Osteocalcin and Its Potential Functions for Preventing Fatty Liver Hemorrhagic Syndrome in Poultry
Abstract
Simple Summary
Abstract
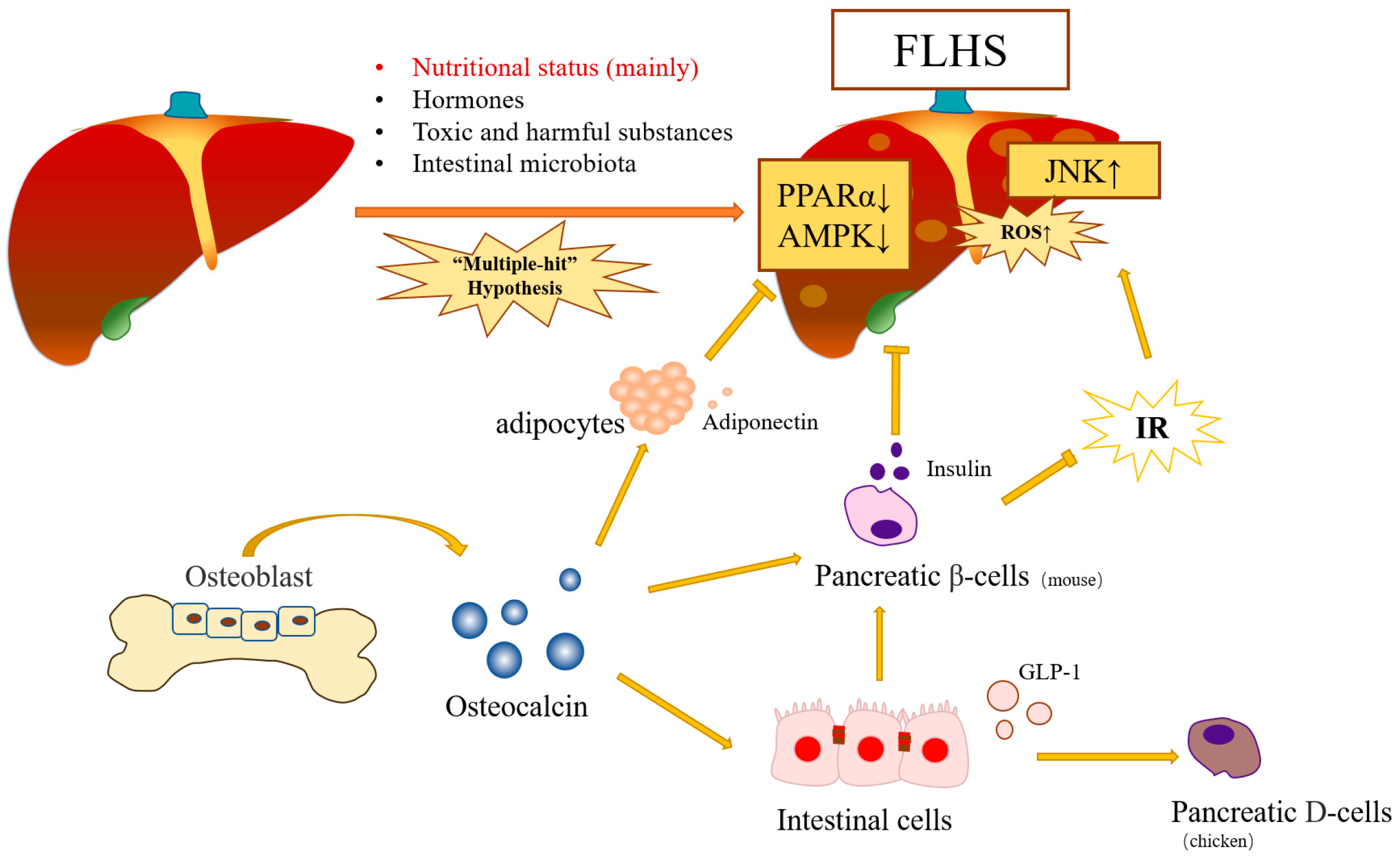
1. Fatty Liver Hemorrhage Syndrome
2. Osteocalcin
2.1. Osteocalcin Gene and Protein
2.2. Osteocalcin Receptor
2.3. The Function of Osteocalcin
3. Molecular Mechanism of Osteocalcin in FLHS Chickens
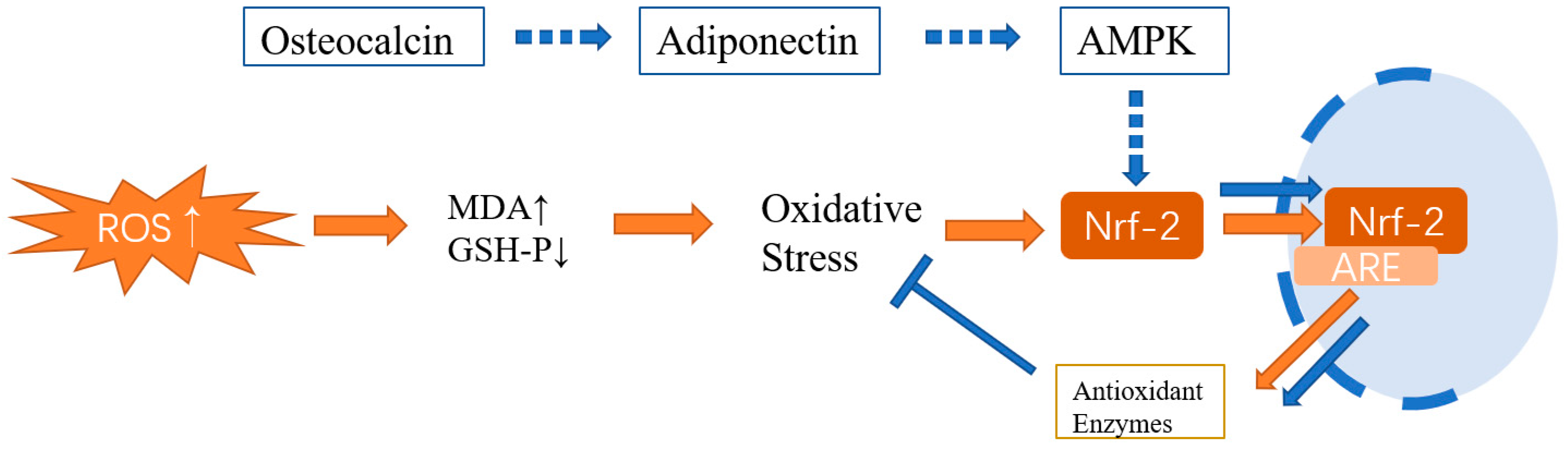
3.1. Osteocalcin Reduces Fat Accumulation and Inflammatory Reaction by Inhibiting the ROS–JNK Signal Pathway
3.2. Osteocalcin Might Prevent Insulin Resistance through the JNK Pathway
3.3. Effect of Adiponectin on Osteocalcin Protecting Poultry from FLHS
3.3.1. Osteocalcin Protects Poultry from FLHS via the ADPN/AMPK Signaling Pathway
3.3.2. Osteocalcin Protects Poultry from FLHS via the ADPN/ PPARα Signaling Pathway
3.4. Leptin
3.5. GLP-1
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wolford, J.H.; Polin, D. Lipid Accumulation and Hemorrhage in Livers of Laying Chickens. A study on fatty liver-hemorrhagic syndrome (FLHS). Poult. Sci. 1972, 51, 1707–1713. [Google Scholar] [CrossRef]
- Yousefi, M.; Shivazad, M.; Sohrabi-Haghdoost, I. Effect of Dietary Factors on Induction of Fatty Liver-Hemorrhagic Syndrome and its Diagnosis Methods with Use of Serum and Liver Parameters in Laying Hens. Int. J. Poult. Sci. 2005, 4, 568–572. [Google Scholar]
- Shini, A.; Shini, S.; Bryden, W.L. Fatty liver haemorrhagic syndrome occurrence in laying hens: Impact of production system. Avian Pathol. 2018, 48, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Tang, H.; Wang, H. The Anti-Oxidation and Mechanism of Essential Oil of Paederia scandens in the NAFLD Model of Chicken. Animals 2019, 9, 850. [Google Scholar] [CrossRef] [PubMed]
- Leveille, G.A.; Romsos, D.R.; Yeh, Y.-Y.; O’hea, E.K. Lipid Biosynthesis in the Chick. A Consideration of Site of Synthesis, Influence of Diet and Possible Regulatory Mechanisms. Poult. Sci. 1975, 54, 1075–1093. [Google Scholar] [CrossRef] [PubMed]
- Seki, Y.; Sato, K.; Kono, T.; Abe, H.; Akiba, Y. Broiler chickens (Ross strain) lack insulin-responsive glucose transporter GLUT4 and have GLUT8 cDNA. Gen. Comp. Endocrinol. 2003, 133, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Byers, M.S.; Howard, C.; Wang, X. Avian and Mammalian Facilitative Glucose Transporters. Microarrays 2017, 6, 7. [Google Scholar] [CrossRef] [PubMed]
- Braun, E.J.; Sweazea, K.L. Glucose regulation in birds. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2008, 151, 1–9. [Google Scholar] [CrossRef]
- Dupont, J.; Métayer-Coustard, S.; Ji, B.; Ramé, C.; Gespach, C.; Voy, B.; Simon, J. Characterization of major elements of insulin signaling cascade in chicken adipose tissue: Apparent insulin refractoriness. Gen. Comp. Endocrinol. 2012, 176, 86–93. [Google Scholar] [CrossRef]
- Choi, Y.I.; Ahn, H.J.; Lee, B.K.; Oh, S.T.; An, B.K.; Kang, C.W. Nutritional and Hormonal Induction of Fatty Liver Syndrome and Effects of Dietary Lipotropic Factors in Egg-type Male Chicks. Asian-Australas. J. Anim. Sci. 2012, 25, 1145–1152. [Google Scholar] [CrossRef]
- Buzzetti, E.; Pinzani, M.; Tsochatzis, E.A. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism 2016, 65, 1038–1048. [Google Scholar] [CrossRef]
- Zhuang, Y.; Xing, C.; Cao, H.; Zhang, C.; Luo, J.; Guo, X.; Hu, G. Insulin resistance and metabonomics analysis of fatty liver haemorrhagic syndrome in laying hens induced by a high-energy low-protein diet. Sci. Rep. 2019, 9, 10141. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Ma, N.; Liu, H.; Liu, J.; Liu, J.; Wang, J.; He, X.; Zhao, X. Untargeted and targeted metabolomics profiling reveals the underlying pathogenesis and abnormal arachidonic acid metabolism in laying hens with fatty liver hemorrhagic syndrome. Poult. Sci. 2021, 100, 101320. [Google Scholar] [CrossRef]
- Yang, F.; Ruan, J.; Wang, T.; Luo, J.; Cao, H.; Song, Y.; Huang, J.; Hu, G. Improving effect of dietary soybean phospholipids supplement on hepatic and serum indexes relevant to fatty liver hemorrhagic syndrome in laying hens. Anim. Sci. J. 2017, 88, 1860–1869. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Jin, N.; Amevor, F.K.; Shu, G.; Du, X.; Kang, X.; Ning, Z.; Deng, X.; Tian, Y.; Zhu, Q.; et al. Dietary supplementation of salidroside alleviates liver lipid metabolism disorder and inflammatory response to promote hepatocyte regeneration via PI3K/AKT/Gsk3-β pathway. Poult. Sci. 2022, 101, 102034. [Google Scholar] [CrossRef]
- Lv, Z.; Xing, K.; Li, G.; Liu, D.; Guo, Y. Dietary Genistein Alleviates Lipid Metabolism Disorder and Inflammatory Response in Laying Hens with Fatty Liver Syndrome. Front. Physiol. 2018, 9, 1493. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhang, X.; Du, P.; Wang, Z.; Luo, P.; Huang, Y.; Liu, Z.; Zhang, H.; Chen, W. Dietary herbaceous mixture supplementation reduced hepatic lipid deposition and improved hepatic health status in post-peak laying hens. Poult. Sci. 2022, 101, 101870. [Google Scholar] [CrossRef]
- Xie, Z.; Zhang, J.; Ma, S.; Huang, X.; Huang, Y. Effect of Chinese herbal medicine treatment on plasma lipid profile and hepatic lipid metabolism in Hetian broiler. Poult. Sci. 2017, 96, 1918–1924. [Google Scholar] [CrossRef] [PubMed]
- Ipsen, D.H.; Lykkesfeldt, J.; Tveden-Nyborg, P. Molecular mechanisms of hepatic lipid accumulation in non-alcoholic fatty liver disease. Cell. Mol. Life Sci. 2018, 75, 3313–3327. [Google Scholar] [CrossRef]
- Hamid, H.; Zhang, J.; Li, W.; Liu, C.; Li, M.; Zhao, L.; Ji, C.; Ma, Q. Interactions between the cecal microbiota and non-alcoholic steatohepatitis using laying hens as the model. Poult. Sci. 2019, 98, 2509–2521. [Google Scholar] [CrossRef]
- Tsai, M.T.; Chen, Y.-J.; Chen, C.-Y.; Tsai, M.-H.; Han, C.-L.; Chen, Y.-J.; Mersmann, H.; Ding, S.-T. Identification of Potential Plasma Biomarkers for Nonalcoholic Fatty Liver Disease by Integrating Transcriptomics and Proteomics in Laying Hens. J. Nutr. 2017, 147, 293–303. [Google Scholar] [CrossRef]
- Qiu, K.; Zhao, Q.; Wang, J.; Qi, G.-H.; Wu, S.-G.; Zhang, H.-J. Effects of Pyrroloquinoline Quinone on Lipid Metabolism and Anti-Oxidative Capacity in a High-Fat-Diet Metabolic Dysfunction-Associated Fatty Liver Disease Chick Model. Int. J. Mol. Sci. 2021, 22, 1458. [Google Scholar] [CrossRef] [PubMed]
- Raza, S.; Rajak, S.; Anjum, B.; Sinha, R.A. Molecular links between non-alcoholic fatty liver disease and hepatocellular carcinoma. Hepatoma Res. 2019, 2019, 42. [Google Scholar] [CrossRef]
- Wu, X.L.; Zou, X.Y.; Zhang, M.; Hu, H.Q.; Wei, X.L.; Jin, M.L.; Cheng, H.W.; Jiang, S. Osteocalcin prevents insulin resistance, hepatic inflammation, and activates autophagy associated with high-fat diet-induced fatty liver hemorrhagic syndrome in aged laying hens. Poult. Sci. 2021, 100, 73–83. [Google Scholar] [CrossRef]
- Zhang, M.; Tu, W.; Zhang, Q.; Wu, X.; Zou, X.; Jiang, S. Osteocalcin reduces fat accumulation and inflammatory reaction by inhibiting ROS-JNK signal pathway in chicken embryonic hepatocytes. Poult. Sci. 2022, 101, 102026. [Google Scholar] [CrossRef] [PubMed]
- Tacey, A.; Hayes, A.; Zulli, A.; Levinger, I. Osteocalcin and vascular function: Is there a cross-talk? Mol. Metab. 2021, 49, 101205. [Google Scholar] [CrossRef] [PubMed]
- Neugebauer, B.M.; Moore, M.A.; Broess, M.; Gerstenfeld, L.; Hauschka, P. Characterization of structural sequences in the chicken osteocalcin gene: Expression of osteocalcin by maturing osteoblasts and by hypertrophic chondrocytes in vitro. J. Bone Miner. Res. 2009, 10, 157–163. [Google Scholar] [CrossRef]
- Diaz-Franco, M.C.; de Leon, R.F.; Villafan-Bernal, J.R. Osteocalcin-GPRC6A: An update of its clinical and biological multi-organic interactions (Review). Mol. Med. Rep. 2018, 19, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Komori, T. Functions of Osteocalcin in Bone, Pancreas, Testis, and Muscle. Int. J. Mol. Sci. 2020, 21, 7513. [Google Scholar] [CrossRef]
- Xia, M.; Rong, S.; Zhu, X.; Yan, H.; Chang, X.; Sun, X.; Zeng, H.; Li, X.; Zhang, L.; Chen, L.; et al. Osteocalcin and Non-Alcoholic Fatty Liver Disease: Lessons from Two Population-Based Cohorts and Animal Models. J. Bone Miner. Res. 2020, 36, 712–728. [Google Scholar] [CrossRef]
- Jiang, S.; Cheng, H.W.; Cui, L.Y.; Zhou, Z.L.; Hou, J.F. Changes of blood parameters associated with bone remodeling following experimentally induced fatty liver disorder in laying hens. Poult. Sci. 2013, 92, 1443–1453. [Google Scholar] [CrossRef] [PubMed]
- Matuszewski, A.; Łukasiewicz, M.; Niemiec, J.; Jaworski, S.; Kamaszewski, M.; Szudrowicz, H.; Puppel, K.; Chwalibog, A.; Sawosz, E. Effect of in ovo application of hydroxyapatite nanoparticles on chicken embryo development, oxidative status and bone characteristics. Arch. Anim. Nutr. 2020, 74, 343–361. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Tong, X.; Yu, Z.; Hu, Y.; Zhang, L.; Liu, Y.; Zhou, Z. Dietary supplementation of total flavonoids from Rhizoma Drynariae improves bone health in older caged laying hens. Poult. Sci. 2020, 99, 5047–5054. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Shao, D.; Sheng, Z.; Wang, Q.; Shi, S. A mixture of daidzein and Chinese herbs increases egg production and eggshell strength as well as blood plasma Ca, P, antioxidative enzymes, and luteinizing hormone levels in post-peak, brown laying hens. Poult. Sci. 2019, 98, 3298–3303. [Google Scholar] [CrossRef] [PubMed]
- Ferron, M.; Wei, J.; Yoshizawa, T.; Ducy, P.; Karsenty, G. An ELISA-based method to quantify osteocalcin carboxylation in mice. Biochem. Biophys. Res. Commun. 2010, 397, 691–696. [Google Scholar] [CrossRef]
- Zhang, M.; Nie, X.; Yuan, Y.; Wang, Y.; Ma, X.; Yin, J.; Bao, Y. Osteocalcin Alleviates Nonalcoholic Fatty Liver Disease in Mice through GPRC6A. Int. J. Endocrinol. 2021, 2021, 9178616. [Google Scholar] [CrossRef]
- Clemmensen, C.; Smajilovic, S.; Wellendorph, P.; Bräuner-Osborne, H. The GPCR, class C, group 6, subtype A (GPRC6A) receptor: From cloning to physiological function. Br. J. Pharmacol. 2014, 171, 1129–1141. [Google Scholar] [CrossRef]
- Li, X.; Hua, J.; Wang, S.; Hu, Z.; Wen, A.; Yang, B. Genes and Signaling Pathways Involved in the Regulation of Selenium-Enriched Yeast on Liver Metabolism and Health of Broiler (Gallus gallus). Biol. Trace Element Res. 2022, 201, 387–402. [Google Scholar] [CrossRef]
- Oury, F.; Sumara, G.; Sumara, O.; Ferron, M.; Chang, H.; Smith, C.E.; Hermo, L.; Suarez, S.; Roth, B.L.; Ducy, P.; et al. Endocrine Regulation of Male Fertility by the Skeleton. Cell 2011, 144, 796–809. [Google Scholar] [CrossRef]
- Pi, M.; Nishimoto, S.K.; Quarles, L.D. Explaining Divergent Observations Regarding Osteocalcin/GPRC6A Endocrine Signaling. Endocrinology 2021, 162, bqab011. [Google Scholar] [CrossRef] [PubMed]
- Pi, M.; Kapoor, K.; Ye, R.; Nishimoto, S.K.; Smith, J.C.; Baudry, J.; Quarles, L.D. Evidence for Osteocalcin Binding and Activation of GPRC6A in β-Cells. Endocrinology 2016, 157, 1866–1880. [Google Scholar] [CrossRef]
- Teng, B.; Huang, C.; Cheng, C.-L.; Udduttula, A.; Yu, X.-F.; Liu, C.; Li, J.; Yao, Z.-Y.; Long, J.; Miao, L.-F.; et al. Newly identified peptide hormone inhibits intestinal fat absorption and improves NAFLD through its receptor GPRC6A. J. Hepatol. 2020, 73, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, C.V.; Bräuner-Osborne, H. Pharmacology and physiological function of the orphan GPRC6A receptor. Basic Clin. Pharmacol. Toxicol. 2020, 126, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Jeong, E.; Kim, Y.; Jeong, J.; Cho, Y. Structure of the class C orphan GPCR GPR158 in complex with RGS7-Gβ5. Nat. Commun. 2021, 12, 6805. [Google Scholar] [CrossRef]
- Khrimian, L.; Obri, A.; Ramos-Brossier, M.; Rousseaud, A.; Moriceau, S.; Nicot, A.-S.; Mera, P.; Kosmidis, S.; Karnavas, T.; Saudou, F.; et al. Gpr158 mediates osteocalcin’s regulation of cognition. J. Exp. Med. 2017, 214, 2859–2873. [Google Scholar] [CrossRef]
- Watkins, L.R.; Orlandi, C. Orphan G Protein Coupled Receptors in Affective Disorders. Genes 2020, 11, 694. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Wei, S.; Wang, T.; Fan, H.; Zhang, Y.; Da Costa, C.; Brandner, S.; Yang, G.; Pan, Y.; He, Y.; et al. Research Status of the Orphan G Protein Coupled Receptor 158 and Future Perspectives. Cells 2022, 11, 1334. [Google Scholar] [CrossRef] [PubMed]
- Dumontet, T.; Hammer, G.D. Bones and adrenal organogenesis: How embryonic osteocalcin influences lifelong adrenal function. J. Clin. Investig. 2022, 132, e157200. [Google Scholar] [CrossRef]
- Nakamura, M.; Imaoka, M.; Takeda, M. Interaction of bone and brain: Osteocalcin and cognition. Int. J. Neurosci. 2020, 131, 1115–1123. [Google Scholar] [CrossRef]
- Komori, T. What is the function of osteocalcin? J. Oral Biosci. 2020, 62, 223–227. [Google Scholar] [CrossRef]
- Mera, P.; Laue, K.; Ferron, M.; Confavreux, C.; Wei, J.; Galán-Díez, M.; Lacampagne, A.; Mitchell, S.J.; Mattison, J.A.; Chen, Y.; et al. Osteocalcin Signaling in Myofibers Is Necessary and Sufficient for Optimum Adaptation to Exercise. Cell Metab. 2016, 23, 1078–1092. [Google Scholar] [CrossRef] [PubMed]
- Diegel, C.R.; Hann, S.; Ayturk, U.M.; Hu, J.C.W.; Lim, K.-E.; Droscha, C.J.; Madaj, Z.B.; Foxa, G.E.; Izaguirre, I.; Core, V.V.A.T.; et al. An osteocalcin-deficient mouse strain without endocrine abnormalities. PLoS Genet. 2020, 16, e1008361. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Zhang, L.; Wang, Z.; Zhao, X.; Zou, J. Endocrine Regulation of Extra-skeletal Organs by Bone-derived Secreted Protein and the effect of Mechanical Stimulation. Front. Cell Dev. Biol. 2021, 9, 778015. [Google Scholar] [CrossRef] [PubMed]
- Mizokami, A.; Yasutake, Y.; Gao, J.; Matsuda, M.; Takahashi, I.; Takeuchi, H.; Hirata, M. Osteocalcin Induces Release of Glucagon-Like Peptide-1 and Thereby Stimulates Insulin Secretion in Mice. PLoS ONE 2013, 8, e57375. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, P.; Kimondo, J.W. Adiponectin and osteocalcin: Relation to insulin sensitivity. Biochem. Cell Biol. 2012, 90, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Teng, X.; Zhang, W.; Xu, D.; Liu, Z.; Yang, N.; Luo, D.; Wang, H.; Ge, M.; Zhang, R. Effects of low dietary phosphorus on tibia quality and metabolism in caged laying hens. Prev. Vet. Med. 2020, 181, 105049. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Chen, Y.; Nian, H.; Wang, J.; Liu, Y.; Wang, J.; Yang, K.; Zhao, Q.; Zhang, R.; Bao, J. Abnormal Bone Metabolism May Be a Primary Causative Factor of Keel Bone Fractures in Laying Hens. Animals 2021, 11, 3133. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Luo, D.; Teng, X.; Liu, Z.; Wang, H.; Ge, M.; Zhang, R. Study on the morphological and metabolic changes of femur in laying hens with hypophosphatemia. Res. Vet. Sci. 2020, 134, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Mizokami, A.; Kawakubo-Yasukochi, T.; Hirata, M. Osteocalcin and its endocrine functions. Biochem. Pharmacol. 2017, 132, 1–8. [Google Scholar] [CrossRef]
- Miao, Y.; Gao, X.; Xu, D.; Li, M.; Gao, Z.; Tang, Z.; Mhlambi, N.; Wang, W.; Fan, W.; Shi, X.; et al. Protective effect of the new prepared Atractylodes macrocephala Koidz polysaccharide on fatty liver hemorrhagic syndrome in laying hens. Poult. Sci. 2020, 100, 938–948. [Google Scholar] [CrossRef]
- Lin, C.-W.; Huang, T.-W.; Peng, Y.-J.; Lin, Y.-Y.; Mersmann, H.J.; Ding, S.-T. A novel chicken model of fatty liver disease induced by high cholesterol and low choline diets. Poult. Sci. 2020, 100, 100869. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Mao, H.; Peng, G.; Zeng, Q.; Wei, Q.; Ruan, J.; Huang, J. Effect of JAK-STAT pathway in regulation of fatty liver hemorrhagic syndrome in chickens. Anim. Biosci. 2021, 34, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Amin, S.; El Amrousy, D.; Elrifaey, S.; Gamal, R.; Hodeib, H. Serum Osteocalcin Levels in Children with Nonalcoholic Fatty Liver Disease. J. Craniofacial Surg. 2018, 66, 117–121. [Google Scholar] [CrossRef]
- Machado, M.V.; Diehl, A.M. The hedgehog pathway in nonalcoholic fatty liver disease. Crit. Rev. Biochem. Mol. Biol. 2018, 53, 264–278. [Google Scholar] [CrossRef]
- Zeng, L.; Tang, W.J.; Yin, J.J.; Zhou, B.J. Signal transductions and nonalcoholic fatty liver: A mini-review. Int. J. Clin. Exp. Med. 2014, 7, 1624–1631. [Google Scholar]
- Zhang, D.; Zhang, Y.; Wang, Z.; Lei, L. Thymoquinone attenuates hepatic lipid accumulation by inducing autophagy via AMPK/mTOR/ULK1-dependent pathway in nonalcoholic fatty liver disease. Phytother. Res. 2023, 37, 781–797. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; You, H.; Qiu, S.; Yu, D.; Bai, Y.; He, J.; Cao, H.; Che, Q.; Guo, J.; Su, Z. A new perspective on NAFLD: Focusing on the crosstalk between peroxisome proliferator-activated receptor alpha (PPARα) and farnesoid X receptor (FXR). Biomed. Pharmacother. 2022, 154, 113577. [Google Scholar] [CrossRef]
- Chavez, C.P.; Cusi, K.; Kadiyala, S. The Emerging Role of Glucagon-like Peptide-1 Receptor Agonists for the Management of NAFLD. J. Clin. Endocrinol. Metab. 2021, 107, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Tan, J.-X.; He, Y.; Bai, F.; Li, S.-W.; Hou, Y.-W.; Ji, L.-S.; Gao, Y.-T.; Zhang, X.; Zhou, Z.-H.; et al. Atractylenolide III ameliorates Non-Alcoholic Fatty Liver Disease by activating Hepatic Adiponectin Receptor 1-Mediated AMPK Pathway. Int. J. Biol. Sci. 2022, 18, 1594–1611. [Google Scholar] [CrossRef]
- You, M.; Zhang, S.; Shen, Y.; Zhao, X.; Chen, L.; Liu, J.; Ma, N. Quantitative lipidomics reveals lipid perturbation in the liver of fatty liver hemorrhagic syndrome in laying hens. Poult. Sci. 2023, 102, 102352. [Google Scholar] [CrossRef]
- Rozenboim, I.; Mahato, J.; Cohen, N.A.; Tirosh, O. Low protein and high-energy diet: A possible natural cause of fatty liver hemorrhagic syndrome in caged White Leghorn laying hens. Poult. Sci. 2016, 95, 612–621. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, L.; Zhang, Y.; Yan, S.; Huang, L. Improvement of Lipotoxicity-Induced Islet β Cellular Insulin Secretion Disorder by Osteocalcin. J. Diabetes Res. 2022, 2022, 3025538. [Google Scholar] [CrossRef]
- Du, J.; Zhang, M.; Lu, J.; Zhang, X.; Xiong, Q.; Xu, Y.; Bao, Y.; Jia, W. Osteocalcin improves nonalcoholic fatty liver disease in mice through activation of Nrf2 and inhibition of JNK. Endocrine 2016, 53, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Tian, R.; She, Z.; Cai, J.; Li, H. Role of oxidative stress in the pathogenesis of nonalcoholic fatty liver disease. Free Radic. Biol. Med. 2020, 152, 116–141. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.; Zhao, H.; Lu, J.; He, F.; Liu, W.; Yu, W.; Wang, Q.; Hisatome, I.; Yamamoto, T.; Koyama, H.; et al. High uric acid induces liver fat accumulation via ROS/JNK/AP-1 signaling. Am. J. Physiol. Metab. 2021, 320, E1032–E1043. [Google Scholar] [CrossRef] [PubMed]
- Ruan, L.; Ruan, L.; Li, F.; Li, S.; Zhang, M.; Wang, F.; Lv, X.; Liu, Q. Effect of Different Exercise Intensities on Hepatocyte Apoptosis in HFD-Induced NAFLD in Rats: The Possible Role of Endoplasmic Reticulum Stress through the Regulation of the IRE1/JNK and eIF2α/CHOP Signal Pathways. Oxidative Med. Cell. Longev. 2021, 2021, 6378568. [Google Scholar] [CrossRef]
- Hirosumi, J.; Tuncman, G.; Chang, L.; Görgün, C.Z.; Uysal, K.T.; Maeda, K.; Karin, M.; Hotamisligil, G.S. A central role for JNK in obesity and insulin resistance. Nature 2002, 420, 333–336. [Google Scholar] [CrossRef]
- Joshi-Barve, S.; Barve, S.S.; Amancherla, K.; Gobejishvili, L.; Hill, D.; Cave, M.; Hote, P.; McClain, C.J. Palmitic acid induces production of proinflammatory cytokine interleukin-8 from hepatocytes. Hepatology 2007, 46, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.; Chao, X.; Williams, J.; Fulte, S.; Li, T.; Yang, L.; Ding, W.-X. Autophagy in liver diseases: A review. Mol. Asp. Med. 2021, 82, 100973. [Google Scholar] [CrossRef]
- Chang, L.; Karin, M. Mammalian MAP kinase signalling cascades. Nature 2001, 410, 37–40. [Google Scholar] [CrossRef]
- Yue, C.; Chen, J.; Hou, R.; Tian, W.; Liu, K.; Wang, D.; Lu, Y.; Liu, J.; Wu, Y.; Hu, Y. The antioxidant action and mechanism of selenizing Schisandra chinensis polysaccharide in chicken embryo hepatocyte. Int. J. Biol. Macromol. 2017, 98, 506–514. [Google Scholar] [CrossRef]
- Liu, P.; Shi, L.; Cang, X.; Huang, J.; Wu, X.; Yan, J.; Chen, L.; Cui, S.; Ye, X. CtBP2 ameliorates palmitate-induced insulin resistance in HepG2 cells through ROS mediated JNK pathway. Gen. Comp. Endocrinol. 2017, 247, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Dupont, J.; Tesseraud, S.; Simon, J. Insulin signaling in chicken liver and muscle. Gen. Comp. Endocrinol. 2008, 163, 52–57. [Google Scholar] [CrossRef]
- Wang, A.; Jiang, H.; Liu, Y.; Chen, J.; Zhou, X.; Zhao, C.; Chen, X.; Lin, M. Rhein induces liver cancer cells apoptosis via activating ROS-dependent JNK/Jun/caspase-3 signaling pathway. J. Cancer 2020, 11, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Yazıcı, D.; Sezer, H. Insulin Resistance, Obesity and Lipotoxicity. Adv. Exp. Med. Biol. 2017, 960, 277–304. [Google Scholar] [CrossRef]
- Yung, J.H.M.; Giacca, A. Role of c-Jun N-terminal Kinase (JNK) in Obesity and Type 2 Diabetes. Cells 2020, 9, 706. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.K.; Sowa, H.; Hinoi, E.; Ferron, M.; Ahn, J.D.; Confavreux, C.; Dacquin, R.; Mee, P.J.; McKee, M.D.; Jung, D.Y.; et al. Endocrine Regulation of Energy Metabolism by the Skeleton. Cell 2007, 130, 456–469. [Google Scholar] [CrossRef]
- Cruz-Pineda, W.D.; Parra-Rojas, I.; Rodríguez-Ruíz, H.A.; Illades-Aguiar, B.; Matia-García, I.; Garibay-Cerdenares, O.L. The regulatory role of insulin in energy metabolism and leukocyte functions. J. Leukoc. Biol. 2021, 111, 197–208. [Google Scholar] [CrossRef]
- Saltiel, A.R.; Kahn, C.R. Insulin signalling and the regulation of glucose and lipid metabolism. Nature 2001, 414, 799–806. [Google Scholar] [CrossRef]
- Lebovitz, H.E. Insulin resistance: Definition and consequences. Exp. Clin. Endocrinol. Diabetes 2001, 109 (Suppl. 2), S135–S148. [Google Scholar] [CrossRef]
- Gamberi, T.; Magherini, F.; Modesti, A.; Fiaschi, T. Adiponectin Signaling Pathways in Liver Diseases. Biomedicines 2018, 6, 52. [Google Scholar] [CrossRef] [PubMed]
- Ocón-Grove, O.M.; Krzysik-Walker, S.M.; Maddineni, S.R.; Hendricks, G.L.; Ramachandran, R. Adiponectin and its receptors are expressed in the chicken testis: Influence of sexual maturation on testicular ADIPOR1 and ADIPOR2 mRNA abundance. Reproduction 2008, 136, 627–638. [Google Scholar] [CrossRef]
- Fang, H.; Judd, R.L. Adiponectin Regulation and Function. Compr. Physiol. 2018, 8, 1031–1063. [Google Scholar] [PubMed]
- El Amrousy, D.; El-Afify, D. Osteocalcin and osteoprotegerin levels and their relationship with adipokines and proinflammatory cytokines in children with nonalcoholic fatty liver disease. Cytokine 2020, 135, 155215. [Google Scholar] [CrossRef] [PubMed]
- Vella, A.; Kumar, R. Osteocalcin and the Regulation of Glucose Metabolism. Clin. Rev. Bone Miner. Metab. 2012, 11, 11–16. [Google Scholar] [CrossRef]
- Hu, Q.; Wang, D.; Lin, H.; Li, H.; Zhao, J.; Jiao, H.C.; Wang, X. Adiponectin Reduces Lipid Content in Chicken Myoblasts by Activating AMPK Signaling Pathway. Biosci. Rep. 2022, 42, BSR20212549. [Google Scholar] [CrossRef]
- Gan, L.; Yan, J.; Liu, Z.; Feng, M.; Sun, C. Adiponectin Prevents Reduction of Lipid-Induced Mitochondrial Biogenesis via AMPK/ACC2 Pathway in Chicken Adipocyte. J. Cell. Biochem. 2015, 116, 1090–1100. [Google Scholar] [CrossRef]
- Hendricks, G.L., 3rd; Hadley, J.; Krzysik-Walker, S.; Prabhu, S.; Vasilatos-Younken, R.; Ramachandran, R. Unique profile of chicken adiponectin, a predominantly heavy molecular weight multimer, and relationship to visceral adiposity. Endocrinology 2009, 150, 3092–3100. [Google Scholar] [CrossRef]
- Zhuang, Y.-R.; Lin, Y.-Y. Chicken recombinant adiponectin enhances fatty acid metabolism in oleic acid- and palmitic acid-treated LMH cells. Rev. Bras. Zootec. 2022, 51, e20220087. [Google Scholar] [CrossRef]
- Lian, K.; Feng, Y.-N.; Li, R.; Liu, H.-L.; Han, P.; Zhou, L.; Li, C.-X.; Wang, Q. Middle- and high-molecular weight adiponectin levels in relation to nonalcoholic fatty liver disease. J. Clin. Lab. Anal. 2019, 34, e23148. [Google Scholar] [CrossRef] [PubMed]
- Mavilia, M.G.; Wu, G.Y. Liver and serum adiponectin levels in non-alcoholic fatty liver disease. J. Dig. Dis. 2021, 22, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Zhuang, Q.; Ye, X.; Ning, M.; Wu, S.; Lu, L.; Wan, X. Adiponectin Inhibits NLRP3 Inflammasome Activation in Nonalcoholic Steatohepatitis via AMPK-JNK/ErK1/2-NFκB/ROS Signaling Pathways. Front. Med. 2020, 7, 546445. [Google Scholar] [CrossRef]
- Ma, W.; Zhang, S.; Li, Y.; Chen, T.; Yang, Q.; Feng, X. Adiponectin alleviates non-alcoholic fatty liver injury via regulating oxidative stress in liver cells. Minerva Med. 2023, 113, 990–999. [Google Scholar] [CrossRef]
- Cao, Z.; Ma, B.; Cui, C.; Zhao, J.; Liu, S.; Qiu, Y.; Zheng, Y.; Gao, M.; Luan, X. Protective effects of AdipoRon on the liver of Huoyan goose fed a high-fat diet. Poult. Sci. 2022, 101, 101708. [Google Scholar] [CrossRef]
- Bordoloi, J.; Ozah, D.; Bora, T.; Kalita, J.; Manna, P. Gamma-glutamyl carboxylated Gas6 mediates the beneficial effect of vitamin K on lowering hyperlipidemia via regulating the AMPK/SREBP1/PPARα signaling cascade of lipid metabolism. J. Nutr. Biochem. 2019, 70, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Saltiel, A.R. From overnutrition to liver injury: AMP-activated protein kinase in nonalcoholic fatty liver diseases. J. Biol. Chem. 2020, 295, 12279–12289. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Li, L.; Wang, H.; Yang, Y.; Ma, H. Activated AMP-activated protein kinase prevents hepatic steatosis, oxidative stress and inflammation in primary chicken hepatocytes. Front. Physiol. 2022, 13, 974825. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, H.; Yang, Y.; Jiang, Z.; Ma, H. Dehydroepiandrosterone activates the GPER-mediated AMPK signaling pathway to alleviate the oxidative stress and inflammatory response in laying hens fed with high-energy and low-protein diets. Life Sci. 2022, 308, 120926. [Google Scholar] [CrossRef]
- Gao, X.; Liu, P.; Wu, C.; Wang, T.; Liu, G.; Cao, H.; Zhang, C.; Hu, G.; Guo, X. Effects of fatty liver hemorrhagic syndrome on the AMP-activated protein kinase signaling pathway in laying hens. Poult. Sci. 2019, 98, 2201–2210. [Google Scholar] [CrossRef]
- Zhang, K.; Shi, Y.; Huang, C.; Huang, C.; Xu, P.; Zhou, C.; Liu, P.; Hu, R.; Zhuang, Y.; Li, G.; et al. Activation of AMP-activated protein kinase signaling pathway ameliorates steatosis in laying hen hepatocytes. Poult. Sci. 2020, 100, 100805. [Google Scholar] [CrossRef]
- Fu, C.; Zhang, Y.; Yao, Q.; Wei, X.; Shi, T.; Yan, P.; Liu, X. Maternal conjugated linoleic acid alters hepatic lipid metabolism via the AMPK signaling pathway in chick embryos. Poult. Sci. 2020, 99, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.K.K.; Zhang, L.; Chan, M.T.V. Autophagy, NAFLD and NAFLD-Related HCC. Adv. Exp. Med. Biol. 2018, 1061, 127–138. [Google Scholar] [CrossRef]
- Wang, X.; Xing, C.; Yang, F.; Zhou, S.; Li, G.; Zhang, C.; Cao, H.; Hu, G. Abnormal expression of liver autophagy and apoptosis-related mRNA in fatty liver haemorrhagic syndrome and improvement function of resveratrol in laying hens. Avian Pathol. 2020, 49, 171–178. [Google Scholar] [CrossRef]
- Zhou, F.; Ding, M.; Gu, Y.; Fan, G.; Liu, C.; Li, Y.; Sun, R.; Wu, J.; Li, J.; Xue, X.; et al. Aurantio-Obtusin Attenuates Non-Alcoholic Fatty Liver Disease Through AMPK-Mediated Autophagy and Fatty Acid Oxidation Pathways. Front. Pharmacol. 2022, 12, 826628. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Zhu, W.; Wen, T.; Mukhamejanova, Z.; Xu, F.; Xiang, Q.; Pang, J. Xyloketal B Reverses Nutritional Hepatic Steatosis, Steatohepatitis, and Liver Fibrosis through Activation of the PPARα/PGC1α Signaling Pathway. J. Nat. Prod. 2022, 85, 1738–1750. [Google Scholar] [CrossRef]
- Zhang, J.; Du, H.; Shen, M.; Zhao, Z.; Ye, X. Kangtaizhi Granule Alleviated Nonalcoholic Fatty Liver Disease in High-Fat Diet-Fed Rats and HepG2 Cells via AMPK/mTOR Signaling Pathway. J. Immunol. Res. 2020, 2020, 3413186. [Google Scholar] [CrossRef]
- Shi, C.; Xue, W.; Han, B.; Yang, F.; Yin, Y.; Hu, C. Acetaminophen aggravates fat accumulation in NAFLD by inhibiting autophagy via the AMPK/mTOR pathway. Eur. J. Pharmacol. 2019, 850, 15–22. [Google Scholar] [CrossRef]
- Colakoglu, H.E.; Yazlik, M.O.; Kaya, U.; Colakoglu, E.C.; Kurt, S.; Oz, B.; Bayramoglu, R.; Vural, M.R.; Kuplulu, S. MDA and GSH-Px activity in transition dairy cows under seasonal variations and their relationship with reproductive performance. J. Vet. Res. 2017, 61, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Dou, W.; Ni, Z.; Wen, Q.; Zhang, R.; Qin, M.; Wang, X.; Tang, H.; Cao, Y.; Wang, J.; et al. Deletion of Nrf2 leads to hepatic insulin resistance via the activation of NF-κB in mice fed a high-fat diet. Mol. Med. Rep. 2016, 14, 1323–1331. [Google Scholar] [CrossRef]
- Ding, X.; Jian, T.; Li, J.; Lv, H.; Tong, B.; Li, J.; Meng, X.; Ren, B.; Chen, J. Chicoric Acid Ameliorates Nonalcoholic Fatty Liver Disease via the AMPK/Nrf2/NFκB Signaling Pathway and Restores Gut Microbiota in High-Fat-Diet-Fed Mice. Oxidative Med. Cell. Longev. 2020, 2020, 9734560. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, H.; Yang, Y.; Jiang, Z.; Ma, H. Dehydroepiandrosterone protects against oleic acid-triggered mitochondrial dysfunction to relieve oxidative stress and inflammation via activation of the AMPK-Nrf2 axis by targeting GPR30 in hepatocytes. Mol. Immunol. 2023, 155, 110–123. [Google Scholar] [CrossRef] [PubMed]
- Rives, C.; Fougerat, A.; Ellero-Simatos, S.; Loiseau, N.; Guillou, H.; Gamet-Payrastre, L.; Wahli, W. Oxidative Stress in NAFLD: Role of Nutrients and Food Contaminants. Biomolecules 2020, 10, 1702. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Fan, H.; Zhang, B.; Ning, C.; Xing, K.; Guo, Y. Dietary genistein supplementation in laying broiler breeder hens alters the development and metabolism of offspring embryos as revealed by hepatic transcriptome analysis. FASEB J. 2018, 32, 4214–4228. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Shi, Y.; Li, G.; Huang, C.; Zhuang, Y.; Shu, B.; Cao, X.; Li, Z.; Hu, G.; Liu, P.; et al. Preparation of the peroxisome proliferator-activated receptor α polyclonal antibody: Its application in fatty liver hemorrhagic syndrome. Int. J. Biol. Macromol. 2021, 182, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zeng, Q.; Li, F.; Fang, H.; Zhou, Z.; Jiang, T.; Yin, C.; Wei, Q.; Wang, Y.; Ruan, J.; et al. Dysregulated H3K27 Acetylation Is Implicated in Fatty Liver Hemorrhagic Syndrome in Chickens. Front. Genet. 2021, 11, 574167. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.; Jeong, I.-K.; Ahn, K.J.; Chung, H.Y.; Hwang, Y.-C. Fenofibrate, a PPARα agonist, reduces hepatic fat accumulation through the upregulation of TFEB-mediated lipophagy. Metabolism 2021, 120, 154798. [Google Scholar] [CrossRef]
- Wei, C.C.; Luo, Z.; Hogstrand, C.; Xu, Y.H.; Wu, L.X.; Chen, G.H.; Pan, Y.-X.; Song, Y.F. Zinc reduces hepatic lipid deposition and activates lipophagy via Zn(2+)/MTF-1/PPARα and Ca(2+)/CaMKKβ/AMPK pathways. FASEB J. 2018, 32, 6666–6680. [Google Scholar] [CrossRef]
- Horev, G.; Einat, P.; Aharoni, T.; Eshdat, Y.; Friedman-Einat, M. Molecular cloning and properties of the chicken leptin-receptor (CLEPR) gene. Mol. Cell. Endocrinol. 2000, 162, 95–106. [Google Scholar] [CrossRef]
- Ohkubo, T.; Tanaka, M.; Nakashima, K. Structure and tissue distribution of chicken leptin receptor (cOb-R) mRNA. Biochim. Biophys. Acta 2000, 1491, 303–308. [Google Scholar] [CrossRef]
- Seroussi, E.; Cinnamon, Y.; Yosefi, S.; Genin, O.; Smith, J.G.; Rafati, N.; Bornelöv, S.; Andersson, L.; Friedman-Einat, M. Identification of the Long-Sought Leptin in Chicken and Duck: Expression Pattern of the Highly GC-Rich Avian leptin Fits an Autocrine/Paracrine Rather Than Endocrine Function. Endocrinology 2015, 157, 737–751. [Google Scholar] [CrossRef]
- Seroussi, E.; Knytl, M.; Pitel, F.; Elleder, D.; Krylov, V.; Leroux, S.; Morisson, M.; Yosefi, S.; Miyara, S.; Ganesan, S.; et al. Avian Expression Patterns and Genomic Mapping Implicate Leptin in Digestion and TNF in Immunity, Suggesting That Their Interacting Adipokine Role Has Been Acquired Only in Mammals. Int. J. Mol. Sci. 2019, 20, 4489. [Google Scholar] [CrossRef] [PubMed]
- Friedman-Einat, M.; Seroussi, E. Avian Leptin: Bird’s-Eye View of the Evolution of Vertebrate Energy-Balance Control. Trends Endocrinol. Metab. 2019, 30, 819–832. [Google Scholar] [CrossRef] [PubMed]
- Ferré, P.; Foufelle, F. Hepatic steatosis: A role for de novo lipogenesis and the transcription factor SREBP-1c. Diabetes Obes. Metab. 2010, 12 (Suppl. 2), 83–92. [Google Scholar] [CrossRef] [PubMed]
- Hackl, M.T.; Fürnsinn, C.; Schuh, C.M.; Krssak, M.; Carli, F.; Guerra, S.; Freudenthaler, A.; Baumgartner-Parzer, S.; Helbich, T.H.; Luger, A.; et al. Brain leptin reduces liver lipids by increasing hepatic triglyceride secretion and lowering lipogenesis. Nat. Commun. 2019, 10, 2717. [Google Scholar] [CrossRef]
- Dridi, S.; Buyse, J.; Decuypere, E.; Taouis, M. Potential role of leptin in increase of fatty acid synthase gene expression in chicken liver. Domest. Anim. Endocrinol. 2005, 29, 646–660. [Google Scholar] [CrossRef]
- Shih, P.-H.; Shiue, S.-J.; Chen, C.-N.; Cheng, S.-W.; Lin, H.-Y.; Wu, L.-W.; Wu, M.-S. Fucoidan and Fucoxanthin Attenuate Hepatic Steatosis and Inflammation of NAFLD through Modulation of Leptin/Adiponectin Axis. Mar. Drugs 2021, 19, 148. [Google Scholar] [CrossRef]
- Bernardi, O.; Estienne, A.; Reverchon, M.; Bigot, Y.; Froment, P.; Dupont, J. Adipokines in metabolic and reproductive functions in birds: An overview of current knowns and unknowns. Mol. Cell. Endocrinol. 2021, 534, 111370. [Google Scholar] [CrossRef]
- Zendehdel, M.; Khodadadi, M.; Vosoughi, A.; Mokhtarpouriani, K.; Baghbanzadeh, A. β2 adrenergic receptors and leptin interplay to decrease food intake in chicken. Br. Poult. Sci. 2020, 61, 156–163. [Google Scholar] [CrossRef]
- Li, R.; Hu, Y.; Ni, Y.; Xia, D.; Grossmann, R.; Zhao, R. Leptin stimulates hepatic activation of thyroid hormones and promotes early posthatch growth in the chicken. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2011, 160, 200–206. [Google Scholar] [CrossRef]
- Adachi, H.; Murase, D.; Ohkubo, T. Inhibitory Mechanism of Signal Transduction through Chicken Leptin Receptor by Suppressor of Cytokine Signaling 3 (SOCS3). J. Poult. Sci. 2013, 50, 262–269. [Google Scholar] [CrossRef]
- Piekarski, A.; Nagarajan, G.; Ishola, P.; Flees, J.; Greene, E.S.; Kuenzel, W.J.; Ohkubo, T.; Maier, H.; Bottje, W.G.; Cline, M.A.; et al. AMP-Activated Protein Kinase Mediates the Effect of Leptin on Avian Autophagy in a Tissue-Specific Manner. Front. Physiol. 2018, 9, 541. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Hiramatsu, K.; Nishimura, K.; Ono, T. Glucagon-like Peptide-1 Receptor Expression in the Pancreatic D Cells of Three Avian Species; White Leghorn Chickens, Northern Bobwhites, and Common Ostriches. J. Poult. Sci. 2018, 55, 199–203. [Google Scholar] [CrossRef]
- Huang, G.; Li, J.; Fu, H.; Yan, Z.; Bu, G.; He, X.; Wang, Y. Characterization of glucagon-like peptide 1 receptor (GLP1R) gene in chickens: Functional analysis, tissue distribution, and identification of its transcript variants. Domest. Anim. Endocrinol. 2012, 43, 1–15. [Google Scholar] [CrossRef]
- Hiramatsu, K. Chicken Intestinal L Cells and Glucagon-like Peptide-1 Secretion. J. Poult. Sci. 2020, 57, 1–6. [Google Scholar] [CrossRef]
- Drucker, D.J. GLP-1 physiology informs the pharmacotherapy of obesity. Mol. Metab. 2021, 57, 101351. [Google Scholar] [CrossRef] [PubMed]
- Seino, Y.; Yabe, D. Glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1: Incretin actions beyond the pancreas. J. Diabetes Investig. 2013, 4, 108–130. [Google Scholar] [CrossRef]
- Zhang, J.-M.; Sun, Y.-S.; Zhao, L.-Q.; Chen, T.-T.; Fan, M.-N.; Jiao, H.-C.; Zhao, J.-P.; Wang, X.-J.; Li, F.-C.; Li, H.-F.; et al. SCFAs-Induced GLP-1 Secretion Links the Regulation of Gut Microbiome on Hepatic Lipogenesis in Chickens. Front. Microbiol. 2019, 10, 2176. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, T.; Oikawa, D.; Adachi, N.; Boswell, T.; Furuse, M. Intracerebroventricular injection of glucagon-like peptide-1 changes lipid metabolism in chicks. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2007, 147, 1104–1108. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tu, W.; Zhang, Y.; Jiang, K.; Jiang, S. Osteocalcin and Its Potential Functions for Preventing Fatty Liver Hemorrhagic Syndrome in Poultry. Animals 2023, 13, 1380. https://doi.org/10.3390/ani13081380
Tu W, Zhang Y, Jiang K, Jiang S. Osteocalcin and Its Potential Functions for Preventing Fatty Liver Hemorrhagic Syndrome in Poultry. Animals. 2023; 13(8):1380. https://doi.org/10.3390/ani13081380
Chicago/Turabian StyleTu, Wenjun, Yuhan Zhang, Kunyu Jiang, and Sha Jiang. 2023. "Osteocalcin and Its Potential Functions for Preventing Fatty Liver Hemorrhagic Syndrome in Poultry" Animals 13, no. 8: 1380. https://doi.org/10.3390/ani13081380
APA StyleTu, W., Zhang, Y., Jiang, K., & Jiang, S. (2023). Osteocalcin and Its Potential Functions for Preventing Fatty Liver Hemorrhagic Syndrome in Poultry. Animals, 13(8), 1380. https://doi.org/10.3390/ani13081380




