A Diet Rich in HUFAs Enhances the Energetic and Immune Response Capacities of Larvae of the Scallop Argopecten purpuratus
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Approvals
2.2. Larval Rearing
2.3. Microalgae Cultivation, Proximal Analysis, and Diet Design
2.3.1. Culture Conditions
2.3.2. Proximate Composition
- Carbohydrates: One liter of each microalgal culture was dehydrated at 60 °C until constant mass. The dry mass was pulverized and homogenized with deionized water (1:1 w/v). The phenol–sulfuric acid method was applied [47] to determine the total carbohydrate concentration. Briefly, 5 mL of 20% trichloroacetic acid was mixed with 1.5 mL of the homogenate, heated at 65 °C for 1 h, and centrifuged at 6000× g for 15 min. Then, 1 mL of the supernatant was mixed with 2.5% phenol and concentrated sulfuric acid. The carbohydrate concentration was determined using an EPOCH microplate spectrophotometer (BioTek) at 490 nm, and a 50 µg·mL−1 solution of glycogen in deionized water was used as a standard. The carbohydrate content was expressed in µg·mg−1 dry mass.
- Proteins: Protein content was quantified following Brokordt et al. [48]. Briefly, a 3 mg wet mass of each microalga was homogenized in a buffer containing 32 mM Tris-HCl at pH 7.5, 2% SDS, 1 mM EDTA, 1 mM Pefabloc, and 1 mM protease inhibitor cocktail (Sigma). The obtained homogenates were incubated at 100 °C for 5 min. A second incubation (for 5 min at 100 °C) was applied after resuspending the homogenate in 100 μL of homogenization buffer. The obtained homogenate was centrifuged for 20 min at 10,600× g. A Micro-BCA kit was then used to quantify total protein in an aliquot of the supernatant in an EPOCH microplate spectrophotometer (BioTek) at 660 nm, using a 0.4 mg·mL−1 solution of albumin serum in deionized water as a standard.
- Lipids and fatty acid composition: Lipid extraction was performed according to Folch et al.’s [49] protocols. Fatty acid methyl esters (FAMEs) of total lipids were prepared by transmethylation with 14% BF3 MeOH for 10 min at 60 °C. FAMEs were then obtained by liquid–liquid extraction with hexane and washed with 20% NaCl. The organic phase was roto-evaporated, resuspended in 1 mL of hexane, and filtered (0.22 µm PVDF filter). FAMEs were analyzed by injecting 1 µL of the obtained organic phase in a Clarus 600, PerkinElmer gas chromatograph with an FID detector, using a Supelco Wax 320 Omega column (30 m × 0.25 µm film thickness) and helium as the carrier gas. The temperature ramp started at 140 °C for 5 min, and then the temperature increased at a rate of 2 °C per minute until reaching 240 °C and being maintained at that level for 5 min. Individual FAs were quantified by relative percentage by comparing the retention times (RT) and the peak area of the sample with a FAME standard (Sigma, CRM47885, St. Louis, MO, USA).
2.4. Diet Design
2.5. Membrane Fluidity Analysis in Larvae and Early Juveniles
2.5.1. Decalcification and Incubation
2.5.2. Fluorescence Spectroscopy
2.6. Bacterial Challenge
2.7. Enzymatic Assays
2.7.1. Tissue Homogenization
2.7.2. Pyruvate Kinase (PK)
2.7.3. Citrate Synthase (CS)
2.7.4. Electron Transport System (ETS)
2.8. Oxygen Consumption
2.9. RNA Isolation, cDNA Synthesis, and Relative mRNA Expression of Immune-Associated Genes by RT–qPCR
2.10. Postchallenge Vibrio Quantification
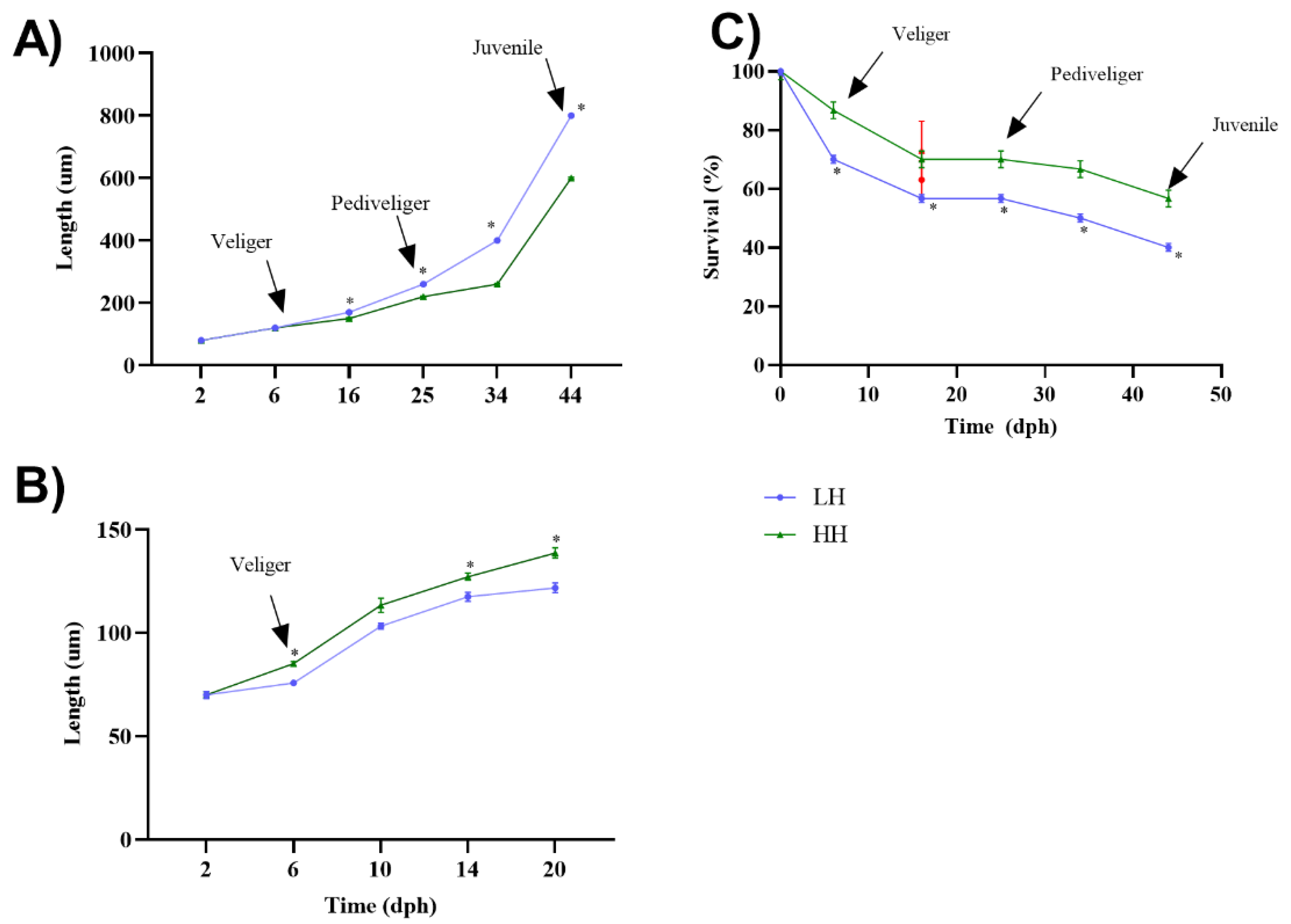
2.11. Growth and Survival
2.12. Statistical Analysis
3. Results
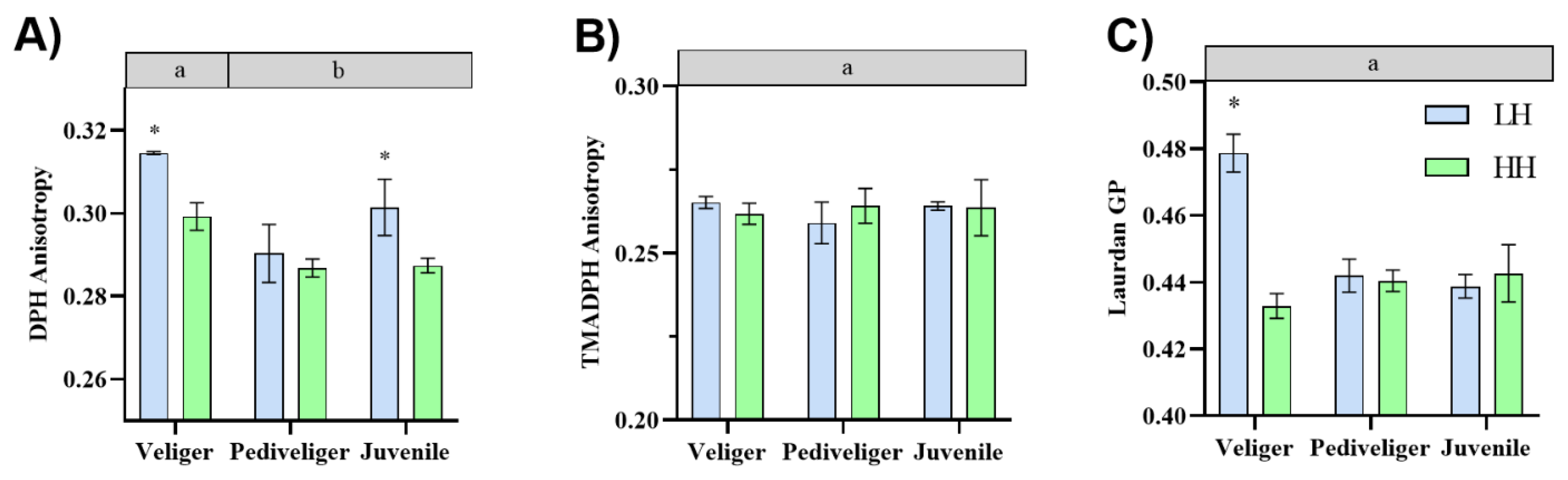
3.1. Membrane Fluidity Analyses
3.2. Enzyme Activity
3.2.1. Pyruvate Kinase (PK)
3.2.2. Citrate Synthase (CS)
3.2.3. Electro Transport System (ETS)
3.2.4. PK:CS
3.2.5. ETS:CS
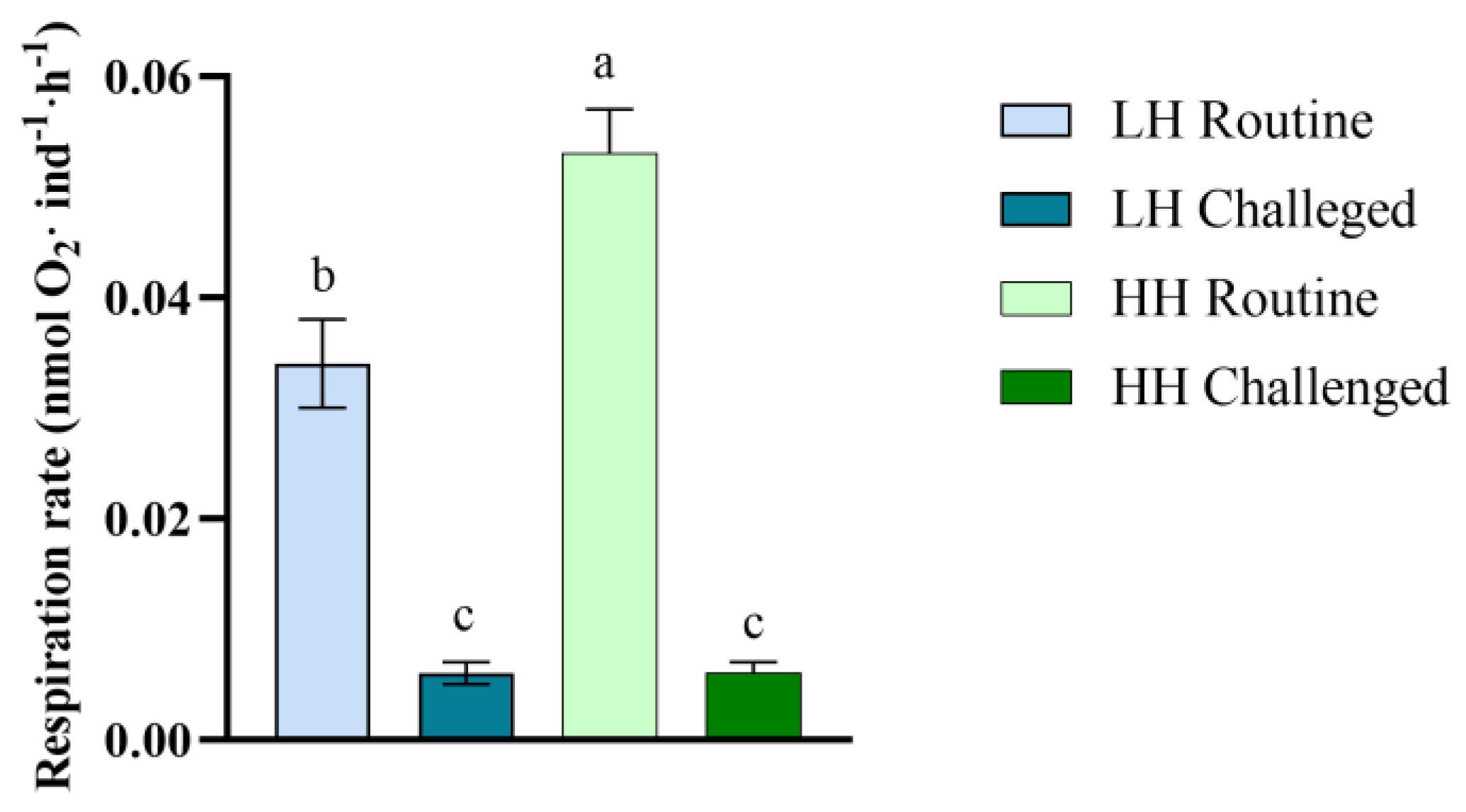
3.3. Respiration Rate
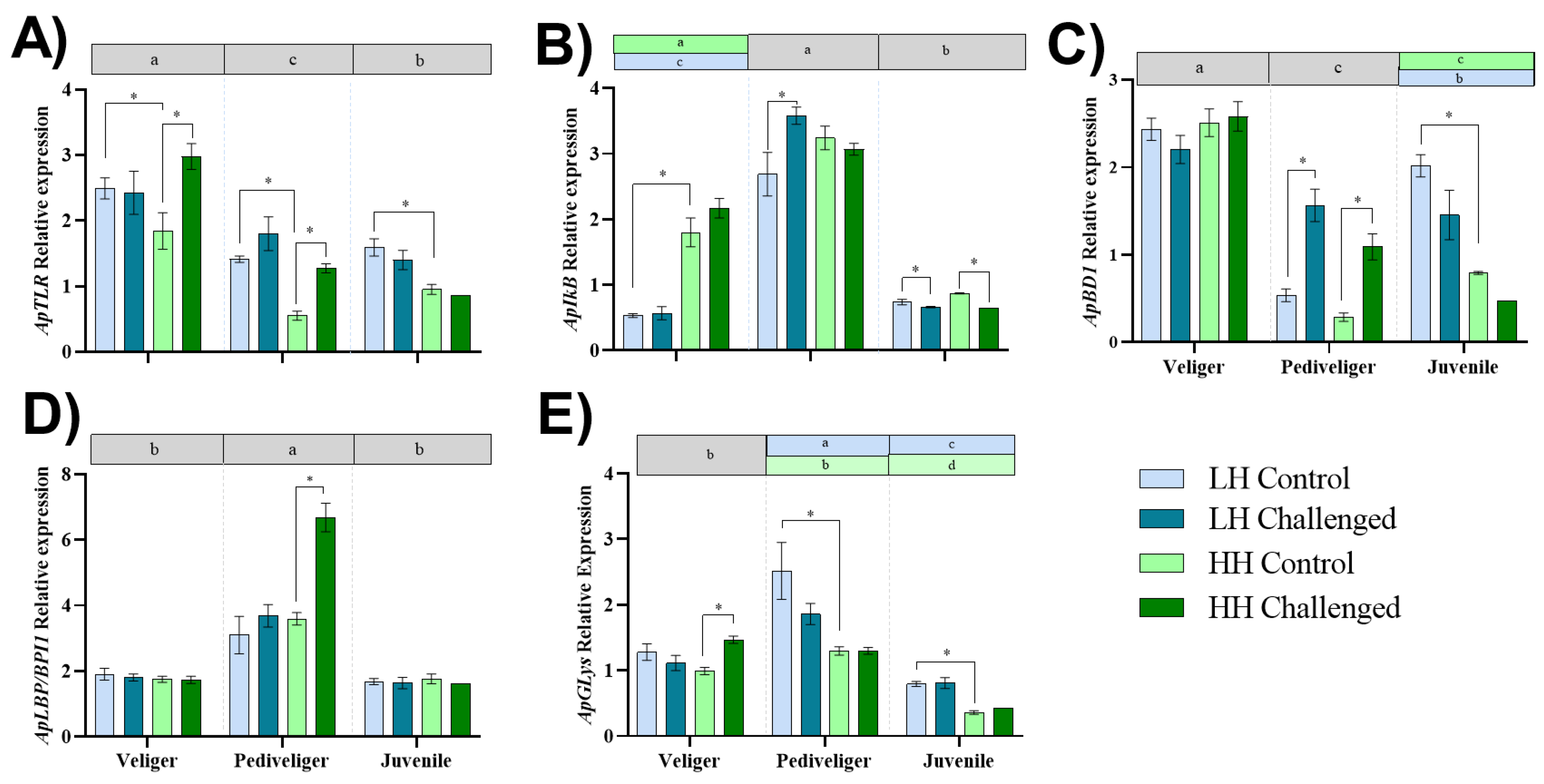
3.4. Expression of Immune-Related Genes
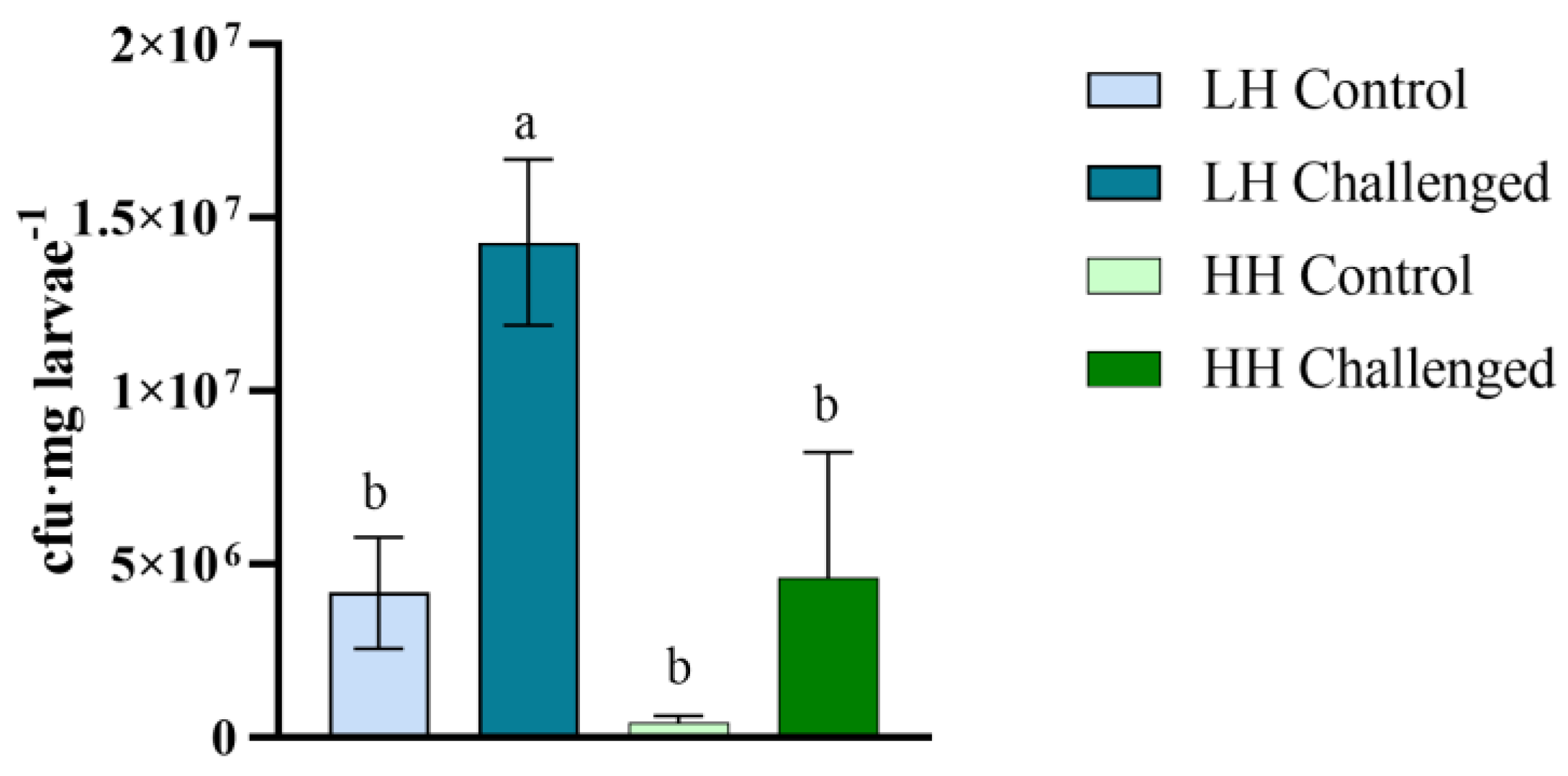
3.5. Vibrio Proliferation
3.6. Growth and Survival
4. Discussion
4.1. Effect of Dietary HUFA Levels on Cellular Membrane Fluidity during Early Development
4.2. Effect of Dietary HUFA Levels on Energy Metabolism during Early Development and Bacterial Challenge
4.3. Effect of Dietary HUFAs on the Expression of Immune-Related Genes in Larvae and Early Juveniles
4.4. Effect of Dietary HUFAs on Vibrio Proliferation in Challenged Veliger Larvae
4.5. Effect of Dietary HUFA Levels on Growth and Survival during Early Development
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. FAO STATS. License: CC BY-NC-SA 3.0 IGO. Available online: https://www.fao.org/fishery/statistics-query/en/aquaculture (accessed on 7 July 2022).
- Cai, J.; Han, Y.; Wang, Z. Isolation of Vibrio parahaemolyticus from abalone (Haliotis diversicolor supertexta L.) postlarvae associated with mass mortalities. Aquaculture 2006, 257, 161–166. [Google Scholar] [CrossRef]
- Liu, R.; Qiu, L.; Yu, Z.; Zi, J.; Yue, F.; Wang, L.; Zhang, H.; Teng, W.; Liu, X.; Song, L. Identification and characterisation of pathogenic Vibrio splendidus from Yesso scallop (Patinopecten yessoensis) cultured in a low temperature environment. J. Invertebr. Pathol. 2013, 114, 144–150. [Google Scholar] [CrossRef]
- Dubert, J.; Romalde, J.L.; Prado, S.; Barja, J.L. Vibrio bivalvicida sp. nov., a novel larval pathogen for bivalve molluscs reared in a hatchery. Syst. Appl. Microbiol. 2016, 39, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Urtubia, R.; Miranda, C.D.; Rodríguez, S.; Dubert, J.; Barja, J.L.; Rojas, R. First Report, Characterization and Pathogenicity of Vibrio chagasii Isolated from Diseased Reared Larvae of Chilean Scallop, Argopecten purpuratus (Lamarck, 1819). Pathogens 2023, 12, 183. [Google Scholar] [CrossRef]
- Riquelme, C.; Hayashida, G.; Toranzo, A.E.; Vilches, J.; Chavez, P. Pathogenicity studies on a Vibrio anguillarum-related (VAR) strain causing an epizootic in Argopecten purpuratus larvae cultured in Chile. Dis. Aquat. Organ. 1995, 22, 135–141. [Google Scholar] [CrossRef]
- Rojas, R.; Miranda, C.D.; Opazo, R.; Romero, J. Characterization and pathogenicity of Vibrio splendidus strains associated with massive mortalities of commercial hatchery-reared larvae of scallop Argopecten purpuratus (Lamarck, 1819). J. Invertebr. Pathol. 2015, 124, 61–69. [Google Scholar] [CrossRef]
- Rojas, R.; Miranda, C.D.; Santander, J.; Romero, J. First report of Vibrio tubiashii associated with a massive larval mortality event in a commercial hatchery of scallop Argopecten purpuratus in Chile. Front. Microbiol. 2016, 7, 1473. [Google Scholar] [CrossRef]
- Garnier, M.; Labreuche, Y.; Garcia, C.; Robert, M.; Nicolas, J.L. Evidence for the involvement of pathogenic bacteria in summer mortalities of the pacific oyster Crassostrea gigas. Microb. Ecol. 2007, 53, 187–196. [Google Scholar] [CrossRef]
- Delaunay, F.; Marty, Y.; Moal, J.; Samain, J.F. Growth and lipid class composition of Pecten maximus (L.) larvae grown under hatchery conditions. J. Exp. Mar. Bio. Ecol. 1992, 163, 209–219. [Google Scholar] [CrossRef]
- Revilla, J.; Márquez, A.; Lodeiros, C.; Sonnenholzner, S. Experimental cultures of giant lion’s paw Nodipecten subnodosus in equatorial waters of the eastern pacific: Progress in larval development and suspended culture. Lat. Am. J. Aquat. Res. 2019, 47, 818–825. [Google Scholar] [CrossRef]
- Genard, B.; Moraga, D.; Pernet, F.; David, É.; Boudry, P.; Tremblay, R. Expression of candidate genes related to metabolism, immunity and cellular stress during massive mortality in the American oyster Crassostrea virginica larvae in relation to biochemical and physiological parameters. Gene 2012, 499, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Yue, F.; Shi, X.; Zhou, Z.; Wang, L.; Wang, M.; Yang, J.; Qiu, L.; Song, L. The expression of immune-related genes during the ontogenesis of scallop Chlamys farreri and their response to bacterial challenge. Fish Shellfish Immunol. 2013, 34, 855–864. [Google Scholar] [CrossRef]
- Rojas, I.; Cárcamo, C.; Stambuk, F.; Mercado, L.; Rojas, R.; Schmitt, P.; Brokordt, K. Expression of immune-related genes during early development of the scallop Argopecten purpuratus after Vibrio splendidus challenge. Aquaculture 2021, 533, 736132. [Google Scholar] [CrossRef]
- Coyne, V.E. The importance of ATP in the immune system of molluscs. Invertebr. Surviv. J. 2011, 8, 48–55. [Google Scholar]
- Cochennec-Laureau, N.; Auffret, M.; Renault, T.; Langlade, A. Changes in circulating and tissue-infiltrating hemocyte parameters of European flat oysters, Ostrea edulis, naturally infected with Bonamia ostreae. J. Invertebr. Pathol. 2003, 83, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Desjardins, M.; Houde, M.; Gagnon, E. Phagocytosis: The convoluted way from nutrition to adaptive immunity. Immunol. Rev. 2005, 207, 158–165. [Google Scholar] [CrossRef]
- Somboonwiwat, K.; Chaikeeratisak, V.; Wang, H.C.; Fang Lo, C.; Tassanakajon, A. Proteomic analysis of differentially expressed proteins in Penaeus monodon hemocytes after Vibrio harveyi infection. Proteome Sci. 2010, 8, 39. [Google Scholar] [CrossRef]
- Rantala, M.J.; Roff, D.A. An analysis of trade-offs in immune function, body size and development time in the Mediterranean Field Cricket, Gryllus bimaculatus. Funct. Ecol. 2005, 19, 323–330. [Google Scholar] [CrossRef]
- Soler, J.J.; De Neve, L.; Pérez-Contreras, T.; Soler, M.; Sorci, G. Trade-off between immunocompetence and growth in magpies: An experimental study. Proc. R. Soc. B Biol. Sci. 2003, 270, 241–248. [Google Scholar] [CrossRef]
- Kraaijeveld, A.R.; Limentani, E.C.; Godfray, H.C.J. Basis of the trade-off between parasitoid resistance and larval competitive ability in Drosophila melanogaster. Proc. R. Soc. B Biol. Sci. 2001, 268, 259–261. [Google Scholar] [CrossRef]
- Brokordt, K.; Defranchi, Y.; Espósito, I.; Cárcamo, C.; Schmitt, P.; Mercado, L.; De la Fuente-Ortega, E.; Rivera-Ingraham, G.A. Reproduction immunity trade-off in a mollusk: Hemocyte energy metabolism underlies cellular and molecular immune responses. Front. Physiol. 2019, 10, 77. [Google Scholar] [CrossRef] [PubMed]
- Farías, A.; Uriarte, I.; Castilla, J.C. A biochemical study of the larval and postlarval stages of the Chilean scallop Argopecten purpuratus. Aquaculture 1998, 166, 37–47. [Google Scholar] [CrossRef]
- García-Esquivel, Z.; Bricelj, V.M.; González-Gómez, M.A. Physiological basis for energy demands and early postlarval mortality in the Pacific oyster, Crassostrea gigas. J. Exp. Mar. Bio. Ecol. 2001, 263, 77–103. [Google Scholar] [CrossRef]
- Rojas, I.; Rivera-ingraham, G.A.; Cárcamo, C.B.; Jeno, K.; De Fuente-ortega, E.; Schmitt, P.; Brokordt, K. Metabolic cost of the immune response during early ontogeny of the scallop Argopecten purpuratus. Front. Physiol. 2021, 12, 718467. [Google Scholar] [CrossRef] [PubMed]
- Riquelme, C.; Hayashida, G.; Vergara, N.; Vasquez, A.; Morales, Y.; Chavez, P. Bacteriology of the scallop Argopecten purpuratus (Lamarck, 1819) cultured in Chile. Science 1995, 138, 49–60. [Google Scholar] [CrossRef]
- Hulbert, A.J.; Turner, N.; Storlien, L.H.; Else, P.L. Dietary fats and membrane function: Implications for metabolism and disease. Biol. Rev. Camb. Philos. Soc. 2005, 80, 155–169. [Google Scholar] [CrossRef]
- Delaunay, F.; Marty, Y.; Moal, J.; Samain, J.F. The effect of monospecific algal diets on growth and fatty acid composition of Pecten maximus (L.) larvae. J. Exp. Mar. Bio. Ecol. 1993, 173, 163–179. [Google Scholar] [CrossRef]
- Delaporte, M.; Soudant, P.; Moal, J.; Lambert, C.; Quéré, C.; Miner, P.; Choquet, G.; Paillard, C.; Samain, J.F. Effect of a mono-specific algal diet on immune functions in two bivalve species—Crassostrea gigas and Ruditapes philippinarum. J. Exp. Biol. 2003, 206, 3053–3064. [Google Scholar] [CrossRef]
- Pernet, F.; Bricelj, V.M.; Parrish, C.C. Effect of varying dietary levels of ω6 polyunsaturated fatty acids during the early ontogeny of the sea scallop, Placopecten magellanicus. J. Exp. Mar. Bio. Ecol. 2005, 327, 115–133. [Google Scholar] [CrossRef]
- Kheder, R.B.; Quéré, C.; Moal, J.; Robert, R. Effect of nutrition on Crassostrea gigas larval development and the evolution of physiological indices. Part A: Quantitative and qualitative diet effects. Aquaculture 2010, 305, 165–173. [Google Scholar] [CrossRef]
- Marty, Y.; Delaunay, F.; Moal, J.; Samain, J.-F. Changes in the fatty acid composition of Pecten maximus (L.) during larval development. J. Exp. Mar. Bio. Ecol. 1992, 163, 221–234. [Google Scholar] [CrossRef]
- Soudant, P.; Marty, Y.; Moal, J.; Masski, H.; Samain, J.-F. Fatty acid composition of polar lipid classes during larval development of scallop Pecten maximus (L.). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 1998, 121, 279–288. [Google Scholar] [CrossRef]
- Caers, M.; Coutteau, P.; Cure, K.; Morales, V.; Gajardo, G.; Sorgeloos, P. The Chilean scallop Argopecten purpuratus (Lamarck, 1819): I. Fatty acid composition and lipid content of six organs. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1999, 123, 89–96. [Google Scholar] [CrossRef]
- Pernet, F.; Bricelj, V.M.; Cartier, S. Lipid class dynamics during larval ontogeny of sea scallops, Placopecten magellanicus, in relation to metamorphic success and response to antibiotics. J. Exp. Mar. Bio. Ecol. 2006, 329, 265–280. [Google Scholar] [CrossRef]
- Palma-Fleming, H.; Navarro, J.M.; Peña, E.; Martínez, G. Effect of three conditioning diets on the fatty acid composition of gonads and muscle of Argopecten purpuratus. New Zeal. J. Mar. Freshw. Res. 2002, 36, 605–620. [Google Scholar] [CrossRef]
- Lehninger, N. Bioquímica. In Lehninger—Principios Bioquim, 5th ed.; Ediciones Omega: Barcelona, Spain, 2009; pp. 184, 201, 202, 203. [Google Scholar]
- Pernet, F.; Tremblay, R.; Demers, E.; Roussy, M. Variation of lipid class and fatty acid composition of Chaetoceros muelleri and Isochrysis sp. Grown in a semicontinuous system. Aquaculture 2003, 221, 393–406. [Google Scholar] [CrossRef]
- MacDonald, B.A. Physiological energetics of Japanese scallop Patinopecten yessoensis larvae. J. Exp. Mar. Bio. Ecol. 1988, 120, 155–170. [Google Scholar] [CrossRef]
- Hernández, Y.K.C.; Ortiz, E.A.; Gómez-León, J. Crecimiento y supervivencia de larvas de Argopecten nucleus alimentadas con diferentes dietas microalgales. Bol. Investig. Mar. Costeras 2012, 41, 103–120. [Google Scholar] [CrossRef]
- Miranda, C.D.; Rojas, R.; Abarca, A.; Hurtado, L. Effect of florfenicol and oxytetracycline treatments on the intensive larval culture of the Chilean scallop Argopecten purpuratus (Lamarck, 1819). Aquac. Res. 2013, 45, 16–30. [Google Scholar] [CrossRef]
- Merino, G.; Uribe, E.; Soria, G.; von Brand, E.A. comparison of larval production of the northern scallop, Argopecten purpuratus, in closed and recirculating culture systems. Aquac. Eng. 2009, 40, 95–103. [Google Scholar] [CrossRef]
- Rivero-Rodríguez, S.; Beaumont, A.R.; Lora-Vilchis, M.C. The effect of microalgal diets on growth, biochemical composition, and fatty acid profile of Crassostrea corteziensis (Hertlein) juveniles. Aquaculture 2007, 263, 199–210. [Google Scholar] [CrossRef]
- Ponis, E.; Parisi, G.; Chini Zittelli, G.; Lavista, F.; Robert, R.; Tredici, M.R. Pavlova lutheri: Production, preservation and use as food for Crassostrea gigas larvae. Aquaculture 2008, 282, 97–103. [Google Scholar] [CrossRef]
- Guillard, R. Culture of some phytoplankton for feeding marine invertebrates. In Culture of Marine Invertebrates; Smith, W., Chanley, M., Eds.; Plenum Press: New York, NY, USA, 1975; pp. 29–61. [Google Scholar]
- Gao, Y.; Yang, M.; Wang, C. Nutrient deprivation enhances lipid content in marine microalgae. Bioresour. Technol. 2013, 147, 484–491. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Brokordt, K.; Pérez, H.; Herrera, C.; Gallardo, A. Reproduction reduces HSP70 expression capacity in Argopecten purpuratus scallops subject to hypoxia and heat stress. Aquat. Biol. 2015, 23, 265–274. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane, G.H. A simple method for the isolation and purification of total lipis from animal tissues. Biochem. Cell Biol. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Lora-Vilchis, M.C.; Maeda-Martínez, A.N. Ingestion and digestion index of catarina scallop Argopecten ventricosus-circularis, Sowerby, 1842, veliger larvae with ten microalgae species. Aquac. Res. 1997, 28, 905–910. [Google Scholar] [CrossRef]
- Gagné, R.; Tremblay, R.; Pernet, F.; Miner, P.; Samain, J.F.; Olivier, F. Lipid requirements of the scallop Pecten maximus (L.) during larval and post-larval development in relation to addition of Rhodomonas salina in diet. Aquaculture 2010, 309, 212–221. [Google Scholar] [CrossRef]
- Duportail, G.; Klymchenko, A.S.; Demchenko, A.P.; Me, Y. Monitoring biophysical properties of lipid membranes by environment-sensitive fluorescent probes. Biophys. J. 2009, 96, 3461–3470. [Google Scholar] [CrossRef]
- Wang, Q.; London, E. Lipid structure and somposition control consequences of interleaflet coupling in asymmetric vesicles. Biophys. J. 2018, 115, 664–678. [Google Scholar] [CrossRef]
- Parasassi, T.; Gratton, E. membrane lipid domains and dynamics as detected by Laurdan Fluorescence. J. Fluoresc. 1995, 5, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Brokordt, K.B.; Himmelman, J.H.; Guderley, H.E. Effect of reproduction on escape responses and muscle metabolic capacities in the scallop Chlamys islandica Muller 1776. J. Exp. Mar. Bio. Ecol. 2000, 251, 205–225. [Google Scholar] [CrossRef]
- Guderley, H.; Gawlicka, A. Qualitative modification of muscle metabolic organization with thermal acclimation of rainbow trout, Oncorhynchus mykiss. Fish Physiol. Biochem. 1992, 10, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Packard, T.T. The measurement of respiratory electron-transport activity in marine phytoplankton. J. Mar. Res. 1971, 29, 234–244. [Google Scholar]
- Larsen, S.; Nielsen, J.; Hansen, C.N.; Nielsen, L.B.; Wibrand, F.; Schroder, H.D.; Boushel, R.; Helge, J.W.; Dela, F. Biomarkers of mitochondrial content in skeletal muscle of healthy young human subjects. J. Physiol. 2012, 14, 3349–3360. [Google Scholar] [CrossRef]
- Coba de la Peña, T.; Cárcamo, C.B.; Díaz, M.I.; Brokordt, K.B.; Winkler, F.M. Molecular characterization of two ferritins of the scallop Argopecten purpuratus and gene expressions in association with early development, immune response and growth rate. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2016, 198, 46–56. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Mair, P.; Wilcox, R. Robust Statistical Methods Using WRS2 package. Behav. Res. Methods 2020, 52, 464–488. [Google Scholar] [CrossRef]
- Miller, R. Simultaneus Statistical Inference, 2nd ed.; Miller, R., Ed.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1981. [Google Scholar]
- Guderley, H.; Brokordt, K.; Pérez, H.M.; Marty, Y.; Kraffe, E. Diet and performance in the scallop, Argopecten purpuratus, force production during escape responses and mitochondrial oxidative capacities.pdf. Aquat. Living Resour. 2011, 24, 261–271. [Google Scholar] [CrossRef]
- Sánchez-Lazo, C.; Martínez-Pita, I. Biochemical and energy dynamics during larval development of the mussel Mytilus galloprovincialis (Lamarck, 1819). Aquaculture 2012, 358–359, 71–78. [Google Scholar] [CrossRef]
- Farías, A.; Bell, J.G.; Uriarte, I.; Sargent, J.R. Polyunsaturated fatty acids in total lipid and phospholipids of Chilean scallop Argopecten purpuratus (L.) larvae: Effects of diet and temperature. Aquaculture 2003, 228, 289–305. [Google Scholar] [CrossRef]
- Calder, P.C.; Bond, J.A.; Harvey, D.J.; Gordon, S.; Newsholme, E.A. Uptake and incorporation of saturated and unsaturated fatty acids into macrophage lipids and their effect upon macrophage adhesion and phagocytosis. Biochem. J. 1990, 269, 807–814. [Google Scholar] [CrossRef] [PubMed]
- Milke, L.M.; Bricelj, V.M.; Parrish, C.C. Growth of postlarval sea scallops, Placopecten magellanicus, on microalgal diets, with emphasis on the nutritional role of lipids and fatty acids. Aquaculture 2004, 234, 293–317. [Google Scholar] [CrossRef]
- Nevejan, N.; Courtens, V.; Hauva, M.; Gajardo, G.; Sorgeloos, P. Effect of lipid emulsions on production and fatty acid composition of eggs of the scallop Argopecten purpuratus. Mar. Biol. 2003, 143, 327–338. [Google Scholar] [CrossRef]
- Langdon, C.J.; Waldock, M.J. The effect of algal and artificial diets on the growth and fatty acid composition of Crassostrea gigas spat. J. Mar. Biol. Assoc. 1981, 61, 431–448. [Google Scholar] [CrossRef]
- Sprung, M. Physiological energetics of mussel larvae (Mytilus edulis). III. Respiration. Mar. Ecol. Prog. Ser. 1984, 18, 171–178. [Google Scholar] [CrossRef]
- Lemos, D.; Salomon, M.; Gomes, V.; Phan, V.N.; Buchholz, F. Citrate synthase and pyruvate kinase activities during early life stages of the shrimp Farfantepenaeus paulensis (Crustacea, Decapoda, Penaeidae): Effects of development and temperature. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2003, 135, 707–719. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, Y.; Zhou, Z.; Zong, Y.; Zheng, Y.; Liu, C.; Kong, N.; Gao, Q.; Wang, L.; Song, L. Metabolomic and transcriptomic profiling reveals the alteration of energy metabolism in oyster larvae during initial shell formation and under experimental ocean acidification. Sci. Rep. 2020, 10, 6111. [Google Scholar] [CrossRef] [PubMed]
- Jeria, E.; Oyanedel, D.; Rojas, R.; Farlora, R.; Lira, G.; Mercado, A.; Muñoz, K.; Destoumieux-Garzón, D.; Brokordt, K.; Schmitt, P. Resistance of Argopecten purpuratus scallop larvae to vibriosis is associated with the front-loading of immune genes and enhanced antimicrobial response. Front. Immunol. 2023, 14, 1150280. [Google Scholar] [CrossRef]
- González, R.; Brokordt, K.; Rojas, R.; Schmitt, P. Molecular characterization and expression patterns of two LPS binding/bactericidal permeability-increasing proteins (LBP/BPIs) from the scallop Argopecten purpuratus. Fish Shellfish Immunol. 2020, 97, 12–17. [Google Scholar] [CrossRef]
- González, R.; Muñoz, K. Scallop Immunology. Ref. Modul. Life Sci. 2019, 1–11. [Google Scholar] [CrossRef]
- Kawasaki, T.; Kawai, T. Toll-like receptor signaling pathways. Front. Immunol. 2014, 5, 461. [Google Scholar] [CrossRef]
- Oyanedel, D.; Gonzalez, R.; Flores-Herrera, P.; Brokordt, K.; Rosa, R.D.; Mercado, L.; Schmitt, P. Molecular characterization of an inhibitor of NF-κB in the scallop Argopecten purpuratus: First insights into its role on antimicrobial peptide regulation in a mollusk. Fish Shellfish Immunol. 2016, 52, 85–93. [Google Scholar] [CrossRef]
- Zannella, C.; Mosca, F.; Mariani, F.; Franci, G.; Folliero, V.; Galdiero, M.; Tiscar, P.G.; Galdiero, M. Microbial diseases of bivalve mollusks: Infections, immunology and antimicrobial defense. Mar. Drugs 2017, 15, 182. [Google Scholar] [CrossRef]
- Hikima, S.; Hikima, J.; Rojtinnakorn, J.; Hirono, I.; Aoki, T. Characterization and function of kuruma shrimp lysozyme possessing lytic activity against Vibrio species. Gene 2003, 316, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Xue, Q.; Hellberg, M.E.; Schey, K.L.; Itoh, N.; Eytan, R.I.; Cooper, R.K.; La Peyre, J.F. A new lysozyme from the eastern oyster, Crassostrea virginica, and a possible evolutionary pathway for i-type lysozymes in bivalves from host defense to digestion. BMC Evol. Biol. 2010, 10, 213. [Google Scholar] [CrossRef] [PubMed]
- Patrzykat, A.; Douglas, S.E. Gone gene fishing: How to catch novel marine antimicrobials. Trends Biotechnol. 2003, 21, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Hancock, R.E.W. Synergistic interactions between mammalian antimicrobial defense peptides. Antimicrob. Agents Chemother. 2001, 45, 1558–1560. [Google Scholar] [CrossRef]
- Liu, B.; Dong, B.; Tang, B.; Zhang, T.; Xiang, J. Effect of stocking density on growth, settlement and survival of clam larvae, Meretrix meretrix. Aquaculture 2006, 258, 344–349. [Google Scholar] [CrossRef]
- Oliva, D.; Abarca, A.; Gutiérrez, R.; Herrera, L.; Celis, Á.; Durán, L.R. Effect of stocking density and food ration on growth and survival of veliger and pediveliger larvae of the taquilla clam Mulinia edulis reared in the laboratory. Rev. Biol. Mar. Oceanogr. 2014, 49, 567–575. [Google Scholar] [CrossRef]
- Laudicella, V.A.; Beveridge, C.; Carboni, S.; Franco, S.C.; Doherty, M.K.; Long, N.; Mitchell, E.; Stanley, M.S.; Whitfield, P.D.; Hughes, A.D. Lipidomics analysis of juveniles’ blue mussels (Mytilus edulis L. 1758), a key economic and ecological species. PLoS ONE 2019, 15, e0223031. [Google Scholar] [CrossRef] [PubMed]
- Pernet, F.; Tremblay, R. Effect of varying levels of dietary essential fatty acid during early ontogeny of the sea scallop Placopecten magellanicus. J. Exp. Mar. Bio. Ecol. 2004, 310, 73–86. [Google Scholar] [CrossRef]
- Feller, S.E.; Gawrisch, K.; Mackerell, A.D. Polyunsaturated fatty acids in lipid bilayers: Intrinsic and environmental contributions to their unique physical properties. J. Am. Chem. Soc. 2002, 124, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.; Else, P.L.; Hulbert, A.J. Docosahexaenoic acid (DHA) content of membranes determines molecular activity of the sodium pump: Implications for disease states and metabolism. Naturwissenschaften 2003, 90, 521–523. [Google Scholar] [CrossRef] [PubMed]
- Pérez, H.M.; Brokordt, K.; Gallardo, A.; Vidal, I.; Guderley, H. A diet rich in polyunsaturated fatty acids improves the capacity for HSP70 synthesis in adult scallop Argopecten purpuratus and their offspring. Mar. Biol. 2016, 163, 193. [Google Scholar] [CrossRef]
- Oppedisano, F.; Maiuolo, J.; Gliozzi, M.; Musolino, V.; Carresi, C.; Nucera, S.; Scicchitano, M.; Scarano, F.; Bosco, F.; Macr, R.; et al. The potential for natural antioxidant supplementation in the early stages of neurodegenerative disorders. Int. J. Mol. Sci. 2020, 21, 2618. [Google Scholar] [CrossRef]
- González, R.; Brokordt, V.; Cárcamo, C.B.; de la Peña, T.C.; Oyanedel, D.; Mercado, L.; Schmitt, P. Molecular characterization and protein localization of the antimicrobial peptide big defensin from the scallop Argopecten purpuratus after Vibrio splen-didus challenge. Fish Shellfish Immunol. 2017, 68, 173–179. [Google Scholar] [CrossRef]
- González, R.; González, D.; Stambuk, F.; Ramírez, F.; Guzmán, F.; Mercado, L.; Rojas, R.; Henríquez, C.; Brokordt, K.; Schmitt, P. A g-type lysozyme from the scallop Argopecten purpuratus participates in the immune response and in the stability of the hemolymph microbiota. Fish Shellfish Immunol. 2022, 123, 324–334. [Google Scholar] [CrossRef]

| Initial Diet (2–8 dpf) | Continuation Diet (from 9 dpf) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Low HUFA | High HUFA | Low HUFA | High HUFA | |||||||
| Microalgae | T-iso | P. lutheri | T-iso * | P. lutheri * | T-iso | P. lutheri | C. gracilis | T-iso * | P. lutheri * | C. gracilis * |
| Microalgae proportion | 0.7 | 0.3 | 0.5 | 0.5 | 0.5 | 0.3 | 0.2 | 0.4 | 0.4 | 0.2 |
| Proteins | 778.09 | 297.27 | 783.45 | 779.78 | 555.78 | 297.27 | NC | 626.76 | 623.82 | NC |
| Carbohydrates | 63.602 | 6.885 | 24.585 | 33.15 | 45.43 | 6.885 | NC | 19.668 | 26.52 | |
| Lipids (% dry mass) | 14.021 | 7.161 | 12.175 | 14.575 | 10.015 | 7.161 | 0.784 | 9.74 | 11.66 | 0.822 |
| FAME (mg) | 7.84 | 3.267 | 18.45 | 8.45 | 5.6 | 3.267 | 0.498 | 14.76 | 6.76 | 0.82 |
| ∑ Saturated fatty acid (mg) | 3.451 | 1.533 | 8.705 | 3.895 | 2.465 | 1.533 | 0.256 | 6.964 | 3.116 | 0.406 |
| ∑ Monounsaturated fatty acid (mg) | 1.512 | 0.69 | 4.865 | 2.405 | 1.08 | 0.69 | 0.186 | 3.892 | 1.924 | 0.296 |
| ∑ Polyunsaturated fatty acid (mg) | 2.66 | 1.05 | 4.95 | 2.2 | 1.9 | 1.05 | 0.056 | 3.96 | 1.76 | 0.118 |
| HUFAs | ||||||||||
| ARA (mg) | NC | NC | 0.0136 | 0.0016 | ||||||
| EPA (mg) | 0.051 | 0.021 | 0.040 | 0.0315 | 0.036 | 0.021 | 0.032 | 0.0324 | 0.025 | 0.072 |
| DHA (mg) | 1.125 | 0.452 | 2.961 | 1.271 | 0.804 | 0.452 | 0.008 | 2.368 | 1.016 | 0.1 |
| Total content in the diet mix | ||||||||||
| Proteins | 633.8 | 781.6 | 367. 1 | 500.2 | ||||||
| Carbohydrates | 46.59 | 28.87 | 24.78 | 18.48 | ||||||
| Lipids (% dry mass) | 11.96 | 13.38 | 7.156 | 8.56 | ||||||
| Total ARA (mg) | NC | NC | 0.013 | 0.001 | ||||||
| Total EPA (mg) | 0.072 | 0.072 | 0.090 | 0.130 | ||||||
| Total DHA (mg) | 1.578 | 4.232 | 1.260 | 3.486 | ||||||
| Total HUFAs (mg) | 1.650 | 4.304 | 1.363 | 3.617 | ||||||
| EPA/DHA | 0.046 | 0.017 | 0.071 | 0.037 | ||||||
| ARA/EPA | - | - | 0.152 | 0.012 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rojas, I.; Cárcamo, C.B.; Defranchi, Y.; Jeno, K.; Rengel, J.; Araya, M.; Tarnok, M.E.; Aguilar, L.; Álvarez, G.; Schmitt, P.; et al. A Diet Rich in HUFAs Enhances the Energetic and Immune Response Capacities of Larvae of the Scallop Argopecten purpuratus. Animals 2023, 13, 1416. https://doi.org/10.3390/ani13081416
Rojas I, Cárcamo CB, Defranchi Y, Jeno K, Rengel J, Araya M, Tarnok ME, Aguilar L, Álvarez G, Schmitt P, et al. A Diet Rich in HUFAs Enhances the Energetic and Immune Response Capacities of Larvae of the Scallop Argopecten purpuratus. Animals. 2023; 13(8):1416. https://doi.org/10.3390/ani13081416
Chicago/Turabian StyleRojas, Isis, Claudia B. Cárcamo, Yohana Defranchi, Katherine Jeno, José Rengel, Michael Araya, María Elena Tarnok, Luis Aguilar, Gonzalo Álvarez, Paulina Schmitt, and et al. 2023. "A Diet Rich in HUFAs Enhances the Energetic and Immune Response Capacities of Larvae of the Scallop Argopecten purpuratus" Animals 13, no. 8: 1416. https://doi.org/10.3390/ani13081416
APA StyleRojas, I., Cárcamo, C. B., Defranchi, Y., Jeno, K., Rengel, J., Araya, M., Tarnok, M. E., Aguilar, L., Álvarez, G., Schmitt, P., & Brokordt, K. (2023). A Diet Rich in HUFAs Enhances the Energetic and Immune Response Capacities of Larvae of the Scallop Argopecten purpuratus. Animals, 13(8), 1416. https://doi.org/10.3390/ani13081416






