Recovery of Spinal Walking in Paraplegic Dogs Using Physiotherapy and Supportive Devices to Maintain the Standing Position
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
- -
- For spasm reduction, the current type was Premod (interferential), the type of emission was continuous, low frequency (10 Hz), and intensity was variable.
- -
- For muscle strengthening, the current type was VMS (vibratory motor stimulation), with continuous emission, phase duration of 200 μs, a ramp of 2 s, frequency of 50 Hz, and variable intensity (Supplementary Materials).
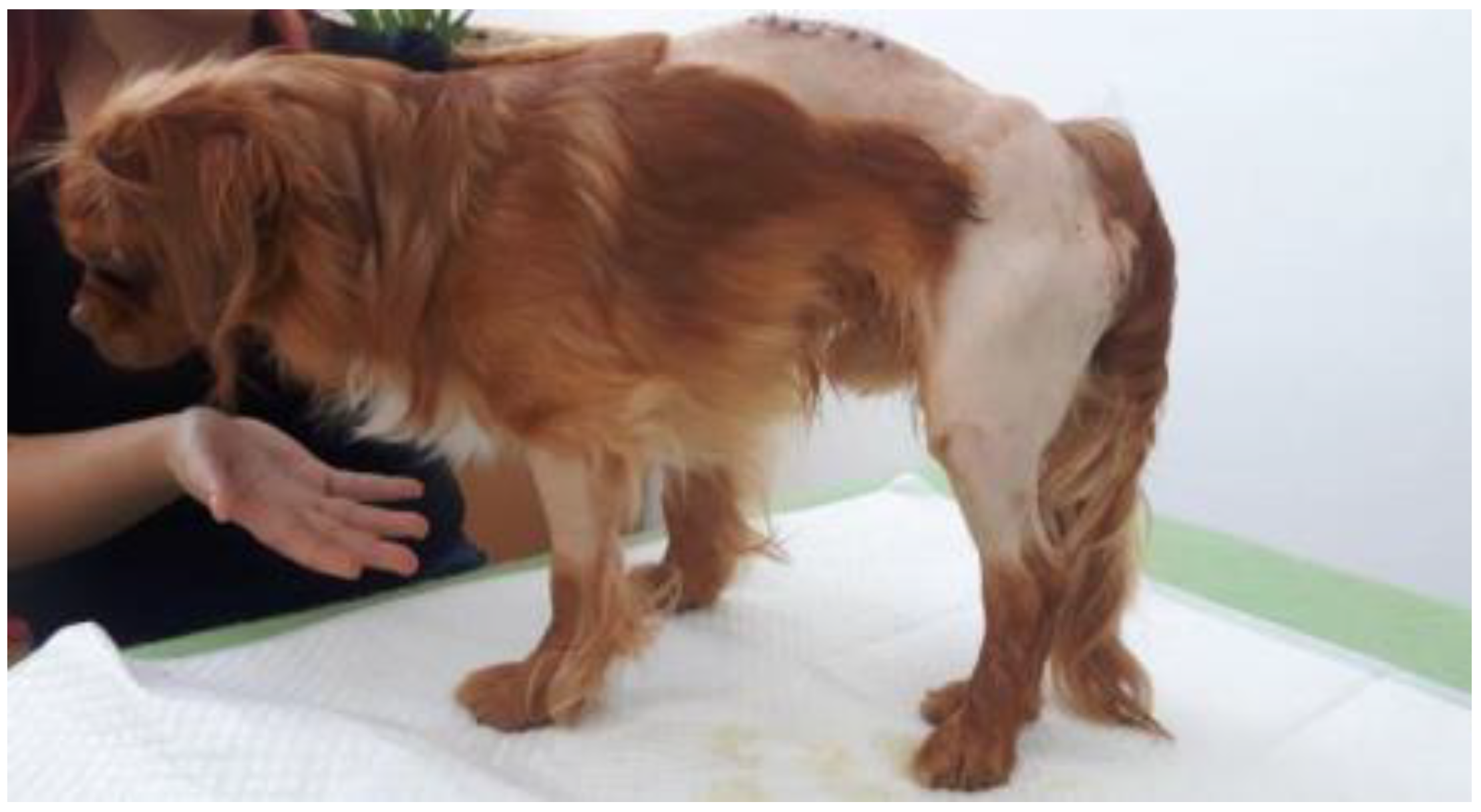
2.1. Total Assistance for Supporting Body Weight and Adopting the Standing Position
2.2. Standing Position Actively Supported Using Trolleys and Straps
2.3. Standing Position Actively Supported with Exercise Rollers
2.4. Minimal Support of the Standing Position
2.5. Recovering of Proprioceptive Sensitivity
2.6. Changing the Center of Gravity
2.7. The Balancing Platform
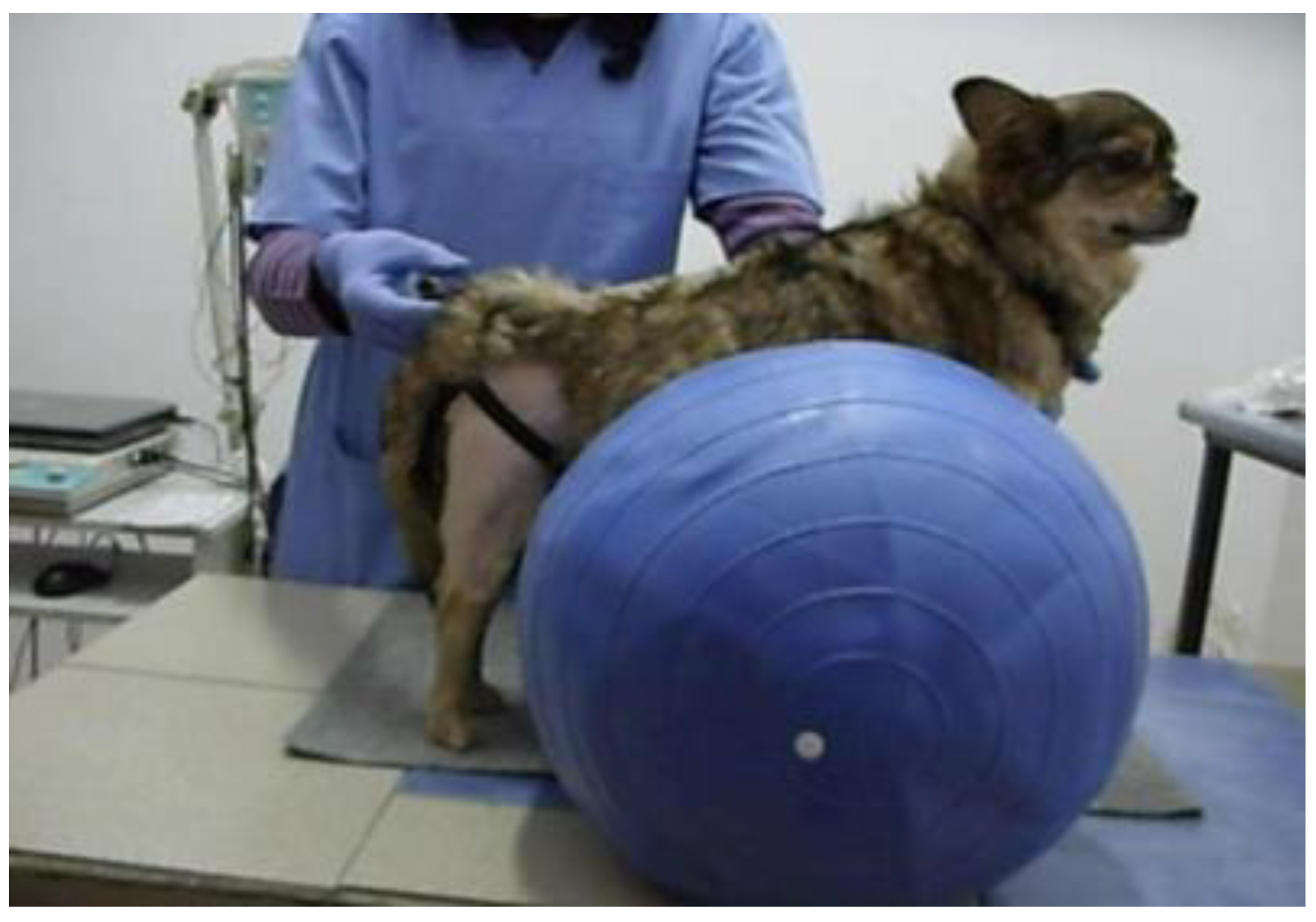
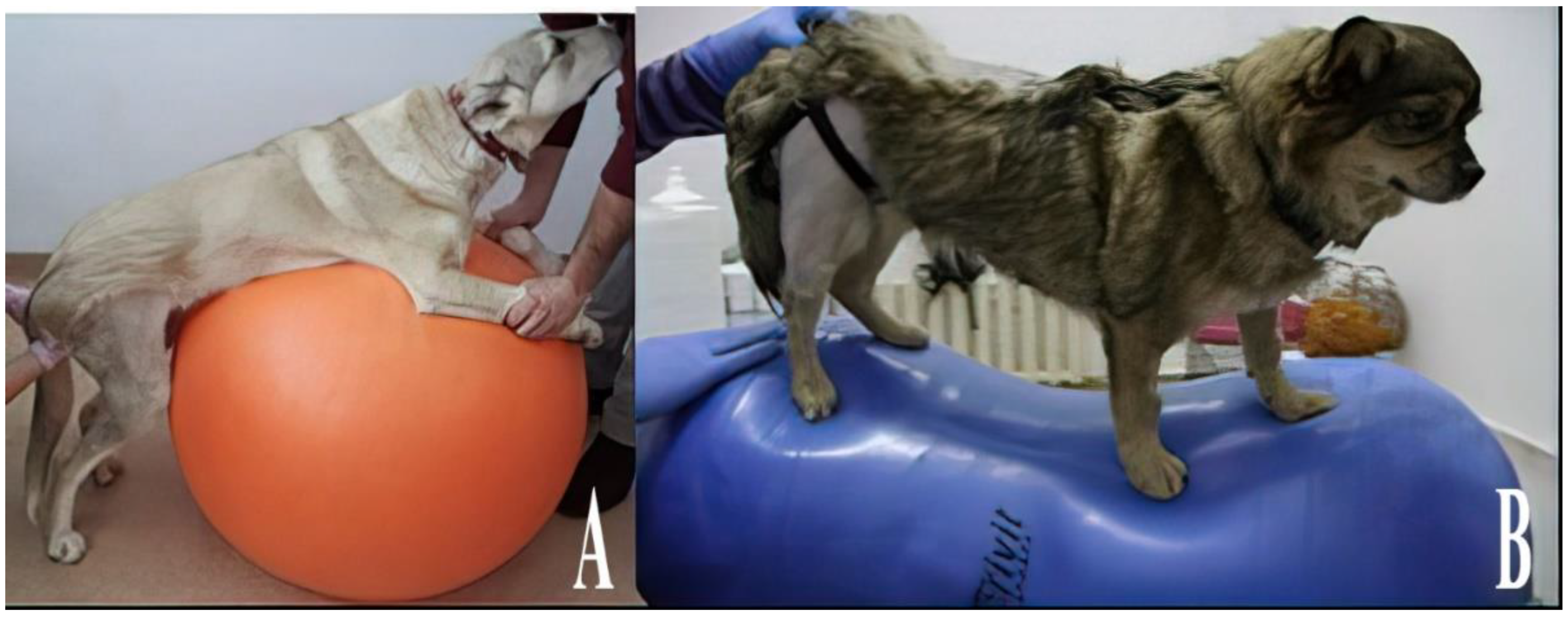
2.8. Physio Balls and Rollers
2.9. Motility Exercises
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Olby, N.J.; Levine, J.; Harris, T.; Muñana, K.; Skeen, T.; Sharp, N. Long-term functional outcome of dogs with severe injuries of the thoracolumbar spinal cord: 87 cases (1996–2001). J. Am. Vet. Med. Assoc. 2003, 222, 762–769. [Google Scholar] [CrossRef] [PubMed]
- Dewey, W.C.; da Costa, R.; Thomas, W.B. Performing the Neurologic Examination. In Practical Guide to Canine and Feline Neurology, 3rd ed.; Dewey, W.C., da Costa, R., Eds.; Wiley Blackwell: Ames, IA, USA, 2016; pp. 9–28. ISBN 978-1-119-94611-3. [Google Scholar]
- Fletcher, D.J.; Dewey, W.C.; da Costa, R. Spinal Trauma Management. In Practical Guide to Canine and Feline Neurology, 3rd ed.; Dewey, W.C., da Costa, R., Eds.; Wiley Blackwell: Ames, IA, USA, 2016; pp. 423–444. ISBN 978-1-119-94611-3. [Google Scholar]
- De Lahunta, A. General sensory system: General proprioception and general somatic afferent. In Veterinary Neuroanatomy and Clinical Neurology, 5th ed.; De Lahunta, A., Glass, E., Kent, M., Eds.; Saunders Elsevier: Philadelphia, PA, USA, 2020; pp. 237–256. ISBN 978-03-236-96111. [Google Scholar]
- Edgerton, V.R.; Tillakaratne, N.J.K.; Bigbee, A.J.; de Leon, R.D.; Roy, R.R. Plasticity of the spinal neural circuitry after injury. Annu. Rev. Neurosci. 2004, 27, 145–167. [Google Scholar] [CrossRef]
- Lewis, M.J.; Jeffery, N.D.; Olby, N.J.; Canine Spinal Cord Injury Consortium (CANSORT-SCI). Ambulation in Dogs with Absent Pain Perception after Acute Thoracolumbar Spinal Cord Injury. Front. Vet. Sci. 2020, 7, 560. [Google Scholar] [CrossRef] [PubMed]
- Gallucci, A.; Dragone, L.; Menchetti, M.; Gagliardo, T.; Pietra, M.; Cardinali, M.; Gandini, G. Acquisition of Involuntary Spinal Locomotion (Spinal Walking) in Dogs with Irreversible Thoracolumbar Spinal Cord Lesion: 81 Dogs. J. Vet. Intern. Med. 2017, 31, 492–497. [Google Scholar] [CrossRef]
- Kiehn, O. Locomotor circuits in the mammalian spinal cord. Annu. Rev. Neurosci. 2006, 29, 279–306. [Google Scholar] [CrossRef] [PubMed]
- Kiehn, O. Development and functional organization of spinal locomotor circuits. Curr. Opin. Neurobiol. 2011, 21, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Steward, O.; Willenberg, R. Rodent spinal cord injury models for studies of axon regeneration. Exp. Neurol. 2017, 287, 374–383. [Google Scholar] [CrossRef]
- Bradbury, E.J.; Moon, L.D.F.; Popat, R.J.; King, V.R.; Bennett, G.S.; Patel, P.N.; Fawcett, J.W.; McMahon, S.B. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature 2002, 416, 636–640. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.C.; Knikou, M. A review on locomotor training after spinal cord injury: Reorganization of spinal neuronal circuits and recovery of motor function. Neural Plast. 2016, 2016, 1216258. [Google Scholar] [CrossRef]
- Moore, S.A.; Early, P.J.; Hettlich, B.F. Practice patterns in the management of acute intervertebral disc herniation in dogs. J. Small Anim. Pract. 2016, 57, 409–415. [Google Scholar] [CrossRef]
- Moore, S.A.; Tipold, A.; Olby, N.J.; Stein, V.; Granger, N.; CANSORT-SCI. Current Approaches to the Management of Acute Thoracolumbar Disc Extrusion in Dogs. Front. Vet. Sci. 2020, 7, 610. [Google Scholar] [CrossRef] [PubMed]
- Levine, J.M.; Levine, G.J.; Boozer, L.; Schatzberg, S.J.; Platt, S.R.; Kent, M.; Kerwin, S.C.; Fosgate, G.T. Adverse effects and outcome associated with dexamethasone administration in dogs with acute thoracolumbar intervertebral disc herniation: 161 cases (2000–2006). J. Am. Vet. Med. Assoc. 2008, 232, 411–417. [Google Scholar] [CrossRef]
- Levine, J.M.; Levine, G.J.; Johnson, S.I.; Kerwin, S.C.; Hettlich, B.F.; Fosgate, G.T. Evaluation of the success of medical management for presumptive thoracolumbar intervertebral disk herniation in dogs. Vet. Surg. 2007, 36, 482–491. [Google Scholar] [CrossRef]
- Scott, H.W.; McKee, W.M. Laminectomy for 34 dogs with thoracolumbar intervertebral disc disease and loss of deep pain perception. J. Small Anim. Pract. 1999, 40, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Skytte, D.; Schmökel, H. Relationship of preoperative neurologic score with intervals to regaining micturition and ambulation following surgical treatment of thoracolumbar disk herniation in dogs. J. Am. Vet. Med. Assoc. 2018, 253, 196–200. [Google Scholar] [CrossRef]
- Guevar, J.; Zidan, N.; Durand, A.; Olby, N.J. Minimally invasive spine surgery in dogs: Evaluation of the safety and feasibility of a thoracolumbar approach to the spinal cord. Vet. Surg. 2020, 49 (Suppl. 1), O76–O85. [Google Scholar] [CrossRef] [PubMed]
- Laitinen, O.M.; Puerto, D.A. Surgical decompression in dogs with thoracolumbar intervertebral disc disease and loss of deep pain perception: A retrospective study of 46 cases. Acta Vet. Scand. 2005, 46, 79–85. [Google Scholar] [CrossRef]
- Dragomir, M.F.; Pestean, C.P.; Melega, I.; Danciu, C.G.; Purdoiu, R.C.; Oana, L. Current Aspects Regarding the Clinical Relevance of Electroacupuncture in Dogs with Spinal Cord Injury: A Literature Review. Animals 2021, 11, 219. [Google Scholar] [CrossRef] [PubMed]
- Han, H.J.; Yoon, H.Y.; Kim, J.Y.; Jang, H.Y.; Lee, B.; Choi, S.H.; Jeong, S.W. Clinical effect of additional electroacupuncture on thoracolumbar intervertebral disc herniation in 80 paraplegic dogs. Am. J. Chin. Med. 2010, 38, 1015–1025. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, A.M.; Matera, J.M.; Fonseca Pinto, A.C. Evaluation of electroacupuncture treatment for thoracolumbar intervertebral disk disease in dogs. J. Am. Vet. Med. Assoc. 2007, 231, 913–918. [Google Scholar] [CrossRef] [PubMed]
- Joaquim, J.G.; Luna, S.P.; Brondani, J.T.; Torelli, S.R.; Rahal, S.C.; de Paula Freitas, F. Comparison of decompressive surgery, electroacupuncture, and decompressive surgery followed by electroacupuncture for the treatment of dogs with intervertebral disk disease with long-standing severe neurologic deficits. J. Am. Vet. Med. Assoc. 2010, 236, 1225–1229. [Google Scholar] [CrossRef]
- Henea, M.E. Applications of Phisiotherapy in Locomotory System Diseases of Companion Carnivora; Ion Ionescu de la Brad: Iași, Romania, 2020; ISBN 978-973-147-379-6. (In Romanian) [Google Scholar]
- Brian, S. Physiotherapy in small animal practice. Practice 2008, 30, 190–199. [Google Scholar] [CrossRef]
- Brian, S. Feline physiotherapy and rehabilitation. Principles and potential. JFMS 2012, 14, 622–632. [Google Scholar] [CrossRef]
- Steiss, J.E. Canine rehabilitation. In Braund’s Clinical Neurology in Small Animal: Localization, Diagnosis and Treatment; Vite, C.H., Ed.; International Veterinary Information Service: Itacha, NY, USA, 2010. [Google Scholar]
- Tefend Campbell, M.; Huntingford, J.L. Nursing care and rehabilitation therapy for patients with Neurologic Disease. In Practical Guide to Canine and Feline Neurology; Dewey, C.W., da Costa, R.C., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2016; pp. 559–584. ISBN 978-1-119-94611-3. [Google Scholar]
- Millis, D.L.; Levine, D. Canine Rehabilitation and Physical Therapy; Saunders Elsevier, Knight: St. Louis, MO, USA, 2013; ISBN 9781437703092. [Google Scholar]
- Pegram, C.; Gray, C.; Packer, R.M.A.; Richards, Y.; Church, D.B.; Brodbelt, D.C.; O’Neill, D.G. Proportion and risk factors for death by euthanasia in dogs in the UK. Sci. Rep. 2021, 11, 9145. [Google Scholar] [CrossRef]
- Olby, N.J.; Moore, S.A.; Brisson, B.; Fenn, J.; Flegel, T.; Kortz, G.; Lewis, M.; Tipold, A. ACVIM consensus statement on diagnosis and management of acute canine thoracolumbar intervertebral disc extrusion. J. Vet. Intern. Med. 2022, 36, 1570–1596. [Google Scholar] [CrossRef]
- Sims, C.; Waldron, R.; Marcellin-Little, D.J. Rehabilitation and Physical Therapy for the Neurologic Veterinary Patient. Vet. Clin. N. Am. Small Anim. Pract. 2015, 45, 123–143. [Google Scholar] [CrossRef] [PubMed]
- Piotti, P.; Albertini, M.; Lavesi, E.; Ferri, A.; Pirrone, F. Physiotherapy Improves Dogs’ Quality of Life Measured with the Milan Pet Quality of Life Scale: Is Pain Involved? Vet. Sci. 2022, 9, 335. [Google Scholar] [CrossRef] [PubMed]
- Levine, J.M.; Budke, C.M.; Levine, G.J.; Kerwin, S.C.; Hettlich, B.F.; Slater, M.R. Owner-perceived, weighted quality-of-life assessments in dogs with spinal cord injuries. J. Am. Vet. Med. Assoc. 2008, 233, 931–935. [Google Scholar] [CrossRef]
- Levine, G.; Levine, J.; Budke, C.M.; Kerwin, S.C.; Au, J.; Vinayak, A.; Hettlich, B.F.; Slater, M.R. Description and repeatability a newly developed spinal cord scale for dogs. Prev. Vet. Med. 2009, 89, 121–127. [Google Scholar] [CrossRef]
- Olby, N.J.; Lim, J.H.; Babb, K.; Bach, K.; Domaracki, C.; Williams, K.; Griffith, E.; Harris, T.; Muguet-Chanoit, A. Gait scoring in dogs with thoracolumbar spinal cord injuries when walking on a treadmill. BMC Vet. Res. 2014, 10, 58. [Google Scholar] [CrossRef] [PubMed]
- Zidan, N.; Fenn, J.; Griffith, E.; Early, P.J.; Mariani, C.; Muñana, K.R.; Guevar, J.; Olby, N.J. The Effect of Electromagnetic Fields on Post-Operative Pain and Locomotor Recovery in Dogs with Acute, Severe Thoracolumbar Intervertebral Disc Extrusion: A Randomized Placebo-Controlled, Prospective Clinical Trial. J. Neurotrauma 2018, 35, 1726–1736. [Google Scholar] [CrossRef]
- Calvert, J.S.; Grahn, P.J.; Zhao, K.D.; Lee, K.H. Emergence of epidural electrical stimulation to facilitate sensorimotor network functionality after spinal cord injury. Neuromodulation 2019, 22, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Goody, P.C. Dog Anatomy—A Pictorial Approach to Canine Structure, 1st ed.; The Crowood Press: London, UK, 1999; ISBN 978-0851316369. [Google Scholar]
- Blauch, B. Spinal reflex walking in the dog. Vet. Med. Small Anim. Clin. 1977, 72, 169–173. [Google Scholar]
- Rousse, C.; Olby, N.; Williams, K.; Harris, T.; Griffith, E.; Mariani, C.; Muñana, K.; Early, P. Recovery of stepping and coordination in dogs following acute thoracolumbar intervertebral disc herniations. Vet. J. 2016, 213, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Zhang, W.; Liu, Y.; Sheng, C.; So, K.F.; Zhou, L.; Zhou, H. The effects and potential mechanisms of locomotor training on improvements of functional recovery after spinal cord injury. Int. Rev. Neurobiol. 2019, 147, 199–217. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Lucke, C.; Käferle, J.; Berner, B.-M.R.; Ahlborg, L.; Hansen, H.M.; Tollefsen, U.S.; Thon, T.; Moen, R.D.; Pekanovic, A.; Tornberg, B.; et al. Effect of assisted walking-movement in patients with genetic and acquired neuromuscular disorders with the motorised Innowalk device: An international case study meta-analysis. PeerJ 2019, 7, e7098. [Google Scholar] [CrossRef] [PubMed]
- Martins, Â.; Gouveia, D.; Cardoso, A.; Coelho, T.; Silva, C.; Viegas, I.; Gamboa, Ó.; Ferreira, A. A controlled clinical study of intensive neurorehabilitation in post-surgical dogs with severe acute intervertebral disc extrusion. Animals 2021, 11, 3034. [Google Scholar] [CrossRef] [PubMed]
- Jeong, I.S.; Piao, Z.; Rahman, M.M.; Kim, S.; Kim, N.S. Canine thoracolumbar intervertebral disk herniation and rehabilitation therapy after surgical decompression: A retrospective study. J. Adv. Vet. Anim. Res. 2019, 6, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.A.; Granger, N.; Olby, N.; Spitzbarth, I.; Jeffery, N.; Tipold, A.; Nout-Lomas, Y.S.; DA Costa, R.C.; Stein, V.M.; Noble-Haeusslein, L.J.; et al. Targeting Translational Successes through CANSORT-SCI: Using Pet Dogs to Identify Effective Treatments for Spinal Cord Injury. J. Neurotrauma 2017, 34, 2007–2018. [Google Scholar] [CrossRef] [PubMed]
- Brand, R.V.D.; Heutschi, J.; Barraud, Q.; DiGiovanna, J.; Bartholdi, K.; Huerlimann, M.; Friedli, L.; Vollenweider, I.; Moraud, E.M.; Duis, S.; et al. Restoring voluntary control of locomotion after paralyzing spinal cord injury. Science 2012, 336, 1182–1185. [Google Scholar] [CrossRef]
- Zhang, S.X.; Huang, F.; Gates, M.; Shen, X.; Holmberg, E.G. Early application of tail nerve electrical stimulation-induced walking training promotes locomotor recovery in rats with spinal cord injury. Spinal Cord 2016, 54, 942–946. [Google Scholar] [CrossRef]
- Rejc, E.; Angelia, C.; Harkema, S. Effects of lumbosacral spinal cord epidural stimulation for standing after chronic complete paralysis in humans. PLoS ONE 2015, 10, e0133998. [Google Scholar] [CrossRef] [PubMed]
- Backus, D.; Cordo, P.; Gillott, A.; Kandilakis, C.; Mori, M.; Raslan, A.M. Assisted movement with proprioceptive stimulation reduces impairment and restores function in incomplete spinal cord injury. Arch. Phys. Med. Rehabil. 2014, 95, 1447–1453. [Google Scholar] [CrossRef]
- Beekhuizen, K.S.; Field-Fote, E.C. Sensory stimulation augments the effects of massed practice training in persons with tetraplegia. Arch. Phys. Med. Rehabil. 2008, 89, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Cragg, J.J.; Haefeli, J.; Jutzeler, C.R.; Röhrich, F.; Weidner, N.; Saur, M.; Maier, D.D.; Kalke, Y.B.; Schuld, C.; Curt, A.; et al. Effects of pain and pain management on motor recovery of spinal cord injured patients: A longitudinal study. Neurorehabilit. Neural Repair 2016, 30, 753–761. [Google Scholar] [CrossRef]
- Harkema, S.J.; Hilyer, J.; Schmidt-Read, M.; Ardolino, E.; Sisto, S.A.; Behrman, A.L. Locomotor training: As a treatment of spinal cord injury and in the progression of neurologic rehabilitation. Arch. Phys. Med. Rehabil. 2012, 93, 1588–1597. [Google Scholar] [CrossRef] [PubMed]
- Ichiyama, R.M.; Gerasimenko, Y.P.; Zhong, H.; Roy, R.R.; Edgerton, V.R. Hindlimb stepping movements in complete spinal rats induced by epidural spinal cord stimulation. Neurosci. Lett. 2005, 383, 339–344. [Google Scholar] [CrossRef]
- James, N.D.; McMahon, S.B.; Field-Fote, E.C.; Bradbury, E.J. Neuromodulation in the restoration of function after spinal cord injury. Lancet 2018, 17, 905–917. [Google Scholar] [CrossRef] [PubMed]
- Courtine, G.; Sofroniew, M.V. Spinal cord repair: Advances in biology and technology. Nat. Med. 2019, 25, 898–908. [Google Scholar] [CrossRef]
- Alluin, O.; Delivet-Mongrain, H.; Gauthier, M.K.; Fehlings, M.G.; Rossignol, S.; Karimi-Abdolrezaee, S. Examination of the combined effects of chondroitinase ABC, growth factors and locomotor training following compressive spinal cord injury on neuroanatomical plasticity and kinematics. PLoS ONE 2015, 9, e111072. [Google Scholar] [CrossRef]





| Site of Lesion | Number of Cases | % | IVDE | Trauma | ||
|---|---|---|---|---|---|---|
| Number of Cases | % | Number of Cases | % | |||
| Th9–Th10 | 2 | 3.33 | 2 | 3.33 | ||
| Th10–Th11 | 6 | 10 | 4 | 6.67 | 2 | 3.33 |
| Th11–Th12 | 22 | 36.67 | 20 | 33.33 | 2 | 3.33 |
| Th13–L1 | 29 | 48.33 | 26 | 43.33 | 3 | 5.00 |
| L1–L2 | 1 | 1.67 | 1 | 1.67 | ||
| Total | 60 | 100 | 53 | 88.33 | 7 | 11.67 |
| Spinal Walking Group | No Spinal Walking Group | |
|---|---|---|
| Dogs | 35 (58.33%) | 25 (41.67%) |
| Breeds most represented | Mixed breed (n = 9; 25.71%) Teckel (n = 4; 11.43%) Bichon (n = 5; 14.28%) Pekingese (n = 4; 11.43%) Caniche (n = 2; 5.71%) | Mixed breed (n = 16; 64%) Teckel (n = 4; 16%) Bichon (n = 2; 8%) Caniche (n = 1; 4%) Pug (n = 1; 4%) |
| Age | m: 54.85 months (range: 3–126) | m: 66.96 months (range: 27–129) |
| Weight | 6.83 kg (range: 1.5–15.7) * | 15.59 kg (range: 5.5–45.2) |
| Dogs with IVDH | 32 (91.42%) | 21 (84%) |
| Dogs with traumatic injuries | 3 (8.57%) | 4 (16%) |
| Dogs with lesion T9–T10 | 2 (5.71%) | 0 |
| Dogs with lesion T10–T11 | 2 (5.71%) | 4 (40%) |
| Dogs with lesion T11–T12 | 7 (20%) | 15 (16%) |
| Dogs with lesion T13–L1 | 23 (65.71%) | 6 (24%) |
| Dogs with lesion L1–L2 | 1 (2.85%) | 0 |
| No. of Physiotherapy Sessions | Deep Pain and Proprioception | Reflectivity | Motility Recuperation/Description | Gait Score * [37] |
|---|---|---|---|---|
| Initial (t0) | absent | absent | absent | 0 |
| 40 | absent | absent in 12/60 patients (20%) | 47/60 (78.33%) of patients stand up fourfold when drinking water or eating and remain in this position for about 30 s to one minute | 5.8 ± 1.44 ** |
| 80 | absent | absent in 12/60 patients (20%) | 47/60 (78.33%) patients stand up quadrupedally and can take at least 10 consecutive steps without falling | 8.7 ± 1.31 ** |
| 125–320 | absent | absent in 12/60 patients (20%) | 35/60 (58.33%) developed spinal walking, being able to walk without falling, or fell only sometimes in the case of a quick look, with a lack of coordination between the thoracic and pelvic limbs and difficulties in turning, especially when changing direction, but with recovery of the quadrupedal position in less than 30 s | 11.6 ± 1.57 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Henea, M.E.; Șindilar, E.V.; Burtan, L.C.; Mihai, I.; Grecu, M.; Anton, A.; Solcan, G. Recovery of Spinal Walking in Paraplegic Dogs Using Physiotherapy and Supportive Devices to Maintain the Standing Position. Animals 2023, 13, 1398. https://doi.org/10.3390/ani13081398
Henea ME, Șindilar EV, Burtan LC, Mihai I, Grecu M, Anton A, Solcan G. Recovery of Spinal Walking in Paraplegic Dogs Using Physiotherapy and Supportive Devices to Maintain the Standing Position. Animals. 2023; 13(8):1398. https://doi.org/10.3390/ani13081398
Chicago/Turabian StyleHenea, Mădălina Elena, Eusebiu Viorel Șindilar, Liviu Cătălin Burtan, Iuliana Mihai, Mariana Grecu, Alina Anton, and Gheorghe Solcan. 2023. "Recovery of Spinal Walking in Paraplegic Dogs Using Physiotherapy and Supportive Devices to Maintain the Standing Position" Animals 13, no. 8: 1398. https://doi.org/10.3390/ani13081398
APA StyleHenea, M. E., Șindilar, E. V., Burtan, L. C., Mihai, I., Grecu, M., Anton, A., & Solcan, G. (2023). Recovery of Spinal Walking in Paraplegic Dogs Using Physiotherapy and Supportive Devices to Maintain the Standing Position. Animals, 13(8), 1398. https://doi.org/10.3390/ani13081398






