Dairy Sheep Grazing Management and Pasture Botanical Composition Affect Milk Macro and Micro Components: A Methodological Approach to Assess the Main Managerial Factors at Farm Level
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Dairy Farming System and Pasture Botanical Characteristics
2.2. Milk, Herbage and Feedstuff Chemical Characteristics
2.3. Statistical Analysis
3. Results and Discussion
3.1. Dairy Farming System and Feeding Managements
3.2. Grassland Botanical and Chemical Composition
3.3. Milk Chemical Composition and Relationship with Structural and Managerial Factors
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gutiérrez-Peña, R.; Avilés, C.; Galán-Soldevilla, H.; Polvillo, O.; Ruiz Pérez-Cacho, P.; Guzmán, J.L.; Horcada, A.; Delgado-Pertíñez, M. Physicochemical Composition, Antioxidant Status, Fatty Acid Profile, and Volatile Compounds of Milk and Fresh and Ripened Ewes’ Cheese from a Sustainable Part-Time Grazing System. Foods 2021, 10, 80. [Google Scholar] [CrossRef] [PubMed]
- Joubran, A.M.; Pierce, K.M.; Garvey, N.; Shalloo, L.; O’Callaghan, T.F. Invited review: A 2020 perspective on pasture-based dairy systems and products. J. Dairy Sci. 2020, 104, 7364–7382. [Google Scholar] [CrossRef] [PubMed]
- Claps, S.; Mecca, M.; Di Trana, A.; Sepe, L. Local Small Ruminant Grazing in the Monti Foy Area (Italy): The Relationship Between Grassland Biodiversity Maintenance and Added-Value Dairy Products. Front. Vet. Sci. 2020, 7, 951. [Google Scholar] [CrossRef]
- Cabiddu, A.; Decandia, M.; Molle, G. Formaggi da latte di pecora: Aspetti zootecnici: Legame al territorio. In I Georgofili Quaderni, 3rd ed.; Nardone, A., Piva, G., Eds.; Edizioni Polistampa: Firenz, Italia, 2014; pp. 75–88. [Google Scholar]
- Hennessy, D.; Delaby, L.; Van den Pol-van Dasselaar, A.; Shalloo, L. Possibilities and constraints for grazing in high output dairy systems. In 18 Symposium of the European Grassland Federation, Wageningen, Netherlands; Van den Pol-van Dasselaar, A., Aarts, H.F.M., De Vliegher, A., Elgersma, A., Reheul, D., Reijneveld, J.A., Verloop, J., Hopkins, A., Eds.; Wageningen Academic Publishers: Wageningen, Netherlands, 2015; p. 151162. [Google Scholar]
- Pulina, G.; Milán, M.J.; Lavín, M.P.; Theodoridis, A.; Morin, E.; Capote, J.; Thomas, D.L.; Francesconi, A.H.D.; Caja, G. Current production trends, farm structures, and economics of the dairy sheep and goat sectors. J. Dairy Sci. 2018, 101, 6715–6729. [Google Scholar] [CrossRef]
- Salis, L.; Puddu, R.; Fanni, S.; Vargiu, M. Vegetational and Pedological characterization of a grazing land in central Sardinia and first proposals for improvement and rational use. Ital. J. Agron. 2007, 4, 383–392. [Google Scholar] [CrossRef]
- Sitzia, M.; Bonanno, A.; Todaro, M.; Cannas, A.; Atzori, A.S.; Francesconi, A.H.D.; Trabalza-Marinucci, M. Feeding and management techniques to favour summer sheep milk and cheese production in the Mediterranean environment. Small Rum. Res. 2015, 126, 43–58. [Google Scholar] [CrossRef]
- Agradi, S.; Curone, G.; Negroni, D.; Vigo, D.; Brecchia, G.; Bronzo, V.; Panseri, S.; Chiesa, L.M.; Peric, T.; Danes, D.; et al. Determination of Fatty Acids Profile in Original Brown Cows Dairy Products and Relationship with Alpine Pasture Farming System. Animals 2020, 10, 1231. [Google Scholar] [CrossRef]
- Cabiddu, A.; Carta, G.; Molle, G.; Decandia, M.; Addis, M.; Piredda, G.; Delogu, A.; Pirisi, A.; Lai, V.; Cera, V.; et al. Relationship between feeding regimen and content of conjugated linoleic acid in sheep milk and cheese. Opt. Méd. 2005, 67, 171–175. [Google Scholar]
- Addis, M.; Cabiddu, A.; Decandia, M.; Fiori, M.; Spada, S.; Bulleddu, C.; Cammelli, R.; Caria, A.; Lai, V.; Lutzoni, G.; et al. A survey on the milk fatty acid composition of forty dairy sheep flocks in Sardinia. Ital. J. Anim. Sci. 2006, 6, 532–534. [Google Scholar] [CrossRef]
- Nudda, A.; Cannas, A.; Correddu, F.; Atzori, A.S.; Lunesu, M.F.; Battacone, G.; Pulina, G. Sheep and Goats Respond Differently to feeding strategies directed to improve the fatty acid profile of milk fat. Animals 2020, 10, 1290. [Google Scholar] [CrossRef]
- Cabiddu, A.; Peratoner, G.; Valenti, B.; Monteils, V.; Martin, B.; Coppa, M. A quantitative review of on-farm feeding practices to enhance the quality of grassland-based ruminant dairy and meat products. Animal 2021, 16, 100375. [Google Scholar] [CrossRef]
- Cabiddu, A.; Wencelová, M.; Bomboi, G.; Decandia, M.; Molle, G.; Salis, L. Fatty acid profile in two berseem clover (Trifolium alexandrinum L.) cultivars: Preliminary study of the effect of part of plant and phenological stage. Grassl. Sci. 2017, 63, 101–110. [Google Scholar] [CrossRef]
- Cabiddu, A.; Delgadillo-Puga, C.; Decandia, M.; Molle, G. Extensive Ruminant Production Systems and Milk Quality with Emphasis on Unsaturated Fatty Acids, Volatile Compounds, Antioxidant Protection Degree and Phenol Content. Animals 2019, 9, 771. [Google Scholar] [CrossRef]
- Prache, S.; Martin, B.; Coppa, M. Review: Authentication of grass-fed meat and dairy products from cattle and sheep. Animal 2020, 14, 854–863. [Google Scholar] [CrossRef] [PubMed]
- Molle, G.; Cabiddu, A.; Decandia, M.; Acciaro, M.; Scanu, G.; Addis, M.; Fiori, M.; Caredda, M. A Note on the tracing of herbage contribution to grazing sheep diet using milk and feces biomarkers. Front. Vet. Sci. 2021, 8, 50. [Google Scholar] [CrossRef] [PubMed]
- Molle, G.; Cabiddu, A.; Decandia, M.; Sitzia, M.; Ibba, I.; Giovanetti, V.; Scanu, G.; Addis, M.; Caredda, M. Can FT-mid-infrared spectroscopy of milk samples discriminate different dietary regimens of sheep grazing with restricted access time? Front. Vet. Sci. 2021, 8, 211. [Google Scholar] [CrossRef] [PubMed]
- Mele, M.; Macciotta, N.P.P.; Cecchinato, A.; Conte, G.; Schiavon, S.; Bittante, G. Multivariate factor analysis of detailed milk fatty acid profile: Effects of dairy system, feeding, herd, parity, and stage of lactation. J. Dairy Sci. 2016, 99, 9820–9833. [Google Scholar] [CrossRef] [PubMed]
- Correddu, F.; Murgia, M.A.; Mangia, N.P.; Lunesu, M.F.; Cesarani, A.; Deiana, P.; Pulina, G.; Nudda, A. Effect of altitude of flock location, season of milk production and ripening time on the fatty acid profile of Pecorino Sardo cheese. Int. Dairy J. 2021, 113, 104895. [Google Scholar] [CrossRef]
- Correddu, F.; Cesarani, A.; Dimauro, C.; Gaspa, G.; Macciotta, N.P.P. Principal component and multivariate factor analysis of detailed sheep milk fatty acid profile. J. Dairy Sci. 2021, 104, 5079–5094. [Google Scholar] [CrossRef]
- Cabiddu, A.; Dattena, M.; Decandia, M.; Molle, G.; Lopreiato, V.; Minuti, A.; Trevisi, E. The effect of parity number on the metabolism, inflammation, and oxidative status of dairy sheep during the transition period. J. Dairy Sci. 2020, 103, 8564–8575. [Google Scholar] [CrossRef]
- Cannas, A.; Tedeschi, L.O.; Fox, D.G.; Pell, A.N.; Van Soest, P.J. A mechanistic model for predicting the nutrient requirements and feed biological values for sheep. J. Anim. Sci. 2004, 82, 149–169. [Google Scholar] [CrossRef]
- Hess, M.; Barralis, G.; Bleiholder, H.; Buhr, L.; Eggers, T.H.; Hack, H.; Stauss, R. Use of the extended BBCH-scale-general for the description of the growth stages of mono- and dicotyledonous weed species. Weed Res. 1997, 37, 433–441. [Google Scholar] [CrossRef]
- Meier, U. Growth Stages of Mono and Dicotyledonous Plants. In BBCH Monograph; Federal Biological Research Centre for Agriculture and Forestry: Bonn, Germany, 2001. [Google Scholar] [CrossRef]
- Murphy, J.J.; McNeill, G.P.; Connolly, J.F.; Gleeson, P.A. Effect on cow performance and milk fat composition of including full fat soyabeans and rapeseeds in the concentrate mixture for lactating dairy cows. J. Dairy Res. 1990, 57, 295–306. [Google Scholar] [CrossRef]
- Fiori, M.; Scintu, M.F.; Addis, M. Characterization of the Lipid Fraction in Lamb Meat: Comparison of Different Lipid Extraction Methods. Food Anal. Met. 2013, 6, 1648–1656. [Google Scholar] [CrossRef]
- Kramer, J.K.G.; Hernandez, M.; Cruz-Hernandez Kraft, J.; Dugan, M.E.R. Combining results of two GC separation partly achieves determination of all cis and trans 16:1 18:1, 18.2 and 18:3 except CLA isomers of milk fat as demonstrated using Ag-Ion SPE fractionation. Lipids 2008, 43, 259–273. [Google Scholar] [CrossRef]
- Panfili, G.; Manzi, P.; Pizzoferratto, L. High Performance Liquid Chromatography for the Simultaneous Determination of Tocopherols, Carotenes, and Retinols and its Isomers in Italian Cheeses. Analyst 1994, 119, 1161–1165. [Google Scholar] [CrossRef] [PubMed]
- Pizzoferrato, L.; Manzi, P.; Marconi, S.; Fedele, V.; Claps, S.; Rubino, R. Degree of antioxidant protection. A parameter to trace the origin and quality of goat’s milk and cheese. J. Dairy Sci. 2007, 10, 4569–4574. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Amamcharla, J.K.; Metzger, L.E. Modification of the ferric reducing antioxidant power (FRAP) assay to determine the susceptibility of raw milk to oxidation. Int. Dairy J. 2014, 34, 177–179. [Google Scholar] [CrossRef]
- Velázquez Vázquez, C.; Guadalupe Villa Rojas, M.; Alvarez Ramírez, C.; Chávez-Servín, J.L.; García-Gasca, T.; Ferriz Martínez, R.A.; García, O.P.; Rosado, J.L.; López-Sabater, C.M.; Castellote, A.I.; et al. Total phenolic compounds in milk from different species. Design of an extraction technique for quantification using the Folin–Ciocalteu method. Food Chem. 2015, 176, 480–486. [Google Scholar] [CrossRef]
- Rothwell, J.A.; Perez-Jimenez, J.; Neveu, V.; Medina-Remón, A.; M’Hiri, N.; García-Lobato, P.; Manach, C.; Knox, C.; Eisner, R.; Wishart, D.S.; et al. Phenol-Explorer 3.0: A major update of the Phenol-Explorer database to incorporate data on the effects of food processing on polyphenol content. Database 2013, 2013, bat070. [Google Scholar] [CrossRef] [PubMed]
- Lucini, L.; Rocchetti, G.; Kane, D.; Trevisan, M. Phenolic fingerprint allows discriminating processed tomato products and tracing different processing sites. Food Control 2017, 73, 696–703. [Google Scholar] [CrossRef]
- Rocchetti, G.; Chiodelli, G.; Giuberti, G.; Masoero, F.; Trevisan, M.; Lucini, L. Evaluation of phenolic profile and antioxidant capacity in gluten-free flours. Food Chem. 2017, 228, 367–373. [Google Scholar] [CrossRef]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Cabiddu, A.; Salis, L.; Tweed, J.; Molle, G.; Decandia, M.; Lee, M. The influence of plant polyphenols on lipolysis and biohydrogenation in dried forages at different phenological stages: In vitro study. J. Sci. Food Agric. 2010, 90, 829–835. [Google Scholar] [CrossRef] [PubMed]
- Vlaeminck, B.; Fievez, V.; Cabrita, A.R.J.; Fonseca, A.J.M.; Dewhurst, R.J. Factors affecting odd- and branched-chain fatty acids in milk: A review. Anim. Feed Sci. Tech. 2006, 131, 389–417. [Google Scholar] [CrossRef]
- Abilleira, E.; Virto, M.; Isabel Nájera, A.; Albisu, M.; Pérez-Elortondo, F.J.; Ruiz de Gordoa, J.C.; de Renobales, M.; Barron, L.J.R. Effects of seasonal changes in feeding management under part-time grazing on terpene concentrations of ewes’ milk. J. Dairy Res. 2011, 78, 129–135. [Google Scholar] [CrossRef]
- Larcher, W. Physiological Plant Ecology, 4th ed.; Springer: Berlin/Heidelberg, Germany, 2003; p. 514. [Google Scholar]
- Mariaca, R.G.; Berger, T.F.H.; Gauch, R.; Imhof, M.I.; Jeangros, B.; Bosset, J.O. Occurrence of volatile mono- and sesquiterpenoids in highland and 795 lowland plant species as possible precursors for flavor compounds in milk and 796 dairy products. J. Agric. Food Chem. 1997, 45, 4423–4434. [Google Scholar] [CrossRef]
- Cabiddu, A.; Decandia, M.; Addis, M.; Piredda, G.; Pirisi, A.; Molle, G. Managing mediterranean pastures in order to enhance the level of beneficial fatty acids in sheep milk. Small Rum. Res. 2005, 59, 169–180. [Google Scholar] [CrossRef]
- Cabiddu, A.; Addis, M.; Fiori, M.; Spada, S.; Decandia, M.; Molle, G. Pros and cons of the supplementation with oilseed enriched concentrates on milk fatty acid profile of dairy sheep grazing Mediterranean pastures. Small Rumin. Res. 2017, 147, 63–72. [Google Scholar] [CrossRef]
- Churakov, M.; Karlsson, J.; Edvardsson Rasmussen, A.; Holtenius, K. Milk fatty acids as indicators of negative energy balance of dairy cows in early lactation. Animal 2021, 15, 100253. [Google Scholar] [CrossRef] [PubMed]
- Chilliard, Y.; Ferlay, A.; Rouel, J.; Lamberet, G. A review of nutritional and physiological factors affecting goat milk lipid synthesis and lipolysis. J. Dairy Sci. 2003, 86, 1751–1770. [Google Scholar] [CrossRef]
- Puppel, K.; Gołebiewski, M.; Solarczyk, P.; Grodkowski, G.; Slósarz, J.; Kunowska-Slósarz, M.; Balcerak, M.; Przysucha, T.; Kalinska, A.; Kuczynska, B. The relationship between plasma β-hydroxybutyric acid and conjugated linoleic acid in milk as a biomarker for early diagnosis of ketosis in postpartum Polish Holstein-Friesian cows. BMC Vet. Res. 2019, 15, 367. [Google Scholar] [CrossRef] [PubMed]
- Coppa, M.; Chassaing, C.; Ferlay, A.; Agabriel, C.; Laurent, C.; Borreani, G.; Barcarolo, R.; Baars, T.; Kusche, D.; Harstad, O.M.; et al. Potential of milk fatty acid composition to predict diet composition and authenticate feeding systems and altitude origin of European bulk milk. J. Dairy Sci. 2015, 98, 1539–1551. [Google Scholar] [CrossRef] [PubMed]
- Díaz, M.; Álvarez, I.; De la Fuente, J.; Sañudo, C.; Campo, M.; Oliver, M.; i Furnols, M.F.; Montossi, F.; Julián, R.S.; Nute, G.; et al. Fatty acid composition of meat from typical lamb production systems of Spain, United Kingdom, Germany and Uruguay. Meat Sci. 2005, 71, 256–263. [Google Scholar] [CrossRef]
- Manzocchi, E.; Martin, B.; Bord, C.; Verdier-Metz, I.; Bouchon, M.; De Marchi, M.; Constant, I.; Giller, K.; Kreuzer, M.; Berard, J.; et al. Feeding cows with hay, silage, or fresh herbage on pasture or indoors affects sensory properties and chemical composition of milk and cheese. J. Dairy Sci. 2021, 104, 5285–5302. [Google Scholar] [CrossRef]
- Chilliard, Y.; Ferlay, A.; Mansbridge, R.M.; Doreau, M. Ruminant milk fat plasticity: Nutritional control of saturated. polyunsaturated. trans and conjugated fatty acids. Ann. Zootech. 2000, 49, 181–205. [Google Scholar] [CrossRef]
- Correddu, F.; Serdino, J.; Manca, M.G.; Cosenza, G.; Pauciullo, A.; Ramunno, L.; Macciotta, N.P.P. Use of multivariate factor analysis to characterize the fatty acid profile of buffalo milk. J. Food Comp. Anal. 2017, 60, 25–31. [Google Scholar] [CrossRef]
- Renna, M.; Ferlay, A.; Lussiana, C.; Bany, D.; Graulet, B.; Wyss, U.; Enri, S.R.; Battaglini, L.M.; Coppa, M.; San, Z.; et al. Relative hierarchy of farming practices affecting the fatty acid composition of permanent grasslands and of the derived bulk milk. Anim. Feed Sci. Tech. 2020, 267, 114561. [Google Scholar] [CrossRef]
- Molle, G.; Decandia, M.; Fois, N.; Ligios, S.; Cabiddu, A.; Sitzia, M. The performance of Mediterranean dairy sheep given access to sulla (Hedysarum coronarium L.) and annual ryegrass (Lolium rigidum Gaudin) pastures in different time proportions. Small Rumin. Res. 2003, 49, 319–328. [Google Scholar] [CrossRef]
- Barry, T.N.; McNeill, D.M.; McNabb, W.C. Plant secondary compounds; their impact on forage nutritive value and upon animal production. In Proceedings of the XIX International Grassland Congress, São Pedro, São Paulo, Brazil, 11–21 February 2001; da Silva, S.C., Mattos, W.R.S., Eds.; Fundacao de Estudos Agrarios Luiz de Queiroz: São Paulo, Brazil, 2001; pp. 445–452. [Google Scholar]
- Elgersma, A. Grazing increases the unsaturated fatty acid concentration of milk from grass-fed cows: A review of the contributing factors, challenges and future perspectives. Eur. J. Lipid Sci. Tech. 2015, 117, 1345–1369. [Google Scholar] [CrossRef]
- Buccioni, A.; Decandia, M.; Minieri, S.; Molle, G.; Cabiddu, A. Lipid metabolism in the rumen: New insights on lipolysis and biohydrogenation with an emphasis on the role of endogenous plant factors. Anim. Feed Sci. Technol. 2012, 174, 1–25. [Google Scholar] [CrossRef]
- Jung, H.G. Forage Digestibility: The Intersection of Cell Wall Lignification and Plant Tissue Anatomy. Available online: http://dairy.ifas.ufl.edu/rns/2012/12jungrns2012.pdf (accessed on 21 September 2022).
- Singleton, V.L.; Kratzer, F.H. Toxicity and related physiological activity of phenolic substances of plant origin. J. Agric. Food Chem. 1969, 17, 497–512. [Google Scholar] [CrossRef]
- Chesson, A.; Stewart, C.S.; Wallace, R.J. Influence of plant phenolic acids on growth and cellulolytic activity of rumen bacteria. Appl. Environ. Microbiol. 1982, 44, 597–603. Available online: https://www.sciencedirect.com/science/article/pii/S095869462030265X (accessed on 10 April 2019). [CrossRef] [PubMed]
- Griinari, J.M.; Dwyer, D.A.; McGuire, M.A.; Bauman, D.E.; Palmquist, D.; Nurmela, K.V.V. Trans octadecanoic acids and milk fat depression in lactating dairy cows. J. Dairy Sci. 1998, 81, 1251–1261. [Google Scholar] [CrossRef]
- Dewanckele, L.; Toral, P.G.; Vlaeminck, B.; Fievez, V. Invited review: Role of rumen biohydrogenation intermediates and rumen microbes in diet-induced milk fat depression. J. Dairy Sci. 2019, 103, 7655–7681. [Google Scholar] [CrossRef]
- Milovanovic, B.; Djekic, I.; Miocinovic, J.; Djordjevic, V.; Lorenzo, J.M.; Barba, F.J.; Mörlein, D.; Tomasevic, I. What Is the Color of Milk and Dairy Products and How Is It Measured? Foods 2020, 9, 1629. [Google Scholar] [CrossRef]
- Priolo, A.; Lanza, M.; Barbagallo, D.; Finocchiaro, L.; Biondi, L. Can the reflectance spectrum be used to trace grass feeding in ewe milk? Small Rum. Res. 2003, 48, 103–107. [Google Scholar] [CrossRef]
- Nozière, P.; Graulet, B.; Lucas, A.; Martin, B.; Grolier, P.; Doreau, M. Carotenoids for ruminants, from forages to dairy products. Anim. Feed Sci. Tech. 2006, 131, 418–450. [Google Scholar] [CrossRef]
- Andargie, M.; Vinas, M.; Rathgeb, A.; Möller, E.; Karlovsky, P. Lignans of Sesame (Sesamum indicum L.): A Comprehensive Review. Molecules 2021, 26, 883. [Google Scholar] [CrossRef]
- Serrapica, F.; Masucci, F.; Di Francia, A.; Napolitano, F.; Braghieri, A.; Esposito, G.; Romano, R. Seasonal Variation of Chemical Composition, Fatty Acid Profile, and Sensory Properties of a Mountain Pecorino Cheese. Foods 2020, 9, 1091. [Google Scholar] [CrossRef]
| n | Average | SEM | Min | Max | |
|---|---|---|---|---|---|
| Flock (n. sheep) | 42 | 204.143 | 26.388 | 20 | 700 |
| UAA (Ha) | 42 | 67.714 | 6.376 | 14 | 132 |
| Sampling date (SD) | 42 | 2.548 | 0.174 | 1 | 4 |
| Plant phenological stage | 42 | 1.262 | 0.069 | 1 | 2 |
| Herbage intake (g DM head−1 day−1) | 42 | 883.622 | 106.577 | 0 | 1777 |
| Total supplementation (g DM head−1 day−1) | 42 | 566.500 | 73.723 | 100 | 1850 |
| Concentrate intake (g DM head−1 day−1) | 42 | 381.690 | 33.376 | 100 | 1300 |
| Hay intake (g DM head−1 day−1) | 42 | 184.810 | 53.059 | 0 | 1350 |
| Milk yield (l head−1 day−1) | 42 | 0.879 | 0.058 | 0.32 | 1.8 |
| Milk fat (%) | 42 | 6.184 | 0.134 | 3.73 | 7.88 |
| Milk protein (%) | 42 | 5.409 | 0.051 | 4.66 | 6.07 |
| Milk lactose (%) | 42 | 4.604 | 0.040 | 3.92 | 4.95 |
| SCC | 42 | 1530.905 | 143.389 | 236 | 3959 |
| Casein (%) | 42 | 4.131 | 0.045 | 3.47 | 4.72 |
| Milk vit A (% fat) | 42 | 1.337 | 0.040 | 0.904 | 1.878 |
| Milk vit E (% fat) | 42 | 3.983 | 0.278 | 1.569 | 11.450 |
| Cholesterol (ppm) | 42 | 211.020 | 8.160 | 121.070 | 453.810 |
| DAP | 42 | 10.281 | 0.568 | 4.249 | 25.441 |
| FRAP (µmol L−1 FeSO4*7H2O) | 42 | 191.901 | 18.659 | 12.3 | 498 |
| b* | 42 | 6.20 | 0.16 | 4.60 | 8.81 |
| a* | 42 | −3.13 | 0.07 | −3.88 | −0.92 |
| L* | 42 | 70.39 | 0.52 | 62.38 | 76.68 |
| Cyanidin (mg/L) | 42 | 0.372 | 0.017 | 0.116 | 0.641 |
| Luteolin (mg/L) | 42 | 1.708 | 0.100 | 0.383 | 3.074 |
| Flavonoids (mg/L) | 42 | 2.080 | 0.117 | 0.499 | 3.716 |
| Ferulic acid (mg/L) | 42 | 14.636 | 1.016 | 5.236 | 30.322 |
| Tyrosol (mg/L) | 42 | 15.468 | 0.439 | 9.665 | 21.448 |
| Ferulate (mg/L) | 42 | 30.104 | 1.123 | 20.127 | 50.301 |
| Sesamin (mg/L) | 42 | 29.641 | 0.574 | 22.247 | 39.545 |
| Non-flavonoids (mg/L) | 42 | 59.932 | 1.351 | 43.554 | 81.090 |
| TP (mg/L) | 42 | 62.012 | 1.342 | 45.746 | 83.801 |
| GAE (mg/L) | 42 | 329.603 | 21.426 | 70 | 879 |
| SCFA | |||||
| C4:0 (% FAME) | 42 | 4.056 | 0.059 | 3.373 | 4.731 |
| C6:0 | 42 | 2.709 | 0.095 | 1.599 | 3.576 |
| C7:0 | 42 | 0.025 | 0.002 | 0.006 | 0.053 |
| C8:0 | 42 | 2.174 | 0.102 | 1.061 | 3.221 |
| C10:0 | 42 | 6.335 | 0.310 | 2.669 | 9.883 |
| C11:0 | 42 | 0.303 | 0.012 | 0.132 | 0.454 |
| MCFA | |||||
| C12:0 (% FAME) | 42 | 3.388 | 0.137 | 1.808 | 4.977 |
| C13:0i | 42 | 0.031 | 0.002 | 0.015 | 0.061 |
| C13:0ai | 42 | 0.038 | 0.001 | 0.028 | 0.052 |
| C14:0i | 42 | 0.157 | 0.009 | 0.048 | 0.297 |
| C14:0 | 42 | 9.956 | 0.167 | 6.550 | 11.851 |
| C14:1 c9 | 42 | 0.180 | 0.007 | 0.110 | 0.294 |
| C15:0i | 42 | 0.075 | 0.002 | 0.059 | 0.105 |
| C15:0ai | 42 | 0.318 | 0.014 | 0.181 | 0.536 |
| C15:0 | 42 | 1.185 | 0.032 | 0.634 | 1.675 |
| C16:0i | 42 | 0.382 | 0.014 | 0.202 | 0.601 |
| C16:0 | 42 | 24.963 | 0.487 | 21.198 | 33.062 |
| C16:1 t9 | 42 | 0.200 | 0.013 | 0.062 | 0.390 |
| C16:1 c9 | 42 | 0.829 | 0.031 | 0.574 | 1.322 |
| C16:1 c7 | 42 | 0.289 | 0.012 | 0.075 | 0.445 |
| C17:0 | 42 | 0.783 | 0.027 | 0.557 | 1.255 |
| C17:0i | 42 | 0.505 | 0.010 | 0.380 | 0.618 |
| C17:0ai | 42 | 0.562 | 0.016 | 0.298 | 0.764 |
| LCFA | |||||
| C18:0 (% FAME) | 42 | 9.268 | 0.246 | 6.481 | 13.939 |
| C18:0i | 42 | 0.074 | 0.003 | 0.041 | 0.118 |
| C18:1 t4 | 42 | 0.013 | 0.002 | 0.004 | 0.071 |
| C18: t5 | 42 | 0.015 | 0.002 | 0.004 | 0.069 |
| C18:1 t6 + t8 | 42 | 0.251 | 0.017 | 0.116 | 0.793 |
| C18:1 t9 | 42 | 0.282 | 0.011 | 0.191 | 0.611 |
| C18:1 t10 | 42 | 0.416 | 0.030 | 0.174 | 0.952 |
| C18:1 t11 | 42 | 2.378 | 0.163 | 0.617 | 4.736 |
| C18:1 t12 | 42 | 0.446 | 0.021 | 0.204 | 0.711 |
| C18:1 t13 + t14 | 42 | 1.150 | 0.071 | 0.364 | 2.128 |
| C18:1 c9 | 42 | 17.015 | 0.544 | 10.586 | 25.075 |
| C18:1 t15 + c10c | 42 | 0.364 | 0.035 | 0.075 | 1.216 |
| C18:1 c11c | 42 | 0.387 | 0.009 | 0.300 | 0.556 |
| C18:1 c12 | 42 | 0.199 | 0.010 | 0.118 | 0.402 |
| C18:1 c13 | 42 | 0.096 | 0.004 | 0.048 | 0.143 |
| C18:1 c14 + t16 | 42 | 0.552 | 0.025 | 0.224 | 0.763 |
| C18:2 t9t12 | 42 | 0.042 | 0.006 | 0.004 | 0.155 |
| C18:2 c9t13 | 42 | 0.492 | 0.024 | 0.201 | 0.804 |
| C18:2 c9t12t8c12n6 | 42 | 0.174 | 0.009 | 0.066 | 0.300 |
| C18:1 c16 | 42 | 0.130 | 0.005 | 0.079 | 0.230 |
| C18:2 t9c12n6 | 42 | 0.027 | 0.003 | 0.006 | 0.078 |
| C18:2 t11c15n3 | 42 | 0.427 | 0.037 | 0.053 | 1.175 |
| C18:2 c9c12n6 | 42 | 2.035 | 0.057 | 1.285 | 2.957 |
| C18:2 c9c15n3 | 42 | 0.020 | 0.002 | 0.003 | 0.055 |
| CLA c9t11 | 42 | 1.498 | 0.079 | 0.619 | 2.677 |
| CLA t9c11 | 42 | 0.110 | 0.005 | 0.064 | 0.170 |
| CLA c9c11 | 42 | 0.057 | 0.005 | 0.011 | 0.142 |
| CLA t12t14c11c13 | 42 | 0.027 | 0.003 | 0.000 | 0.131 |
| CLA t11t13 | 42 | 0.044 | 0.006 | 0.000 | 0.276 |
| CLA t9t11 | 42 | 0.029 | 0.001 | 0.020 | 0.057 |
| C18:3 c6c9c12n6 | 42 | 0.035 | 0.001 | 0.011 | 0.050 |
| C18:3 c9c12c15n3 | 42 | 1.029 | 0.040 | 0.339 | 1.698 |
| C20:0 | 42 | 0.342 | 0.025 | 0.178 | 0.820 |
| C20:1 c9 | 42 | 0.009 | 0.001 | 0.000 | 0.018 |
| C20:1 c11 | 42 | 0.037 | 0.002 | 0.015 | 0.057 |
| C20:2 c11c14n6 | 42 | 0.018 | 0.001 | 0.008 | 0.030 |
| C20:3 c5c8c11 | 42 | 0.063 | 0.012 | 0.000 | 0.283 |
| C20:4 c5c8c11c14n6 | 42 | 0.143 | 0.006 | 0.096 | 0.259 |
| C20:5 c5c8c11c14c17n3 | 42 | 0.074 | 0.002 | 0.050 | 0.127 |
| C22:0 | 42 | 0.167 | 0.008 | 0.090 | 0.293 |
| C22:5 c7c10c13c16c19n3 | 42 | 0.170 | 0.005 | 0.116 | 0.275 |
| C22:6 c4c7c10c13c16c19n3 | 42 | 0.064 | 0.004 | 0.032 | 0.133 |
| C23:0 | 42 | 0.071 | 0.004 | 0.037 | 0.142 |
| C24:0 | 42 | 0.077 | 0.004 | 0.037 | 0.143 |
| C26:0 | 42 | 0.044 | 0.002 | 0.015 | 0.091 |
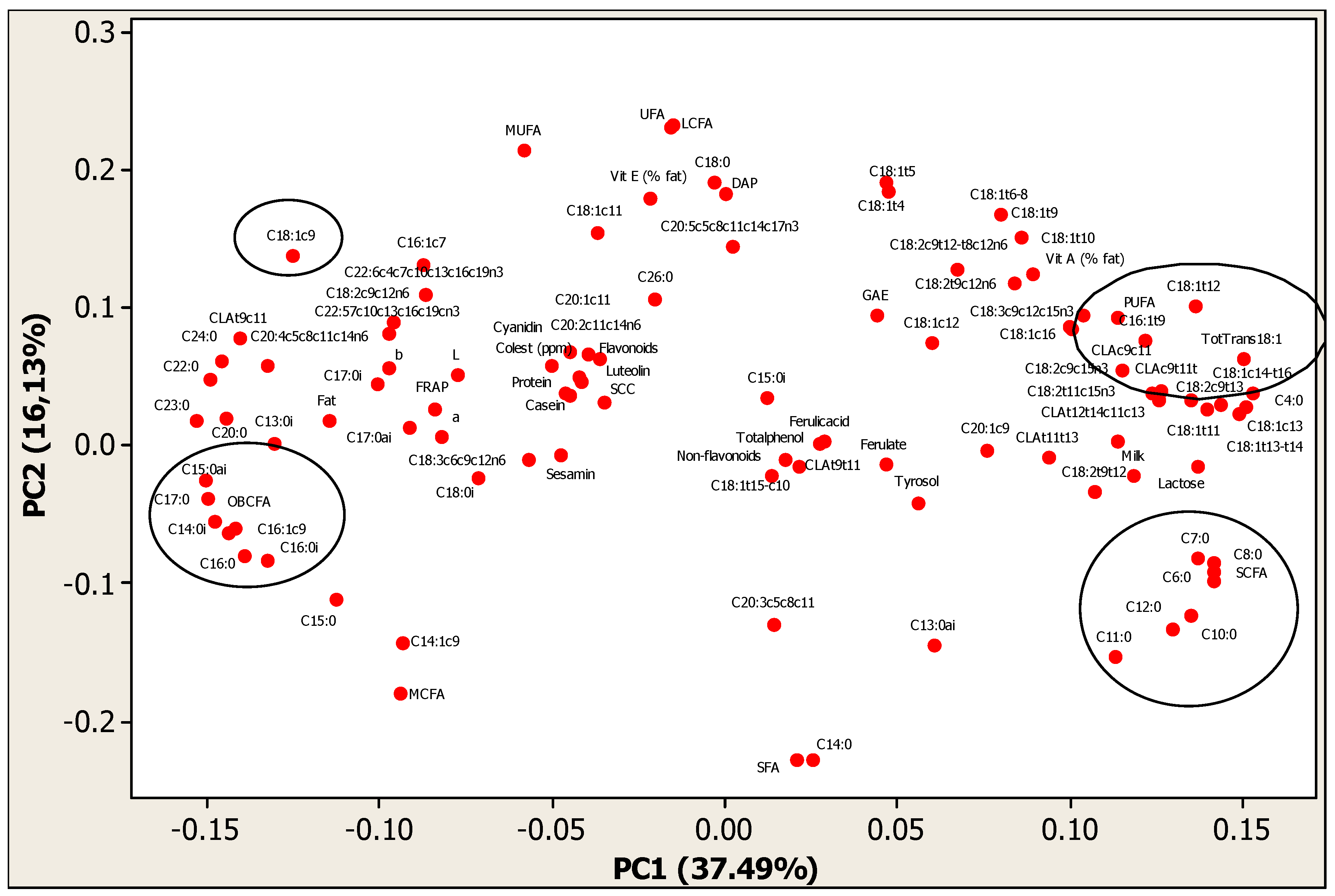
| Var | PC1 | PC2 | PC3 | PC4 | PC5 |
|---|---|---|---|---|---|
| Milk yield and macro-composition | |||||
| Milk yield | 0.118 | −0.021 | −0.127 | −0.089 | 0.040 |
| Milk fat | −0.115 | 0.018 | 0.114 | 0.174 | 0.027 |
| Milk protein | −0.045 | 0.037 | 0.200 | 0.263 | 0.119 |
| Milk lactose | 0.136 | −0.015 | −0.065 | 0.026 | 0.014 |
| SCC | −0.035 | 0.033 | −0.065 | −0.145 | −0.037 |
| Casein | −0.046 | 0.039 | 0.183 | 0.274 | 0.126 |
| Milk vit A | 0.083 | 0.119 | −0.090 | −0.085 | 0.138 |
| Milk vit E | −0.022 | 0.180 | 0.173 | −0.007 | 0.031 |
| Cholesterol | −0.050 | 0.058 | 0.240 | 0.041 | 0.135 |
| DAP | 0.000 | 0.183 | 0.065 | 0.024 | −0.052 |
| FRAP | −0.084 | 0.028 | 0.018 | −0.039 | 0.004 |
| Colours parameters | |||||
| b * | −0.097 | 0.057 | 0.128 | 0.025 | −0.090 |
| a * | −0.082 | 0.007 | 0.086 | 0.083 | 0.162 |
| L * | −0.077 | 0.051 | 0.067 | 0.084 | 0.196 |
| Phenols content | |||||
| Cyanidin | −0.045 | 0.068 | −0.123 | 0.052 | −0.260 |
| Luteolin | −0.042 | 0.047 | −0.150 | 0.073 | −0.241 |
| Flavonoids | −0.042 | 0.050 | −0.146 | 0.070 | −0.245 |
| Ferulic acid | 0.027 | 0.003 | 0.307 | −0.018 | −0.049 |
| Tyrosol | 0.056 | −0.041 | 0.065 | −0.093 | −0.117 |
| Ferulate | 0.046 | −0.013 | 0.303 | −0.052 | −0.090 |
| Sesamin | −0.048 | −0.006 | 0.117 | 0.122 | −0.114 |
| Non-flavonoids | 0.021 | −0.015 | 0.303 | 0.006 | −0.120 |
| TP | 0.018 | −0.010 | 0.292 | 0.012 | −0.143 |
| GAE | 0.044 | 0.095 | 0.115 | 0.019 | 0.063 |
| SCFA | |||||
| C4:0 | 0.123 | 0.038 | −0.098 | −0.093 | 0.070 |
| C6:0 | 0.141 | −0.084 | −0.040 | −0.048 | 0.095 |
| C7:0 | 0.136 | −0.081 | −0.015 | 0.123 | 0.070 |
| C8:0 | 0.141 | −0.091 | −0.024 | −0.027 | 0.085 |
| C10:0 | 0.134 | −0.123 | 0.002 | 0.005 | 0.068 |
| C11:0 | 0.113 | −0.153 | 0.019 | 0.035 | 0.054 |
| MCFA | |||||
| C12:0 | 0.130 | −0.132 | 0.019 | 0.033 | 0.052 |
| C13:0i | −0.131 | 0.002 | 0.110 | −0.094 | 0.017 |
| C13:0ai | 0.060 | −0.145 | 0.064 | 0.099 | 0.066 |
| C14:0 | 0.025 | −0.228 | 0.054 | 0.024 | 0.008 |
| C14:0i | −0.144 | −0.063 | 0.040 | −0.052 | 0.015 |
| C14:1 c9 | −0.093 | −0.143 | 0.082 | 0.116 | −0.033 |
| C15:0 | −0.112 | −0.111 | 0.056 | 0.043 | −0.022 |
| C15:0i | 0.012 | 0.035 | −0.130 | 0.035 | 0.197 |
| C15:0ai | −0.150 | −0.038 | 0.034 | −0.030 | 0.024 |
| C16:0 | −0.139 | −0.080 | 0.017 | 0.023 | −0.014 |
| C16:0i | −0.132 | −0.082 | 0.003 | 0.003 | 0.051 |
| C16:1 c7 | −0.088 | 0.132 | −0.028 | 0.078 | 0.220 |
| C16:1 c9 | −0.142 | −0.060 | 0.024 | 0.096 | −0.005 |
| C16:1 t9 | 0.121 | 0.076 | 0.136 | −0.113 | 0.003 |
| C17:0 | −0.151 | −0.025 | 0.021 | 0.038 | 0.001 |
| C17:0i | −0.101 | 0.046 | −0.012 | 0.003 | 0.131 |
| C17:0ai | −0.092 | 0.013 | −0.044 | 0.102 | 0.161 |
| LCFA | |||||
| C18:0 | −0.003 | 0.191 | −0.074 | −0.057 | −0.032 |
| C18:0i | −0.072 | −0.022 | 0.085 | −0.053 | −0.101 |
| C18:1 t4 | 0.047 | 0.184 | −0.006 | 0.126 | −0.089 |
| C18: t5 | 0.046 | 0.192 | 0.017 | 0.134 | −0.053 |
| C18:1 t6 + t8 | 0.080 | 0.168 | 0.016 | 0.155 | −0.079 |
| C18:1 t9 | 0.086 | 0.152 | 0.027 | 0.158 | −0.119 |
| C18:1 t10 | 0.089 | 0.125 | −0.059 | 0.197 | 0.054 |
| C18:1 t11 | 0.139 | 0.027 | 0.104 | −0.103 | −0.050 |
| C18:1 t12 | 0.136 | 0.101 | −0.018 | 0.077 | −0.029 |
| C18:1 t13 + t14 | 0.151 | 0.028 | −0.001 | 0.103 | 0.016 |
| C18:1 c9 | −0.125 | 0.138 | −0.050 | 0.006 | −0.056 |
| C18:1 t15 + c10 | 0.014 | −0.021 | 0.069 | 0.040 | −0.118 |
| C18:1 c11 | −0.037 | 0.155 | −0.028 | 0.077 | 0.115 |
| C18:1 c12 | 0.060 | 0.074 | −0.104 | 0.190 | 0.112 |
| C18:1 c13 | 0.148 | 0.024 | 0.036 | 0.073 | −0.034 |
| C18:1 c14 + t16 | 0.153 | 0.039 | −0.020 | 0.004 | −0.007 |
| C18:1 c16 | 0.099 | 0.087 | −0.049 | −0.043 | −0.068 |
| C18:2 t9t12 | 0.107 | −0.034 | 0.002 | 0.003 | −0.097 |
| C18:2 c9t13 | 0.143 | 0.030 | 0.022 | 0.096 | 0.003 |
| C18:2 c9t12t8c12n6 | 0.067 | 0.128 | −0.039 | −0.032 | 0.088 |
| C18:2 t9c12n6 | 0.103 | 0.095 | 0.022 | −0.106 | 0.152 |
| C18:2 t11c15n3 | 0.135 | 0.033 | 0.096 | 0.021 | 0.060 |
| C18:2 c9c12n6 | −0.096 | 0.089 | −0.125 | −0.029 | 0.034 |
| C18:2 c9c15n3 | 0.126 | 0.041 | 0.014 | 0.162 | 0.099 |
| CLA c9t11 | 0.126 | 0.033 | 0.141 | −0.116 | −0.065 |
| CLA t9c11 | −0.141 | 0.078 | 0.021 | 0.050 | 0.051 |
| CLA c9c11 | 0.115 | 0.055 | 0.162 | −0.089 | 0.086 |
| CLA t12t14c11c13 | 0.113 | 0.004 | −0.002 | 0.108 | 0.095 |
| CLA t11t13 | 0.094 | −0.009 | 0.021 | 0.149 | 0.091 |
| CLA t9t11 | 0.028 | 0.004 | 0.010 | 0.225 | −0.149 |
| C18:3 c6c9c12n6 | −0.057 | −0.009 | −0.049 | 0.104 | 0.236 |
| C18:3 c9c12c15n3 | 0.100 | 0.085 | 0.112 | −0.156 | 0.037 |
| C20:0 | −0.144 | 0.021 | −0.024 | 0.026 | −0.092 |
| C20:1 c9 | 0.076 | −0.003 | −0.029 | 0.174 | −0.009 |
| C20:1 c11 | −0.039 | 0.067 | −0.080 | 0.213 | 0.077 |
| C20:2 c11c14n6 | −0.037 | 0.063 | −0.076 | −0.077 | 0.105 |
| C20:3 c5c8c11 | 0.014 | −0.129 | 0.080 | 0.142 | −0.085 |
| C20:4 c5c8c11c14n6 | −0.133 | 0.058 | −0.034 | −0.013 | 0.135 |
| C20:5 c5c8c11c14c17n3 | 0.002 | 0.145 | 0.130 | −0.185 | 0.169 |
| C22:5 c7c10c13c16c19n3 | −0.149 | 0.049 | −0.008 | −0.017 | −0.043 |
| C22:6 c4c7c10c13c16c19n3 | −0.097 | 0.082 | 0.130 | −0.133 | 0.129 |
| C23:0 | −0.087 | 0.110 | 0.108 | −0.159 | 0.120 |
| C24:0 | −0.153 | 0.019 | −0.004 | −0.006 | 0.003 |
| C26:0 | −0.020 | 0.107 | −0.003 | −0.172 | 0.053 |
| SCFA | 0.141 | −0.098 | −0.021 | −0.020 | 0.079 |
| MCFA | −0.094 | −0.178 | 0.044 | 0.037 | 0.010 |
| LCFA | −0.015 | 0.232 | −0.022 | −0.024 | −0.070 |
| SFA | 0.021 | −0.228 | −0.015 | −0.004 | 0.083 |
| UFA | −0.016 | 0.231 | 0.013 | 0.003 | −0.074 |
| MUFA | −0.058 | 0.214 | −0.020 | 0.032 | −0.090 |
| PUFA | 0.114 | 0.094 | 0.093 | −0.080 | 0.025 |
| OBCFA | −0.148 | −0.055 | 0.023 | 0.030 | 0.045 |
| totaltrans18:1 | 0.150 | 0.063 | 0.055 | 0.011 | −0.033 |
| Eigenvalue | 37.87 | 16.13 | 7.30 | 4.93 | 4.44 |
| Total Variance explained (%) | 37.49 | 53.62 | 60.92 | 65.85 | 70.28 |
| FACTORS | Score PC1 | SE | DF | t Value | p | |
|---|---|---|---|---|---|---|
| HeI | High | −2.8344 | 1.1007 | 14 | −2.58 | 0.0220 |
| Medium | −5.9794 | 1.5394 | 14 | −3.88 | 0.0017 | |
| Zero | −2.6156 | 0.9691 | 14 | −2.70 | 0.0173 | |
| PPS | GW/FW | 2.7864 | 0.7330 | 10 | 3.80 | 0.0035 |
| MS | −10.4060 | 1.4720 | 10 | −7.07 | <0.0001 | |
| BC | Grass | −4.2524 | 0.7118 | 10 | −5.97 | 0.0001 |
| (forbs + legumes) | −3.3672 | 0.8183 | 10 | −4.11 | 0.0021 | |
| FACTORS | Score PC2 | SE | DF | t Value | p | |
|---|---|---|---|---|---|---|
| HeI | High | −1.1492 | 1.6990 | 14 | −0.68 | 0.5098 |
| Medium | 0.5833 | 2.3762 | 14 | 0.25 | 0.8096 | |
| Zero | 0.4316 | 1.4959 | 14 | 0.29 | 0.7772 | |
| PPS | GW/FW | 0.9079 | 1.1314 | 10 | 0.80 | 0.4409 |
| MS | −0.9974 | 2.2722 | 10 | −0.44 | 0.6700 | |
| BC | Grass | 1.2054 | 1.0988 | 10 | 1.10 | 0.2983 |
| (forbs + legumes) | −1.2949 | 1.2632 | 10 | −1.03 | 0.3294 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabiddu, A.; Carrillo, S.; Contini, S.; Spada, S.; Acciaro, M.; Giovanetti, V.; Decandia, M.; Lucini, L.; Bertuzzi, T.; Gallo, A.; et al. Dairy Sheep Grazing Management and Pasture Botanical Composition Affect Milk Macro and Micro Components: A Methodological Approach to Assess the Main Managerial Factors at Farm Level. Animals 2022, 12, 2675. https://doi.org/10.3390/ani12192675
Cabiddu A, Carrillo S, Contini S, Spada S, Acciaro M, Giovanetti V, Decandia M, Lucini L, Bertuzzi T, Gallo A, et al. Dairy Sheep Grazing Management and Pasture Botanical Composition Affect Milk Macro and Micro Components: A Methodological Approach to Assess the Main Managerial Factors at Farm Level. Animals. 2022; 12(19):2675. https://doi.org/10.3390/ani12192675
Chicago/Turabian StyleCabiddu, Andrea, Sebastian Carrillo, Salvatore Contini, Simona Spada, Marco Acciaro, Valeria Giovanetti, Mauro Decandia, Luigi Lucini, Terenzio Bertuzzi, Antonio Gallo, and et al. 2022. "Dairy Sheep Grazing Management and Pasture Botanical Composition Affect Milk Macro and Micro Components: A Methodological Approach to Assess the Main Managerial Factors at Farm Level" Animals 12, no. 19: 2675. https://doi.org/10.3390/ani12192675
APA StyleCabiddu, A., Carrillo, S., Contini, S., Spada, S., Acciaro, M., Giovanetti, V., Decandia, M., Lucini, L., Bertuzzi, T., Gallo, A., & Salis, L. (2022). Dairy Sheep Grazing Management and Pasture Botanical Composition Affect Milk Macro and Micro Components: A Methodological Approach to Assess the Main Managerial Factors at Farm Level. Animals, 12(19), 2675. https://doi.org/10.3390/ani12192675










