RNA Sequencing (RNA-Seq) Analysis Reveals Liver Lipid Metabolism Divergent Adaptive Response to Low- and High-Salinity Stress in Spotted Scat (Scatophagus argus)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Salinity Stress, Fish Sampling, and Ethics Statement
2.2. RNA Extraction, Complementary DNA (cDNA) Library Construction, and Sequencing
2.3. Differential Gene Expression Analysis and Functional Enrichment
2.4. Data Validation and Expression of Genes Related to Lipid Metabolism
2.5. RT-qPCR and Statistical Analysis
3. Results
3.1. Illumina Sequencing and Reads Mapping
3.2. Differential Expression Analysis
3.3. RNA-Seq Validation
3.4. KEGG Enrichment Analysis
3.5. Expression Levels of Lipid Metabolism-Related Genes
4. Discussion
4.1. Steroid Biosynthesis
4.2. Steroid Hormone Biosynthesis
4.3. Glycerophospholipid Metabolism
4.4. Biosynthesis of Unsaturated Fatty Acids
4.5. Adipocytokine Signaling Pathway
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sørensen, S.R.; Butts, I.A.E.; Munk, P.; Tomkiewicz, J. Effects of salinity and sea salt type on egg activation, fertilization, buoyancy and early embryology of European eel, Anguilla anguilla. Zygote 2016, 24, 121–138. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Liu, J.; Lu, Z.; Xu, L.; Ji, C.; Wang, Q.; Zhao, J. Metabolite and gene expression responses in juvenile flounder Paralichthys olivaceus exposed to reduced salinities. Fish Shellfish Immunol. 2017, 63, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.; Lui, E.; Li, Z.; Cai, S.; Sung, W.; Mathavan, S.; Lam, T.; Ip, Y. Differential transcriptomic analyses revealed genes and signaling pathways involved in iono-osmoregulation and cellular remodeling in the gills of euryhaline Mozambique tilapia, Oreochromis mossambicus. BMC Genom. 2014, 15, 921. [Google Scholar] [CrossRef] [PubMed]
- Bozza, D.C.; Freire, C.A.; Prodocimo, V. Osmo-ionic regulation and carbonic anhydrase, Na+/K+-ATPase and V-H+-ATPase activities in gills of the ancient freshwater crustacean Aegla schmitti (Anomura) exposed to high salinities. Comp. Biochem. Physiol. Part A 2019, 231, 201–208. [Google Scholar] [CrossRef]
- Kokou, F.; Con, P.; Barki, A.; Nitzan, T.; Slosman, T.; Mizrahi, I.; Cnaani, A. Short- and long-term low-salinity acclimation effects on the branchial and intestinal gene expression in the European seabass (Dicentrarchus labrax). Comp. Biochem. Physiol. Part A 2019, 231, 11–18. [Google Scholar] [CrossRef]
- Ahmmed, M.K.; Ahmmed, F.; Kabir, K.A.; Faisal, M.; Ahmed, S.I.; Ahsan, M.N. Biochemical impacts of salinity on the catfish, Heteropneustes fossilis (Bloch, 1794), and possibility of their farming at low saline water. Aquac. Res. 2017, 48, 4251–4261. [Google Scholar] [CrossRef]
- Guo, T.; Yang, Y.; Meng, F.; Wang, S.; Xia, S.; Qian, Y.; Li, M.; Wang, R. Effects of low salinity on gill and liver glycogen metabolism of great blue-spotted mudskippers (Boleophthalmus pectinirostris). Comp. Biochem. Physiol. Part C 2020, 230, 108709. [Google Scholar] [CrossRef]
- Larsen, E.H.; Deaton, L.E.; Onken, H.; O’Donnell, M.; Grosell, M.; Dantzler, W.H.; Weihrauch, D. Osmoregulation and excretion. Compr. Physiol. 2011, 4, 405–573. [Google Scholar] [CrossRef]
- Fettiplace, R.; Haydon, D.A. Water permeability of lipid membranes. Physiol. Rev. 1980, 60, 510–550. [Google Scholar] [CrossRef]
- Frallicciardi, J.; Melcr, J.; Siginou, P.; Marrink, S.J.; Poolman, B. Membrane thickness, lipid phase and sterol type are determining factors in the permeability of membranes to small solutes. Nat. Commun. 2022, 13, 1605. [Google Scholar] [CrossRef]
- Si, Y.; Wen, H.; Li, Y.; He, F.; Li, J.; Li, S.; He, H. Liver transcriptome analysis reveals extensive transcriptional plasticity during acclimation to low salinity in Cynoglossus semilaevis. BMC Genom. 2018, 19, 464. [Google Scholar] [CrossRef]
- Tseng, Y.; Hwang, P. Some insights into energy metabolism for osmoregulation in fish. Comp. Biochem. Physiol. Part C 2008, 148, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Sangiao-Alvarellos, S.; Laiz-Carrión, R.; Guzmán, J.M.; Martiín Del Riío, M.P.; Miguez, J.M.; Mancera, J.M.; Soengas, J.L. Acclimation of S. aurata to various salinities alters energy metabolism of osmoregulatory and nonosmoregulatory organs. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 285, R897–R907. [Google Scholar] [CrossRef] [PubMed]
- Sangiao-Alvarellos, S.; Arjona, F.J.; Del Río, M.P.M.; Míguez, J.M.; Mancera, J.M.; Soengas, J.L. Time course of osmoregulatory and metabolic changes during osmotic acclimation in Sparus auratus. J. Exp. Biol. 2005, 208, 4291–4304. [Google Scholar] [CrossRef]
- Ronkin, D.; Seroussi, E.; Nitzan, T.; Doron-Faigenboim, A.; Cnaani, A. Intestinal transcriptome analysis revealed differential salinity adaptation between two tilapiine species. Comp. Biochem. Physiol. Part D 2015, 13, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.H.; Piermarini, P.M.; Choe, K.P. The multifunctional fish gill: Dominant site of gas exchange, osmoregulation, acid-base regulation, and excretion of nitrogenous waste. Physiol. Rev. 2005, 85, 97–177. [Google Scholar] [CrossRef]
- Gupta, S. An overview on morphology, biology, and culture of spotted scat Scatophagus argus (Linnaeus 1766). Rev. Fish. Sci. Aquac. 2016, 24, 203–212. [Google Scholar] [CrossRef]
- Su, M.; Duan, Z.; Shi, H.; Zhang, J. The effects of salinity on reproductive development and egg and larvae survival in the spotted scat Scatophagus argus under controlled conditions. Aquac. Res. 2019, 50, 1782–1794. [Google Scholar] [CrossRef]
- Mookkan, M.; Muniyandi, K.; Rengasamy, T.A.; Premkumar. Influence of salinity on survival and growth of early juveniles of Spotted scat Scatophagus argus (Linnaeus, 1766). Indian J. Innov. Dev. 2014, 3, 23–29. [Google Scholar]
- Assan, D.; Huang, Y.; Mustapha, U.F.; Addah, M.N.; Li, G.; Chen, H. Fish Feed Intake, Feeding Behavior, and the Physiological Response of Apelin to Fasting and Refeeding. Front. Endocrinol. 2021, 12, 798903. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−ΔΔ C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, R.J. The physiology of hyper-salinity tolerance in teleost fish: A review. J. Comp. Physiol. B 2012, 182, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Harshini, V.; Shukla, N.; Raval, I.; Kumar, S.; Shrivastava, V.; Patel, A.K.; Joshi, C.G. Kidney transcriptome response to salinity adaptation in Labeo rohita. Front. Physiol. 2022, 13, 991366. [Google Scholar] [CrossRef]
- Xu, Z.; Gan, L.; Li, T.; Xu, C.; Chen, K.; Wang, X.; Qin, J.G.; Chen, L.; Li, E. Transcriptome Profiling and Molecular Pathway Analysis of Genes in Association with Salinity Adaptation in Nile Tilapia Oreochromis niloticus. PLoS ONE 2015, 10, e0136506. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.; Stevenson, J.; Kristiana, I.; Brown, A.J. Cholesterol-dependent degradation of squalene monooxygenase, a control point in cholesterol synthesis beyond HMG-CoA reductase. Cell Metab. 2011, 13, 260–273. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, Y.; Aoyama, Y.; Noshiro, M.; Gotoh, O. Sterol 14-demethylase P450 (CYP51) provides a breakthrough for the discussion on the evolution of cytochrome P450 gene superfamily. Biochem. Biophys. Res. Commun. 2000, 273, 799–804. [Google Scholar] [CrossRef]
- Nes, W.D. Biosynthesis of cholesterol and other sterols. Chem. Rev. 2011, 111, 6423–6451. [Google Scholar] [CrossRef] [PubMed]
- Luu, W.; Hart-Smith, G.; Sharpe, L.J.; Brown, A.J. The terminal enzymes of cholesterol synthesis, DHCR24 and DHCR7, interact physically and functionally[S]. J. Lipid Res. 2015, 56, 888–897. [Google Scholar] [CrossRef]
- Fahy, E.; Subramaniam, S.; Murphy, R.C.; Nishijima, M.; Raetz, C.R.H.; Shimizu, T.; Spener, F.; van Meer, G.; Wakelam, M.J.O.; Dennis, E.A. Update of the LIPID MAPS comprehensive classification system for lipids1. J. Lipid Res. 2009, 50, S9–S14. [Google Scholar] [CrossRef]
- Ranasinghe, N.; Lin, C.; Lee, T. Cholesterol accumulation in livers of Indian medaka, Oryzias dancena, acclimated to fresh water and seawater. Front. Mar. Sci. 2022, 9, 891706. [Google Scholar] [CrossRef]
- Luke, A.R.; Davis, D.A.; Saoud, I.P. Effects of lecithin and cholesterol supplementation to practical diets for Litopenaeus vannamei reared in low salinity waters. Aquaculture 2006, 257, 446–452. [Google Scholar] [CrossRef]
- Itäaho, K.; Mackenzie, P.I.; Ikushiro, S.; Miners, J.O.; Finel, M. The configuration of the 17-hydroxy group variably influences the glucuronidation of β-estradiol and epiestradiol by human UDP-glucuronosyltransferases. Drug Metab. Dispos. 2008, 36, 2307–2315. [Google Scholar] [CrossRef] [PubMed]
- Sneitz, N.; Vahermo, M.; Mosorin, J.; Laakkonen, L.; Poirier, D.; Finel, M. Regiospecificity and stereospecificity of human UDP-glucuronosyltransferases in the glucuronidation of estriol, 16-epiestriol, 17-epiestriol, and 13-epiestradiol. Drug Metab. Dispos. 2013, 41, 582–591. [Google Scholar] [CrossRef] [PubMed]
- Udomuksorn, W.; Elliot, D.J.; Lewis, B.C.; Mackenzie, P.I.; Yoovathaworn, K.; Miners, J.O. Influence of mutations associated with Gilbert and Crigler–Najjar type II syndromes on the glucuronidation kinetics of bilirubin and other UDP-glucuronosyltransferase 1A substrates. Pharmacogenet. Genom. 2007, 17, 1017–1029. [Google Scholar] [CrossRef]
- Schults, M.A.; Sanen, K.; Godschalk, R.W.; Theys, J.; van Schooten, F.J.; Chiu, R.K. Hypoxia diminishes the detoxification of the environmental mutagen benzo [a] pyrene. Mutagenesis 2014, 29, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Zheng, L.; Deng, T.; Li, C.; He, Y.; Li, H.; Li, P. Stilbene glucoside inhibits the glucuronidation of emodin in rats through the down-regulation of UDP-glucuronosyltransferases 1A8: Application to a drug–drug interaction study in Radix Polygoni Multiflori. J. Ethnopharmacol. 2013, 147, 335–340. [Google Scholar] [CrossRef]
- Xu, J.; Kulkarni, S.R.; Li, L.; Slitt, A.L. UDP-glucuronosyltransferase expression in mouse liver is increased in obesity- and fasting-induced steatosis. Drug Metab. Dispos. 2012, 40, 259–266. [Google Scholar] [CrossRef]
- van Meer, G.; Voelker, D.R.; Feigenson, G.W. Membrane lipids: Where they are and how they behave. Nat. Rev. Mol. Cell Biol. 2008, 9, 112–124. [Google Scholar] [CrossRef] [PubMed]
- Uyama, T.; Ichi, I.; Kono, N.; Inoue, A.; Tsuboi, K.; Jin, X.; Araki, N.; Aoki, J.; Arai, H.; Ueda, N. Regulation of peroxisomal lipid metabolism by catalytic activity of tumor suppressor H-rev107. J. Biol. Chem. 2012, 287, 2706–2718. [Google Scholar] [CrossRef]
- Wang, B.; Tontonoz, P. Phospholipid Remodeling in Physiology and Disease. Annu. Rev. Physiol. 2019, 81, 165–188. [Google Scholar] [CrossRef]
- Gibellini, F.; Smith, T.K. The Kennedy pathway-De novo synthesis of phosphatidylethanolamine and phosphatidylcholine. IUBMB Life 2010, 62, 414–428. [Google Scholar] [CrossRef] [PubMed]
- Tomohiro, S.; Kawaguti, A.; Kawabe, Y.; Kitada, S.; Kuge, O. Purification and characterization of human phosphatidylserine synthases 1 and 2. Biochem. J. 2009, 418, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yu, C.; Zhuang, J.; Qi, W.; Jiang, J.; Liu, X.; Zhao, W.; Cao, Y.; Wu, H.; Qi, J.; et al. The role of phosphatidylserine on the membrane in immunity and blood coagulation. Biomark. Res. 2022, 10, 4. [Google Scholar] [CrossRef]
- Goren, M.A.; Fox, B.G. Wheat germ cell-free translation, purification, and assembly of a functional human stearoyl-CoA desaturase complex. Protein Expr. Purif. 2008, 62, 171–178. [Google Scholar] [CrossRef]
- Lebovitz, H.E. Insulin resistance: Definition and consequences. Exp. Clin. Endocrinol. Diabet. 2001, 109 (Suppl. S2), S135. [Google Scholar] [CrossRef]
- Song, H.; Yang, R.; Zhang, J.; Sun, P.; Xing, X.; Wang, L.; Sairijima, T.; Hu, Y.; Liu, Y.; Cheng, H.; et al. Oleic acid-induced steatosis model establishment in LMH cells and its effect on lipid metabolism. Poult. Sci. 2022, 102, 102297. [Google Scholar] [CrossRef]
- Pettem, C.M.; Briens, J.M.; Janz, D.M.; Weber, L.P. Cardiometabolic response of juvenile rainbow trout exposed to dietary selenomethionine. Aquat. Toxicol. 2018, 198, 175–189. [Google Scholar] [CrossRef]
- Rossi, A.; Bacchetta, C.; Cazenave, J. Effect of thermal stress on metabolic and oxidative stress biomarkers of Hoplosternum littorale (Teleostei, Callichthyidae). Ecol. Indic. 2017, 79, 361–370. [Google Scholar] [CrossRef]
- Rossi, A.; Cazenave, J.; Bacchetta, C.; Campana, M.; Parma, M.J. Physiological and metabolic adjustments of Hoplosternum littorale (Teleostei, Callichthyidae) during starvation. Ecol. Indic. 2015, 56, 161–170. [Google Scholar] [CrossRef]
- Tallima, H.; El Ridi, R. Arachidonic acid: Physiological roles and potential health benefits—A review. J. Adv. Res. 2018, 11, 33–41. [Google Scholar] [CrossRef]
- Procaccini, C.; De Rosa, V.; Galgani, M.; Carbone, F.; La Rocca, C.; Formisano, L.; Matarese, G. Role of adipokines signaling in the modulation of T cells function. Front. Immunol. 2013, 4, 332. [Google Scholar] [CrossRef]
- Tanabe, H.; Fujii, Y.; Okada-Iwabu, M.; Iwabu, M.; Nakamura, Y.; Hosaka, T.; Motoyama, K.; Ikeda, M.; Wakiyama, M.; Terada, T.; et al. Crystal structures of the human adiponectin receptors. Nature 2015, 520, 312–316. [Google Scholar] [CrossRef]
- Ghoshal, K.; Bhattacharyya, M. Adiponectin: Probe of the molecular paradigm associating diabetes and obesity. World J. Diabetes 2015, 6, 151. [Google Scholar] [CrossRef] [PubMed]
- Pi, D.; Wang, J.; Zhao, M.; Liu, M.; Zhang, Y.; Qin, C.; Yang, L.; Yan, X.; Nie, G. Adiponectin and adiponectin receptors in common carp (Cyprinus carpio): Tissue distribution and their expressions in response to high-carbohydrate and high-lipid diets. Aquacult. Rep. 2022, 27, 101341. [Google Scholar] [CrossRef]
- Huang, L.; Cheng, Y.; Huang, K.; Zhou, Y.; Ma, Y.; Zhang, M. Ameliorative effect of Sedum sarmentosum Bunge extract on Tilapia fatty liver via the PPAR and P53 signaling pathway. Sci. Rep. 2018, 8, 8456. [Google Scholar] [CrossRef]
- Chou, J.Y.; Mansfield, B.C. Mutations in the glucose-6-phosphatase-α (G6PC) gene that cause type Ia glycogen storage disease. Hum. Mutat. 2008, 29, 921–930. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.R.; Yuan, H.; Holyoak, T.; Arajs, K.H.; Hakimi, P.; Markello, T.C.; Wolfe, L.A.; Vilboux, T.; Burton, B.K.; Fajardo, K.F. Three rare diseases in one Sib pair: RAI1, PCK1, GRIN2B mutations associated with Smith–Magenis Syndrome, cytosolic PEPCK deficiency and NMDA receptor glutamate insensitivity. Mol. Genet. Metab. 2014, 113, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Latorre-Muro, P.; Baeza, J.; Armstrong, E.A.; Hurtado-Guerrero, R.; Corzana, F.; Wu, L.E.; Sinclair, D.A.; López-Buesa, P.; Carrodeguas, J.A.; Denu, J.M. Dynamic acetylation of phosphoenolpyruvate carboxykinase toggles enzyme activity between gluconeogenic and anaplerotic reactions. Mol. Cell 2018, 71, 718–732.e9. [Google Scholar] [CrossRef]
- Valeri, M.; Raffatellu, M. Cytokines IL-17 and IL-22 in the host response to infection. Pathog. Dis. 2016, 74, ftw111. [Google Scholar] [CrossRef] [PubMed]
- Qiao, R.; Xie, Y.; Liu, Y.; Gao, S.; Li, X.; Li, J.; Zhu, P.; Wei, Y.; Qi, Z. Molecular characterization and expression analysis of suppressors of cytokine signalling from golden pompano (Trachinotus ovatus). Aquac. Res. 2021, 52, 6087–6097. [Google Scholar] [CrossRef]
- Frobøse, H.; Groth Rønn, S.; Heding, P.E.; Mendoza, H.; Cohen, P.; Mandrup-Poulsen, T.; Billestrup, N. Suppressor of cytokine signaling-3 inhibits interleukin-1 signaling by targeting the TRAF-6/TAK1 complex. Mol. Endocrinol. 2006, 20, 1587–1596. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; He, A.; Chen, L.; Limbu, S.M.; Wang, Y.; Zhang, M.; Du, Z. Molecular characterization and immune response to lipopolysaccharide (LPS) of the suppressor of cytokine signaling (SOCS)-1, 2 and 3 genes in Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2016, 50, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Zhang, Y.; Yang, L.; Yang, X.; Wu, Y.; Liu, D.; Chen, W.; Weng, S.; Yu, X.; He, J. The JAK and STAT family members of the mandarin fish Siniperca chuatsi: Molecular cloning, tissues distribution and immunobiological activity. Fish Shellfish Immunol. 2009, 27, 349–359. [Google Scholar] [CrossRef]
- Srivastava, V.; Vashishta, M.; Gupta, S.; Singla, R.; Singla, N.; Behera, D.; Natarajan, K. Suppressors of cytokine signaling inhibit effector T cell responses during Mycobacterium tuberculosis Infection. Immunol. Cell Biol. 2011, 89, 786–791. [Google Scholar] [CrossRef]
- Weiss, D.J.; Evanson, O.A.; Souza, C.D. Expression of interleukin-10 and suppressor of cytokine signaling-3 associated with susceptibility of cattle to infection with Mycobacterium avium subsp paratuberculosis. Am. J. Vet. Res. 2005, 66, 1114. [Google Scholar] [CrossRef]

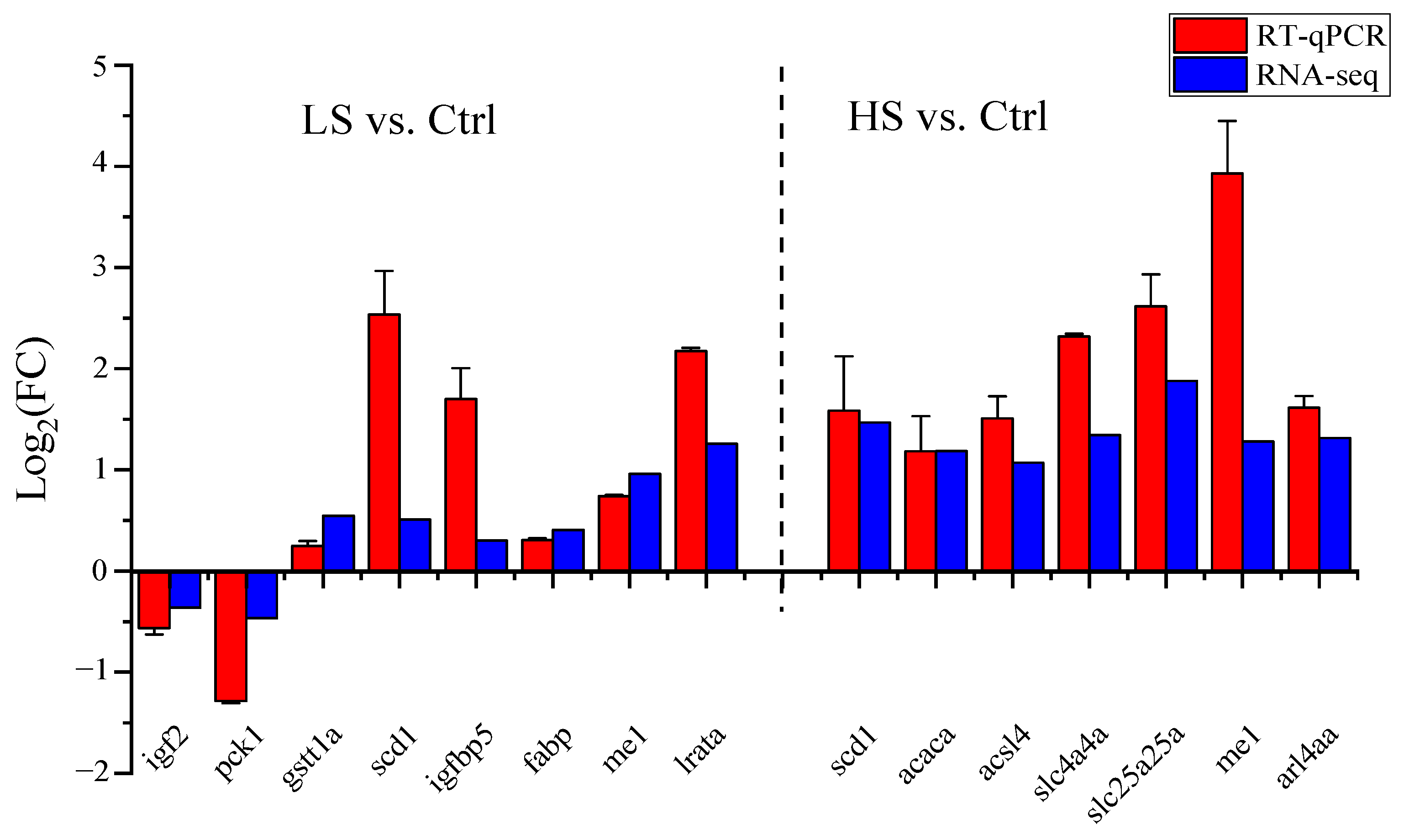
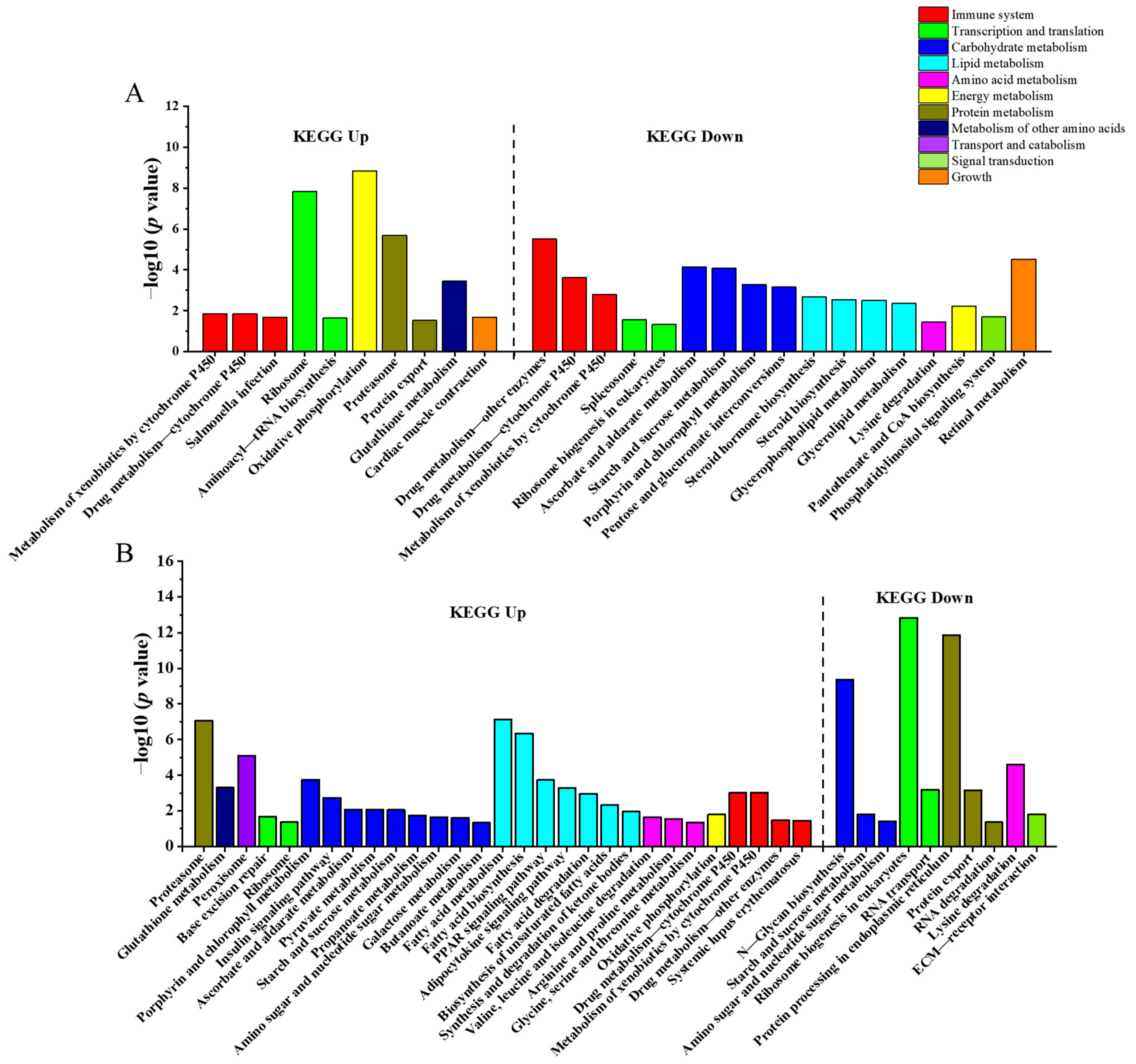
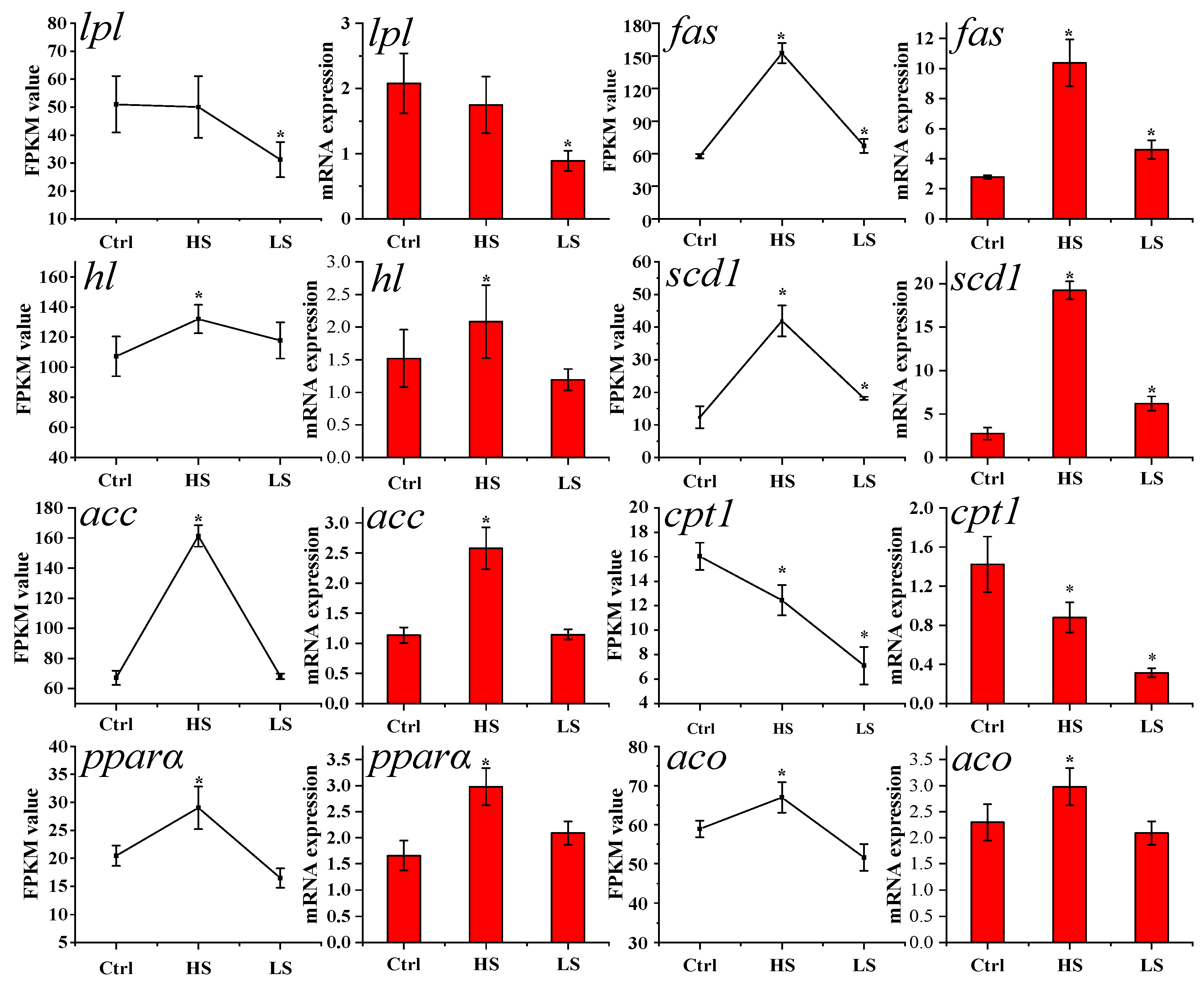
| Genes Name | Forward Primer (5′-3′) | Reverse Primer (5′-3′) | Application |
|---|---|---|---|
| β-actin | GAGAGGTTCCGTTGCCCAGAG | CAGACAGCACAGTGTTGGCGT | validation, lipid metabolism |
| lpl | GCCAATCAAACTGTTGCCAAAT | ACATACCACTCAGCGTCCATCC | lipid metabolism |
| fas | ACTCCTTGATGGGCGTTGA | TGGCTCTGTGCCGTGTTG | lipid metabolism |
| hl | GGAGGAGACTTCCAACCAGG | AGAGTTTGTAAGGCATCCGAGA | lipid metabolism |
| scd1 | GTGGCTGCTGGTGCGTAAA | ACCGTCTCGTGGGCAACTC | validation, lipid metabolism |
| acc | GTTGAAGTTCGGGGCCTAC | TGACGAGGGTTGATGGTTGG | validation, lipid metabolism |
| cpt1 | GCCGTGGTAAGAACAAGCAAT | CAGTGACCCTCCTCAGTATAGCC | lipid metabolism |
| pparα | TGCCAATACTGCCGCTTCC | GTGCTGGTCTTGCCCGTGA | lipid metabolism |
| aco | CATTGCGGACCCAGAAG | CGGTGGGACTGTTCAAGAC | lipid metabolism |
| igf2 | GAGCAGCAGAATGAAGGTC | TCTGCCGCACCTCGTATT | validation |
| igfbp5 | TACAAACCTGCCCACCCG | CGCTGCTTGCCTTGCTTC | validation |
| gstt1a | CTCTACAGGGCAGCATTACA | CTCTAGCAGGTTGAGCGACT | validation |
| fabp | AGACCACGCCTGATGACC | TGCCTGGACTCCCTCAAA | validation |
| me1 | GCCTCCTTGTTTCATCAGTC | AAAGTTTCTCGTTGTGGTCC | validation |
| lrata | GCCTGCTGTGGAATAACTGTGAA | AAGGGAATAAGGATTGTGGGTAA | validation |
| acsl4 | CTATCTGCCTCTGGCTCACG | GTCCGCAACCTCTGTAATGG | validation |
| slc4a4a | TGAGCGAGAACACTTCGGAC | TCGAACTTGATCCACCTGGC | validation |
| slc25a25a | CTGTGGGTTCGTGGAGTC | CTGCCAAGTATTCTTTAGGG | validation |
| arl4a | TTCACATCGCCATCCTCG | CTCCCGCAGTCCATCTCC | validation |
| Group | Ctrl | LS | HS | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Sample name a | 25ppt-1 | 25ppt-2 | 25ppt-3 | 5ppt-1 | 5ppt-2 | 5ppt-3 | 35ppt-1 | 35ppt-2 | 35ppt-3 |
| Clean reads (×106) | 22.00 | 20.07 | 21.46 | 21.31 | 21.57 | 21.46 | 20.90 | 21.42 | 22.31 |
| Clean bases (G) | 6.56 | 5.98 | 6.39 | 6.36 | 6.43 | 6.41 | 6.21 | 6.40 | 6.65 |
| Q20 (%) b | 96.8 | 96.78 | 96.83 | 96.79 | 96.68 | 96.75 | 96.58 | 96.54 | 96.63 |
| Q30 (%) c | 91.92 | 91.78 | 91.86 | 91.90 | 91.65 | 91.79 | 91.41 | 91.36 | 91.50 |
| Total mapped (×106) d | 43.95 | 40.17 | 42.92 | 42.63 | 42.13 | 42.91 | 41.79 | 42.85 | 44.63 |
| Mapping rate (%) | 87.68% | 88.46% | 88.18% | 88.39% | 87.14% | 87.92% | 87.92% | 87.15% | 87.82% |
| Uniquely mapped (×106) | 36.83 | 34.04 | 36.32 | 35.89 | 36.10 | 36.08 | 35.19 | 35.74 | 37.48 |
| Uniquely mapped rate (%) | 83.82% | 84.82% | 84.63% | 84.19% | 83.68% | 84.09% | 84.21% | 83.42% | 83.97% |
| Gene ID a | Gene Name | Log2 FC | p Value | Gene Function |
|---|---|---|---|---|
| LS vs. Ctrl | ||||
| EVM0003401 | ugt2a1, UDP-glucuronosyltransferase 2A1 | −0.552813189 | 4.90 × 10−3 | Steroid hormone biosynthesis |
| EVM0003565 | ugt2a2, UDP-glucuronosyltransferase 2A2-like | −0.466258335 | 3.22 × 10−2 | |
| EVM0005100 | ugt2b31, UDP-glucuronosyltransferase 2B31-like | −0.871147633 | 9.77 × 10−5 | |
| EVM0019647 | ugt2b20, UDP-glucuronosyltransferase 2B20-like | −0.457260075 | 2.07 × 10−2 | |
| EVM0006384 | sqle; squalene epoxidase | −0.622426552 | 7.39 × 10−3 | Steroid biosynthesis |
| EVM0010040 | dhcr7; 7-dehydrocholesterol reductase | −0.507776896 | 2.00 × 10−2 | |
| EVM0023884 | cyp51a1, lanosterol 14-alpha demethylase | −0.625469837 | 9.21 × 10−3 | |
| EVM0000521 | cept1; choline/ethanolamine phosphotransferase 1 | −0.53137678 | 1.67 × 10−2 | Glycerophospholipid metabolism |
| EVM0012014 | pla2g4a, phospholipase A2-like | −0.802009596 | 6.68 × 10−4 | |
| EVM0018902 | dgki; diacylglycerol kinase iota | −0.632031625 | 8.94 × 10−3 | |
| EVM0022818 | ptdss2; phosphatidylserine synthase 2 | −0.379058107 | 3.49 × 10−2 | |
| EVM0006432 | dgkd; diacylglycerol kinase delta-like | −0.990811261 | 2.75 × 10−4 | Glycerolipid metabolism |
| EVM0000633 | scd1, delta-9-desaturase 1 | 0.558057248 | 6.52 × 10−3 | Biosynthesis of unsaturated fatty acids |
| EVM0009831 | pck1; phosphoenolpyruvate carboxykinase 1 | −0.466918319 | 3.95 × 10−2 | Adipocytokine signaling pathway |
| HS vs. Ctrl | ||||
| EVM0006069 | pecr; peroxisomal trans-2-enoyl-CoA reductase | 0.381801622 | 4.13 × 10−8 | Biosynthesis of unsaturated fatty acids |
| EVM0002080 | elovl1; ELOVL fatty acid elongase 1 | 0.451575173 | 6.64 × 10−3 | |
| EVM0020874 | fads2, fatty acid desaturase 2-like | 0.938107743 | 1.88 × 10−4 | |
| EVM0000633 | scd1, delta-9-desaturase 1 | 1.468072624 | 9.61 × 10−8 | |
| EVM0009139 | fas, fatty acid synthase | 1.243001461 | 4.06 × 10−9 | Fatty acid biosynthesis |
| EVM0023488 | acaca; acetyl-CoA carboxylase alpha | 1.185429522 | 2.97 × 10−11 | |
| EVM0001796 | g6pc1, glucose-6-phosphatase | 1.173745732 | 4.81 × 10−4 | Adipocytokine signaling pathway |
| EVM0001859 | socs1, suppressor of cytokine signaling | 1.179125942 | 4.51 × 10−4 | |
| EVM0003316 | socs3, suppressor of cytokine signaling 3 | 1.313341782 | 1.90 × 10−7 | |
| EVM0006476 | adipor2, adiponectin receptor protein 2 | 0.430474726 | 8.40 × 10−3 | |
| EVM0009831 | pck1; phosphoenolpyruvate carboxykinase 1 | 0.518387425 | 4.80 × 10−2 | |
| EVM0021145 | ppara; peroxisome proliferator activated receptor alpha | 0.481586273 | 4.83 × 10−2 | |
| EVM0000521 | cept1; choline/ethanolamine phosphotransferase 1 | −0.709767922 | 6.67 × 10−3 | Glycerophospholipid metabolism (ko00564) |
| EVM0012014 | pla2g4a; phospholipase A2-like | −0.548207575 | 4.77 × 10−2 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Cai, B.; Tian, C.; Jiang, D.; Shi, H.; Huang, Y.; Zhu, C.; Li, G.; Deng, S. RNA Sequencing (RNA-Seq) Analysis Reveals Liver Lipid Metabolism Divergent Adaptive Response to Low- and High-Salinity Stress in Spotted Scat (Scatophagus argus). Animals 2023, 13, 1503. https://doi.org/10.3390/ani13091503
Chen J, Cai B, Tian C, Jiang D, Shi H, Huang Y, Zhu C, Li G, Deng S. RNA Sequencing (RNA-Seq) Analysis Reveals Liver Lipid Metabolism Divergent Adaptive Response to Low- and High-Salinity Stress in Spotted Scat (Scatophagus argus). Animals. 2023; 13(9):1503. https://doi.org/10.3390/ani13091503
Chicago/Turabian StyleChen, Jieqing, Bosheng Cai, Changxu Tian, Dongneng Jiang, Hongjuan Shi, Yang Huang, Chunhua Zhu, Guangli Li, and Siping Deng. 2023. "RNA Sequencing (RNA-Seq) Analysis Reveals Liver Lipid Metabolism Divergent Adaptive Response to Low- and High-Salinity Stress in Spotted Scat (Scatophagus argus)" Animals 13, no. 9: 1503. https://doi.org/10.3390/ani13091503
APA StyleChen, J., Cai, B., Tian, C., Jiang, D., Shi, H., Huang, Y., Zhu, C., Li, G., & Deng, S. (2023). RNA Sequencing (RNA-Seq) Analysis Reveals Liver Lipid Metabolism Divergent Adaptive Response to Low- and High-Salinity Stress in Spotted Scat (Scatophagus argus). Animals, 13(9), 1503. https://doi.org/10.3390/ani13091503








