Vitamin B12 in Cats: Nutrition, Metabolism, and Disease
Abstract
Simple Summary
Abstract
1. Introduction
2. Cobalamin in Cats’ Diet
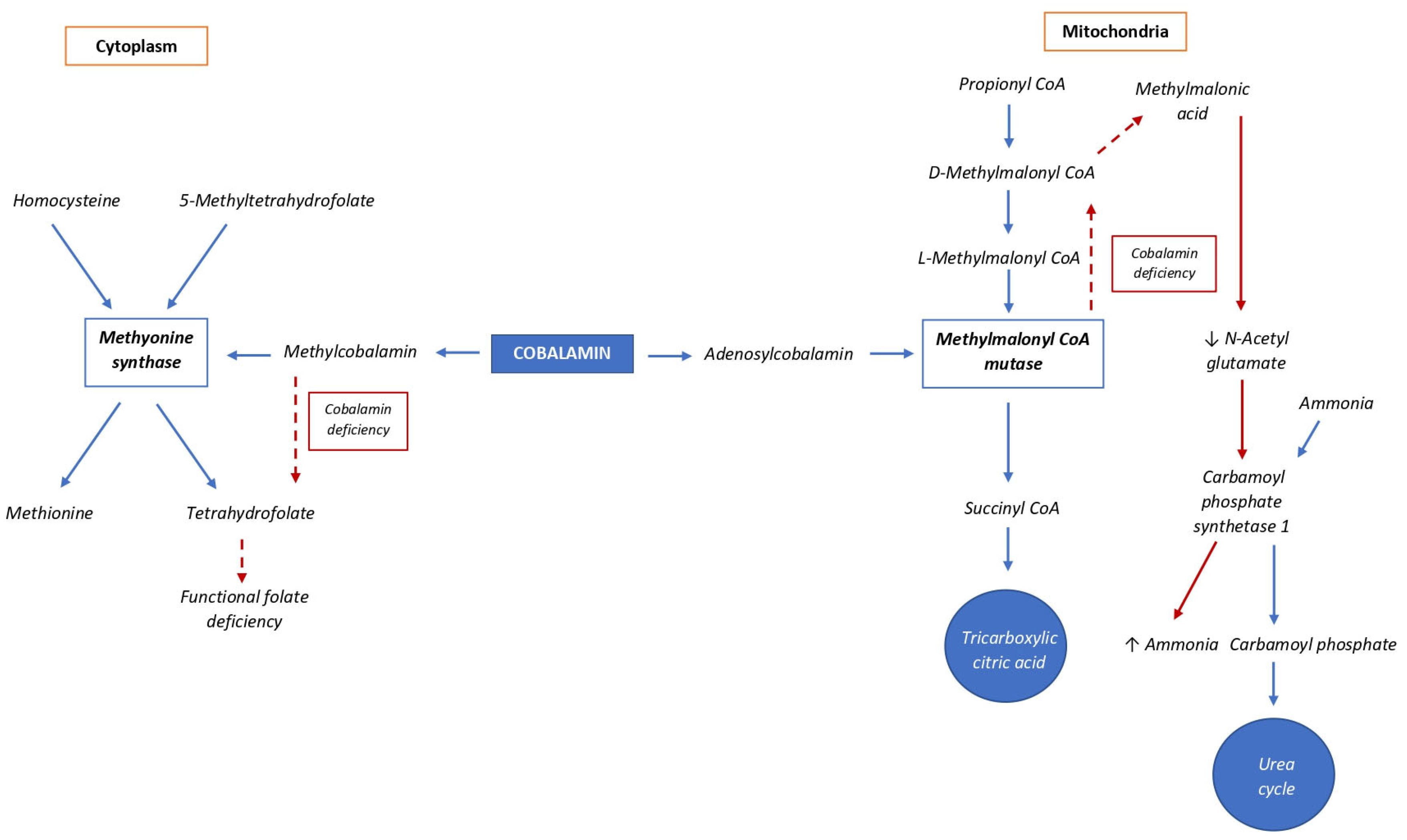
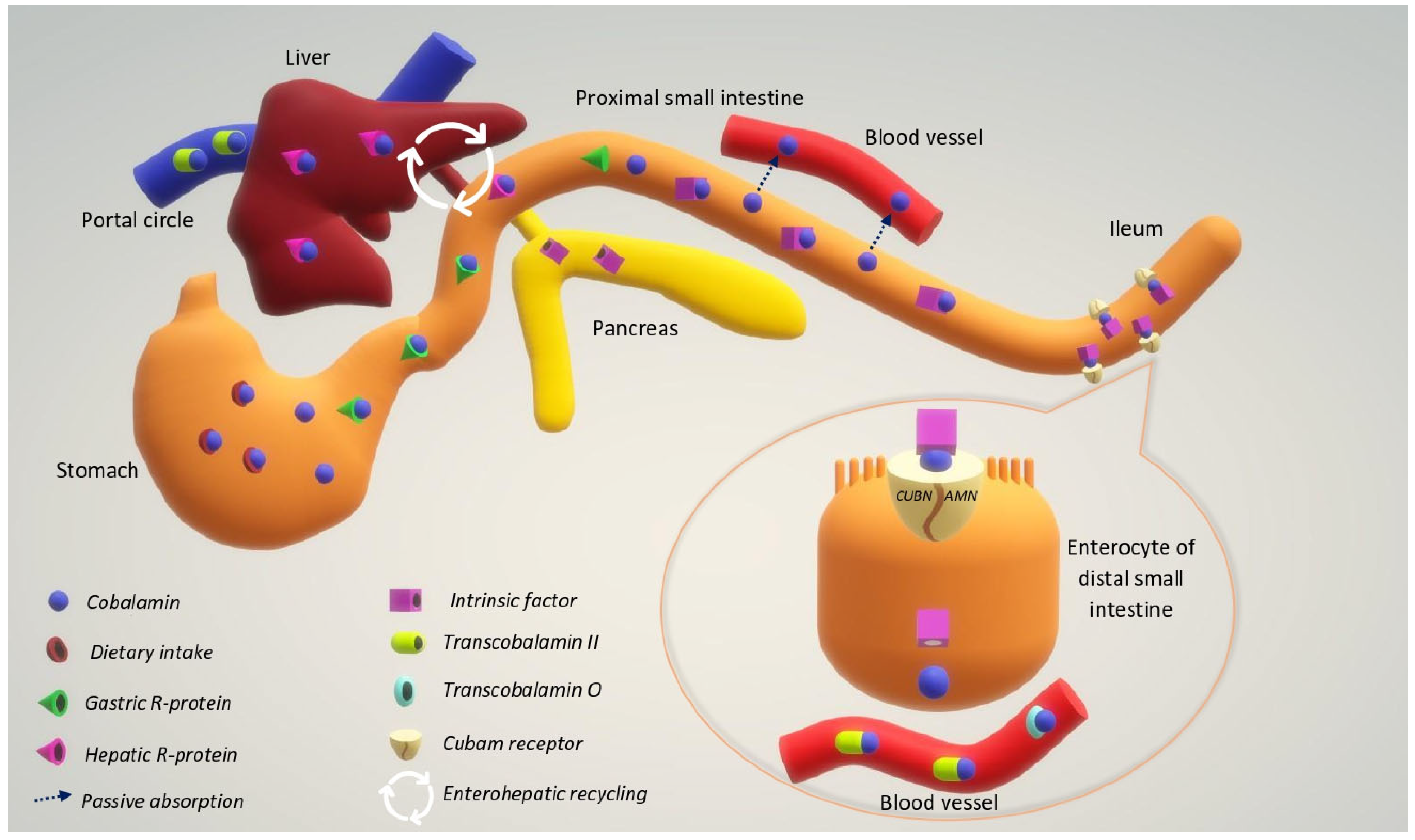
3. Metabolism
4. Evaluation of Cobalamin Status and Deficiency in Cats
4.1. Interpretation of Cobalamin Status and Diseases
4.1.1. Hypocobalaminemia
4.1.2. Hypercobalaminemia
4.1.3. Gastrointestinal Disease
4.1.4. Exocrine Pancreatic Insufficiency
4.1.5. Pancreatitis and Triaditis
4.1.6. Dysbiosis
4.1.7. Hepatic Lipidosis
4.1.8. Hyperthyroidism
4.1.9. Cardiomyopathy and Arterial Thromboembolism
4.1.10. Neurological Diseases
5. Cobalamin Supplementation
5.1. Parenteral Cobalamin Supplementation
5.2. Oral Cobalamin Supplementation
6. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Beck, W.S. Cobalamin (Vitamin B12). In Handbook of Vitamins, 3rd ed.; Rucker, R.B., Zempleni, J., Suttie, J.W., McCormick, D.B., Machlin, L.J., Eds.; Marcel Dekker: New York, NY, USA, 2001; pp. 463–512. [Google Scholar]
- National Research Council. Nutrient Requirements of Dogs and Cats; The National Academies Press: Washington, DC, USA, 2006.
- Kempf, J.; Hersberger, M.; Melliger, R.H.; Reusch, C.E.; Kook, P.H. Effects of 6 Weeks of Parenteral Cobalamin Supplementation on Clinical and Biochemical Variables in Cats with Gastrointestinal Disease. J. Vet. Intern. Med. 2017, 31, 1664–1672. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.G. The Essentiality of Biotin and Vitamin B-12 for the Cat. Proceedings of Kal Kan Symposium for the Treatment of Dog and Cat Diseases, Columbus, OH, USA, 19–20 September 1977; Kal Kan Foods Inc.: Trenton, NY, USA, 1977; pp. 15–18. [Google Scholar]
- Stabler, S.; Lindenbaum, J.; Savage, D.; Allen, R. Elevation of Serum Cystathionine Levels in Patients with Cobalamin and Folate Deficiency. Blood 1993, 81, 3404–3413. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, R.; Ragsdale, S.W. The Many Faces of Vitamin B12: Catalysis by Cobalamin-Dependent Enzymes. Annu. Rev. Biochem. 2003, 72, 209–247. [Google Scholar] [CrossRef] [PubMed]
- Dodd, S.A.S.; Adolphe, J.L.; Verbrugghe, A. Plant-Based Diets for Dogs. J. Am. Vet. Med. Assoc. 2018, 253, 1425–1432. [Google Scholar] [CrossRef] [PubMed]
- Laflamme, D.P. Understanding the Nutritional Needs of Healthy Cats and Those with Diet-Sensitive Conditions. Vet. Clin. N. Am. Small Anim. Pract. 2020, 50, 905–924. [Google Scholar] [CrossRef]
- Kang, M.-H.; Morris, J.G.; Rogers, Q.R. Effect of Concentration of Some Dietary Amino Acids and Protein on Plasma Urea Nitrogen Concentration in Growing Kittens. J. Nutr. 1987, 117, 1689–1696. [Google Scholar] [CrossRef]
- FEDIAF. Nutritional Guidelines for Complete and Complementary Pet Food for Cats and Dogs; The European Pet Food Industry Federation: Bruxelles, Belgium, 2021. [Google Scholar]
- Dzanis, D.A. The Association of American Feed Control Officials Dog and Cat Food Nutrient Profiles: Substantiation of Nutritional Adequacy of Complete and Balanced Pet Foods in the United States. J. Nutr. 1994, 124, 2535S–2539S. [Google Scholar] [CrossRef] [PubMed]
- Molonon, B.R.; Bowers, J.A.; Dayton, A.D. Vitamin B12 and Folic Acid Content of Raw and Cooked Turkey Muscle. Poult. Sci. 1980, 59, 303–307. [Google Scholar] [CrossRef]
- Watanabe, F.; Abe, K.; Fujita, T.; Goto, M.; Hiemori, M.; Nakano, Y. Effects of Microwave Heating on the Loss of Vitamin B12 in Foods. J. Agric. Food Chem. 1998, 46, 206–210. [Google Scholar] [CrossRef]
- Nishioka, M.; Kanosue, F.; Yabuta, Y.; Watanabe, F. Loss of Vitamin B12 in Fish (Round Herring) Meats during Various Cooking Treatments. J. Nutr. Sci. Vitaminol. 2011, 57, 432–436. [Google Scholar] [CrossRef]
- Hanisch, F.; Toresson, L.; Spillmann, T. Cobalaminmangel Bei Hund Und Katze. Tierarztl. Prax. Ausg. K. Kleintiere Heimtiere 2018, 46, 309–314. [Google Scholar] [CrossRef]
- Regulation (EC) No 767/2009 of the European Parliament and of the Council of 13 July 2009 on the Placing on the Market and Use of Feed, Amending European Parliament and Council Regulation (EC) No 1831/2003 and Repealing Council Directive 79/373/EEC, Commission Directive 80/511/EEC, Council Directives 82/471/EEC, 83/228/EEC, 93/74/EEC, 93/113/EC and 96/25/EC and Commission Decision 2004/217/EC OJ L 229/1. Available online: https://eur-lex.europa.eu/eli/reg/2009/767/oj (accessed on 18 April 2023).
- Kather, S.; Grützner, N.; Kook, P.H.; Dengler, F.; Heilmann, R.M. Review of Cobalamin Status and Disorders of Cobalamin Metabolism in Dogs. J. Vet. Intern. Med. 2020, 34, 13–28. [Google Scholar] [CrossRef]
- Fyfe, J.C. Feline Intrinsic Factor (IF) Is Pancreatic in Origin and Mediates Ileal Cobalamin (CBL) Absorption. J. Vet. Intern. Med. 1993, 7, 133. [Google Scholar]
- Batt, R.M.; Horadagoda, N.U. Gastric and Pancreatic Intrinsic Factor-Mediated Absorption of Cobalamin in the Dog. Am. J. Physiol. Gastrointest. 1989, 257, G344–G349. [Google Scholar] [CrossRef] [PubMed]
- Batt, R.M.; Horadagoda, N.U.; McLean, L.; Morton, D.B.; Simpson, K.W. Identification and Characterization of a Pancreatic Intrinsic Factor in the Dog. Am. J. Physiol. Gastrointest. 1989, 256, G517–G523. [Google Scholar] [CrossRef]
- Vaillant, C.; Horadagoda, N.U.; Batt, R.M. Cellular Localization of Intrinsic Factor in Pancreas and Stomach of the Dog. Cell. Tissue Res. 1990, 260, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Levine, J.S.; Allen, R.H.; Alpers, D.H.; Seetharam, B. Immunocytochemical Localization of the Intrinsic Factor-Cobalamin Receptor in Dog-Ileum: Distribution of Intracellular Receptor during Cell Maturation. J. Cell. Biol. 1984, 98, 1111–1118. [Google Scholar] [CrossRef]
- Gazet, J.-C.; McColl, I. Absorption of Vitamin B12 from the Small Intestine. Study in Man, Monkey, Cat, and Dog. Br. J. Surg. 2005, 54, 128–131. [Google Scholar] [CrossRef]
- Fyfe, J.C.; Madsen, M.; Højrup, P.; Christensen, E.I.; Tanner, S.M.; de la Chapelle, A.; He, Q.; Moestrup, S.K. The Functional Cobalamin (Vitamin B12)–Intrinsic Factor Receptor Is a Novel Complex of Cubilin and Amnionless. Blood 2004, 103, 1573–1579. [Google Scholar] [CrossRef]
- Hornbuckle, E.H.; Simpson, K.W.; Tennant, B.C. Gastrointestinal Function. In Clinical Biochemistry of Domestic Animals; Kaneko, J.J., Harvey, J.W., Bruss, M.L., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 432–433. [Google Scholar]
- Quadros, E.M.; Matthews, D.A.; Wise, I.J.; Linnell, J.C. Tissue Distribution of Endogenous Cobalamins and Other Corrins in the Rat, Cat and Guinea Pig. Biochim. Biophys. Acta 1976, 421, 141–152. [Google Scholar] [CrossRef]
- Rappazzo, M.; Hall, C. Cyanocobalamin Transport Proteins in Canine Plasma. Am. J. Physiol. Leg. Content 1972, 222, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Linnell, J.C.; Collings, L.; Down, M.C.; England, J.M. Distribution of Endogenous Cobalamin between the Transcobalamins in Various Mammals. Clin. Sci. 1979, 57, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Ruaux, C.G.; Steiner, J.M.; Williams, D.A. Metabolism of Amino Acids in Cats with Severe Cobalamin Deficiency. Am. J. Vet. Res. 2001, 62, 1852–1858. [Google Scholar] [CrossRef]
- Simpson, K.W.; Fyfe, J.; Cornetta, A.; Sachs, A.; Strauss-Ayali, D.; Lamb, S.V.; Reimers, T.J. Subnormal Concentrations of Serum Cobalamin (Vitamin B12) in Cats with Gastrointestinal Disease. J. Vet. Intern. Med. 2001, 15, 26. [Google Scholar] [CrossRef]
- Hall, E.; German, A. Disease of the Small Intestine. In Textbook of Veterinary Internal Medicine; Ettinger, S., Feldman, E., Eds.; Saunders Elsevier: St. Louis, MI, USA, 2010; pp. 1526–1572. [Google Scholar]
- Luhby, A.L.; Cooperman, J.M.; Donnenfeld, A.M. Placental Transfer and Biological Half-Life of Radioactive Vit. B12 in the Dog. Exp. Biol. Med. 1959, 100, 214–217. [Google Scholar] [CrossRef]
- Birn, H.; Willnow, T.E.; Nielsen, R.; Norden, A.G.W.; Bönsch, C.; Moestrup, S.K.; Nexø, E.; Christensen, E.I. Megalin Is Essential for Renal Proximal Tubule Reabsorption and Accumulation of Transcobalamin-B12. Am. J. Physiol. Ren. Physiol. 2002, 282, F408–F416. [Google Scholar] [CrossRef]
- He, Q. Amnionless Function Is Required for Cubilin Brush-Border Expression and Intrinsic Factor-Cobalamin (Vitamin B12) Absorption in Vivo. Blood 2005, 106, 1447–1453. [Google Scholar] [CrossRef]
- Maunder, C.L.; Day, M.J.; Hibbert, A.; Steiner, J.M.; Suchodolski, J.S.; Hall, E.J. Serum Cobalamin Concentrations in Cats with Gastrointestinal Signs: Correlation with Histopathological Findings and Duration of Clinical Signs. J. Feline Med. Surg. 2012, 14, 686–693. [Google Scholar] [CrossRef]
- McLeish, S.A.; Burt, K.; Papasouliotis, K. Analytical Quality Assessment and Method Comparison of Immunoassays for the Measurement of Serum Cobalamin and Folate in Dogs and Cats. J. Vet. Diagn. Investig. 2019, 31, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Ruaux, C.G.; Steiner, J.M.; Williams, D.A. Relationships between Low Serum Cobalamin Concentrations and Methlymalonic Acidemia in Cats. J. Vet. Intern. Med. 2009, 23, 472–475. [Google Scholar] [CrossRef] [PubMed]
- Kempf, J.; Melliger, R.H.; Reusch, C.E.; Kook, P.H. Effects of Storage Conditions and Duration on Cobalamin Concentration in Serum Samples from Cats and Dogs. J. Am. Vet. Med. Assoc. 2018, 252, 1368–1371. [Google Scholar] [CrossRef]
- Hill, S.A.; Cave, N.J.; Forsyth, S. Effect of Age, Sex and Body Weight on the Serum Concentrations of Cobalamin and Folate in Cats Consuming a Consistent Diet. J. Feline Med. Surg. 2018, 20, 135–141. [Google Scholar] [CrossRef]
- Salas, A.; Manuelian, C.L.; Garganté, M.; Sanchez, N.; Fernández, S.; Compagnucci, M.; Ceròn, J.J.; Jeusette, I.; Vilaseca, L.; Torre, C. Fat Digestibility Is Reduced in Old Cats With Subnormal Cobalamin Concentrations. J. Nutr. Sci. 2014, 3, e62. [Google Scholar] [CrossRef] [PubMed]
- Stavroulaki, E.M.; Kokkinaki, K.C.G.; Saridomichelakis, M.N.; Steiner, J.M.; Lidbury, J.A.; Xenoulis, P.G. Serial Measurement of Serum Pancreatic Lipase Immunoreactivity, Feline Trypsin-like Immunoreactivity, and Cobalamin Concentrations in Kittens. Vet. Sci. 2022, 9, 469. [Google Scholar] [CrossRef]
- Ibarrola, P.; Blackwood, L.; Graham, P.A.; Evans, H.; German, A.J. Hypocobalaminaemia Is Uncommon in Cats in the United Kingdom. J. Feline Med. Surg. 2005, 7, 341–348. [Google Scholar] [CrossRef]
- Trehy, M.R.; German, A.J.; Silvestrini, P.; Serrano, G.; Batchelor, D.J. Hypercobalaminaemia Is Associated with Hepatic and Neoplastic Disease in Cats: A Cross Sectional Study. BMC Vet. Res. 2014, 10, 175. [Google Scholar] [CrossRef]
- Barron, P.M.; Mackie, J.T.; Evans, N.A.; Langer, N. Serum Cobalamin Concentrations in Healthy Cats And Cats With Non-Alimentary Tract Illness In Australia. Aust. Vet. J. 2009, 87, 280–283. [Google Scholar] [CrossRef]
- Armstrong, P.J.; Blanchard, G. Hepatic Lipidosis in Cats. Vet. Clin. N. Am. Small Anim. 2009, 39, 599–616. [Google Scholar] [CrossRef] [PubMed]
- Ruaux, C.G. Cobalamin in Companion Animals: Diagnostic Marker, Deficiency States and Therapeutic Implications. Vet. J. 2013, 196, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Carmel, R.; Green, R.; Rosenblatt, D.S.; Watkins, D. Update on Cobalamin, Folate, and Homocysteine. Hematology 2003, 2003, 62–81. [Google Scholar] [CrossRef]
- Solomon, L.R. Disorders of Cobalamin (Vitamin B12) Metabolism: Emerging Concepts in Pathophysiology, Diagnosis and Treatment. Blood Rev. 2007, 21, 113–130. [Google Scholar] [CrossRef] [PubMed]
- Berghoff, N.; Suchodolski, J.S.; Steiner, J.M. Association between Serum Cobalamin and Methylmalonic Acid Concentrations in Dogs. Vet. J. 2012, 191, 306–311. [Google Scholar] [CrossRef]
- Watanabe, T.; Hoshi, K.; Zhang, C.; Ishida, Y.; Sakata, I. Hyperammonaemia Due to Cobalamin Malabsorption in a Cat with Exocrine Pancreatic Insufficiency. J. Feline Med. Surg. 2012, 14, 942–945. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, J.D. Methionine Metabolism in Mammals. J. Nutr. Biochem. 1990, 1, 228–237. [Google Scholar] [CrossRef]
- Acharya, U.; Gau, J.-T.; Horvath, W.; Ventura, P.; Hsueh, C.-T.; Carlsen, W. Hemolysis and Hyperhomocysteinemia Caused by Cobalamin Deficiency: Three Case Reports and Review of the Literature. J. Hematol. Oncol. 2008, 1, 26. [Google Scholar] [CrossRef]
- Grützner, N.; Heilmann, R.M.; Stupka, K.C.; Rangachari, V.R.; Weber, K.; Holzenburg, A.; Suchodolski, J.S.; Steiner, J.M. Serum Homocysteine and Methylmalonic Acid Concentrations in Chinese Shar-Pei Dogs with Cobalamin Deficiency. Vet. J. 2013, 197, 420–426. [Google Scholar] [CrossRef]
- Patterson, B.E.; Barr, J.W.; Fosgate, G.T.; Berghoff, N.; Steiner, J.M.; Suchodolski, J.S.; Black, D.M. Homocysteine in Dogs with Systemic Inflammatory Response Syndrome. J. Small Anim. Pract. 2013, 54, 620–624. [Google Scholar] [CrossRef]
- Ruaux, C.G.; Steiner, J.M.; Williams, D.A. Early Biochemical and Clinical Responses to Cobalamin Supplementation in Cats with Signs of Gastrointestinal Disease and Severe Hypocobalaminemia. J. Vet. Intern. Med. 2005, 19, 155. [Google Scholar] [CrossRef]
- Mulreany, L.M.; Ramsay, E.C.; Cushing, A.C.; Suchodolski, J.S.; Lidbury, J.; Steiner, J.M. Exocrine Pancreatic Insufficiency-Like Syndrome in Four Captive Tigers (Panthera tigris). J. Zoo. Wildl. Med. 2021, 52, 1079–1083. [Google Scholar] [CrossRef] [PubMed]
- Fox, L.; Haefele, H.; Uelmen, J.; Hoppes, S.; Swenson, J.; Tolbert, K.; Suchodolski, J.S.; Lidbury, J.; Steiner, J.M. Biomarkers of Gastrointestinal Disease in Cheetahs (Acinonyx jubatus). J. Zoo. Wildl. Med. 2021, 52, 886–892. [Google Scholar] [CrossRef]
- Cook, A.K.; Suchodolski, J.S.; Steiner, J.M.; Robertson, J.E. The Prevalence of Hypocobalaminaemia in Cats with Spontaneous Hyperthyroidism. J. Small Anim. Pract. 2011, 52, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Battersby, I.A.; Giger, U.; Hall, E.J. Hyperammonaemic Encephalopathy Secondary to Selective Cobalamin Deficiency in a Juvenile Border Collie. J. Small Anim. Pract. 2005, 46, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Fyfe, J.C.; Giger, U.; Hall, C.A.; Jezyk, P.F.; Klumpp, S.A.; Levine, J.S.; Patterson, D.F. Inherited Selective Intestinal Cobalamin Malabsorption and Cobalamin Deficiency in Dogs. Pediatr. Res. 1991, 29, 24–31. [Google Scholar] [CrossRef]
- Stanley, E.; Eatroff, A. Hypocobalaminaemia as a Cause of Bone Marrow Failure and Pancytopenia in a Cat. Aust. Vet. J. 2017, 95, 156–160. [Google Scholar] [CrossRef]
- Kather, S.; Sielski, L.; Dengler, F.; Jirasek, A.; Heilmann, R.M. Prevalence and Clinical Relevance of Hypercobalaminaemia in Dogs and Cats. Vet. J. 2020, 265, 105547. [Google Scholar] [CrossRef]
- Sysel, A.M.; Valli, V.E.; Bauer, J.A. Immunohistochemical Quantification of the Cobalamin Transport Protein, Cell Surface Receptor and Ki-67 in Naturally Occurring Canine and Feline Malignant Tumors and in Adjacent Normal Tissues. Oncotarget 2015, 6, 2331. [Google Scholar] [CrossRef]
- Center, S.A. Feline Hepatic Lipidosis. Vet. Clin. N. Am. Small Anim. 2005, 35, 225–269. [Google Scholar] [CrossRef]
- Kook, P.H.; Lutz, S.; Sewell, A.C.; Bigler, B.; Reusch, C.E. Untersuchungen Zur Serumcobalaminkonzentration Bei Katzen Mit Gastrointestinaler Symptomatik. Schweiz. Arch. Tierheilkd. 2012, 154, 479–486. [Google Scholar] [CrossRef]
- Reed, N.; Gunn-Moore, D.; Simpson, K. Cobalamin, Folate and Inorganic Phosphate Abnormalities in Ill Cats. J. Feline Med. Surg. 2007, 9, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Kiselow, M.A.; Rassnick, K.M.; McDonough, S.P.; Goldstein, R.E.; Simpson, K.W.; Weinkle, T.K.; Erb, H.N. Outcome of Cats with Low-Grade Lymphocytic Lymphoma: 41 Cases (1995–2005). J. Am. Vet. Med. Assoc. 2008, 232, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Gianella, P.; Pietra, M.; Crisi, P.E.; Bergamini, P.F.; Fracassi, F.; Morini, M.; Boari, A. Evaluation of Clinicopathological Features in Cats With Chronic Gastrointestinal Signs. Pol. J. Vet. Sci. 2017, 20, 403–410. [Google Scholar] [CrossRef]
- Jugan, M.C.; August, J.R. Serum Cobalamin Concentrations and Small Intestinal Ultrasound Changes in 75 Cats with Clinical Signs of Gastrointestinal Disease: A Retrospective Study. J. Feline Med. Surg. 2017, 19, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Hunt, A.; Jugan, M.C. Anemia, Iron Deficiency, And Cobalamin Deficiency in Cats with Chronic Gastrointestinal Disease. J. Vet. Intern. Med. 2021, 35, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Thompson, K.A.; Parnell, N.K.; Hohenhaus, A.E.; Moore, G.E.; Rondeau, M.P. Feline Exocrine Pancreatic Insufficiency: 16 Cases (1992–2007). J. Feline Med. Surg. 2009, 11, 935–940. [Google Scholar] [CrossRef]
- Kook, H.P.; Zerbe, P.; Reusch, E.C. Exokrine Pankreasinsuffizienz Bei Der Katze. Schweiz. Arch. Tierheilkd. 2011, 153, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Steiner, J.M. Exocrine Pancreatic Insufficiency in the Cat. Top. Companion Anim. Med. 2012, 27, 113–116. [Google Scholar] [CrossRef]
- Xenoulis, P.G.; Zoran, D.L.; Fosgate, G.T.; Suchodolski, J.S.; Steiner, J.M. Feline Exocrine Pancreatic Insufficiency: A Retrospective Study of 150 Cases. J. Vet. Intern. Med. 2016, 30, 1790–1797. [Google Scholar] [CrossRef] [PubMed]
- Simpson, K.W. Pancreatitis and Triaditis in Cats: Causes and Treatment. J. Small Anim. Pract. 2015, 56, 40–49. [Google Scholar] [CrossRef]
- Festen, H.P.M. Intrinsic Factor Secretion and Cobalamin Absorption: Physiology and Pathophysiology in the Gastrointestinal Tract. Scand. J. Gastroenterol. 1991, 26, 1–7. [Google Scholar] [CrossRef]
- Guetterman, H.M.; Huey, S.L.; Knight, R.; Fox, A.M.; Mehta, S.; Finkelstein, J.L. Vitamin B-12 and the Gastrointestinal Microbiome: A Systematic Review. Adv. Nutr. 2022, 13, 530–558. [Google Scholar] [CrossRef]
- Degnan, P.H.; Taga, M.E.; Goodman, A.L. Vitamin B12 as a Modulator of Gut Microbial Ecology. Cell. Metab. 2014, 20, 769–778. [Google Scholar] [CrossRef]
- Quadros, E.V.; Sequeira, J.M. Cellular Uptake of Cobalamin: Transcobalamin and the TCblR/CD320 Receptor. Biochimie 2013, 95, 1008–1018. [Google Scholar] [CrossRef] [PubMed]
- Geesaman, B.M.; Whitehouse, W.H.; Viviano, K.R. Serum Cobalamin and Methylmalonic Acid Concentrations in Hyperthyroid Cats Before and After Radioiodine Treatment. J. Vet. Intern. Med. 2016, 30, 560–565. [Google Scholar] [CrossRef] [PubMed]
- McMichael, M.A.; Freeman, L.M.; Selhub, J.; Rozanski, E.A.; Brown, D.J.; Nadeau, M.R.; Rush, J.E. Plasma Homocysteine, B Vitamins, and Amino Acid Concentrations in Cats with Cardiomyopathy and Arterial Thromboembolism. J. Vet. Intern. Med. 2000, 14, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, E. Vitamin B12, Folic Acid, and the Nervous System. Lancet Neurol. 2006, 5, 949–960. [Google Scholar] [CrossRef]
- Salvadori, C.; Cantile, C.; de Ambrogi, G.; Arispici, M. Degenerative Myelopathy Associated with Cobalamin Deficiency in a Cat. J. Vet. Med. A 2003, 50, 292–296. [Google Scholar] [CrossRef] [PubMed]
- Simpson, K.; Battersby, I.; Lowrie, M. Suspected Acquired Hypocobalaminaemic Encephalopathy in a Cat: Resolution of Encephalopathic Signs and MRI Lesions Subsequent to Cobalamin Supplementation. J. Feline Med. Surg. 2012, 14, 350–355. [Google Scholar] [CrossRef]
- Nielsen, M.J.; Rasmussen, M.R.; Andersen, C.B.F.; Nexø, E.; Moestrup, S.K. Vitamin B12 Transport from Food to the Body’s Cells—A Sophisticated, Multistep Pathway. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 345–354. [Google Scholar] [CrossRef]
- Kook, P.H.; Melliger, R.H.; Hersberger, M. Efficacy of Intramuscular Hydroxocobalamin Supplementation in Cats with Cobalamin Deficiency and Gastrointestinal Disease. J. Vet. Intern. Med. 2020, 34, 1872–1878. [Google Scholar] [CrossRef]
- Worhunsky, P.; Toulza, O.; Rishniw, M.; Berghoff, N.; Ruaux, C.G.; Steiner, J.M.; Simpson, K.W. The Relationship of Serum Cobalamin to Methylmalonic Acid Concentrations and Clinical Variables in Cats. J. Vet. Intern. Med. 2013, 27, 1056–1063. [Google Scholar] [CrossRef]
- Toresson, L.; Steiner, J.M.; Olmedal, G.; Larsen, M.; Suchodolski, J.S.; Spillmann, T. Oral Cobalamin Supplementation in Cats with Hypocobalaminaemia: A Retrospective Study. J. Feline Med. Surg. 2017, 19, 1302–1306. [Google Scholar] [CrossRef] [PubMed]
| Parenteral Administration a [15,37,55] | Parenteral Administration a [86] | Oral Administration a [88] |
|---|---|---|
| 250 μg SC/IM every 7 days for 6 weeks, then 250 μg SC/IM every 14 days for 6 weeks | 300 μg hydroxocobalamin IM every 14 days 3–4 times | 250 μg cyanocobalamin every day until serum concentration exceeds range |
| If value is in normal range, 250 μg SC/IM once a month; if value exceeds range, reduce dose and continue treatment until primary disease is resolved |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siani, G.; Mercaldo, B.; Alterisio, M.C.; Di Loria, A. Vitamin B12 in Cats: Nutrition, Metabolism, and Disease. Animals 2023, 13, 1474. https://doi.org/10.3390/ani13091474
Siani G, Mercaldo B, Alterisio MC, Di Loria A. Vitamin B12 in Cats: Nutrition, Metabolism, and Disease. Animals. 2023; 13(9):1474. https://doi.org/10.3390/ani13091474
Chicago/Turabian StyleSiani, Gerardo, Beatrice Mercaldo, Maria Chiara Alterisio, and Antonio Di Loria. 2023. "Vitamin B12 in Cats: Nutrition, Metabolism, and Disease" Animals 13, no. 9: 1474. https://doi.org/10.3390/ani13091474
APA StyleSiani, G., Mercaldo, B., Alterisio, M. C., & Di Loria, A. (2023). Vitamin B12 in Cats: Nutrition, Metabolism, and Disease. Animals, 13(9), 1474. https://doi.org/10.3390/ani13091474







