Mental Experiences in Wild Animals: Scientifically Validating Measurable Welfare Indicators in Free-Roaming Horses
Abstract
Simple Summary
Abstract
1. Introduction
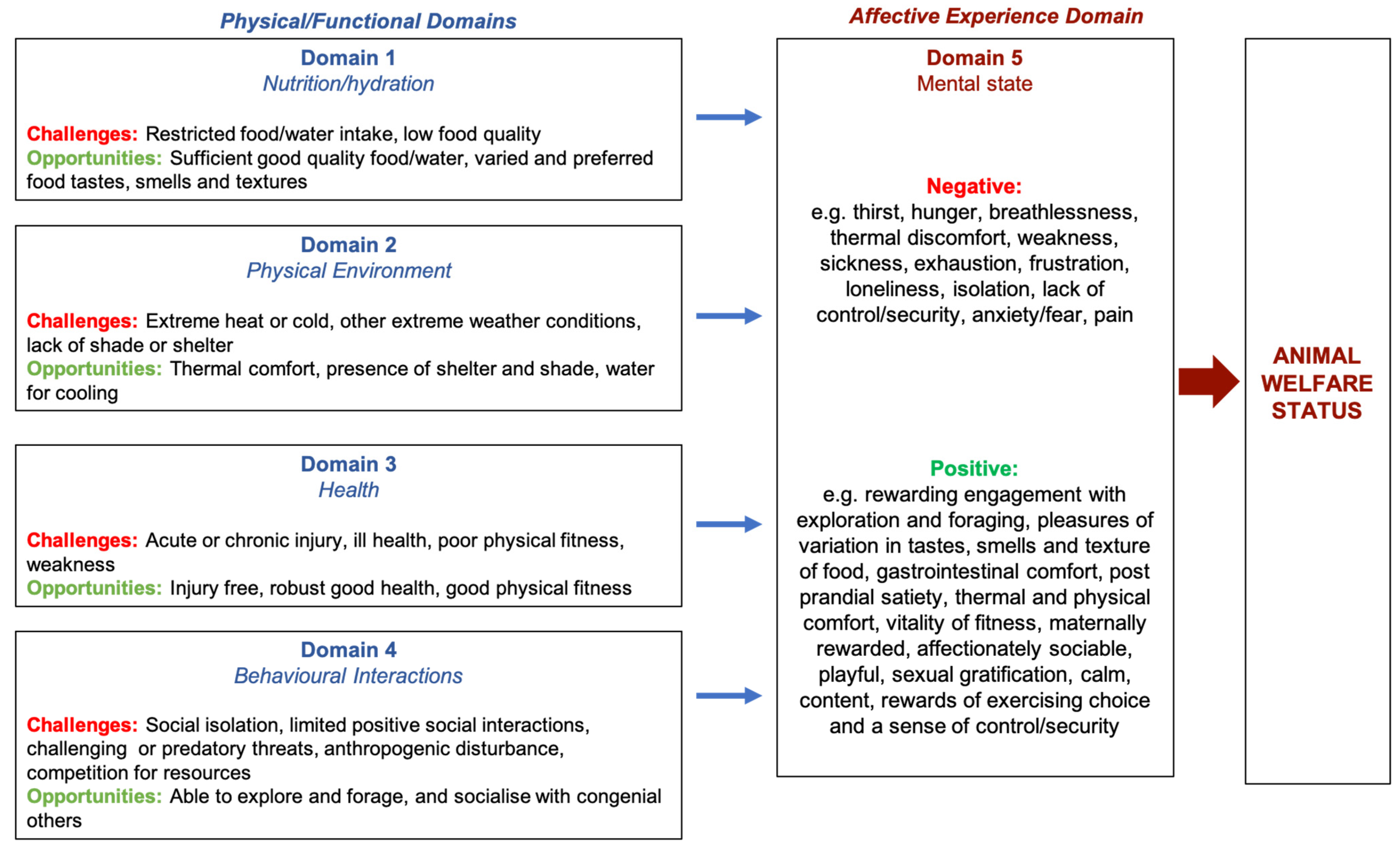
2. Selecting Welfare Indicators
3. Scientific Validation of Welfare Status Indicators
3.1. Indicators of Welfare Compromise (Negative Mental Experiences)
3.1.1. Domain 1: Nutrition
- Indicators of thirst
- Indicators of hunger
3.1.2. Domain 2: Physical Environment
- Indicators of cold and heat discomfort
3.1.3. Domain 3: Health
- General Indicators of Pain
- Indicators of Localised Pain
- Cutaneous pain: wounds
- Mouth pain: Quidding and food pouching
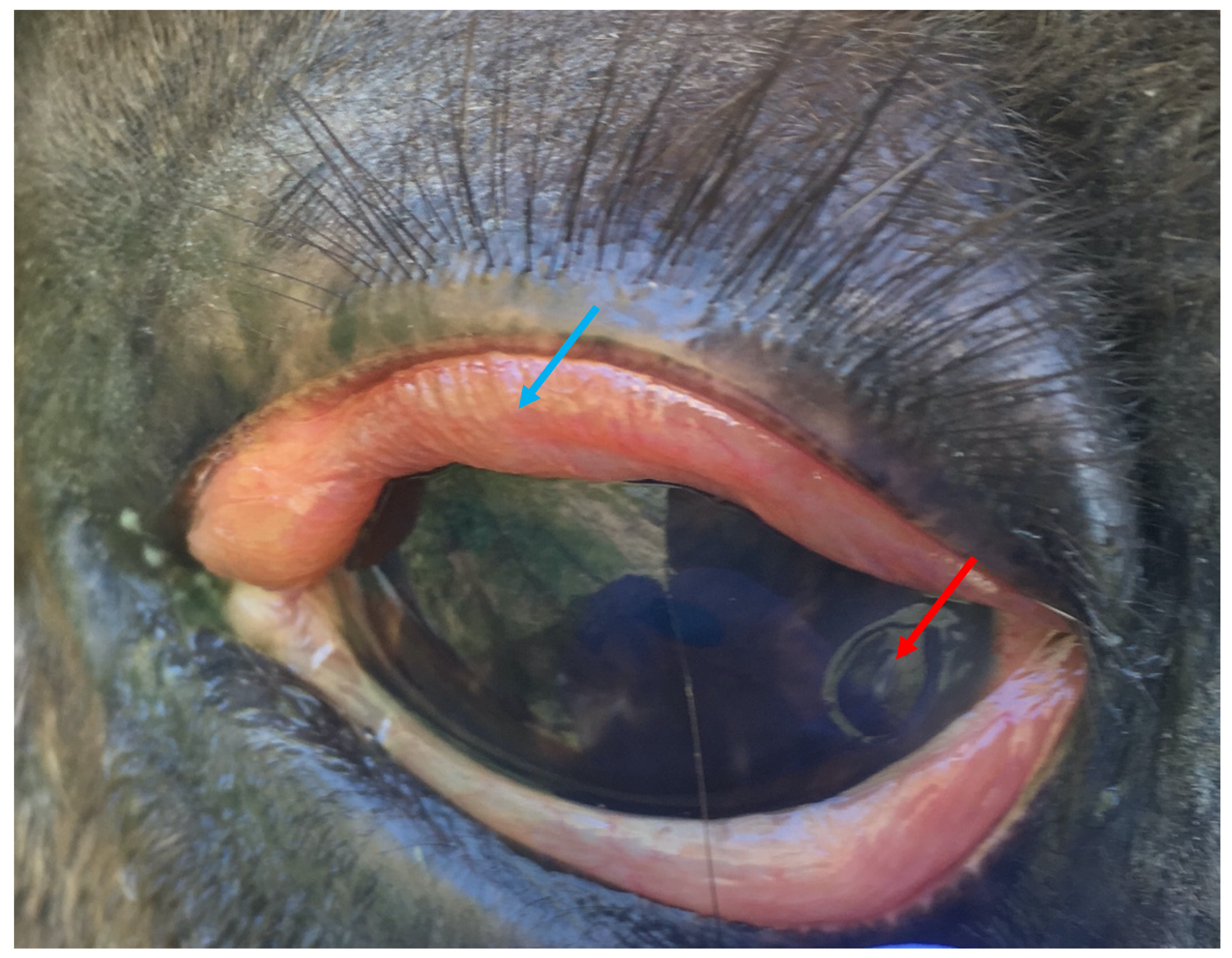
- Ocular pain: blepharospasm
- Limb/foot pain: lameness
- Indicators of Non-Specific Chronic Pain/Malaise/Fatigue/Exhaustion
- Body and ear posture
- Facial grimace
- Reduced alertness/dullness/apathy
- Very thin/emaciated body condition
- Muscular weakness
- Indicators of Breathlessness: Increased Respiratory Rate and/or Effort
3.1.4. Domain 4: Behavioural Interactions
3.2. Indicators of Welfare Enhancement (Positive Mental Experiences)
4. Welfare Alerting Indicators
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Beausoleil, N.J.; Mellor, D.J.; Baker, L.; Baker, S.E.; Bellio, M.; Clarke, A.S.; Dale, A.; Garlick, S.; Jones, B.; Harvey, A.M.; et al. “Feelings and Fitness” Not “Feelings or Fitness”—The Raison d’être of Conservation Welfare, Which Aligns Conservation and Animal Welfare Objectives. Front. Vet. Sci. 2018, 5, 296. [Google Scholar] [CrossRef] [PubMed]
- Harvey, A.M.; Beausoleil, N.J.; Ramp, D.; Mellor, D.J. A Ten-Stage Protocol for Assessing the Welfare of Individual Non-Captive Wild Animals: Free-Roaming Horses (Equus Ferus Caballus) as an Example. Animals 2020, 10, 148. [Google Scholar] [CrossRef] [PubMed]
- Mellor, D.J.; Beausoleil, N.J.; Littlewood, K.E.; McLean, A.N.; McGreevy, P.D.; Jones, B.; Wilkins, C. The 2020 Five Domains Model: Including Human–Animal Interactions in Assessments of Animal Welfare. Animals 2020, 10, 1870. [Google Scholar] [CrossRef] [PubMed]
- Mellor, D.J.; Beausoleil, N.J. Extending the ‘Five Domains’ model for animal welfare assessment to incorporate positive welfare states. Anim. Welf. 2015, 24, 241–253. [Google Scholar] [CrossRef]
- Mellor, D.J. Updating animal welfare thinking: Moving beyond the “Five Freedoms” towards “A Life worth Living”. Animals 2016, 6, 21. [Google Scholar] [CrossRef]
- Littlewood, K.E.; Mellor, D.J. Changes in the Welfare of an Injured Working Farm Dog Assessed Using the Five Domains Model. Animals 2016, 6, 58. [Google Scholar] [CrossRef]
- Mellor, D.J. Operational details of the Five Domains Model and its key applications to the assessment and management of animal welfare. Animals 2017, 7, 60. [Google Scholar] [CrossRef]
- Harvey, A.M.; Ramp, D.; Mellor, D.J. Review of the Foundational Knowledge Required for Assessing Horse Welfare. Animals 2022, 12, 3385. [Google Scholar] [CrossRef]
- Harvey, A.M.; Morton, J.M.; Mellor, D.J.; Russell, V.; Chapple, R.S.; Ramp, D. Use of Remote Camera Traps to Evaluate Animal-Based Welfare Indicators in Individual Free-Roaming Wild Horses. Animals 2021, 11, 2101. [Google Scholar] [CrossRef]
- Harvey, A.M.; Meggiolaro, M.N.; Hall, E.; Watts, E.T.; Ramp, D.; Šlapeta, J. Wild horse populations in south-east Australia have a high prevalence of Strongylus vulgaris and may act as a reservoir of infection for domestic horses. Int. J. Parasitol. Parasites Wildl. 2019, 8, 156–163. [Google Scholar] [CrossRef]
- Hockenhull, J.; Whay, H.R. A review of approaches to assessing equine welfare. Equine Vet. Educ. 2014, 26, 159–166. [Google Scholar] [CrossRef]
- Beausoleil, N.J.; Mellor, D.J. Validating indicators of sheep welfare. In Achieving Sustainable Production of Sheep; Greyling, J., Ed.; Burleigh Dodds Science Publishing: London, UK, 2017. [Google Scholar]
- Denton, D.A.; McKinley, M.J.; Farrell, M.; Egan, G.F. The role of primordial emotions in the evolutionary origin of consciousness. Conscious. Cogn. 2009, 18, 500–514. [Google Scholar] [CrossRef] [PubMed]
- Sufit, E.; Houpt, K.A.; Sweeting, M. Physiological stimuli of thirst and drinking patterns in ponies. Equine Vet. J. 1985, 17, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, J.C.; Burn, C.C.; Barr, A.R. Validity of indicators of dehydration in working horses: A longitudinal study of changes in skin tent duration, mucous membrane dryness and drinking behaviour. Equine Vet. J. 2008, 40, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Mullan, S.; Szmaraged, C.; Hotchkiss, I.; Whay, H.R. The welfare of long-line tethered and free-ranging horses kept on public grazing land in South Wales. Anim. Welf. 2014, 23, 25–37. [Google Scholar] [CrossRef]
- Dalla Costa, E.; Murray, L.; Dai, F.; Canali, E.; Minero, M. Equine on-farm welfare assessment: A review of animal-based indicators. Anim Welf. 2014, 23, 323–341. [Google Scholar]
- McKiernan, F.; Houchins, J.A.; Mattes, R.D. Relationships between human thirst, hunger, drinking and feeding. Physiol. Behav. 2008, 94, 700–708. [Google Scholar] [CrossRef]
- Carrol, C.; Huntington, P. Body condition scoring and weight estimation of horses. Equine Vet. J. 1988, 20, 41–45. [Google Scholar] [CrossRef]
- Burkholder, W.J. Use of body condition scores in clinical assessment of the provision of optimal nutrition. J. Am. Vet. Med. Assoc. 2000, 217, 650–654. [Google Scholar] [CrossRef]
- Pritchard, J.C.; Lindberg, A.C.; Main, D.C.J.; Whay, H.R. Assessment of the welfare of working horses, mules and donkeys, using health and behaviour parameters. Prev. Vet. Med. 2005, 69, 265–283. [Google Scholar] [CrossRef]
- Burn, C.; Pritchard, J.; Whay, H. Observer reliability for working equine welfare assessment: Problems with high prevalence’s of certain results. Anim. Welf. 2009, 18, 177–187. [Google Scholar] [CrossRef]
- Burn, C.C.; Dennison, T.L.; Whay, H.R. Relationships between behaviour and health in working horses, donkeys, and mules in developing countries. Appl. Anim. Behav. Sci. 2010, 126, 109–118. [Google Scholar] [CrossRef]
- Henneke, D.R.; Potter, G.D.; Kreider, J.L.; Yeates, B.F. Relationship between condition score, physical measurements and body fat percentage in mares. Equine Vet. J. 1983, 15, 371–372. [Google Scholar] [CrossRef]
- Dugdale, A.H.A.; Grove-White, D.; Curtis, G.C.; Harris, P.A.; Argo, C.M. Body condition scoring as a predictor of body fat in horses and ponies. Vet. J. 2012, 194, 173–178. [Google Scholar] [CrossRef]
- Kirkden, R.D.; Pajor, E.A. Using preference, motivation and aversion tests to ask scientific questions about animals’ feelings. Appl. Anim. Behav. Sci. 2006, 100, 29–47. [Google Scholar] [CrossRef]
- Toates, F.M. Motivational Systems; Cambridge University Press: Cambridge, UK, 1986. [Google Scholar]
- Alexander, G. Cold thermogenesis. Int. Rev. Physiol. Environ. Physiol. III 1979, 20, 43–155. [Google Scholar]
- Cymbaluk, N.F.; Christison, G.I. Environmental effects of thermoregulation and nutrition of horses. Vet. Clin. N. Am. Equine Pract. 1990, 6, 355–372. [Google Scholar] [CrossRef]
- Cymbaluk, N.F. Thermoregulation of horses in cold, winter weather: A review. Livest. Prod. Sci. 1994, 40, 15–71. [Google Scholar] [CrossRef]
- Evans, C.L.; Smith, D.F.G. Sweating responses in the horse. Proc. R. Soc. B 1956, 145, 61–83. [Google Scholar]
- Ashley, F.H.; Waterman-Pearson, A.E.; Whay, H.R. Behavioural assessment of pain in horses and donkeys: Application to clinical practice and future studies. Equine Vet. J. 2005, 37, 565–575. [Google Scholar] [CrossRef]
- Mejdell, C.M.; Bøe, K.E. Responses to climatic variables of horses housed outdoors under Nordic winter conditions. Can. J. Anim. Sci. 2005, 85, 307–308. [Google Scholar] [CrossRef]
- Heleski, C.R.; Murtazashvili, I. Daytime shelter-seeking behavior in domestic horses. J. Vet. Behav. 2010, 5, 276–282. [Google Scholar] [CrossRef]
- Hausberger, M.; Fureix, C.; Lesimple, C. Detecting horses’ sickness: In search of visible signs. Appl. Anim. Behav. Sci. 2016, 175, 41–49. [Google Scholar] [CrossRef]
- IASP. International Association for the Study of Pain. Pain 1983, 16, 109–110. [Google Scholar]
- Guthrie, D.M. Neuroethology: An Introduction; Blackwell Scientific Publications: Oxford, UK, 1980. [Google Scholar]
- Murrell, J.C.; Johnson, C.B. Neurophysiological techniques to assess pain in animals. J. Vet. Pharmacol. Ther. 2006, 29, 325–335. [Google Scholar] [CrossRef]
- Gregory, N.G. Physiology and Behaviour of Animal Suffering; Blackwell Science: Oxford, UK, 2004. [Google Scholar] [CrossRef]
- Mellor, D.J.; Cook, C.J.; Stafford, K.J. Quantifying some responses to pain as a stressor. In The Biology of Animal Stress: Basic Principles and Implications for Animal Welfare; Moberg, G.P., Mench, J.A., Eds.; CABI: Wallingford, UK, 2000; pp. 171–198. [Google Scholar]
- Redua, M.A.; Valadao, C.A.; Duque, J.C.; Balestrero, L.T. The pre-emptive effect of epidural ketamine on wound sensitivity in horses tested by Von Frey filaments. Vet. Anaesth. Analg. 2002, 29, 200–206. [Google Scholar] [CrossRef]
- Olbrich, V.H.; Mosing, M. A comparison of the analgesic effects of caudal epidural methadone and lidocaine in the horse. Vet. Anaesth. Analg. 2003, 30, 156–164. [Google Scholar] [CrossRef]
- Mellor, D.J. Mouth pain in horses: Physiological foundations, behavioural indices, welfare implications, and a suggested solution. Animals 2020, 10, 572. [Google Scholar] [CrossRef]
- Lane, J.G. A review of dental disorders of the horse, their treatment, and possible fresh approaches to management. Equine Vet. Educ. 1994, 6, 13–21. [Google Scholar] [CrossRef]
- Easley, J.K. Dental and oral examination. In Equine Dentistry; Baker, G.J., Easley, J.K., Eds.; W.B Saunders Co.: London, UK, 1999; pp. 114–117. [Google Scholar]
- Graham, B.P. Dental care in the older horse. Vet. Clin. N. Am. Equine Pract. 2002, 18, 509–522. [Google Scholar] [CrossRef]
- Coneglian, M.M.; Borges, T.D.; Weber, S.H.; Bertagnon, H.G.; Michelotto, P.V. Use of the horse grimace scale to identify and quantify pain due to dental disorders in horses. Appl. Anim. Behav. Sci. 2020, 225, 104970. [Google Scholar] [CrossRef]
- Haggard, P.; de Boer, L. Oral somatosensory awareness. Neurosci. Biobehav. Rev. 2014, 47, 469–484. [Google Scholar] [CrossRef]
- Mantyh, P.W. The neurobiology of skeletal pain. Eur. J. Neurosci. 2014, 39, 508–519. [Google Scholar] [CrossRef]
- Dalla Costa, E.; Minero, M.; Lebelt, D.; Stucke, D.; Canali, E.; Leach, M.C. Development of the Horse Grimace Scale (HGS) as a Pain Assessment Tool in Horses Undergoing Routine Castration. PLoS ONE 2014, 9, e92281. [Google Scholar] [CrossRef]
- Gleerup, K.B.; Forkman, B.; Lindegaard, C.; Andersen, P.H. An equine pain face. Vet. Anaesth. Analg. 2015, 42, 103–114. [Google Scholar] [CrossRef]
- Yang, A.Y.; Chow, J.; Liu, J. Corneal innervation and sensation: The eye and beyond. Yale J. Biol. Med. 2018, 91, 13–21. [Google Scholar]
- Brooks, D.E.; Matthews, A.G. Chapter 25: Equine Ophthalmology. In Veterinary Ophthalmology, 4th ed.; Gelatt, K.N., Ed.; Blackwell Pub: Ames, IA, USA, 2007; pp. 1165–1274. [Google Scholar]
- Hood, D.M.; Wagner, I.P.; Taylor, D.D.; Brumbaugh, G.W.; Chaffin, K. Voluntary limb-load distribution in horses with acute and chronic laminitis. Am. J. Vet. Res. 2001, 62, 1393–1398. [Google Scholar] [CrossRef]
- DuPreez, P. Pelvic fractures: Diagnosis and management. In Proceedings of the 41st British Equine Veterinary Association Congress; Equine Veterinary Journal Ltd.: Newmarket, ON, Canada, 2002; pp. 71–72. [Google Scholar]
- Dyson, S. It’s just the way I walk! A review of gait characteristics. In Proceedings of the 41st British Equine Veterinary Association Congress; Equine Veterinary Journal Ltd.: Newmarket, ON, Canada, 2002; p. 69. [Google Scholar]
- Dyson, S.; Marks, D. Foot pain and the elusive diagnosis. Vet. Clin. N. Am. Equine Pract. 2003, 19, 531–565. [Google Scholar] [CrossRef]
- Dyson, S.J.; Murray, R. Pain associated with the sacroiliac joint region: A clinical study of 74 horses. Equine Vet. J. 2003, 35, 240–245. [Google Scholar] [CrossRef]
- Dabareiner, R.M.; Carter, G.K. Diagnosis, treatment and farriery for horses with chronic heel pain. Vet. Clin. N. Am. Equine Pract. 2003, 19, 417–441. [Google Scholar] [CrossRef]
- Hewetson, M. Reproducibility of a visual analogue scale, numerical rating scale and verbal rating scale for the assessment of lameness in the horse. In Proceedings of the 42nd British Equine Veterinary Association Congress; Equine Veterinary Journal Ltd.: Newmarket, ON, Canada, 2003; p. 295. [Google Scholar]
- Bussières, G.; Jacques, C.; Lainay, O.; Beauchamp, G.; Leblond, A.; Cadore, J.L.; Desmaizières, L.-M.; Cuvelliez, S.; Troncy, E. Development of a composite orthopaedic pain scale in horses. Res. Vet. Sci. 2008, 85, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Stashak, T.S. Examination for lameness. In Adams’ Lameness in Horses, 5th ed.; Stashak, T.S., Ed.; Lippincott, Williams and Wilkins: Philadelphia, PA, USA, 2002; pp. 113–183. [Google Scholar]
- Owens, J.G.; Kamerling, S.G.; Stanton, S.R.; Keowen, M.L. Effects of ketoprofen and phenylbutazone on chronic hoof pain and lameness in the horse. Equine Vet. J. 1995, 27, 296–300. [Google Scholar] [CrossRef] [PubMed]
- Graubner, C.; Gerber, V.; Doherr, M.; Spadavecchia, C. Clinical application and reliability of a post abdominal surgery pain assessment scale (PASPAS) in horses. Vet. J. 2011, 188, 178–183. [Google Scholar] [CrossRef] [PubMed]
- De Grauw, J.C.; van Loon, J.P.A.M. Systematic pain assessment in horses. Vet. J. 2016, 209, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Gregory, N.G. Physiological mechanisms causing sickness behaviour and suffering in diseased animals. Anim. Welf. 1998, 7, 293–305. [Google Scholar] [CrossRef]
- Fureix, C.; Menguy, H.; Hausberger, M. Partners with bad temper: Reject or cure? A study of chronic pain and aggression in horses. PLoS ONE 2010, 5, e12434. [Google Scholar] [CrossRef]
- Broom, D.M. Welfare, stress, and the evolution of feelings. Adv. Study Behav. 1998, 27, 371–403. [Google Scholar] [CrossRef]
- Ledger, R.A.; Mellor, D.J. Forensic Use of the Five Domains Model for Assessing Suffering in Cases of Animal Cruelty. Animals 2018, 8, 101. [Google Scholar] [CrossRef]
- Wagner, A.E. Effects of stress on pain in horses and incorporating pain scales for equine practice. Vet. Clin. N. Am. Equine Pract. 2010, 26, 481–492. [Google Scholar] [CrossRef]
- Taylor, P.M.; Pascoe, P.J.; Mama, K.R. Diagnosing and treating pain in the horse; where are we today? Vet. Clin. N. Am. Equine Pract. 2002, 18, 1–19. [Google Scholar] [CrossRef]
- Ransom, J.I.; Cade, B.S. Quantifying equid behavior: A research ethogram for free-roaming feral horses. In U.S. Geological Survey Techniques and Methods Report 2-A9; United States Geological Survey: Reston, VA, USA, 2009. [Google Scholar]
- McDonnell, S.M. The Equid Ethogram: A Practical Field Guide to Horse Behaviour; CAB International: Cambridge, MA, USA, 2003. [Google Scholar]
- Kiley-Worthington, M. The tail of ungulates, canids, and felids with particular reference to their causation and function as displays. Behavior 1976, 56, 69–114. [Google Scholar] [CrossRef]
- Wolff, A.; Hausberger, M.; LeScolan, N. Experimental tests to assess emotionality in horses. Behav. Proc. 1997, 40, 209–221. [Google Scholar] [CrossRef]
- Waring, G. Horse Behavior, 2nd ed.; Noyes Publications: Norwich, NY, USA; William Andrew Publishing: Norwich, NY, USA, 2003. [Google Scholar]
- Torcivia, C.; McDonnell, S. Equine Discomfort Ethogram. Animals 2021, 11, 580. [Google Scholar] [CrossRef]
- Price, J.; Catriona, S.; Welsh, E.M.; Waran, N.K. Preliminary evaluation of a behaviour- based system for assessment of post-operative pain in horses following arthroscopic surgery. Vet. Anaesth. Analg. 2003, 30, 124–137. [Google Scholar] [CrossRef]
- Pritchett, L.C.; Ulibarri, C.; Roberts, M.C.; Schneider, R.K.; Sellon, D.C. Identification of potential physiological and behavioral indicators of postoperative pain in horses after exploratory celiotomy for colic. Appl. Anim. Behav. Sci. 2003, 80, 31–43. [Google Scholar] [CrossRef]
- Viñuela-Fernandez, I.; Jones, E.; Chase-Topping, M.E.; Price, J. Comparison of 1045 subjective scoring systems used to evaluate equine laminitis. Vet. J. 2011, 188, 171–177. [Google Scholar] [CrossRef]
- Mellor, D.J.; Patterson-Kane, E.; Stafford, K.J. Sleep, Sleep Deprivation and the Welfare of Mature Animals. In The Sciences of Animal Welfare; Wiley-Blackwell: Oxford, UK, 2009; pp. 181–185. [Google Scholar]
- Fleming, P. Non-traditional approaches to pain management. Vet. Clin. N. Am. Equine Pract. 2002, 18, 83–105. [Google Scholar] [CrossRef]
- Dyson, S.; Berger, J.M.; Ellis, A.D.; Mullard, J. Can the presence of musculoskeletal pain be determined from the facial expressions of ridden horses (FEReq)? J. Vet. Behav. 2017, 19, 78–89. [Google Scholar] [CrossRef]
- Wemelsfelder, F. The scientific validity of subjective concepts in models of animal welfare. Appl. Anim. Behav. Sci. 1997, 53, 75–88. [Google Scholar] [CrossRef]
- Popescu, S.; Diugan, E.A. The relationship between behavioral and other welfare indicators of working horses. J. Equine Vet. Sci. 2013, 33, 1–12. [Google Scholar] [CrossRef]
- Lesimple, C. Indicators of Horse Welfare: State-of-the-Art. Animals 2020, 10, 294. [Google Scholar] [CrossRef] [PubMed]
- Fureix, C.; Jego, P.; Henry, S.; Lansade, L.; Hausberger, M. Towards an Ethological Animal Model of Depression? A Study on Horses. PLoS ONE 2012, 7, e39280. [Google Scholar] [CrossRef] [PubMed]
- Henneke, D.R.; Potter, G.D.; Kreiger, J.L. Body condition during pregnancy and lactation, and reproductive efficiency of mares. Theriogenology 1984, 21, 897–909. [Google Scholar] [CrossRef]
- De Kloet, E.R.; Melly, S.; Oitzl, M.S.; Schöbitz, B. Cytokines and the brain corticosteroid receptor balance: Relevance to pathophysiology of neuroendocrine-immune communication. Psychoneuroendocrinology 1994, 19, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Beausoleil, N.J.; Mellor, D.J. Introducing breathlessness as a significant animal welfare issue. N. Z. Vet. J. 2015, 63, 44–51. [Google Scholar] [CrossRef]
- Mellor, D.J.; Beausoleil, N.J. Equine welfare during exercise: An evaluation of breathing, breathlessness and bridles. Animals 2017, 7, 41. [Google Scholar] [CrossRef]
- Lansing, R.W.; Gracely, R.H.; Banzett, R.B. The multiple dimensions of dyspnea: Review and hypotheses. Respir. Physiol. Neurobiol. 2009, 167, 53–60. [Google Scholar] [CrossRef]
- Parshall, M.B.; Schwartzstein, R.M.; Adams, L.; Banzett, R.B.; Manning, H.L.; Bourbeau, J.; Calverley, P.M.; Gift, A.G.; Harver, A.; Lareau, S.C.; et al. An official American Thoracic Society statement: Update on the mechanisms, assessment, and management of dyspnea. Am. J. Respir. Crit. Care Med. 2012, 185, 435–452. [Google Scholar] [CrossRef]
- Mellema, M.S. The neurophysiology of dyspnea. J. Vet. Emerg. Crit. Care 2008, 18, 561–571. [Google Scholar] [CrossRef]
- Banzett, R.B.; Pedersen, S.H.; Schwartzstein, R.M.; Lansing, R.W. The affective dimension of laboratory dyspnea: Air hunger is more unpleasant than work/effort. Am. J. Respir. Crit. Care Med. 2008, 177, 1384–1390. [Google Scholar] [CrossRef]
- Gilbert, F.F.; Gofton, N. Terminal dives in mink, muskrat and beaver. Physiol. Behav. 1982, 28, 835–840. [Google Scholar] [CrossRef]
- Nehashi, S.; Nishino, T.; Ide, T. Inhaled furosemide inhibits behavioral response to airway occlusion in anesthetized cats. Anesthesiology 2001, 95, 1234–1237. [Google Scholar] [CrossRef]
- Niel, L.; Weary, D.M. Rats avoid exposure to carbon dioxide and argon. Appl. Anim. Behav. Sci. 2007, 107, 100–109. [Google Scholar] [CrossRef]
- O’Donnell, D.E.; Ora, J.; Webb, K.A.; Laveneziana, P.; Jensen, D. Mechanisms of activity-related dyspnea in pulmonary diseases. Respir. Physiol. Neurobiol. 2009, 167, 116–132. [Google Scholar] [CrossRef]
- Dalmau, A.; Rodriguez, P.; Llonch, P.; Velarde, A. Stunning pigs with different gas mixtures: Aversion in pigs. Anim. Welf. 2010, 19, 325–333. [Google Scholar] [CrossRef]
- Packer, R.M.A.; Hendricks, A.; Burn, C.C. Do dog owners perceive the clinical signs related to conformation inherited disorders as ’normal’ for the breed? A potential constraint to improving canine welfare. Anim. Welf. 2012, 21, 81–93. [Google Scholar] [CrossRef]
- Buchanan, G.F.; Richerson, G.B. Role of chemoreceptors in mediating dyspnea. Respir. Physiol. Neurobiol. 2009, 167, 9–19. [Google Scholar] [CrossRef]
- Davenport, P.W.; Vovk, A. Cortical and subcortical central neural pathways in respiratory sensations. Respir. Physiol. Neurobiol. 2009, 167, 72–86. [Google Scholar] [CrossRef]
- Evans, K.C. Cortico-limbic circuitry and the airways: Insights from functional neuroimaging of respiratory afferents and efferents. Biol. Psychol. 2010, 84, 13–25. [Google Scholar] [CrossRef]
- Banzett, R.B.; Mulnier, H.E.; Murphy, K.; Rosen, S.D.; Wise, R.J.S.; Adams, L. Breathlessness in humans activates insular cortex. Neuroreport 2000, 11, 2117–2120. [Google Scholar] [CrossRef]
- Evans, K.C.; Banzett, R.B.; Adams, L.; McKay, L.; Frackowiak, R.S.; Corfield, D.R. BOLD fMRI identified limbic, paralimbic, and cerebellar activation during air hunger. J. Neurophysiol. 2002, 88, 1500–1511. [Google Scholar] [CrossRef]
- Peiffer, C. Dsypnea and emotion: What can we learn from functional brain imaging? Am. J. Respir. Crit. Care Med. 2008, 177, 937–939. [Google Scholar] [CrossRef] [PubMed]
- von Leupoldt, A.; Sommer, T.; Kegat, S.; Baumann, H.J.; Klose, H.; Dahme, B.; Büchel, C. The unpleasantness of perceived dyspnea is processed in the anterior insula and amygdala. Am. J. Respir. Crit. Care Med. 2008, 177, 1026–1032. [Google Scholar] [CrossRef] [PubMed]
- Mellor, D.J.; Beausoleil, N.J. Ch 5: Moving beyond a problem-based focus on poor welfare towards creating opportunities to have positive welfare experiences. In Mental Health and Well-Being in Animals, 2nd ed.; McMillan, F.D., Ed.; CAB International: Wallingford, UK, 2020; pp. 50–66. [Google Scholar]
- McMillan, F.D. The psychobiology of social pain: Evidence for a neurocognitive overlap with physical pain and welfare implications for social animals with special attention to the domestic dog (Canis familiaris). Physiol. Behav. 2016, 167, 154–171. [Google Scholar] [CrossRef]
- Alexander, R.D. The evolution of social behavior. Annu. Rev. Ecol. Syst. 1974, 5, 325–383. [Google Scholar] [CrossRef]
- Cacioppo, S.; Capitanio, J.P.; Cacioppo, J.T. Toward a neurology of loneliness. Psychol. Bull. 2014, 140, 1464–1504. [Google Scholar] [CrossRef]
- Sondergaard, E.; Jensen, M.B.; Nical, C.J. Motivation for social contact in horses measured by operant conditioning. Appl. Anim. Behav. Sci. 2011, 132, 131–137. [Google Scholar] [CrossRef]
- Schatzmann, U. Winter pasturing of sport horses in Switzerland: An experimental study. Equine Vet. J. Suppl. 1998, 27, 53–54. [Google Scholar]
- Panksepp, J. Affective consciousness: Core emotional feelings in animals and humans. Conscious. Cogn. 2005, 14, 30–80. [Google Scholar] [CrossRef]
- Boissy, A.; Manteuffel, G.; Jensen, M.B.; Moe, R.O.; Spruijt, B.; Keeling, L.J.; Winckler, C.; Forkman, B.; Dimitrov, I.; Langbein, J.; et al. Assessment of positive emotions in animals to improve their welfare. Physiol. Behav. 2007, 92, 375–397. [Google Scholar] [CrossRef]
- Mellor, D.J. Welfare-aligned sentience: Enhanced capacities to experience, interact, anticipate, choose and survive. Animals 2019, 9, 440. [Google Scholar] [CrossRef]
- Mellor, D.J. Positive animal welfare states and promoting environment-focused and animal-animal interactive behaviours. N. Z. Vet. J. 2015, 63, 9–16. [Google Scholar] [CrossRef]
- Mellor, D.J. Enhancing animal welfare by creating opportunities for ‘positive affective engagement’. N. Z. Vet. J. 2015, 63, 3–8. [Google Scholar] [CrossRef]
- Fraser, D.; Duncan, I.J.H. ‘Pleasures’, ‘pains’ and animal welfare; towards a natural history of affect. Anim. Welf. 1998, 7, 383–396. [Google Scholar] [CrossRef]
- Ikemoto, S.; Panksepp, J. The role of the nucleus accumbens dopamine in motivated behaviour: A unifying interpretation with special reference to reward-seeking. Brain Res. Rev. 1999, 31, 6–41. [Google Scholar] [CrossRef]
- Spinka, M.; Newberry, R.C.; Bekoff, M. Mammalian play: Training for the unexpected. Q. Rev. Biol. 2001, 76, 141–168. [Google Scholar] [CrossRef]
- Scherer, K.R.; Dan, E.S.; Flykt, A. What determines a feeling’s position in affective space? A case for appraisal. Cogn. Emot. 2006, 20, 92–113. [Google Scholar] [CrossRef]
- Yeates, J.W.; Main, D.C.J. Assessment of positive welfare: A review. Vet. J. 2008, 175, 293–300. [Google Scholar] [CrossRef]
- Held, S.D.E.; Spinka, M. Animal play and animal welfare. Anim. Behav. 2011, 81, 891–899. [Google Scholar] [CrossRef]
- Grill, H.J.; Norgren, R. The taste reactivity test I. Mimetic responses to gustatory stimuli in neurologically normal rats. Brain Res. 1978, 143, 263–279. [Google Scholar] [CrossRef]
- Berridge, K.C. Food reward: Brain substrates of wanting and liking. Neurosci. Biobehav. Rev. 1996, 20, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Deag, J.M. Behavioural ecology and the welfare of extensively farmed animals. Appl. Anim. Behav. Sci. 1996, 49, 9–22. [Google Scholar] [CrossRef]
- Steiner, J.E.; Glaser, D.; Hawilo, M.E.; Berridge, K.C. Comparative expression of hedonic impact: Affective reactions to taste by human infants and other primates. Neurosci. Biobehav. Rev. 2001, 25, 53–74. [Google Scholar] [CrossRef] [PubMed]
- Kelley, A.E.; Baldo, B.A.; Pratt, W.E.; Will, M.J. Corticostriatal- hypothalamic circuitry and food motivation: Integration of energy, action and reward. Physiol. Behav. 2005, 86, 773–795. [Google Scholar] [CrossRef]
- Balcombe, J.P. Animal pleasure and its moral significance. Appl. Anim. Behav. Sci. 2009, 118, 208–216. [Google Scholar] [CrossRef]
- Ninomiya, S.; Sato, S.; Kusunose, R.; Mitumasu, T.; Obara, Y. A note on a behavioural indicator of satisfaction in stabled horses. Appl. Anim. Behav. Sci. 2007, 106, 184–189. [Google Scholar] [CrossRef]
- Ninomiya, S.; Kusunose, R.; Obara, Y.; Sato, S. Effect of an open window and conspecifics within view on the welfare of stabled horses, estimated on the basis of positive and negative behavioural indicators. Anim. Welf. 2008, 17, 351–354. [Google Scholar] [CrossRef]
- Cabanac, M. The experience of pleasure in animals. In Mental Health and Well-Being in Animals; McMillan, F.D., Ed.; Blackwell: Clive, IA, USA, 2005; pp. 29–46. [Google Scholar]
- Freire, R.; Buckley, P.; Cooper, J.J. Effects of different forms of exercise on post inhibitory rebound and unwanted behaviour in stabled horses. Equine Vet. J. 2009, 41, 487–492. [Google Scholar] [CrossRef]
- Chaplin, S.J.; Gretgrix, L. Effect of housing conditions on activity and lying behaviour of horses. Animals 2010, 4, 792–795. [Google Scholar] [CrossRef]
- Špinka, M. Animal agency, animal awareness and animal welfare. Anim. Welf. 2019, 28, 11–20. [Google Scholar] [CrossRef]
- Spinka, M.; Wemelsfelder, F. Environmental challenge and animal agency. In Animal Welfare, 2nd ed.; Appleby, M.C., Mench, J.A., Olsson, I.A.S., Hughes, B.O., Eds.; CAB International: Wallingford, UK, 2011; pp. 27–43. [Google Scholar]
- Panksepp, J.; Zellner, M.R. Towards a neurologically based unified theory of aggression. Int. Rev. Soc. Psychol. 2004, 17, 37–61. [Google Scholar]
- Nelson, E.; Panksepp, J. Brain substrates of infant–mother attachment: Contributions of opioids, oxytocin, and norepinepherine. Neurosci. Biobehav. Rev. 1998, 22, 437–452. [Google Scholar] [CrossRef]
- Carter, C.S.; Keverne, E.B. The neurobiology of social affiliation and pair bonding. Horm. Brain Behav. 2002, 1, 299–337. [Google Scholar]
- Lim, M.M.; Young, L.J. Neuropeptidergic regulation of affiliative behavior and social bonding in animals. Horm. Behav. 2006, 50, 506–517. [Google Scholar] [CrossRef]
- Pfaff, D.W. Drive: Neurobiological and Molecular Mechanisms of Sexual Behavior; MIT Press: Cambridge, MA, USA, 1999. [Google Scholar]
- Fisher, H.E.; Aron, A.; Brown, L.L. Romantic love: A mammalian brain system for mate choice. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2006, 361, 2173–2186. [Google Scholar] [CrossRef]
- Panksepp, J. Emotional endophenotypes in evolutionary psychiatry. Prog. Neuropsychopharmacol. Biol. Psychiatry 2006, 30, 774–784. [Google Scholar] [CrossRef]
- Vanderschuren, L.; Niesnik, R.; Van Ree, J. The neurobiology of social play behavior in rats. Neurosci. Biobehav. Rev. 1997, 3, 309–326. [Google Scholar] [CrossRef]
- Burgdorf, J.; Panksepp, J. The neurobiology of positive emotions. Neurosci. Biobehav. Rev. 2006, 30, 173–187. [Google Scholar] [CrossRef]
- Mellor, D.J. Positive welfare states and reference standards for welfare assessment. N. Z. Vet. J. 2015, 63, 17–23. [Google Scholar] [CrossRef]
- McMillan, F.D. Mental health and well-being benefits of social contact and social support in animals. In Mental Health and Well-Being in Animals, 2nd ed.; McMillan, F.D., Ed.; CAB International: Wallingford, UK, 2019; pp. 96–110. [Google Scholar]
- Hausberger, M.; Lerch, N.; Guilbaud, E.; Stomp, M.; Grandgeorge, M.; Henry, S.; Lesimple, C. On-farm welfare assessment of horses: The risks of putting the cart before the horse. Animals 2020, 10, 371. [Google Scholar] [CrossRef]
- Harley, J.J.; Stack, J.D.; Braid, H.; McLennan, K.M.; Stanley, C.R. Evaluation of the Feasibility, Reliability, and Repeatability of Welfare Indicators in Free-Roaming Horses: A Pilot Study. Animals 2021, 11, 1981. [Google Scholar] [CrossRef] [PubMed]
- Harvey, A.M. Wild Horse Welfare: Assessment and Associations with Population and Behavioural Ecology. Ph.D. Thesis, University of Technology Sydney, Sydney, NSW, Australia, 2022. [Google Scholar]












|
| Domain | Welfare Status Indicators | Quasi-Welfare Status Indicators | Welfare-Alerting Indicators |
|---|---|---|---|
| Body condition score | Foraging and water availability (quality and quantity) * | Foraging and water availability (quality and quantity) Reproductive status |
| Sweating Shivering | Ambient temperature and other weather conditions (e.g., humidity, sun exposure, wind, rain) in combination with shelter/shade * | Ambient temperature |
| Other weather conditions (e.g., humidity, sun exposure, wind, rain, snow, hail) | |||
| Shelter/shade | |||
| Body condition score | |||
| Wounds | Body condition score | |
| Quidding/food pouching | Hoof condition | ||
| Blepharospasm | Coat condition | ||
| Lameness | Skin lesions | ||
| Body posture | Nasal discharge | ||
| Facial grimace | Ocular discharge | ||
| Weakness | Faecal egg count | ||
| Qualitative assessment of behaviour (relaxed, alert, dull, apathetic) | Limb pathology | ||
| Body condition score | |||
| Respiratory rate and effort | |||
| Close spatial proximity with other horses and other affiliative interactions | Aspects of population dynamics and social organisation |
| Domain | Welfare Compromise (Status and Quasi-Status) Indicator | Potential Physical/Functional Impacts (Domains 1–4) | Potential Negative Mental Experience Inferred (Domain 5) |
|---|---|---|---|
| Domain 1: Nutrition | Low body condition score (±Food-seeking behaviour) | Long-term energy deficit | Long-term hunger Physical exhaustion |
| * Low food availability | Short-term energy deficit | Short-term hunger | |
| * Water-seeking behaviour/water availability | Dehydration | Thirst | |
| Domain 2: Physical Environment | Sweating | Hyperthermia | Heat discomfort |
| * Ambient temperature above higher critical temperature ± low water availability ± humidity, ±insufficient shade | |||
| Shivering | Hypothermia | Cold discomfort | |
| * Ambient temperature below lower critical temperature, ±rainfall/wind, ±lack of shelter, ±low food availability and other factors impacting thermoregulation (e.g., very low body condition, wet coat) | |||
| Domain 3: Health | Wounds | Disruption of integument/muscle/joints or bones | Skin/muscle/orthopaedic pain |
| Quidding/food pouching | Oral cavity pathology | Mouth pain | |
| Blepharospasm | Ocular pathology | Ocular pain | |
| Lameness | Limb pathology and consequent impaired ambulatory ability | Limb pain | |
| Head-lower-than-withers body posture | Impaired musculoskeletal activity | Malaise, pain, exhaustion | |
| Facial grimace | N/A | Any pain | |
| Weakness | Impaired musculoskeletal activity | Malaise, exhaustion | |
| Reduced alertness, dull, apathetic | Systemic pathology, metabolic disturbances | Malaise, pain, exhaustion | |
| Very thin/emaciated body condition | Muscular weakness, metabolic disturbances | Malaise, exhaustion | |
| Increased respiratory rate +/or effort | Respiratory dysfunction/ impairment | Breathlessness | |
| Domain 4: Behavioural Interactions | Isolation from other horses, no or minimal affiliative interactions | Social isolation | Loneliness, insecurity, anxiety |
| Step 1: Validation of the links between observed indicators and physical/functional impacts (Domains 1–4) | Scientific understanding of the pathophysiology and aetiology of disease |
| Scientific understanding of the mechanisms related to deficiency, dysfunction, disruption, or homeostatic imbalance | |
| Absence of elimination of the indicator using a method known to prevent or remove the underlying causative process (i.e., physical/functional impact) | |
| Coherence between multiple indicators in different modalities (e.g., behavioural, physiological) measured in the same situation | |
| Step 2: Validation of the links between physical/functional impacts (Domains 1–4) and mental experiences (Domain 5) | Scientific understanding of the neurophysiological mechanisms underlying the mental experience in species with similar neurological capacity (i.e., affective neuroscience evidence) |
| Comparison with mental experiences reported by humans in similar situations or with similar physical/functional impacts | |
| Elimination or reduction in a mental experience reported by humans using a method known to prevent or alleviate the physical/functional impact |
| Domain | Opportunity (Resource-Based Measure) | Utilisation (Animal-Based Observation) | Potential Positive Mental Experience Inferred (Domain 5) |
|---|---|---|---|
| 1. Nutrition | Sources of good-quality water readily available | Able to access and utilise water sources | Oral wetting and quenching pleasures |
| Range of quality vegetation available | Observed foraging range of vegetation | Rewarding engagement of foraging, pleasures of eating a variety of food, satiety, gastrointestinal comfort | |
| 2. Physical environment | Appropriate ambient temperatures for thermal comfort, available shelter and shade Radiant sun, e.g., after a cool night. Available water source for immersion on a hot day | Utilising available shelter and shade Observed resting under radiant sun on a cool day, or immersing in water on a hot day | Thermal comfort Pleasures of radiant heat Cooling effects of immersion in cool water |
| 3. Health | Injury free, good health and physical fitness | Observed actively engaging with environment at a range of gaits, alert to surroundings | Vitality of fitness |
| 4. Behavioural interactions | Multiple con-specifics, both sexes, range of ages | Observed engaging in a range of affiliative social interactions on a regular basis | Affectionately sociable, maternally rewarded, contented, sense of security, social comfort |
| Domain | Welfare Alerting Indicator | Alerts to the Risk of Future Physical/Functional Impacts (Domains 1–4) |
|---|---|---|
| Food availability | Energy/nutritional deficit |
| Water-seeking behaviour/water availability | Dehydration | |
| Sweating | Dehydration if combined with lack of water |
| Very low body condition score | Hypothermia if combined with low ambient temperatures | |
| Low ambient temperature, heavy rain, inadequate shelter | Hypothermia | |
| High ambient temperature and humidity, inadequate shade | Hyperthermia | |
| Very low body condition score | Illness, reduced fertility, weakness, exhaustion |
| Poor coat condition | Indicators of illness | |
| Poor hoof condition | Impaired ambulatory ability | |
| High faecal egg count | Gastrointestinal pathology | |
| Positive S. vulgaris PCR | ||
| Small band size Low reproductive rate | Social isolation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harvey, A.M.; Beausoleil, N.J.; Ramp, D.; Mellor, D.J. Mental Experiences in Wild Animals: Scientifically Validating Measurable Welfare Indicators in Free-Roaming Horses. Animals 2023, 13, 1507. https://doi.org/10.3390/ani13091507
Harvey AM, Beausoleil NJ, Ramp D, Mellor DJ. Mental Experiences in Wild Animals: Scientifically Validating Measurable Welfare Indicators in Free-Roaming Horses. Animals. 2023; 13(9):1507. https://doi.org/10.3390/ani13091507
Chicago/Turabian StyleHarvey, Andrea M., Ngaio J. Beausoleil, Daniel Ramp, and David J. Mellor. 2023. "Mental Experiences in Wild Animals: Scientifically Validating Measurable Welfare Indicators in Free-Roaming Horses" Animals 13, no. 9: 1507. https://doi.org/10.3390/ani13091507
APA StyleHarvey, A. M., Beausoleil, N. J., Ramp, D., & Mellor, D. J. (2023). Mental Experiences in Wild Animals: Scientifically Validating Measurable Welfare Indicators in Free-Roaming Horses. Animals, 13(9), 1507. https://doi.org/10.3390/ani13091507






