Modulation of Apoptosis and Autophagy by Melatonin in Juglone-Exposed Bovine Oocytes
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Setup
2.2. Reagents and Eithical Statement
2.3. Oocytes Aspiration and Collection
2.4. In Vitro Maturation (IVM) and Biochemical Treatment
2.5. In Vitro Fertilization and In Vitro Embryo Development
2.6. Quantification of Reactive Oxygen Species (ROS) and Glutathione (GSH) Content
2.7. Evaluation of Mitochondrial Distribution
2.8. Total RNA Extraction and cDNA Synthesis
2.9. Quantitative Reverse Transcription PCR (RT-qPCR)
2.10. Statistical Analysis
3. Results
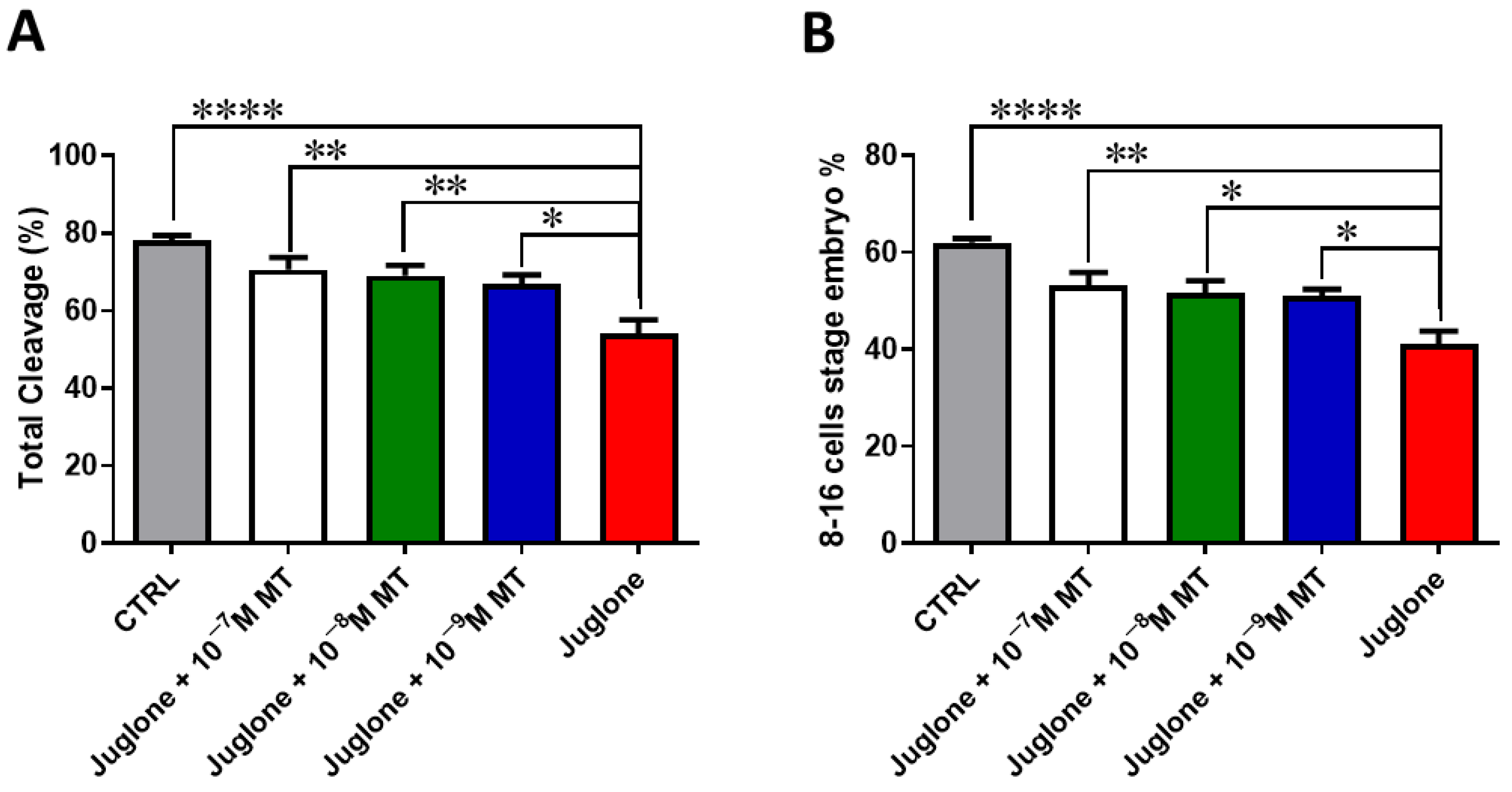
3.1. Melatonin Application during IVM Improves the Developmental Competence of Juglone-Treated Oocytes
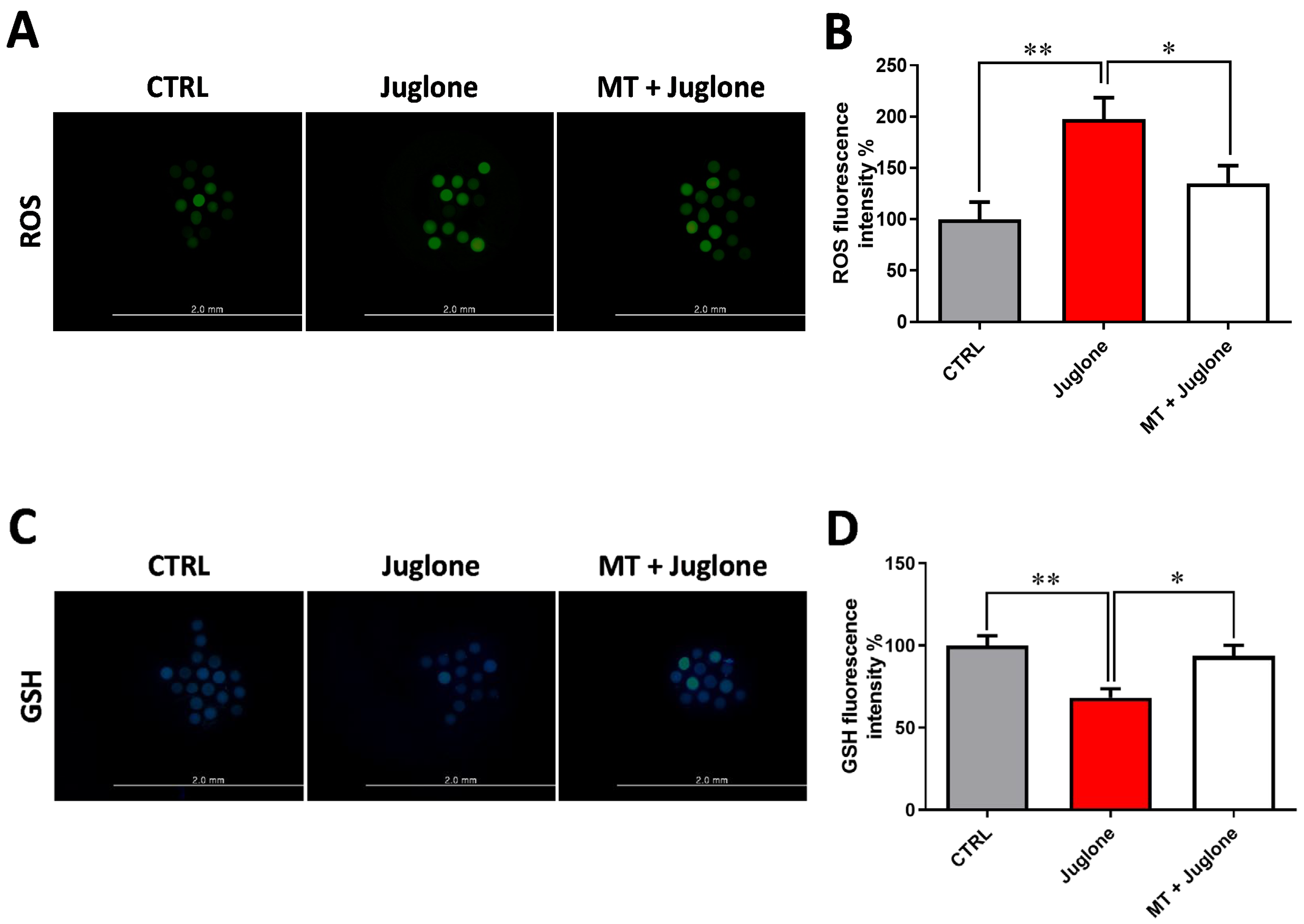
3.2. Juglone Treatment Negatively Affects, while Melatonin Abrogates, Oxidative Stress in Matured Oocytes
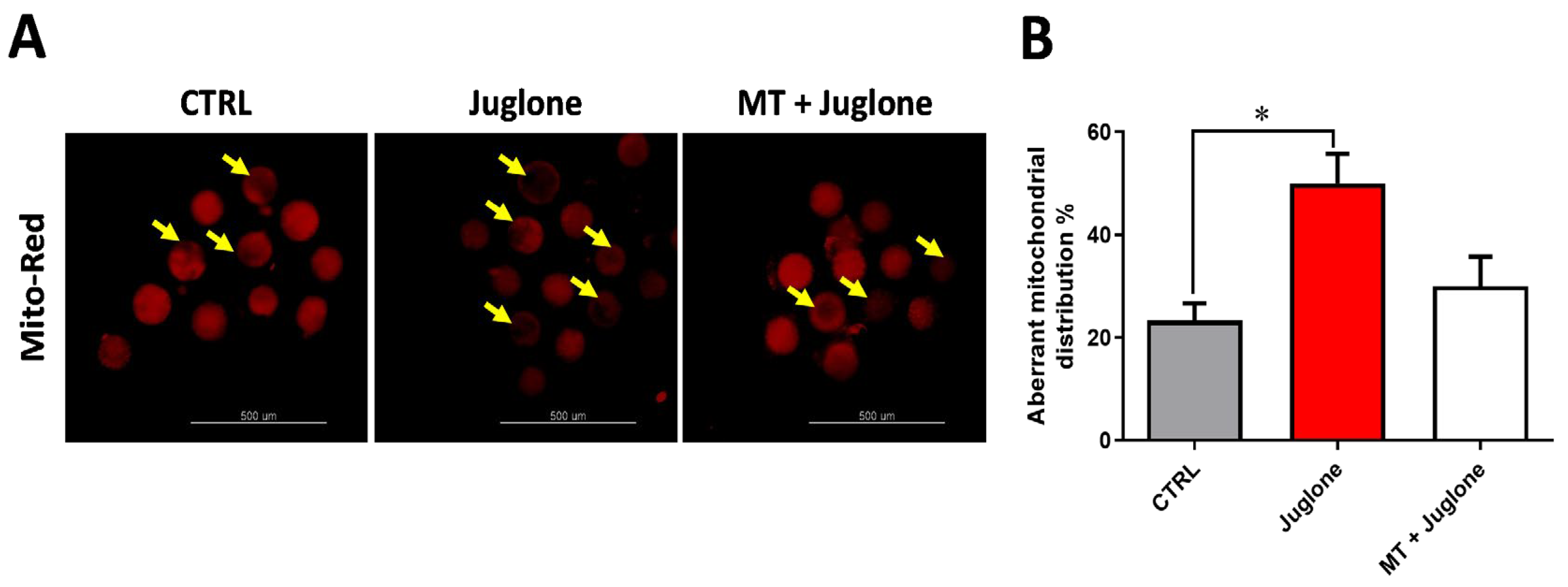
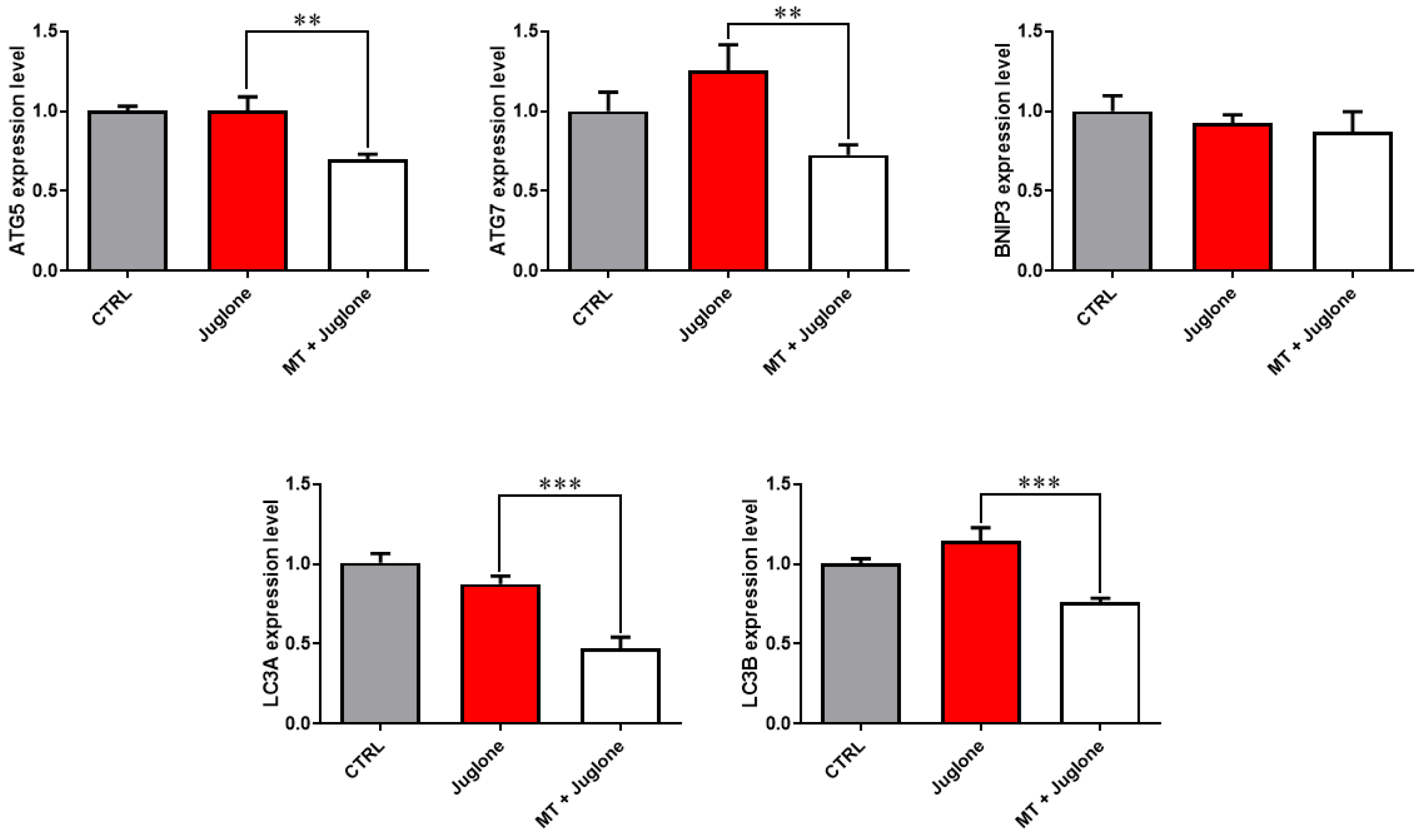
3.3. Juglone Treatment Induces, While Melatonin Restores, Mitochondrial Dysfunction, Apoptosis, and Autophagy in Matured Oocytes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferre, L.B.; Kjelland, M.E.; Strobech, L.B.; Hyttel, P.; Mermillod, P.; Ross, P.J. Review: Recent advances in bovine in vitro embryo production: Reproductive biotechnology history and methods. Animal 2020, 14, 991–1004. [Google Scholar] [CrossRef] [PubMed]
- Tesfaye, D.; Ghanem, N.; Carter, F.; Fair, T.; Sirard, M.A.; Hoelker, M.; Schellander, K.; Lonergan, P. Gene expression profile of cumulus cells derived from cumulus-oocyte complexes matured either in vivo or in vitro. Reprod. Fertil. Dev. 2009, 21, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Thorburn, A. Apoptosis and autophagy: Regulatory connections between two supposedly different processes. Apoptosis 2008, 13, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Van der Reest, J.; Nardini Cecchino, G.; Haigis, M.C.; Kordowitzki, P. Mitochondria: Their relevance during oocyte ageing. Ageing Res. Rev. 2021, 70, 101378. [Google Scholar] [CrossRef]
- Heimlich, G.; McKinnon, A.D.; Bernardo, K.; Brdiczka, D.; Reed, J.C.; Kain, R.; Kronke, M.; Jurgensmeier, J.M. Bax-induced cytochrome c release from mitochondria depends on alpha-helices-5 and -6. Biochem. J. 2004, 378, 247–255. [Google Scholar] [CrossRef]
- Jin, J.Y.; Wei, X.X.; Zhi, X.L.; Wang, X.H.; Meng, D. Drp1-dependent mitochondrial fission in cardiovascular disease. Acta Pharmacol. Sin. 2021, 42, 655–664. [Google Scholar] [CrossRef]
- Lee, Y.J.; Jeong, S.Y.; Karbowski, M.; Smith, C.L.; Youle, R.J. Roles of the mammalian mitochondrial fission and fusion mediators Fis1, Drp1, and Opa1 in apoptosis. Mol. Biol. Cell 2004, 15, 5001–5011. [Google Scholar] [CrossRef]
- Frank, S.; Gaume, B.; Bergmann-Leitner, E.S.; Leitner, W.W.; Robert, E.G.; Catez, F.; Smith, C.L.; Youle, R.J. The role of dynamin-related protein 1, a mediator of mitochondrial fission, in apoptosis. Dev. Cell 2001, 1, 515–525. [Google Scholar] [CrossRef]
- Montessuit, S.; Somasekharan, S.P.; Terrones, O.; Lucken-Ardjomande, S.; Herzig, S.; Schwarzenbacher, R.; Manstein, D.J.; Bossy-Wetzel, E.; Basanez, G.; Meda, P.; et al. Membrane remodeling induced by the dynamin-related protein Drp1 stimulates Bax oligomerization. Cell 2010, 142, 889–901. [Google Scholar] [CrossRef]
- El Sheikh, M.; Mesalam, A.; Mesalam, A.A.; Idrees, M.; Lee, K.L.; Kong, I.K. Melatonin Abrogates the Anti-Developmental Effect of the AKT Inhibitor SH6 in Bovine Oocytes and Embryos. Int. J. Mol. Sci. 2019, 20, 2956. [Google Scholar] [CrossRef]
- El-Sheikh, M.; Mesalam, A.A.; Song, S.H.; Ko, J.; Kong, I.K. Melatonin Alleviates the Toxicity of High Nicotinamide Concentrations in Oocytes: Potential Interaction with Nicotinamide Methylation Signaling. Oxidative Med. Cell. Longev. 2021, 2021, 5573357. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Li, L.; Chen, K.; Li, C.; Wang, Y.; Wang, G. Melatonin alleviates beta-zearalenol and HT-2 toxin-induced apoptosis and oxidative stress in bovine ovarian granulosa cells. Environ. Toxicol. Pharmacol. 2019, 68, 52–60. [Google Scholar] [CrossRef] [PubMed]
- El-Raey, M.; Geshi, M.; Somfai, T.; Kaneda, M.; Hirako, M.; Abdel-Ghaffar, A.E.; Sosa, G.A.; El-Roos, M.E.; Nagai, T. Evidence of melatonin synthesis in the cumulus oocyte complexes and its role in enhancing oocyte maturation in vitro in cattle. Mol. Reprod. Dev. 2011, 78, 250–262. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Tao, J.; Chai, M.; Wu, H.; Wang, J.; Li, G.; He, C.; Xie, L.; Ji, P.; Dai, Y.; et al. Melatonin Improves the Quality of Inferior Bovine Oocytes and Promoted Their Subsequent IVF Embryo Development: Mechanisms and Results. Molecules 2017, 22, 2059. [Google Scholar] [CrossRef] [PubMed]
- Yong, W.; Ma, H.; Na, M.; Gao, T.; Zhang, Y.; Hao, L.; Yu, H.; Yang, H.; Deng, X. Roles of melatonin in the field of reproductive medicine. Biomed. Pharmacother. 2021, 144, 112001. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.T.; Li, Y.; Chu, P.; Ma, X.D.; Tang, Z.Y.; Sun, Z.L. Molecular biological mechanism of action in cancer therapies: Juglone and its derivatives, the future of development. Biomed. Pharmacother. 2022, 148, 112785. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, T.; Suzuki, Y.J. Juglone in Oxidative Stress and Cell Signaling. Antioxidants 2019, 8, 91. [Google Scholar] [CrossRef] [PubMed]
- El-Sheikh, M.; Mesalam, A.; Khalil, A.A.K.; Idrees, M.; Ahn, M.J.; Mesalam, A.A.; Kong, I.K. Downregulation of PI3K/AKT/mTOR Pathway in Juglone-Treated Bovine Oocytes. Antioxidants 2023, 12, 114. [Google Scholar] [CrossRef]
- Mesalam, A.A.; El-Sheikh, M.; Joo, M.D.; Khalil, A.A.K.; Mesalam, A.; Ahn, M.J.; Kong, I.K. Induction of Oxidative Stress and Mitochondrial Dysfunction by Juglone Affects the Development of Bovine Oocytes. Int. J. Mol. Sci. 2020, 22, 168. [Google Scholar] [CrossRef]
- El Sheikh, M.; Mesalam, A.A.; Idrees, M.; Sidrat, T.; Mesalam, A.; Lee, K.L.; Kong, I.K. Nicotinamide Supplementation during the In Vitro Maturation of Oocytes Improves the Developmental Competence of Preimplantation Embryos: Potential Link to SIRT1/AKT Signaling. Cells 2020, 9, 1550. [Google Scholar] [CrossRef]
- Takasaki, A.; Nakamura, Y.; Tamura, H.; Shimamura, K.; Morioka, H. Melatonin as a new drug for improving oocyte quality. Reprod. Med. Biol. 2003, 2, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues-Cunha, M.C.; Mesquita, L.G.; Bressan, F.; Collado, M.D.; Balieiro, J.C.; Schwarz, K.R.; de Castro, F.C.; Watanabe, O.Y.; Watanabe, Y.F.; de Alencar Coelho, L.; et al. Effects of melatonin during IVM in defined medium on oocyte meiosis, oxidative stress, and subsequent embryo development. Theriogenology 2016, 86, 1685–1694. [Google Scholar] [CrossRef]
- Zhao, X.M.; Min, J.T.; Du, W.H.; Hao, H.S.; Liu, Y.; Qin, T.; Wang, D.; Zhu, H.B. Melatonin enhances the in vitro maturation and developmental potential of bovine oocytes denuded of the cumulus oophorus. Zygote 2015, 23, 525–536. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.W.; Jiang, X.L.; Wang, Y.C.; Wang, Y.Y.; Hao, H.S.; Zhao, S.J.; Du, W.H.; Zhao, X.M.; Wang, L.; Zhu, H.B. Melatonin protects against paraquat-induced damage during in vitro maturation of bovine oocytes. J. Pineal Res. 2019, 66, e12532. [Google Scholar] [CrossRef]
- Park, H.J.; Park, S.Y.; Kim, J.W.; Yang, S.G.; Kim, M.J.; Jegal, H.G.; Kim, I.S.; Choo, Y.K.; Koo, D.B. Melatonin Improves Oocyte Maturation and Mitochondrial Functions by Reducing Bisphenol A-Derived Superoxide in Porcine Oocytes In Vitro. Int. J. Mol. Sci. 2018, 19, 3422. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Qin, Y.; Hu, X.; Ren, L.; Zhang, C.; Wang, X.; Wang, W.; Zhang, Z.; Hao, J.; Guo, M.; et al. Melatonin protects in vitro matured porcine oocytes from toxicity of Aflatoxin B1. J. Pineal Res. 2018, 66, e12543. [Google Scholar] [CrossRef]
- De Matos, D.G.; Furnus, C.C.; Moses, D.F. Glutathione synthesis during in vitro maturation of bovine oocytes: Role of cumulus cells. Biol. Reprod. 1997, 57, 1420–1425. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Ding, D.; Yang, H.; Zou, W.; Yang, D.; Wang, K.; Zhang, C.; Chen, B.; Ji, D.; Hao, Y.; et al. Melatonin Protects Mitochondrial Function and Inhibits Oxidative Damage against the Decline of Human Oocytes Development Caused by Prolonged Cryopreservation. Cells 2022, 11, 4018. [Google Scholar] [CrossRef]
- Qu, J.; Wang, Q.; Niu, H.; Sun, X.; Ji, D.; Li, Y. Melatonin protects oocytes from cadmium exposure-induced meiosis defects by changing epigenetic modification and enhancing mitochondrial morphology in the mouse. Ecotoxicol. Environ. Saf. 2022, 248, 114311. [Google Scholar] [CrossRef]
- Wang, P.; Gao, C.; Wang, W.; Yao, L.P.; Zhang, J.; Zhang, S.D.; Li, J.; Fang, S.H.; Fu, Y.J. Juglone induces apoptosis and autophagy via modulation of mitogen-activated protein kinase pathways in human hepatocellular carcinoma cells. Food Chem. Toxicol. 2018, 116, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Balboula, A.Z.; Aboelenain, M.; Fujii, T.; Moriyasu, S.; Bai, H.; Kawahara, M.; Takahashi, M. Effect of autophagy induction and cathepsin B inhibition on developmental competence of poor quality bovine oocytes. J. Reprod. Dev. 2020, 66, 83–91. [Google Scholar] [CrossRef] [PubMed]


| Gene Name | Sequence | Amplicon Size (bp) | Accession Number |
|---|---|---|---|
| ATG5 | F: CCACTGCCGTCATTAAACCT R: TTCCACTCCCTCGAGCTAAA | 212 | XM_027551305.1 |
| ATG7 | F: ATGGCCTTTGAGGAACCTTT R: ATGCCTCCCTTCTGGTTCTT | 210 | XM_010817935.3 |
| BNIP3 | F: GAAGGAATGCCGACACTAGG R: CAAAGCCAGCAGACACTCAG | 138 | XM_027528566.1 |
| LC3A | F: CATGAGCGAGTTGGTCAAAA R: GGGAGGCGTAGACCATGTAG | 170 | XM_027558753.1 |
| LC3B | F: TTATCCGAGAGCAGCATCC R: AGGCTTGATTAGCATTGAGC | 171 | XM_027513856.1 |
| AKT2 | F: CGACTATCTCAAACTCCTGG R: ATCTTCATGGCATAGTAGCG | 90 | NM_001206146.2 |
| BCL2 | F: TGGATGACCGAGTACCTGAA R: CAGCCAGGAGAAATCAAACA | 120 | NM_001166486.1 |
| SOD1 | F: CCATCCACTTCGAGGCAAAG R: TCTCCAAACTGATGGACGTGG | 100 | NM_174615.2 |
| Drp1 | F: AGCCCCTTCTAGAGCAGTGG R: CGGGTGACAATACCAGTGCC | 89 | XM_024992090.1 |
| BAX | F: CACCAAGAAGCTGAGCGAGTGT R: TCGGAAAAAGACCTCTCGGGGA | 118 | NM_173894 |
| NF-KB | F: TGGCGGAATTACCTTCCATAC R: CATCACTCTTGCCACAACTTTC | 110 | DQ464067 |
| GADPH | F: CCCAGAATATCATCCCTGCT R: CTGCTTCACCACCTTCTTGA | 185 | NM_001034034.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Sheikh, M.; Mesalam, A.A.; Kang, S.-M.; Joo, M.-D.; Soliman, S.S.; Khalil, A.A.K.; Ahn, M.-J.; Kong, I.-K. Modulation of Apoptosis and Autophagy by Melatonin in Juglone-Exposed Bovine Oocytes. Animals 2023, 13, 1475. https://doi.org/10.3390/ani13091475
El-Sheikh M, Mesalam AA, Kang S-M, Joo M-D, Soliman SS, Khalil AAK, Ahn M-J, Kong I-K. Modulation of Apoptosis and Autophagy by Melatonin in Juglone-Exposed Bovine Oocytes. Animals. 2023; 13(9):1475. https://doi.org/10.3390/ani13091475
Chicago/Turabian StyleEl-Sheikh, Marwa, Ahmed Atef Mesalam, Seon-Min Kang, Myeong-Don Joo, Seham Samir Soliman, Atif Ali Khan Khalil, Mi-Jeong Ahn, and Il-Keun Kong. 2023. "Modulation of Apoptosis and Autophagy by Melatonin in Juglone-Exposed Bovine Oocytes" Animals 13, no. 9: 1475. https://doi.org/10.3390/ani13091475
APA StyleEl-Sheikh, M., Mesalam, A. A., Kang, S.-M., Joo, M.-D., Soliman, S. S., Khalil, A. A. K., Ahn, M.-J., & Kong, I.-K. (2023). Modulation of Apoptosis and Autophagy by Melatonin in Juglone-Exposed Bovine Oocytes. Animals, 13(9), 1475. https://doi.org/10.3390/ani13091475








