Glucose Starvation Inhibits Ferroptosis by Activating the LKB1/AMPK Signaling Pathway and Promotes the High Speed Linear Motility of Dairy Goat Sperm
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Animals and Semen Collection
2.3. Ethics Statement
2.4. Semen Processing
2.5. Sperm Motility
2.6. Biochemical Assays
2.7. Mitochondrial Permeability Transition Pore (mPTP) Fluorescence Intensity
2.8. Immunofluorescence Assays
2.9. Western Blotting
2.10. Statistical Analysis
3. Results
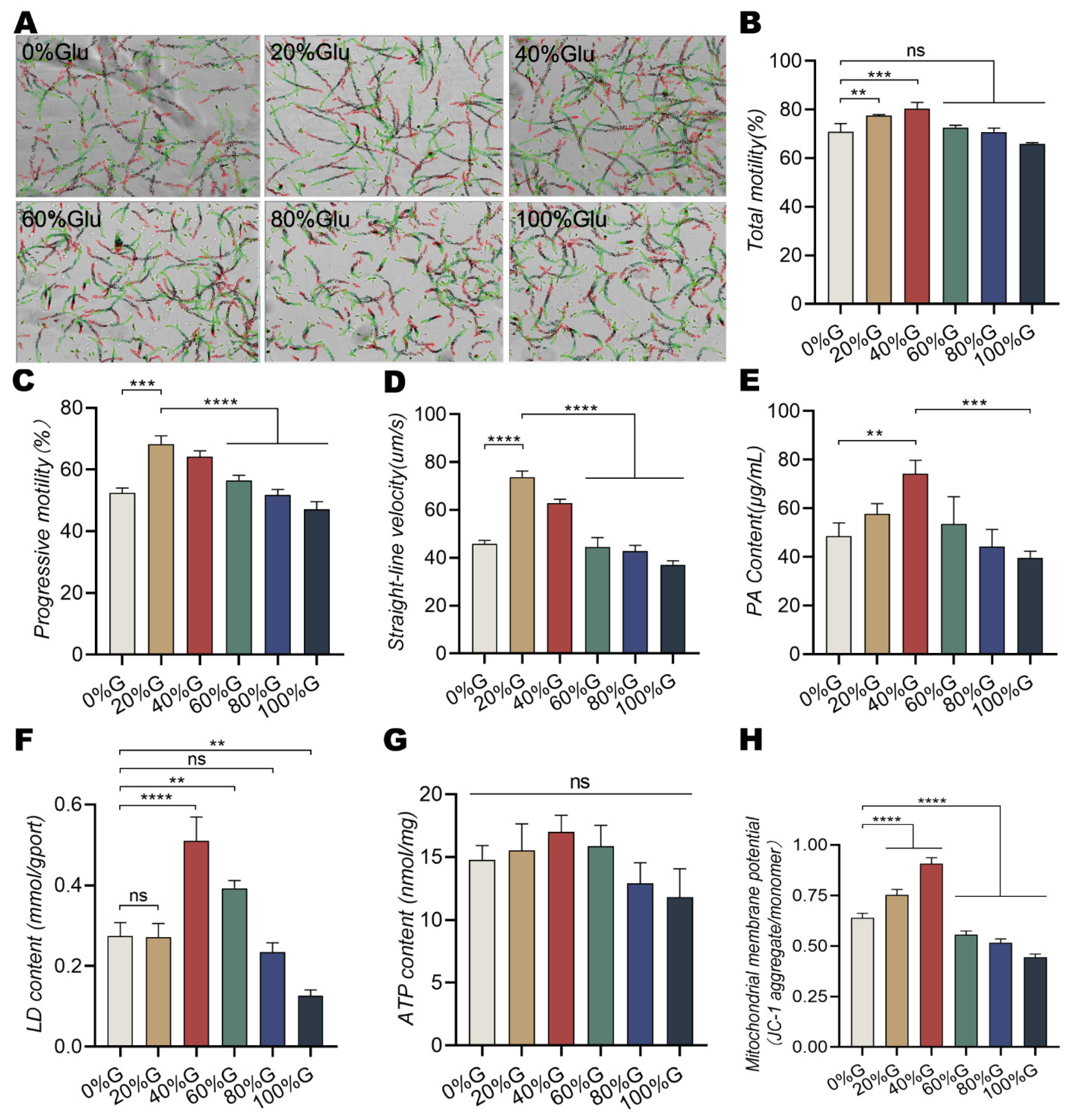
3.1. Sperm Energy Metabolic Pathway and Sperm Motility Patterns Are Changed in Different Concentrations of Glucose
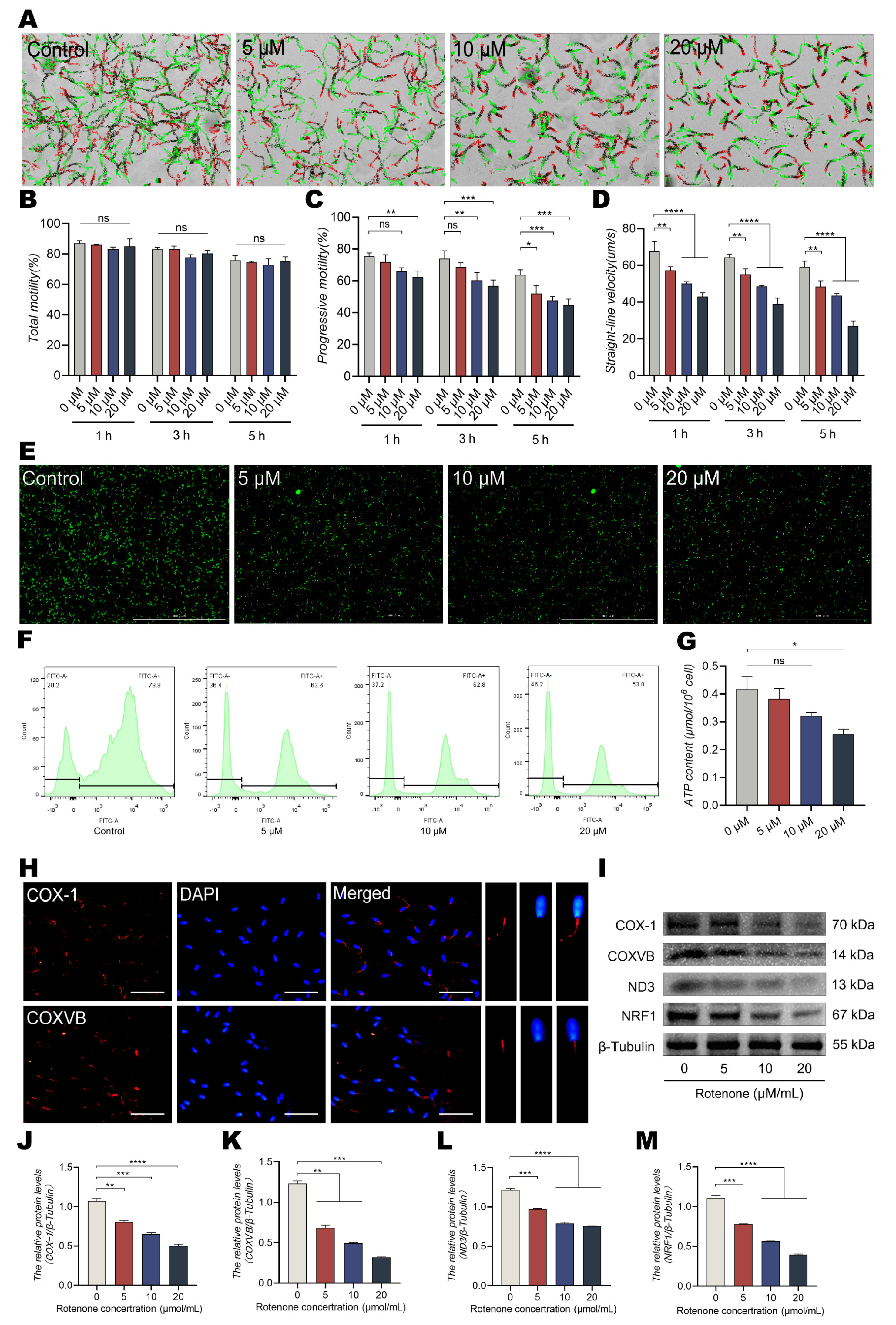
3.2. Addition of Rotenone to the Culture Reduces ATP Content and Straight Line Motility
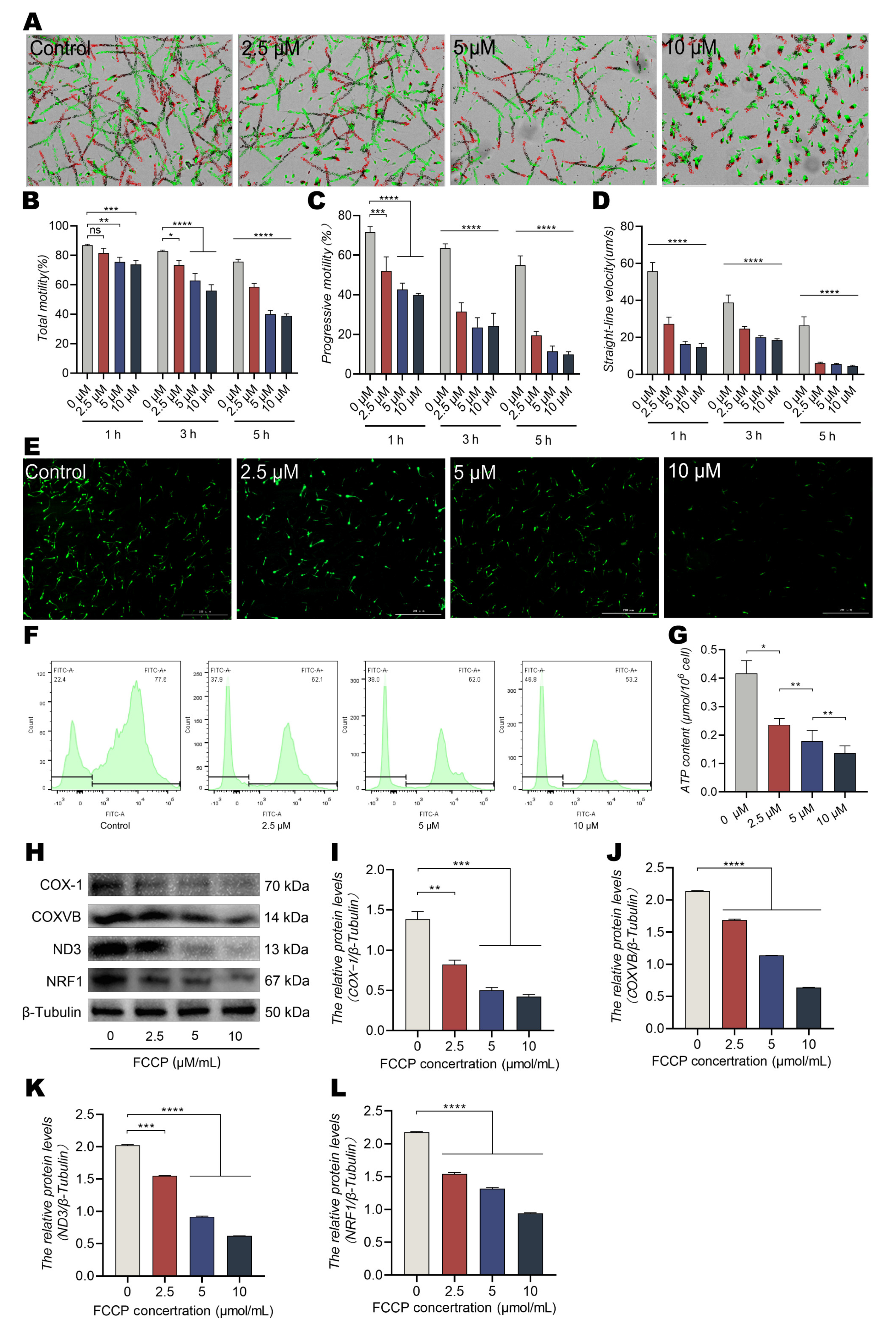
3.3. The OXPHOS Uncoupler FCCP Reduces Sperm Motility and ATP Levels
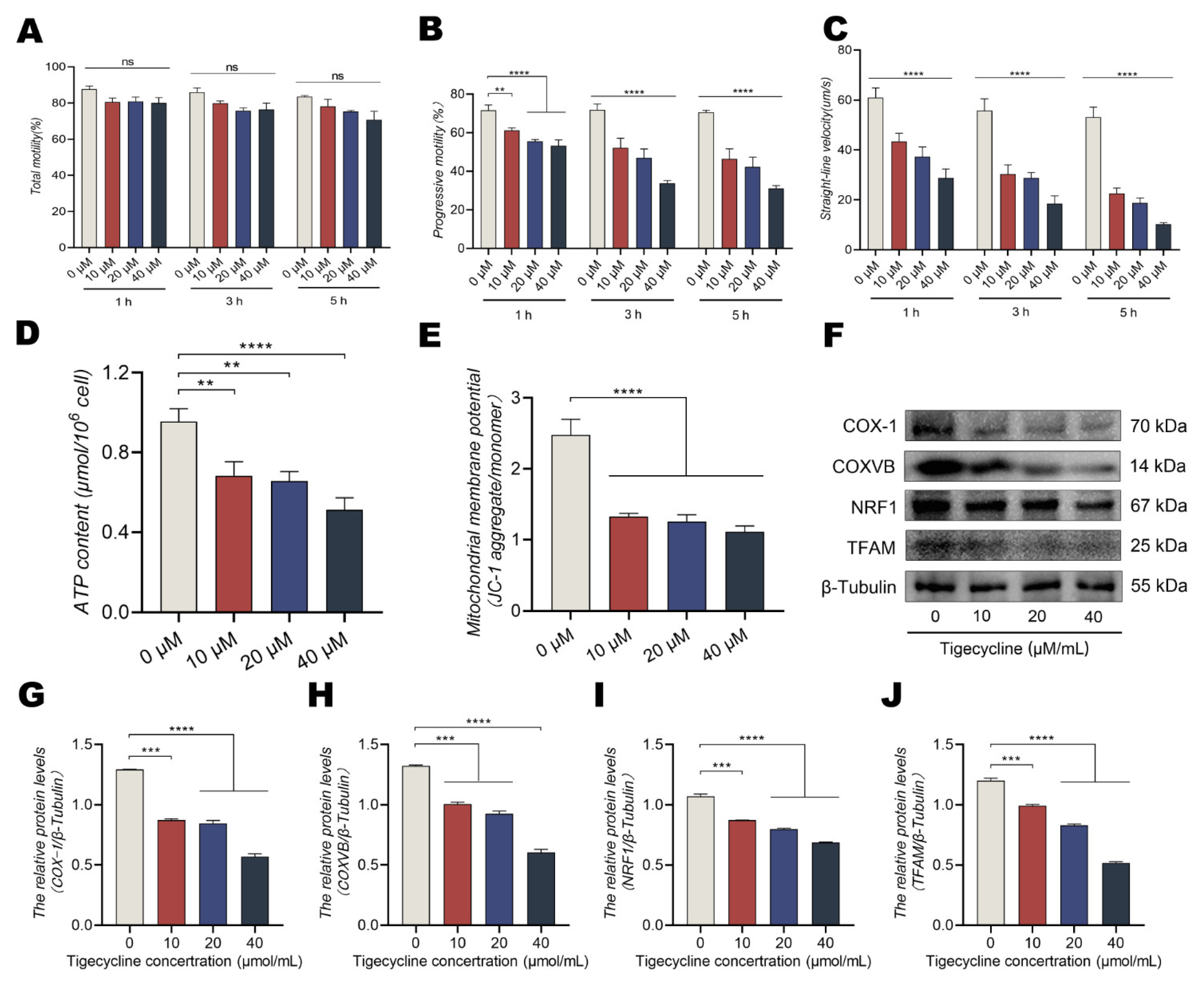
3.4. The Mitochondrial Translational Inhibitor TIG Reduces MMP and ATP Levels in a Dose-Dependent Manner
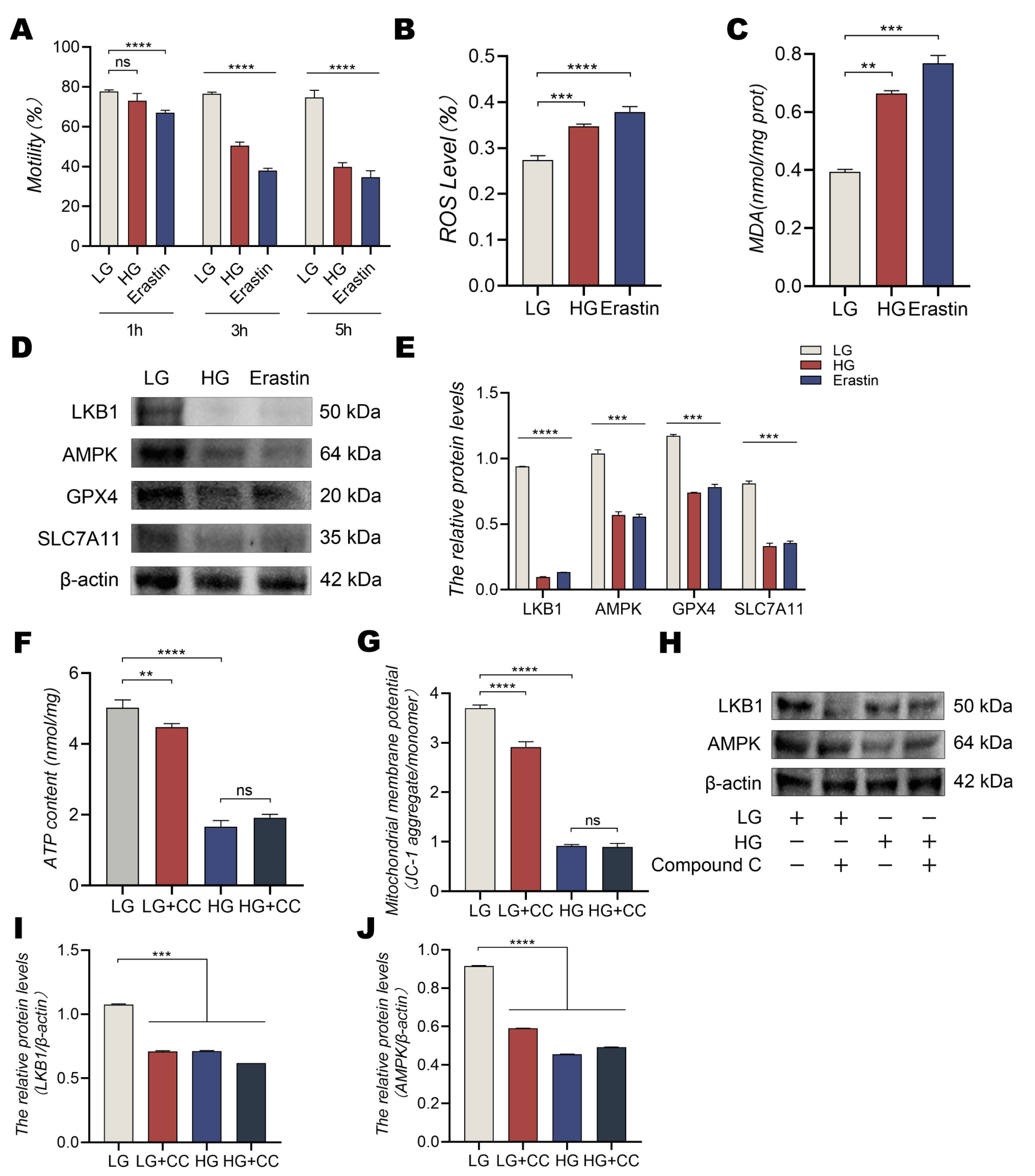
3.5. Low Glucose Conditions Inhibit Ferroptosis-Induced Oxidative Damage by Regulating the Liver Kinase B1 (LKB1)/AMPK Signaling Pathway
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hunter, R.H. Fallopian tube physiology: Preliminaries to monospermic fertilization and cellular events post-fertilization. Ernst Scher. Res. Found. Workshop 2005, 52, 245–261. [Google Scholar] [CrossRef]
- Amaral, A. Energy metabolism in mammalian sperm motility. WIREs Mech. Dis. 2022, 14, e1569. [Google Scholar] [CrossRef]
- Owen, D.H.; Katz, D.F. A review of the physical and chemical properties of human semen and the formulation of a semen simulant. J. Androl. 2005, 26, 459–469. [Google Scholar] [CrossRef]
- Cao, W.; Aghajanian, H.K.; Haig-Ladewig, L.A.; Gerton, G.L. Sorbitol can fuel mouse sperm motility and protein tyrosine phosphorylation via sorbitol dehydrogenase. Biol. Reprod. 2009, 80, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Goodson, S.G.; Qiu, Y.; Sutton, K.A.; Xie, G.; Jia, W.; O’Brien, D.A. Metabolic substrates exhibit differential effects on functional parameters of mouse sperm capacitation. Biol. Reprod. 2012, 87, 75. [Google Scholar] [CrossRef]
- Du Plessis, S.S.; Agarwal, A.; Mohanty, G.; van der Linde, M. Oxidative phosphorylation versus glycolysis: What fuel do spermatozoa use? Asian J. Androl. 2015, 17, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Piomboni, P.; Focarelli, R.; Stendardi, A.; Ferramosca, A.; Zara, V. The role of mitochondria in energy production for human sperm motility. Int. J. Androl. 2012, 35, 109–124. [Google Scholar] [CrossRef]
- Odet, F.; Gabel, S.; London, R.E.; Goldberg, E.; Eddy, E.M. Glycolysis and mitochondrial respiration in mouse LDHC-null sperm. Biol. Reprod. 2013, 88, 95. [Google Scholar] [CrossRef]
- Pasupuleti, V. Role of Glycolysis and Respiration in Sperm Metabolism and Motility. Master’s Thesis, Kent State University, Kent, OH, USA, 2007. [Google Scholar]
- Losano, J.; Angrimani, D.; Dalmazzo, A.; Rui, B.R.; Brito, M.M.; Mendes, C.M.; Kawai, G.; Vannucchi, C.I.; Assumpção, M.; Barnabe, V.H.; et al. Effect of mitochondrial uncoupling and glycolysis inhibition on ram sperm functionality. Reprod. Domest. Anim. Zuchthyg. 2017, 52, 289–297. [Google Scholar] [CrossRef]
- Storey, B.T. Mammalian sperm metabolism: Oxygen and sugar, friend and foe. Int. J. Dev. Biol. 2008, 52, 427–437. [Google Scholar] [CrossRef]
- Gibb, Z.; Aitken, R.J. The Impact of Sperm Metabolism during In Vitro Storage: The Stallion as a Model. BioMed Res. Int. 2016, 2016, 9380609. [Google Scholar] [CrossRef] [PubMed]
- Varner, D.D.; Gibb, Z.; Aitken, R.J. Stallion fertility: A focus on the spermatozoon. Equine Vet. J. 2015, 47, 16–24. [Google Scholar] [CrossRef]
- Bucci, D.; Rodriguez-Gil, J.E.; Vallorani, C.; Spinaci, M.; Galeati, G.; Tamanini, C. GLUTs and mammalian sperm metabolism. J. Androl. 2011, 32, 348–355. [Google Scholar] [CrossRef]
- Davila, M.P.; Muñoz, P.M.; Bolaños, J.M.; Stout, T.A.; Gadella, B.M.; Tapia, J.A.; da Silva, C.B.; Ferrusola, C.O.; Peña, F.J. Mitochondrial ATP is required for the maintenance of membrane integrity in stallion spermatozoa, whereas motility requires both glycolysis and oxidative phosphorylation. Reproduction 2016, 152, 683–694. [Google Scholar] [CrossRef]
- Hardie, D.G.; Hawley, S.A.; Scott, J.W. AMP-activated protein kinase--development of the energy sensor concept. J. Physiol. 2006, 574, 7–15. [Google Scholar] [CrossRef]
- Chhipa, R.R.; Wu, Y.; Mohler, J.L.; Ip, C. Survival advantage of AMPK activation to androgen-independent prostate cancer cells during energy stress. Cell. Signal. 2010, 22, 1554–1561. [Google Scholar] [CrossRef] [PubMed]
- Tosca, L.; Chabrolle, C.; Dupont, J. AMPK: A link between metabolism and reproduction? Med. Sci. M/S 2008, 24, 297–300. [Google Scholar] [CrossRef]
- Huang, J.; Chen, G.; Wang, J.; Liu, S.; Su, J. Platycodin D regulates high glucose-induced ferroptosis of HK-2 cells through glutathione peroxidase 4 (GPX4). Bioengineered 2022, 13, 6627–6637. [Google Scholar] [CrossRef]
- Ren, Y.; Shen, H.M. Critical role of AMPK in redox regulation under glucose starvation. Redox Biol. 2019, 25, 101154. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Jiang, L.; Gu, X.; Huang, S.; Pang, J.; Wu, Y.; Yin, J.; Wang, J. SIRT3 deficiency is resistant to autophagy-dependent ferroptosis by inhibiting the AMPK/mTOR pathway and promoting GPX4 levels. J. Cell. Physiol. 2020, 235, 8839–8851. [Google Scholar] [CrossRef] [PubMed]
- Grahame Hardie, D. AMP-activated protein kinase: A key regulator of energy balance with many roles in human disease. J. Intern. Med. 2014, 276, 543–559. [Google Scholar] [CrossRef] [PubMed]
- Ferramosca, A.; Zara, V. Bioenergetics of mammalian sperm capacitation. BioMed Res. Int. 2014, 2014, 902953. [Google Scholar] [CrossRef]
- Yanagimachi, R. The movement of golden hamster spermatozoa before and after capacitation. J. Reprod. Fertil. 1970, 23, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Stauss, C.R.; Votta, T.J.; Suarez, S.S. Sperm motility hyperactivation facilitates penetration of the hamster zona pellucida. Biol. Reprod. 1995, 53, 1280–1285. [Google Scholar] [CrossRef]
- Keeley, T.P.; Mann, G.E. Defining Physiological Normoxia for Improved Translation of Cell Physiology to Animal Models and Humans. Physiol. Rev. 2019, 99, 161–234. [Google Scholar] [CrossRef]
- Qiu, J.H.; Li, Y.W.; Xie, H.L.; Li, Q.; Dong, H.B.; Sun, M.J.; Gao, W.Q.; Tan, J.H. Effects of glucose metabolism pathways on sperm motility and oxidative status during long-term liquid storage of goat semen. Theriogenology 2016, 86, 839–849. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Li, R.; Wang, L.; Zheng, Y.; Hoque, S.A.M.; Lv, Y.; Zeng, W. Glycogen Synthase Kinase-3 Regulates Sperm Motility and Acrosome Reaction via Affecting Energy Metabolism in Goats. Front. Physiol. 2019, 10, 968. [Google Scholar] [CrossRef]
- Zhu, Z.; Umehara, T.; Okazaki, T.; Goto, M.; Fujita, Y.; Hoque, S.A.M.; Kawai, T.; Zeng, W.; Shimada, M. Gene Expression and Protein Synthesis in Mitochondria Enhance the Duration of High-Speed Linear Motility in Boar Sperm. Front. Physiol. 2019, 10, 252. [Google Scholar] [CrossRef]
- Lin, S.C.; Hardie, D.G. AMPK: Sensing Glucose as well as Cellular Energy Status. Cell Metab. 2018, 27, 299–313. [Google Scholar] [CrossRef]
- Dunn, J.; Grider, M.H. Physiology, Adenosine Triphosphate. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2022. [Google Scholar]
- Nsiah-Sefaa, A.; McKenzie, M. Combined defects in oxidative phosphorylation and fatty acid β-oxidation in mitochondrial disease. Biosci. Rep. 2016, 36, e00313. [Google Scholar] [CrossRef]
- Heo, G.; Sun, M.H.; Jiang, W.J.; Li, X.H.; Lee, S.H.; Guo, J.; Zhou, D.; Cui, X.S. Rotenone causes mitochondrial dysfunction and prevents maturation in porcine oocytes. PLoS ONE 2022, 17, e0277477. [Google Scholar] [CrossRef]
- Blanco-Prieto, O.; Catalán, J.; Trujillo-Rojas, L.; Peña, A.; Rivera Del Álamo, M.M.; Llavanera, M.; Bonet, S.; Fernández-Novell, J.M.; Yeste, M.; Rodríguez-Gil, J.E. Red LED Light Acts on the Mitochondrial Electron Chain of Mammalian Sperm via Light-Time Exposure-Dependent Mechanisms. Cells 2020, 9, 2546. [Google Scholar] [CrossRef]
- Moraes, C.R.; Meyers, S. The sperm mitochondrion: Organelle of many functions. Anim. Reprod. Sci. 2018, 194, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001, 414, 813–820. [Google Scholar] [CrossRef] [PubMed]
- LaRocca, T.J.; Sosunov, S.A.; Shakerley, N.L.; Ten, V.S.; Ratner, A.J. Hyperglycemic Conditions Prime Cells for RIP1-dependent Necroptosis. J. Biol. Chem. 2016, 291, 13753–13761. [Google Scholar] [CrossRef] [PubMed]
- Peña, F.J.; Ortiz-Rodríguez, J.M.; Gaitskell-Phillips, G.L.; Gil, M.C.; Ortega-Ferrusola, C.; Martín-Cano, F.E. An integrated overview on the regulation of sperm metabolism (glycolysis-Krebs cycle-oxidative phosphorylation). Anim. Reprod. Sci. 2022, 246, 106805. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Garcia, D.; Shaw, R.J. AMPK: Mechanisms of Cellular Energy Sensing and Restoration of Metabolic Balance. Mol. Cell 2017, 66, 789–800. [Google Scholar] [CrossRef]
- Fang, X.; Xu, Q.Y.; Jia, C.; Peng, Y.F. Metformin improves epididymal sperm quality and antioxidant function of the testis in diet-induced obesity rats. Zhonghua Nan Ke Xue Natl. J. Androl. 2012, 18, 146–149. [Google Scholar]
- Nguyen, T.M.; Seigneurin, F.; Froment, P.; Combarnous, Y.; Blesbois, E. The 5′-AMP-Activated Protein Kinase (AMPK) Is Involved in the Augmentation of Antioxidant Defenses in Cryopreserved Chicken Sperm. PLoS ONE 2015, 10, e0134420. [Google Scholar] [CrossRef]
- Zhu, Z.; Li, R.; Ma, G.; Bai, W.; Fan, X.; Lv, Y.; Luo, J.; Zeng, W. 5′-AMP-Activated Protein Kinase Regulates Goat Sperm Functions via Energy Metabolism In Vitro. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2018, 47, 2420–2431. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Zhang, G.; Wen, F.; Xian, M.; Guo, S.; Zhang, X.; Feng, X.; Hu, Z.; Hu, J. Glucose Starvation Inhibits Ferroptosis by Activating the LKB1/AMPK Signaling Pathway and Promotes the High Speed Linear Motility of Dairy Goat Sperm. Animals 2023, 13, 1442. https://doi.org/10.3390/ani13091442
Li Y, Zhang G, Wen F, Xian M, Guo S, Zhang X, Feng X, Hu Z, Hu J. Glucose Starvation Inhibits Ferroptosis by Activating the LKB1/AMPK Signaling Pathway and Promotes the High Speed Linear Motility of Dairy Goat Sperm. Animals. 2023; 13(9):1442. https://doi.org/10.3390/ani13091442
Chicago/Turabian StyleLi, Yu, Guangzhi Zhang, Fei Wen, Ming Xian, Songmao Guo, Xing Zhang, Xianzhou Feng, Zhangtao Hu, and Jianhong Hu. 2023. "Glucose Starvation Inhibits Ferroptosis by Activating the LKB1/AMPK Signaling Pathway and Promotes the High Speed Linear Motility of Dairy Goat Sperm" Animals 13, no. 9: 1442. https://doi.org/10.3390/ani13091442
APA StyleLi, Y., Zhang, G., Wen, F., Xian, M., Guo, S., Zhang, X., Feng, X., Hu, Z., & Hu, J. (2023). Glucose Starvation Inhibits Ferroptosis by Activating the LKB1/AMPK Signaling Pathway and Promotes the High Speed Linear Motility of Dairy Goat Sperm. Animals, 13(9), 1442. https://doi.org/10.3390/ani13091442



