness. Biol. Lett. 2007, 3, 642–645. [Google Scholar] [CrossRef]
- Gaston, K.J.; Blackburn, T.M. Pattern and Process in Macroecology; Wiley Online Library: Hoboken, NJ, USA, 2000; Volume 414. [Google Scholar]
- Rominger, A.J.; Miller, T.E.; Collins, S.L. Relative contributions of neutral and niche-based processes to the structure of a desert grassland grasshopper community. Oecologia 2009, 161, 791–800. [Google Scholar] [CrossRef]
- Fisher, C.K.; Mehta, P. The transition between the niche and neutral regimes in ecology. Proc. Natl. Acad. Sci. USA 2014, 111, 13111–13116. [Google Scholar] [CrossRef]
- Caswell, H. Community structure: A neutral model analysis. Ecol. Monogr. 1976, 46, 327–354. [Google Scholar] [CrossRef]
- Gilbert, B.; Lechowicz, M.J. Neutrality, niches, and dispersal in a temperate forest understory. Proc. Natl. Acad. Sci. USA 2004, 101, 7651–7656. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, M.C.; Sanders, N.J.; Normand, S.; Svenning, J.-C.; Ferrier, S.; Gove, A.D.; Dunn, R.R. Environmental and historical imprints on beta diversity: Insights from variation in rates of species turnover along gradients. Proc. R Soc. B Biol. Sci. 2013, 280, 20131201. [Google Scholar] [CrossRef] [PubMed]
- Keith, S.; Baird, A.; Hughes, T.; Madin, J.; Connolly, S. Faunal breaks and species composition of Indo-Pacific corals: The role of plate tectonics, environment and habitat distribution. Proc. R Soc. B Biol. Sci. 2013, 280, 20130818. [Google Scholar] [CrossRef] [PubMed]
- Capinha, C.; Essl, F.; Seebens, H.; Moser, D.; Pereira, H.M. The dispersal of alien species redefines biogeography in the Anthropocene. Science 2015, 348, 1248–1251. [Google Scholar] [CrossRef]
- Wang, X.; Zhong, M.; Yang, S.; Jiang, J.; Hu, J. Multiple β-diversity patterns and the underlying mechanisms across amphibian communities along a subtropical elevational gradient. Divers. Distrib. 2022, 28, 2489–2502. [Google Scholar] [CrossRef]
- Swenson, N.G.; Anglada-Cordero, P.; Barone, J.A. Deterministic tropical tree community turnover: Evidence from patterns of functional beta diversity along an elevational gradient. Proc. R. Soc. B Biol. Sci. 2011, 278, 877–884. [Google Scholar] [CrossRef]
- Rahbek, C. The elevational gradient of species richness: A uniform pattern? Ecography 1995, 18, 200–205. [Google Scholar] [CrossRef]
- Faith, D.P. Conservation evaluation and phylogenetic diversity. Biol. Conserv. 1992, 61, 1–10. [Google Scholar] [CrossRef]
- Graham, C.H.; Fine, P.V. Phylogenetic beta diversity: Linking ecological and evolutionary processes across space in time. Ecol. Lett. 2008, 11, 1265–1277. [Google Scholar] [CrossRef]
- Webb, C.O.; Ackerly, D.D.; McPeek, M.A.; Donoghue, M.J. Phylogenies and community ecology. Annu. Rev. Ecol. Syst. 2002, 33, 475–505. [Google Scholar] [CrossRef]
- Bishop, T.R.; Robertson, M.P.; van Rensburg, B.J.; Parr, C.L. Contrasting species and functional beta diversity in montane ant assemblages. J. Biogeogr. 2015, 42, 1776–1786. [Google Scholar] [CrossRef] [PubMed]
- McGill, B.J.; Enquist, B.J.; Weiher, E.; Westoby, M. Rebuilding community ecology from functional traits. Trends Ecol. Evol. 2006, 21, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Swenson, N.G.; Erickson, D.L.; Mi, X.; Bourg, N.A.; Forero-Montaña, J.; Ge, X.; Howe, R.; Lake, J.K.; Liu, X.; Ma, K. Phylogenetic and functional alpha and beta diversity in temperate and tropical tree communities. Ecology 2012, 93, S112–S125. [Google Scholar] [CrossRef]
- Swenson, N.G.; Enquist, B.J. Opposing assembly mechanisms in a Neotropical dry forest: Implications for phylogenetic and functional community ecology. Ecology 2009, 90, 2161–2170. [Google Scholar] [CrossRef]
- Cavender-Bares, J.; Kozak, K.H.; Fine, P.V.; Kembel, S.W. The merging of community ecology and phylogenetic biology. Ecol. Lett. 2009, 12, 693–715. [Google Scholar] [CrossRef]
- Zhang, Z.; De Clercq, E.; Ou, X.; De Wulf, R.; Verbeke, L. Mapping dominant vegetation communities at Meili Snow Mountain, Yunnan Province, China using satellite imagery and plant community data. Geocarto Int. 2008, 23, 135–153. [Google Scholar] [CrossRef]
- Wen-Fei, M.; Shi-Yin, L.; Yu, Z.; Shi-Mei, D.; Feng-Ze, H. Spatio-temporal differentiation and altitude dependence of temperature and precipitation in Meili Snow Mountains. Adv. Clim. Chang. Res. 2022, 18, 328. [Google Scholar]
- Ming, Q.; Guo, S.; Jiao, Y. High gradient effects of forest biomass energy in mountainous region—A case of meili snow mountain. Procedia Earth Planet. Sci. 2011, 2, 315–320. [Google Scholar] [CrossRef]
- Bibby, C.J.; Burgess, N.D.; Hillis, D.M.; Hill, D.A.; Mustoe, S. Bird Census Techniques; Elsevier: Amsterdam, The Netherlands, 2000. [Google Scholar]
- Yang, L.; Wen, X.; Han, L.; Yang, X.; Shi, W.; Wang, S. The Avifauna of Yunnan, China; Yunnan Science and Technology Press: Yunnan, China, 1995; Volume 1–2. (In Chinese) [Google Scholar]
- Wilman, H.; Belmaker, J.; Simpson, J.; de la Rosa, C.; Rivadeneira, M.M.; Jetz, W. EltonTraits 1.0: Species-level foraging attributes of the world’s birds and mammals: Ecological Archives E095-178. Ecology 2014, 95, 2027. [Google Scholar] [CrossRef]
- Paradis, E.; Schliep, K. ape 5.0: An environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics 2019, 35, 526–528. [Google Scholar] [CrossRef]
- Yu, G.; Smith, D.K.; Zhu, H.; Guan, Y.; Lam, T.T.Y. ggtree: An R package for visualization and annotation of phylogenetic trees with their covariates and other associated data. Methods Ecol. Evol. 2017, 8, 28–36. [Google Scholar] [CrossRef]
- Harmon, L.; Weir, J.; Brock, C.; Glor, R.; Challenger, W.; Hunt, G.; FitzJohn, R.; Pennell, M.; Slater, G.; Brown, J. Package ‘geiger’. R Package Version 2015; Volume 2. Available online: https://cran.r-project.org/web/packages/geiger/index.html (accessed on 10 September 2022).
- Jetz, W.; Thomas, G.H.; Joy, J.B.; Hartmann, K.; Mooers, A.O. The global diversity of birds in space and time. Nature 2012, 491, 444–448. [Google Scholar] [CrossRef] [PubMed]
- Pagel, M. Inferring the historical patterns of biological evolution. Nature 1999, 401, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Freckleton, R.P.; Harvey, P.H.; Pagel, M. Phylogenetic analysis and comparative data: A test and review of evidence. Am. Nat. 2002, 160, 712–726. [Google Scholar] [CrossRef] [PubMed]
- Revell, L.J. Phytools: An R package for phylogenetic comparative biology (and other things). Methods Ecol. Evol. 2012, 3, 217–223. [Google Scholar] [CrossRef]
- Karger, D.N.; Conrad, O.; Böhner, J.; Kawohl, T.; Kreft, H.; Soria-Auza, R.W.; Zimmermann, N.E.; Linder, H.P.; Kessler, M. Climatologies at high resolution for the earth’s land surface areas. Sci. Data 2017, 4, 1–20. [Google Scholar] [CrossRef]
- Trabucco, A.; Zomer, R.J. Global aridity index and potential evapotranspiration (ET0) climate database v2. CGIAR Consort. Spat. Inf. 2018, 10, m9. [Google Scholar]
- Wildlife Conservation Society-WCS; Center for International Earth Science Information Network-CIESIN-Columbia University. Last of the Wild Project, Version 2, 2005 (LWP-2): Global Human Footprint Dataset (Geographic); NASA Socioeconomic Data and Applications Center (SEDAC): Palisades, NY, USA, 2005. [Google Scholar]
- Sorensen, T.A. A method of establishing groups of equal amplitude in plant sociology based on similarity of species content and its application to analyses of the vegetation on Danish commons. Biol. Skar. 1948, 5, 1–34. [Google Scholar]
- Simpson, G.G. Mammals and the nature of continents. Am. J. Sci. 1943, 241, 1–31. [Google Scholar] [CrossRef]
- Villéger, S.; Grenouillet, G.; Brosse, S. Decomposing functional β-diversity reveals that low functional β-diversity is driven by low functional turnover in E uropean fish assemblages. Glob. Ecol. Biogeogr. 2013, 22, 671–681. [Google Scholar] [CrossRef]
- Villéger, S.; Maire, E.; Leprieur, F. On the risks of using dendrograms to measure functional diversity and multidimensional spaces to measure phylogenetic diversity: A comment on Sobral et al.(2016). Ecol. Lett. 2017, 20, 554–557. [Google Scholar] [CrossRef] [PubMed]
- Cornwell, W.K.; Schwilk, D.W.; Ackerly, D.D. A trait-based test for habitat filtering: Convex hull volume. Ecology 2006, 87, 1465–1471. [Google Scholar] [CrossRef] [PubMed]
- Baselga, A.; Orme, D.; Villeger, S.; De Bortoli, J.; Leprieur, F.; Logez, M. Betapart: Partitioning Beta Diversity into Turnover and Nestedness Components. R Package Version 1.5. Consultado 2021; Volume 15. Available online: https://cran.r-project.org/web/packages/betapart/ (accessed on 25 April 2022).
- Dixon, P. VEGAN, a package of R functions for community ecology. J. Veg. Sci. 2003, 14, 927–930. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.; O’hara, R.; Simpson, G.; Solymos, P.; Stevens, M.; Wagner, H. Vegan: Community ecology package. R package version 2.3-0; 2015. Sci. Rep. 2020, 10, 20354. [Google Scholar]
- Walsh, C.; MacNally, R. Hierarchical Partitioning. Online Ref. Version 2015, 1.0-4. Available online: https://www.rdocumentation.org/packages/hier.part/versions/1.0-6 (accessed on 29 May 2019).
- R Core Team. R: A Language and Environment for Statistical Computing. 2013. Available online: https://cran.r-project.org/bin/windows/base/old/4.1.2/ (accessed on 10 September 2018).
- Yang, F.; Liu, Z.; Yang, G.; Feng, G. Dominated taxonomic and phylogenetic turnover but functional nestedness of wetland bird beta diversity in North China. Land 2022, 11, 1090. [Google Scholar] [CrossRef]
- Xu, W.; Dong, W.-J.; Fu, T.-T.; Gao, W.; Lu, C.-Q.; Yan, F.; Wu, Y.-H.; Jiang, K.; Jin, J.-Q.; Chen, H.-M. Herpetological phylogeographic analyses support a Miocene focal point of Himalayan uplift and biological diversification. Natl. Sci. Rev. 2021, 8, nwaa263. [Google Scholar] [CrossRef]
- Lei, F.; Qu, Y.; Song, G.; Alström, P.; Fjeldså, J. The potential drivers in forming avian biodiversity hotspots in the East Himalaya Mountains of Southwest China. Integr. Zool. 2015, 10, 171–181. [Google Scholar] [CrossRef]
- Baselga, A.; Gómez-Rodríguez, C. Assessing the equilibrium between assemblage composition and climate: A directional distance-decay approach. J. Anim. Ecol. 2021, 90, 1906–1918. [Google Scholar] [CrossRef]
- Nekola, J.C.; McGill, B.J. Scale dependency in the functional form of the distance decay relationship. Ecography 2014, 37, 309–320. [Google Scholar] [CrossRef]
- Nekola, J.C.; White, P.S. The distance decay of similarity in biogeography and ecology. J. Biogeogr. 1999, 26, 867–878. [Google Scholar] [CrossRef]
- McKnight, M.W.; White, P.S.; McDonald, R.I.; Lamoreux, J.F.; Sechrest, W.; Ridgely, R.S.; Stuart, S.N. Putting beta-diversity on the map: Broad-scale congruence and coincidence in the extremes. PLoS Biol. 2007, 5, e272. [Google Scholar] [CrossRef] [PubMed]
- Price, T.D.; Hooper, D.M.; Buchanan, C.D.; Johansson, U.S.; Tietze, D.T.; Alström, P.; Olsson, U.; Ghosh-Harihar, M.; Ishtiaq, F.; Gupta, S.K. Niche filling slows the diversification of Himalayan songbirds. Nature 2014, 509, 222–225. [Google Scholar] [CrossRef] [PubMed]


| Taxonomic | Phylogenetic | Functional | |
|---|---|---|---|
| Turnover | 0.68 | 0.54 | 0.25 |
| Nestedness-resultant | 0.06 | 0.1 | 0.24 |
| Total dissimilarities | 0.74 | 0.64 | 0.48 |
| |Z-Score| | p-Values | Patterns | |
|---|---|---|---|
| βsim vs. βsne | 7.36 | <0.001 | βsim > βsne |
| βphylosim vs. βphylosne | 5.4 | <0.001 | βphylosim > βphylosne |
| βfuncsim vs. βfuncsne | 0.24 | 0.21 | / |
| βsim vs. βphylosim | 4.05 | <0.001 | βsim > βphylosim |
| βsim vs. βfuncsim | 5.84 | <0.001 | βsim > βfuncsim |
| βsne vs. βphylosne | 0.71 | 0.48 | / |
| βsne vs. βfuncsne | 1.42 | 0.17 | / |
| βphylosim vs. βfuncsim | 5.88 | <0.001 | βphylosim > βfuncsim |
| βphylosne vs. βfuncsne | 0.77 | 0.44 | / |
| βsor vs. βphylosor | 4.04 | <0.001 | βsor > βphylosor |
| βsor vs. βfuncsor | 6.4 | <0.001 | βsor > βfuncsor |
| βphylosor vs. βfuncsor | 5.66 | <0.001 | βphylosor > βfuncsor |
| Facet | Taxonomic | Phylogenetic | Functional |
|---|---|---|---|
| Turnover | r = 0.88 | r = 0.83 | r = 0.30 |
| Nestedness | r = −0.19 | r = −0.02 | r = −0.05 |
| Total dissimilarities | r = 0.90 | r = 0.85 | r = 0.19 |
| Facet | Taxonomic | Phylogenetic | Functional |
|---|---|---|---|
| Turnover | r = 0.80 | r = 0.73 | r = 0.31 |
| Nestedness | r = 0.19 | r = 0.02 | r = 0.047 |
| Total dissimilarities | r = 0.87 | r = 0.845 | r = 0.194 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, S.; Liu, L.; Shan, P.; Yang, X.; Wu, F. The Elevational Gradient of Bird Beta Diversity in the Meili Snow Mountains, Yunnan Province, China. Animals 2023, 13, 1567. https://doi.org/10.3390/ani13091567
Yao S, Liu L, Shan P, Yang X, Wu F. The Elevational Gradient of Bird Beta Diversity in the Meili Snow Mountains, Yunnan Province, China. Animals. 2023; 13(9):1567. https://doi.org/10.3390/ani13091567
Chicago/Turabian StyleYao, Shunyu, Luming Liu, Pengfei Shan, Xiaojun Yang, and Fei Wu. 2023. "The Elevational Gradient of Bird Beta Diversity in the Meili Snow Mountains, Yunnan Province, China" Animals 13, no. 9: 1567. https://doi.org/10.3390/ani13091567
APA StyleYao, S., Liu, L., Shan, P., Yang, X., & Wu, F. (2023). The Elevational Gradient of Bird Beta Diversity in the Meili Snow Mountains, Yunnan Province, China. Animals, 13(9), 1567. https://doi.org/10.3390/ani13091567





