New Insight into the Quality Traits of Milk and Cheese from Teramana Goats, a Native Italian Breed
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design, Cheesemaking and Sampling
2.2. Fatty Acid Profile of Milk and Cheese
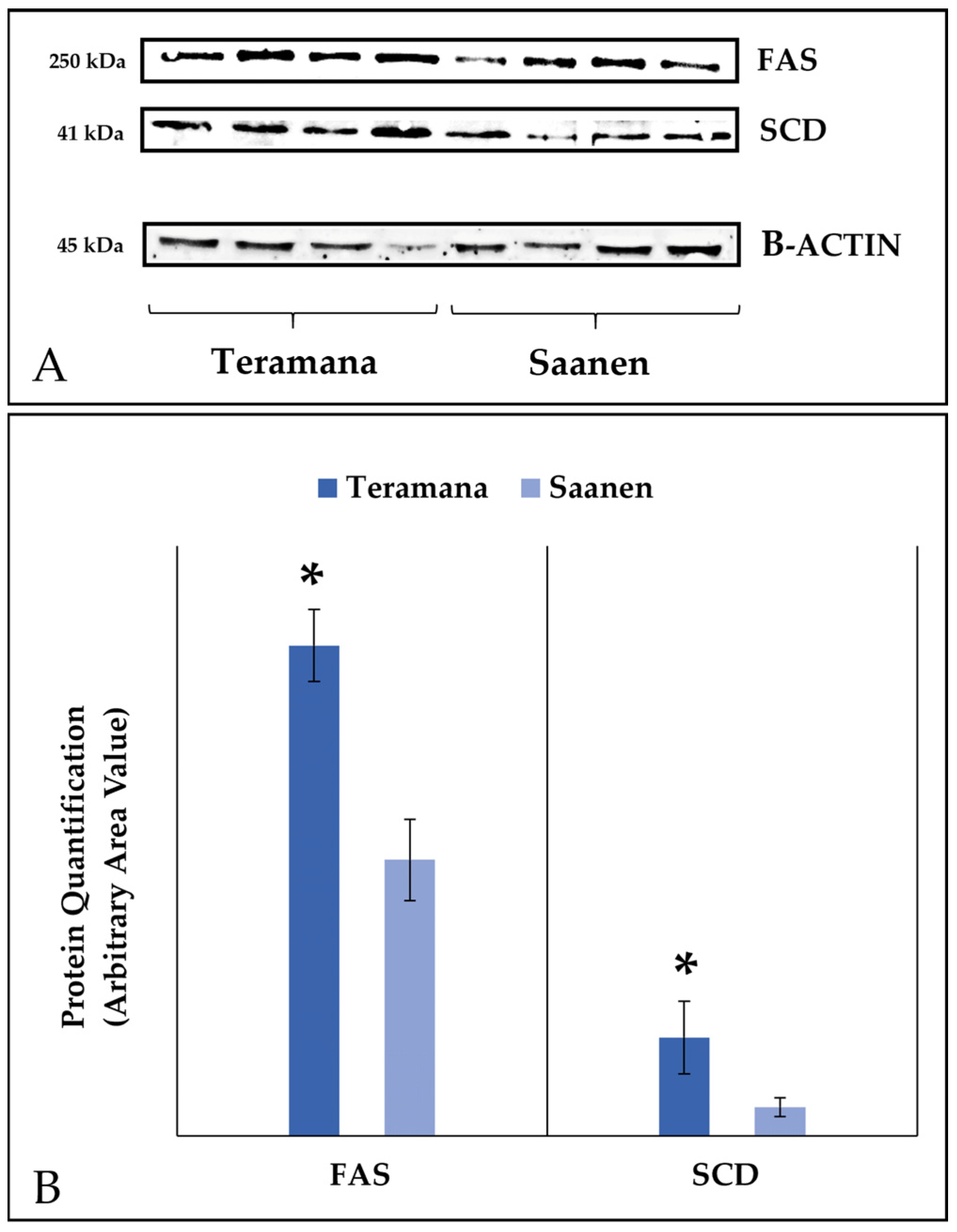
2.3. Western Blot Analysis of Δ9-Desaturase and Fatty Acid Synthase in Somatic Cells
2.4. Cheese Moisture and Total Lipids Content
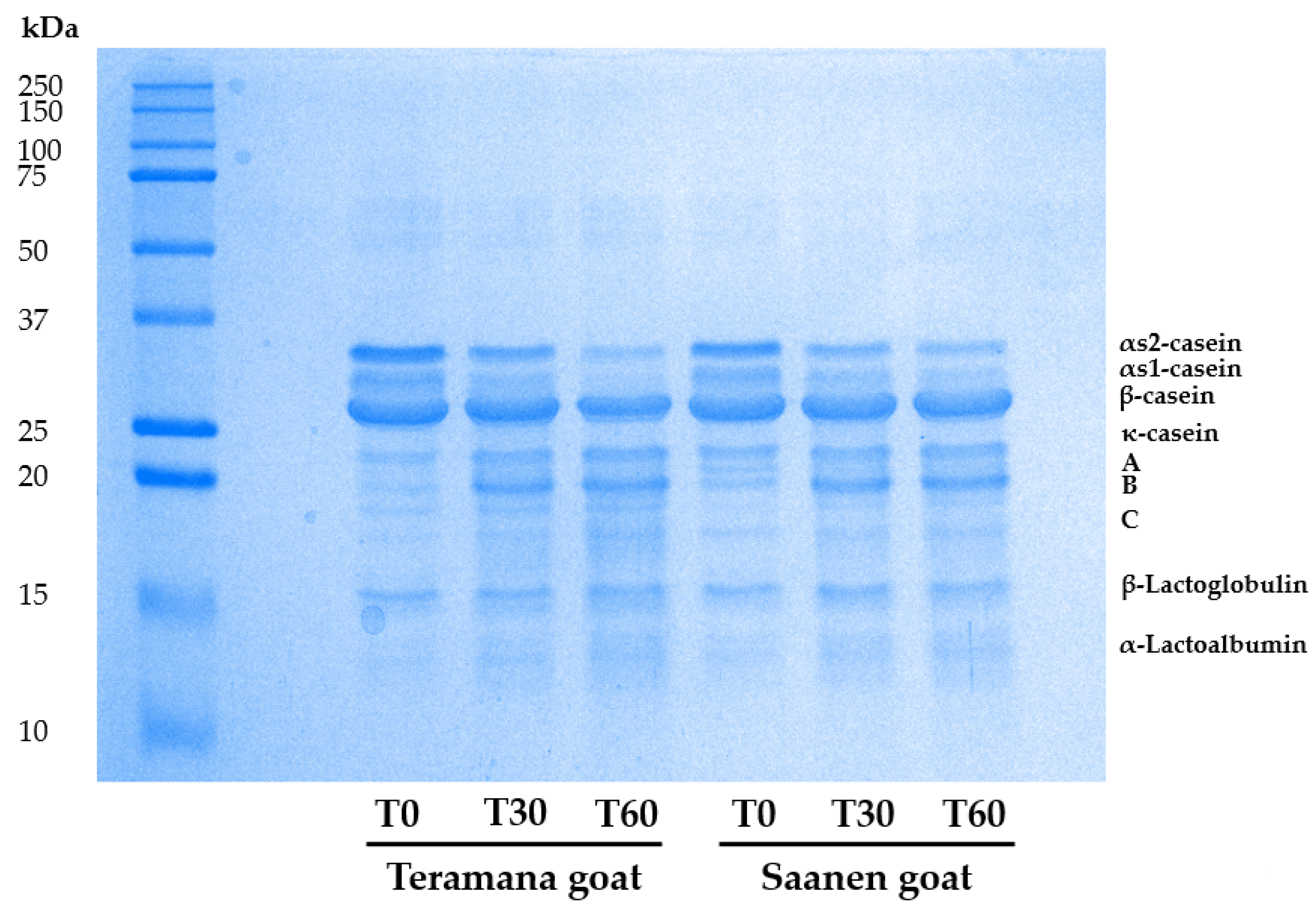
2.5. Cheese Protein Extraction and Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE)
2.6. Cheese Volatile Profile
2.7. Evaluation of Colour and Textural Properties
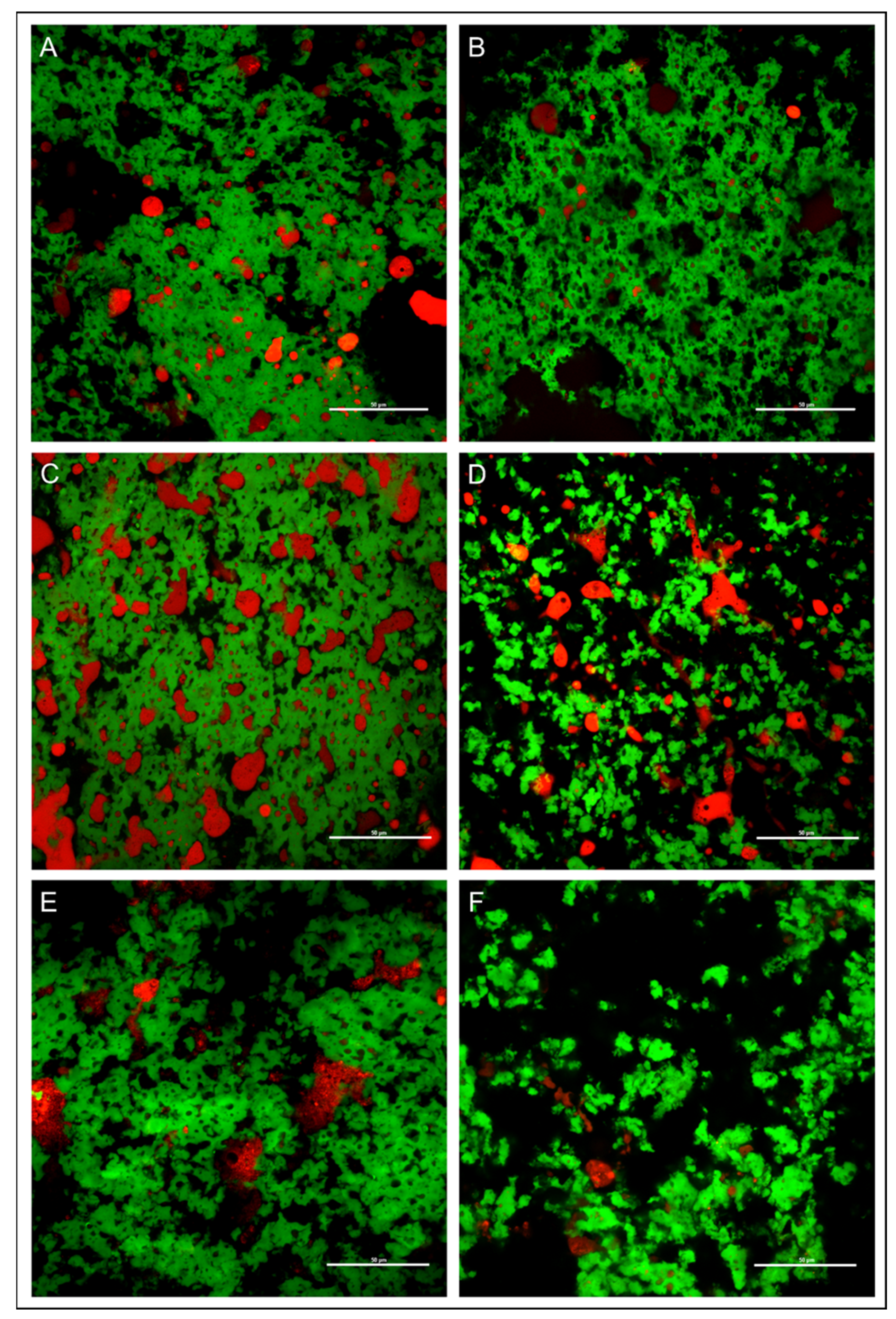
2.8. Confocal Analysis of Cheese
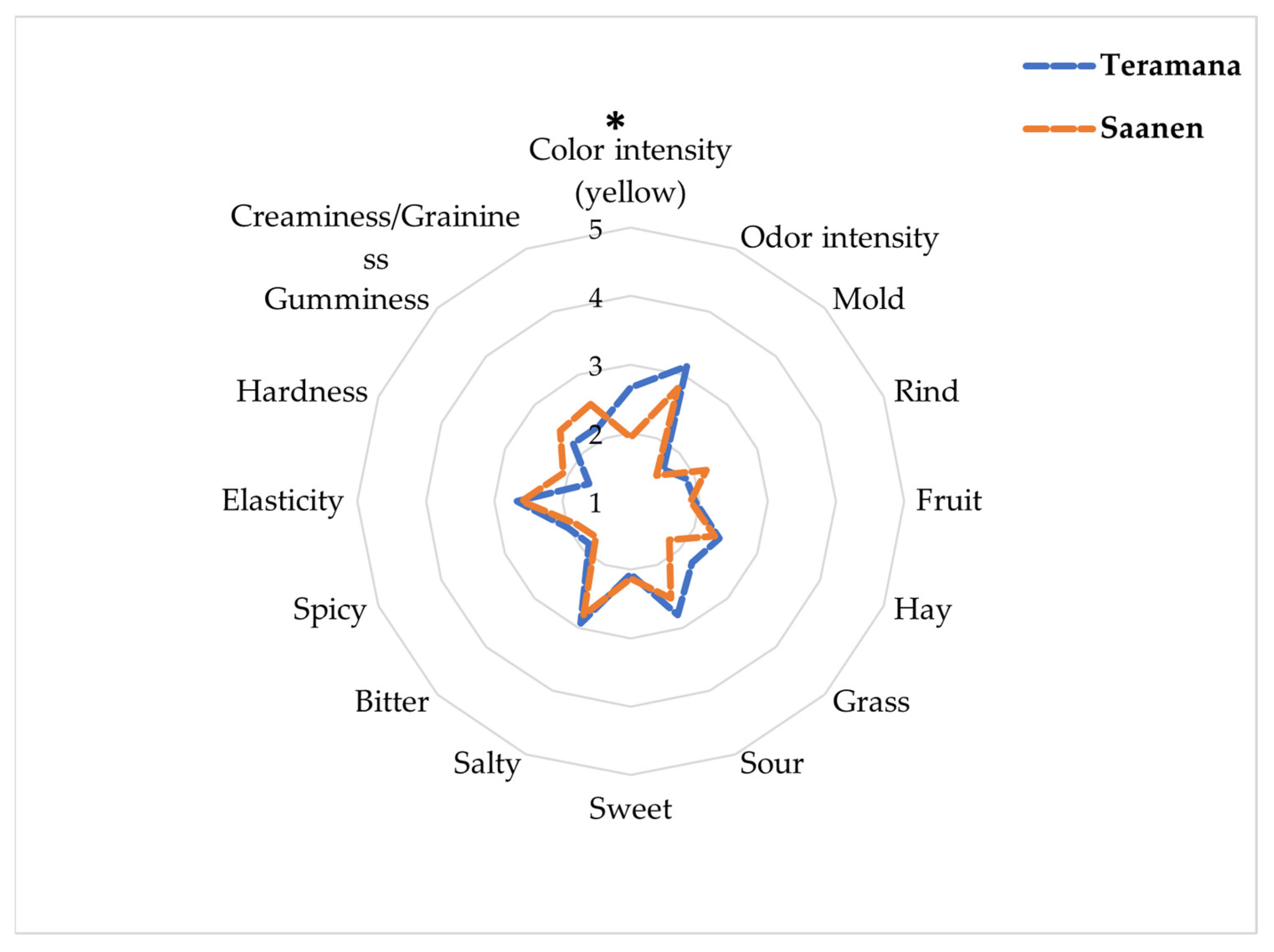
2.9. Sensory Analysis
2.10. Statistical Analysis
3. Results
3.1. Fatty Acid Profile of Milk and Cheese
3.2. Western Blot Analysis of Δ9-Desaturase and Fatty Acid Synthase
3.3. Cheese Moisture, Total Lipids
3.4. Cheese Protein Profile and Proteolysis
3.5. Identification of Volatile Compounds in Cheese
3.6. Confocal Microscopy Image of Cheese
3.7. Physical and Sensorial Properties of Cheese
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- European Commission; Office for Official Publications of the European Communities; Statistical Office of the European Communities; European Union; European Commission. Food: From Farm to Fork Statistics; Publications Office of the European Union: Luxembourg, 2011; ISBN 9789279202391. [Google Scholar]
- Miller, B.A.; Lu, C.D. Special Issue—Current Status of Global Dairy Goat Production: An Overview. Asian-Australas J. Anim. Sci. 2019, 32, 1219–1232. [Google Scholar] [CrossRef] [PubMed]
- Canton, H. Food and Agriculture Organization of the United Nations—FAO. Eur. Dir. Int. Organ. 2021, 2021, 297–305. [Google Scholar] [CrossRef]
- Pulina, G.; Milán, M.J.; Lavín, M.P.; Theodoridis, A.; Morin, E.; Capote, J.; Thomas, D.L.; Francesconi, A.H.D.; Caja, G. Invited Review: Current Production Trends, Farm Structures, and Economics of the Dairy Sheep and Goat Sectors. J. Dairy Sci. 2018, 101, 6715–6729. [Google Scholar] [CrossRef] [PubMed]
- Skeie, S. Characteristics in Milk Influencing the Cheese yield and Cheese Quality. J. Anim. Feed Sci. 2007, 16, 130–142. [Google Scholar] [CrossRef]
- Cosentino, C.; Colonna, M.A.; Musto, M.; Dimotta, A.; Freschi, P.; Tarricone, S.; Ragni, M.; Paolino, R. Effects of Dietary Supplementation with Extruded Linseed and Oregano in Autochthonous Goat Breeds on the Fatty Acid Profile of Milk and Quality of Padraccio Cheese. J. Dairy Sci. 2021, 104, 1445–1453. [Google Scholar] [CrossRef] [PubMed]
- Nicoloso, L.; Bomba, L.; Colli, L.; Negrini, R.; Milanesi, M.; Mazza, R.; Sechi, T.; Frattini, S.; Talenti, A.; Coizet, B.; et al. Genetic Diversity of Italian Goat Breeds Assessed with a Medium-Density SNP Chip. Genet. Sel. Evol. 2015, 47, 62. [Google Scholar] [CrossRef]
- Tarricone, S.; Giannico, F.; Ragni, M.; Colonna, M.A.; Rotondi, P.; Cosentino, C.; Seidavi, A.; Tufarelli, V.; Laudadio, V. Effects of Dietary Extruded Linseed (Linum usitatissimum) and Oregano (Origanum vulgare) on Growth Traits, Carcass Composition and Meat Quality of Grigia Di Potenza Suckling Kids. Int. J. Agric. Biol. 2021, 25, 1147–1152. [Google Scholar] [CrossRef]
- Colonna, M.A.; Giannico, F.; Tufarelli, V.; Laudadio, V.; Selvaggi, M.; De Mastro, G.; Tedone, L. Dietary Supplementation with Camelina Sativa (L. Crantz) Forage in Autochthonous Ionica Goats: Effects on Milk and Caciotta Cheese Chemical, Fatty Acid Composition and Sensory Properties. Animals 2021, 11, 1589. [Google Scholar] [CrossRef]
- Ianni, A.; Bennato, F.; Martino, C.; Di Luca, A.; Martino, G. Qualitative Attributes of Meat from Teramana Goat Kids, an Italian Native Breed of the Abruzzo Region. Anim. Biosci. 2022, 35, 1091–1099. [Google Scholar] [CrossRef]
- Baudoin, A.; Ammouche, L.; Noutary, E.; Lapujade, J.; Ruiz, I.; Tozanli, S.; Hadad-Gauthier, F.F.E. LACTIMED, Cross-Border Cooperation to Promote Typical Dairy Products in the Mediterranean. 2015. Available online: https://anima.coop/en/lactimed-cross-border-cooperation-to-promote-typical-dairy-products-in-the-mediterranean (accessed on 12 April 2023).
- Ianni, A.; Bennato, F.; Martino, C.; Odoardi, M.; Sacchetti, A.; Martino, G. Qualitative Attributes of Commercial Pig Meat from an Italian Native Breed: The Nero d’Abruzzo. Foods 2022, 11, 1297. [Google Scholar] [CrossRef]
- Tedone, L.; Giannico, F.; Tufarelli, V.; Laudadio, V.; Selvaggi, M.; De Mastro, G.; Colonna, M.A. Camelina Sativa (L. Crantz) Fresh Forage Productive Performance and Quality at Different Vegetative Stages: Effects of Dietary Supplementation in Ionica Goats on Milk Quality. Agriculture 2022, 12, 91. [Google Scholar] [CrossRef]
- Bertuzzi, A.S.; McSweeney, P.L.H.; Rea, M.C.; Kilcawley, K.N. Detection of Volatile Compounds of Cheese and Their Contribution to the Flavor Profile of Surface-Ripened Cheese. Compr. Rev. Food Sci. Food Saf. 2018, 17, 371–390. [Google Scholar] [CrossRef] [PubMed]
- Fox, P.F.; Guinee, T.P.; Cogan, T.M.; McSweeney, P.L.H. Biochemistry of Cheese Ripening. Fundam. Cheese Sci. 2017, 57, 391–442. [Google Scholar] [CrossRef]
- Niimi, J.; Eddy, A.I.; Overington, A.R.; Silcock, P.; Bremer, P.J.; Delahunty, C.M. Sensory Interactions between Cheese Aroma and Taste. J. Sens. Stud. 2015, 30, 247–257. [Google Scholar] [CrossRef]
- Zehentbauer, G.; Reineccius, G.A. Determination of Key Aroma Components of Cheddar Cheese Using Dynamic Headspace Dilution Assay. Flavour Fragr. J. 2002, 17, 300–305. [Google Scholar] [CrossRef]
- Drake, M.A.; Delahunty, C.M. Sensory Character of Cheese and Its Evaluation. In Cheese: Chemistry, Physics and Microbiology, 4th ed.; Springer: New York, NY, USA, 2017; Volume 1, pp. 517–545. [Google Scholar] [CrossRef]
- Kilcawley, K.N. Cheese Flavour. In Fundamentals of Cheese Science; Springer: New York, NY, USA, 2017; pp. 443–474. [Google Scholar] [CrossRef]
- AOAC International-Association of Official Analytical Chemists. Offcial Methods of Analysis, 17th ed.; The Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Folch, L.; Less, M.; Stanltey, S.A. Simple Method for the Isolation and Purification of Total Lipids from Animal Tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Florio, M.; Giannone, C.; Ianni, A.; Bennato, F.; Grotta, L.; Martino, G. Seasonal and Feeding System Effects on Qualitative Parameters of Bovine Milk Produced in the Abruzzo Region (Italy). Agriculture 2022, 12, 917. [Google Scholar] [CrossRef]
- Brogna, D.M.R.; Nasri, S.; Salem, H.B.; Mele, M.; Serra, A.; Bella, M.; Priolo, A.; Makkar, H.P.S.; Vasta, V. Effect of Dietary Saponins from Quillaja Saponaria L. on Fatty Acid Composition and Cholesterol Content in Muscle Longissimus Dorsi of Lambs. Animal 2011, 5, 1124–1130. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- AOAC International-Association of Official Analytical Chemists. Official Methods of Analysis, 15th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1990. [Google Scholar]
- Bennato, F.; Ianni, A.; Martino, C.; di Luca, A.; Innosa, D.; Fusco, A.M.; Pomilio, F.; Martino, G. Dietary Supplementation of Saanen Goats with Dried Licorice Root Modifies Chemical and Textural Properties of Dairy Products. J. Dairy Sci. 2020, 103, 52–62. [Google Scholar] [CrossRef]
- Bourne, M.C. Texture Profile Analysis. Food Technol. 1978, 7, 62–66. [Google Scholar]
- Pagliarini, E.; Lembo, P.; Bertuccioli, M. Recent Advancements in Sensory Analysis of Cheese. Ital. J. Food Sci. 2013, 3, 85–99. [Google Scholar]
- Bérodier, F.; Lavanchy, P.; Zannoni, M.; Casals, J.; Herrero, L.; Adamo, C. Guide d’Évaluation Olfacto-Gustative Des Fromages à Pâte Dure et Semi-Dure. LWT Food Sci. Technol. 1997, 30, 653–664. [Google Scholar] [CrossRef]
- Bernard, L.; Leroux, C.; Hayes, H.; Gautier, M.; Chilliard, Y.; Martin, P. Characterization of the Caprine Stearoyl-CoA Desaturase Gene and Its MRNA Showing an Unusually Long 3′-UTR Sequence Arising from a Single Exon. Gene 2001, 281, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Bovo, S.; Ribani, A.; Muñoz, M.; Alves, E.; Araujo, J.P.; Bozzi, R.; Čandek-Potokar, M.; Charneca, R.; di Palma, F.; Etherington, G.; et al. Whole-Genome Sequencing of European Autochthonous and Commercial Pig Breeds Allows the Detection of Signatures of Selection for Adaptation of Genetic Resources to Different Breeding and Production Systems. Genet. Sel. Evol. 2020, 52, 33. [Google Scholar] [CrossRef]
- Conte, G.; Palombo, V.; Serra, A.; Correddu, F.; D’andrea, M.; Macciotta, N.P.P.; Mele, M. Study of the Fatty Acid Profile of Milk in Different Sheep Breeds: Evaluation by Multivariate Factorial Analysis. Animals 2022, 12, 722. [Google Scholar] [CrossRef] [PubMed]
- Wakil, S.J. Fatty Acid Synthase, A Proficient Multifunctional Enzyme. Biochemistry 1989, 28, 4523–4530. [Google Scholar] [CrossRef]
- Green, C.D.; Ozguden-Akkoc, C.G.; Wang, Y.; Jump, D.B.; Olson, L.K. Role of Fatty Acid Elongases in Determination of de Novo Synthesized Monounsaturated Fatty Acid Species. J. Lipid Res. 2010, 51, 1871–1877. [Google Scholar] [CrossRef]
- Destaillats, F.; Trottier, J.P.; Galvez, J.M.G.; Angers, P. Analysis of α-Linolenic Acid Biohydrogenation Intermediates in Milk Fat with Emphasis on Conjugated Linolenic Acids. J. Dairy Sci. 2005, 88, 3231–3239. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The Importance of the Omega-6/Omega-3 Fatty Acid Ratio in Cardiovascular Disease and Other Chronic Diseases. Exp. Biol. Med. 2008, 233, 674–688. [Google Scholar] [CrossRef]
- Collins, Y.F.; McSweeney, P.L.H.; Wilkinson, M.G. Lipolysis and Free Fatty Acid Catabolism in Cheese: A Review of Current Knowledge. Int. Dairy J. 2003, 13, 841–866. [Google Scholar] [CrossRef]
- Thierry, A.; Collins, Y.F.; Abeijón Mukdsi, M.C.; McSweeney, P.L.H.; Wilkinson, M.G.; Spinnler, H.E. Lipolysis and Metabolism of Fatty Acids in Cheese. In Cheese: Chemistry, Physics and Microbiology, 4th ed.; Springer: New York, NY, USA, 2017; Volume 1, pp. 423–444. ISBN 9780122636530. [Google Scholar]
- Park, Y.W.; Juárez, M.; Ramos, M.; Haenlein, G.F.W. Physico-Chemical Characteristics of Goat and Sheep Milk. Small Rumin. Res. 2007, 68, 88–113. [Google Scholar] [CrossRef]
- Sousa, M.J.; Ardö, Y.; McSweeney, P.L.H. Advances in the Study of Proteolysis during Cheese Ripening. Int. Dairy J. 2001, 11, 327–345. [Google Scholar] [CrossRef]
- Lawrence, R.C.; Creamer, L.K.; Gilles, J. Texture Development During Cheese Ripening. J. Dairy Sci. 1987, 70, 1748–1760. [Google Scholar] [CrossRef]
- Barać, M.B.; Smiljanić, M.; Pešic, M.B.; Stanojević, S.P.; Jovanović, S.T.; Maćej, O.D. Primary Proteolysis of White Brined Goat Cheese Monitored by High Molarity Tris Buffer SDS-PAGE System. Mljekarstvo Dairy 2013, 63, 122–131. [Google Scholar]
- Holt, C.; Rogiński, H. Milk Proteins: Biological and Food Aspects of Structure and Function. In Chemical and Functional Properties of Food Proteins; CRC Press: Boca Raton, FL, USA, 2001; pp. 271–334. [Google Scholar]
- Prieto, B.; Franco, I.; Fresno, J.M.; Prieto, J.G.; Bernardo, A.; Carballo, J. Effect of Ripening Time and Type of Rennet (Farmhouse Rennet from Kid or Commercial Calf) on Proteolysis during the Ripening of León Cow Milk Cheese. Food Chem. 2004, 85, 389–398. [Google Scholar] [CrossRef]
- Park, Y.W. Proteolysis and Lipolysis of Goat Milk Cheese. J. Dairy Sci. 2001, 84, E84–E92. [Google Scholar] [CrossRef]
- El-Nimr, A.A.; Eissa, H.A.; El-Abd, M.M.; Mehriz, A.A.; Abbas, H.M.; Bayoumi, H.M. Water Activity, Color Characteristics and Sensory Properties of Egyptian Gouda Cheese during Ripening. J. Am. Sci. 2010, 6, 447–453. [Google Scholar]
- Kondyli, E.; Pappa, E.C.; Svarnas, C. Ripening Changes of the Chemical Composition, Proteolysis, Volatile Fraction and Organoleptic Characteristics of a White-Brined Goat Milk Cheese. Small Rumin. Res. 2016, 145, 1–6. [Google Scholar] [CrossRef]
- Rinaldi, M.; Chiavaro, E.; Massini, R. Pecorino of Appenino Reggiano Cheese: Evaluation of Ripening Time Using Selected Physical Properties. Ital. J. Food Sci. 2010, 22, 54–59. [Google Scholar]
- Casiraghi, E.; Lucisano, M.; Pompei, C. Correlation among Instrumental Texture, Sensory Texture and Chemical Composition of Five Italian Cheeses. Ital. J. Food Sci. 2013, 1, 53–63. [Google Scholar]
- Delgado, F.J.; González-Crespo, J.; Cava, R.; Ramírez, R. Changes in Microbiology, Proteolysis, Texture and Sensory Characteristics of Raw Goat Milk Cheeses Treated by High-Pressure at Different Stages of Maturation. LWT Food Sci. Technol. 2012, 48, 268–275. [Google Scholar] [CrossRef]
- Pino, A.; Prados, F.; Galán, E.; Vivo, R.; Fernández-Salguero, J. Amino Acids Evolution during Ripening of Goats’ Milk Cheese Manufactured with Different Coagulants. Int. J. Food Sci. Technol. 2009, 44, 2062–2069. [Google Scholar] [CrossRef]
- Burgos, L.S.; Pece Azar, N.B.d.C.; Maldonado, S. Proteolysis, Texture and Microstructure of Goat Cheese. Int. J. Eng. Appl. Sci. 2020, 3, 14–19. [Google Scholar]
- Lourenco, A.; Handschuh, S.; Fenelon, M.; Gómez-Mascaraque, L.G. X-Ray Computerized Microtomography and Confocal Raman Microscopy as Complementary Techniques to Conventional Imaging Tools for the Microstructural Characterization of Cheddar Cheese. J. Dairy Sci. 2022, 105, 9387–9403. [Google Scholar] [CrossRef] [PubMed]
- Rocha, R.A.R.; Ribeiro, M.N.; Silva, G.A.; Rocha, L.C.R.; Pinheiro, A.C.M.; Nunes, C.A.; de Deus Souza Carneiro, J. Temporal Profile of Flavor Enhancers MAG, MSG, GMP, and IMP, and Their Ability to Enhance Salty Taste, in Different Reductions of Sodium Chloride. J. Food Sci. 2020, 85, 1565–1575. [Google Scholar] [CrossRef] [PubMed]
| Milk | Cheese | |||||||
|---|---|---|---|---|---|---|---|---|
| Teramana | Saanen | SEM | p-Value | Teramana | Saanen | SEM | p-Value | |
| C4:0 | 1.75 | 1.65 | 0.16 | ns | 2.13 | 1.33 | 0.05 | ns |
| C6:0 | 2.38 | 2.23 | 0.20 | ns | 2.57 | 1.94 | 0.09 | ns |
| C8:0 | 3.22 | 3.02 | 0.26 | ns | 3.22 | 2.86 | 0.22 | ns |
| C10:0 | 11.21 | 11.20 | 0.85 | ns | 10.79 | 11.99 | 3.85 | ns |
| C12:0 | 4.92 | 5.21 | 0.29 | ns | 3.83 | 5.95 | 0.56 | ns |
| C14:0 | 9.84 b | 11.02 a | 0.24 | 0.01 | 9.51 b | 11.41 a | 0.68 | 0.001 |
| C15:0 | 1.23 | 1.06 | 0.01 | ns | 1.00 | 1.10 | 0.01 | ns |
| C16:0 | 26.42 b | 28.77 a | 0.44 | 0.01 | 28.89 b | 29.28 a | 0.91 | 0.001 |
| C18:0 | 11.00 a | 9.83 b | 0.52 | 0.01 | 13.58 a | 11.75 b | 0.96 | 0.001 |
| C20:0 | 0.33 | 0.23 | 0.01 | ns | 0.37 | 0.21 | 0.01 | ns |
| C14:1 | 0.43 | 0.40 | 0.01 | ns | 0.43 | 0.40 | 0.01 | ns |
| C16:1 | 0.35 | 0.26 | 0.02 | ns | 0.96 | 0.74 | 0.01 | ns |
| C18:1,t11 | 1.57 a | 1.10 b | 0.06 | 0.01 | 1.53 a | 0.87 b | 0.02 | 0.001 |
| C18:1,c9 | 17.48 | 16.33 | 0.73 | ns | 14.77 | 14.65 | 2.98 | ns |
| C18:1,c11 | 0.08 | 0.11 | 0.02 | ns | 0.16 | 0.29 | 0.01 | ns |
| C18:2 | 1.22 | 1.31 | 0.04 | ns | 1.88 | 1.31 | 0.06 | ns |
| C18:3 | 1.06 a | 0.93 b | 0.04 | 0.01 | 1.04 a | 0.89 b | 0.01 | 0.01 |
| SFA | 72.50 b | 74.85 a | 0.97 | 0.05 | 73.44 b | 76.75 a | 3.09 | 0.05 |
| MUFA | 19.82 a | 17.90 b | 0.79 | 0.01 | 19.54 | 17.20 | 3.81 | ns |
| PUFA | 4.07 | 3.89 | 0.14 | ns | 3.52 a | 2.81 b | 0.10 | 0.01 |
| CLA | 1.80 a | 1.65 b | 0.07 | 0.001 | 1.79 a | 1.29 b | 0.01 | 0.001 |
| OTHERS | 3.71 | 3.69 | 0.09 | ns | 1.59 | 1.52 | 0.01 | ns |
| DI C14 | 4.19 a | 3.50 b | 0.12 | 0.01 | 4.35 a | 2.95 b | 0.20 | 0.01 |
| DI C16 | 0.94 a | 0.90 b | 0.26 | 0.01 | 2.90 a | 2.47 b | 0.13 | 0.01 |
| DI C18 | 61.38 | 61.99 | 0.45 | ns | 52.65 | 58.48 | 0.44 | ns |
| DI CLA | 44.95 a | 40.00 b | 2.25 | 0.01 | 55.78 a | 44.34 b | 3.31 | 0.01 |
| T0 | T30 | T60 | SEM | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Teramana | Saanen | Teramana | Saanen | Teramana | Saanen | R | T | R × T | ||
| Dry Matter (DM) | 52.06 d | 40.37 e | 73.48 b | 60.08 c | 77.82 a | 72.25 b | 4.00 | 0.001 | 0.001 | 0.001 |
| Lipids % | 23.35 b | 14.54 c | 28.89 a | 16.86 c | 27.77 ab | 18.28 c | 6.51 | 0.001 | 0.001 | ns |
| T0 | T30 | T60 | SEM | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Teramana | Saanen | Teramana | Saanen | Teramana | Saanen | R | T | R × T | ||
| αs2-casein | 28.18 a | 24.00 b | 17.91 c | 14.11 d | 8.89 e | 9.89 e | 2.74 | 0.01 | 0.001 | 0.01 |
| αs1-casein | 9.19 ab | 10.58 a | 7.01 abc | 5.28 bc | 3.28 c | 3.77 c | 3.98 | ns | 0.001 | ns |
| β-casein | 33.19 b | 33.12 b | 39.14 ab | 38.73 ab | 37.77 ab | 40.20 a | 8.55 | ns | 0.001 | ns |
| κ-casein | 7.00 b | 8.00 b | 7.30 b | 8.65 ab | 12.16 a | 10.02 ab | 2.89 | ns | 0.001 | ns |
| A | 3.30 b | 3.63 b | 12.60 a | 10.16 a | 13.51 a | 8.66 a | 4.62 | ns | 0.001 | ns |
| B | 3.29 | 2.54 | 2.55 | 4.46 | 1.80 | 8.29 | 4.24 | ns | ns | ns |
| C | 1.92 b | 3.78 b | 2.86 b | 2.66 b | 5.85 a | 4.07 a | 0.64 | ns | 0.001 | ns |
| β-lactoglobulin | 11.23 | 11.80 | 8.19 | 14.01 | 12.69 | 11.46 | 3.18 | ns | ns | ns |
| α-lactoalbumin | 2.65 | 2.50 | 2.39 | 1.89 | 4.00 | 3.59 | 1.56 | ns | ns | ns |
| Teramana | Saanen | SEM | p-Value | |
|---|---|---|---|---|
| Aldehydes | ||||
| Nonanal | 0.26 | nd | 0.03 | ns |
| Carboxylic Acids | ||||
| Butanoic acid | 2.21 | 5.31 | 1.48 | ns |
| Hexanoic acid | 28.09 | 21.36 | 1.60 | ns |
| Octanoic acid | 35.63 a | 31.78 b | 2.70 | 0.05 |
| Nonanoic acid | 0.40 | 0.18 | 0.07 | ns |
| Decanoic acid | 24.23 | 21.47 | 2.36 | ns |
| Dodecanoic acid | 0.82 | 0.84 | 0.03 | ns |
| Ketones | ||||
| 2-Heptanone | 0.36 b | 7.48 a | 0.69 | 0.01 |
| 2-Nonanone | 6.52 b | 6.96 a | 2.86 | 0.05 |
| 2,7-Octanedione | 0.26 | 0.17 | 0.05 | ns |
| Methyl ester | ||||
| Hexadecanoic acid, methyl ester | 0.05 b | 0.27 a | 0.01 | 0.05 |
| Others | 0.18 | 0.13 | 0.01 | ns |
| T0 | T30 | T60 | SEM | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Teramana | Saanen | Teramana | Saanen | Teramana | Saanen | R | T | R × T | ||
| Color | ||||||||||
| L* | 92.12 a | 88.10 b | 82.36 c | 81.07 c | 83.82 c | 81.54 c | 3.82 | 0.001 | 0.001 | ns |
| b* | 10.46 ab | 7.48 c | 12.07 a | 9.10 bc | 11.44 a | 9.01 bc | 0.95 | 0.001 | 0.001 | ns |
| YI | 16.23 bc | 12.16 d | 21.02 a | 16.04 c | 19.49 ab | 15.78 c | 3.45 | 0.001 | 0.001 | ns |
| Texture | ||||||||||
| Hardness | 4.03 b | 1.49 b | 20.39 a | 22.31 a | 25.27 a | 21.24 a | 25.01 | ns | 0.001 | ns |
| Cohesivity | 0.97 | 0.99 | 0.99 | 0.99 | 0.99 | 0.98 | 0.10 | ns | ns | ns |
| Gumminess | 3.92 b | 1.48 b | 20.34 a | 22.25 a | 25.21 a | 21.06 a | 25.15 | ns | 0.001 | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Florio, M.; Cimini, C.; Ianni, A.; Bennato, F.; Grotta, L.; Valbonetti, L.; Martino, G. New Insight into the Quality Traits of Milk and Cheese from Teramana Goats, a Native Italian Breed. Animals 2023, 13, 1344. https://doi.org/10.3390/ani13081344
Florio M, Cimini C, Ianni A, Bennato F, Grotta L, Valbonetti L, Martino G. New Insight into the Quality Traits of Milk and Cheese from Teramana Goats, a Native Italian Breed. Animals. 2023; 13(8):1344. https://doi.org/10.3390/ani13081344
Chicago/Turabian StyleFlorio, Marco, Costanza Cimini, Andrea Ianni, Francesca Bennato, Lisa Grotta, Luca Valbonetti, and Giuseppe Martino. 2023. "New Insight into the Quality Traits of Milk and Cheese from Teramana Goats, a Native Italian Breed" Animals 13, no. 8: 1344. https://doi.org/10.3390/ani13081344
APA StyleFlorio, M., Cimini, C., Ianni, A., Bennato, F., Grotta, L., Valbonetti, L., & Martino, G. (2023). New Insight into the Quality Traits of Milk and Cheese from Teramana Goats, a Native Italian Breed. Animals, 13(8), 1344. https://doi.org/10.3390/ani13081344






