Microsatellite Instability Assay as a Potential Approach to Evaluate Genotoxicity: Lead Exposure in a Nestling Passerine Bird at the Stage of Intensive Erythropoiesis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
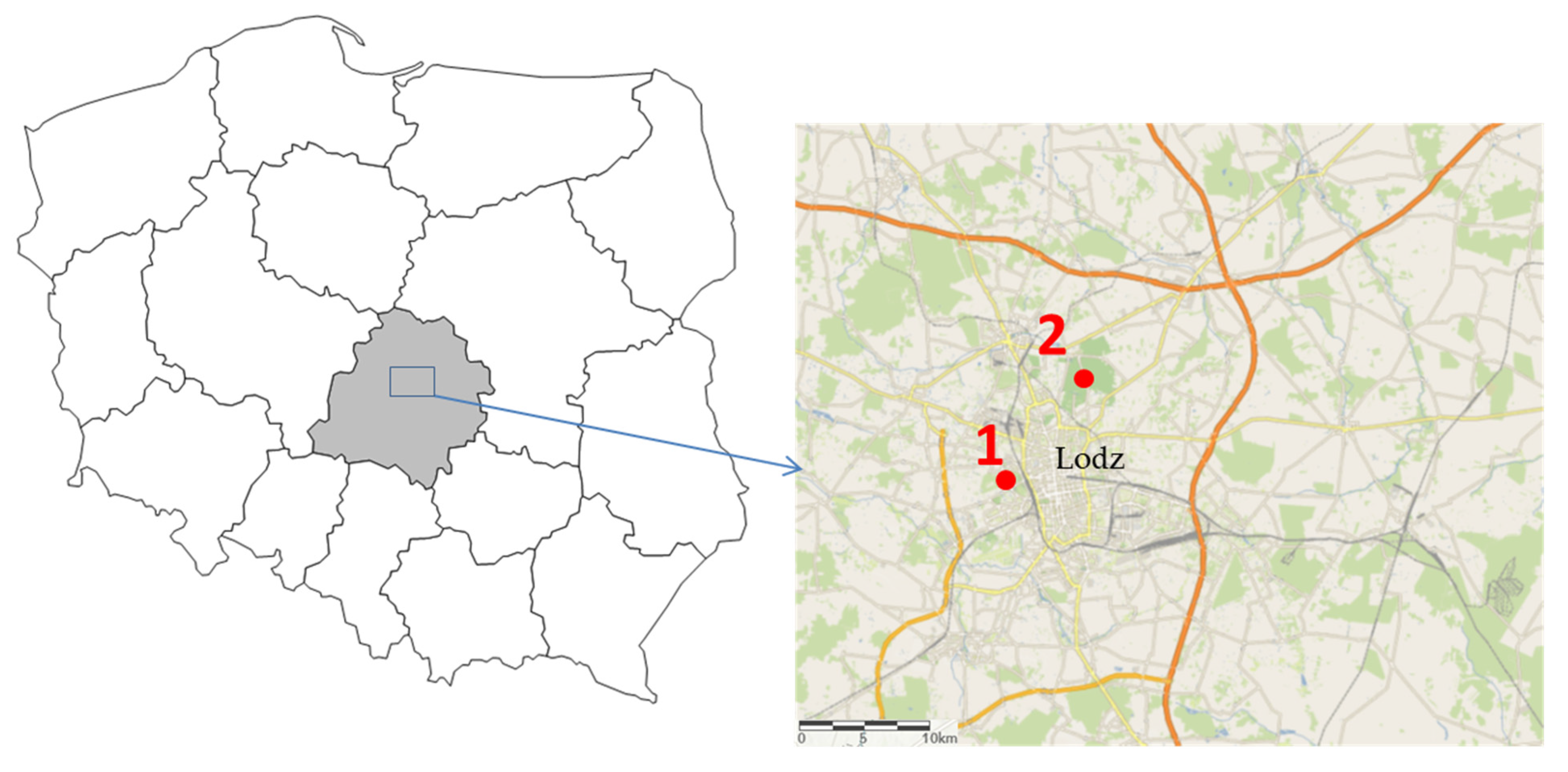
Study Site, Experiment Layout, Sampling, Isolation, Genotyping, and Statistical Analysis
3. Results and Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Franson, J.C.; Pain, D. Lead in birds. In Environmental Contaminants in Biota: Interpreting Tissue Concentrations, 2nd ed.; Beyer, W.N., Meador, J.P., Eds.; Taylor & Francis: Boca Raton, FL, USA, 2011; pp. 563–594. [Google Scholar]
- Haig, S.M.; D’Elia, J.; Eagles-Smith, C.; Fair, J.M.; Gervais, J.; Herring, G.; Rivers, J.W.; Schulz, J.H. The persistent problem of lead poisoning in birds from ammunition and fishing tackle. Condor 2014, 116, 408–428. [Google Scholar] [CrossRef]
- Ferreyra, H.; Beldomenico, P.M.; Marchese, K.; Romano, M.; Caselli, A.; Correa, A.I.; Uhart, M. Lead exposure affects health indices in free-ranging ducks in Argentina. Ecotoxicology 2015, 24, 735–745. [Google Scholar] [CrossRef] [PubMed]
- Provencher, J.F.; Forbes, M.R.; Hennin, H.L.; Love, O.P.; Braune, B.M.; Mallory, M.L.; Gilchrist, H.G. Implications of mercury and lead concentrations on breeding physiology and phenology in Arctic bird. Environ. Pollut. 2016, 218, 1014–1022. [Google Scholar] [CrossRef] [PubMed]
- Cid, F.D.; Fernández, N.C.; Pérez-Chaca, M.V.; Pardo, R.; Caviedes-Vidal, E.; Chediack, J.G. House sparrow biomarkers as lead pollution bioindicators. Evaluation of dose and exposition length on hematological and oxidative stress parameters. Ecotoxicol. Environ. Saf. 2018, 154, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Markowski, M.; Kaliński, A.; Bańbura, M.; Glądalski, M.; Wawrzyniak, J.; Skwarska, J.; Bańbura, J. Effects of experimental lead exposure on physiological indices of nestling great tits Parus major: Haematocrit and heterophile-to-lymphocyte ratio. Conserv. Physiol. 2019, 7, coz067. [Google Scholar] [CrossRef]
- Pain, D.J.; Mateo, R.; Green, R.E. Effects of lead from ammunition on birds and other wildlife: A review and update. Ambio 2019, 48, 935–953. [Google Scholar] [CrossRef] [PubMed]
- García-Lestón, J.; Méndez, J.; Pasaro, E.; Laffon, B. Genotoxic effects of lead: An updated review. Environ. Int. 2010, 36, 623–636. [Google Scholar] [CrossRef]
- Winder, C.; Bonin, T. The genotoxicity of lead. Mutat. Res. 1993, 285, 117–124. [Google Scholar] [CrossRef]
- Johnson, F.M. The genetic effects of environmental lead. Mutat. Res. 1998, 410, 123–140. [Google Scholar] [CrossRef]
- Danadevi, K.; Rozati, R.; Saleha Banu, B.; Hanumanth Rao, P.; Grover, P. DNA damage in workers exposed to lead using comet assay. Toxicology 2003, 187, 183–193. [Google Scholar] [CrossRef]
- Hemmaphan, S.; Bordeerat, N.K. Genotoxic Effects of Lead and Their Impact on the Expression of DNA Repair Genes. Int. J. Environ. Res. Public Health 2022, 19, 4307. [Google Scholar] [CrossRef]
- Ellegren, H.; Lindgren, G.; Primmer, C.R.; Møller, A.P. Fitness loss and germline mutations in barn swallows breeding in Chernobyl. Nature 1997, 389, 593–596. [Google Scholar] [CrossRef] [PubMed]
- Ilyinskikh, N.N.; Ilyinskikh, E.N.; Ksenz, A.S.; Yurkin, A.Y. An assessment of frequencies of micronucleated erythrocytes in peripheral blood of pigeons (Columba livia Gm) living in the polluted radioactive area around the Siberian chemical plant. Environ. Pollut. 1997, 98, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Yauk, C.L.; Fox, G.A.; McCarryc, B.E.; Quinn, J.S. Induced minisatellite germline mutations in herring gulls (Larus argentatus) living near steel mills. Mutat. Res. 2000, 452, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Pastor, N.; López-Lázaro, M.; Tella, J.L.; Baos, R.; Forrero, M.G.; Hiraldo, F.; Cortés, F. DNA Damage in birds after the mining waste spill in Southwestern Spain: A comet assay evaluation. J. Environ. Pathol. Toxicol. Oncol. 2001, 20, 317–324. [Google Scholar] [CrossRef]
- Pastor, N.; López-Lázaro, M.; Tella, J.L.; Baos, R.; Hiraldo, F.; Cortés, F. Assessment of genotoxic damage by the comet assay in white storks Ciconia ciconia after the Donana Ecological Disaster. Mutagenesis 2001, 16, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Pastor, N.; Baos, R.; López-Lázaro, M.; Jovani, R.; Tella, J.L.; Hajji, N.; Hiraldo, F.; Cortés, F. A 4 year follow-up analysis of genotoxic damage in birds of the Doñana area (south west Spain) in the wake of the 1998 mining waste spill. Mutagenesis 2004, 19, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Baos, R.; Jovani, R.; Pastor, N.; Tella, J.L.; Jiménez, B.; Gómez, G.; González, M.; Hiraldo, F. Evaluation of Genotoxic effects of heavy metals and arsenic in wild nestling white storks (Ciconia ciconia) and black kites (Milvus migrans) from southwestern Spain after a mining accident. Environ. Toxicol. Chem. 2006, 25, 2794–2803. [Google Scholar] [CrossRef] [PubMed]
- Eeva, T.; Belskii, E.; Kuranov, B. Environmental pollution affects genetic diversity in wild bird populations. Mutat. Res. 2006, 608, 8–15. [Google Scholar] [CrossRef]
- Quiros, L.; Ruiz, X.; Sanpera, C.; Jover, L.; Pina, B. Analysis of micronucleated erythrocytes in heron nestlings from reference and impacted sites in the Ebro basin (NE Spain). Environ. Pollut. 2008, 155, 81–87. [Google Scholar] [CrossRef]
- Skarphedinsdottir, H.; Hallgrimsson, G.T.; Hansson, T.; Hägerroth, P.A.; Liewenborg, B.; Tjärnlund, U.; Akerman, G.; Barsiene, J.; Balk, L. Genotoxicity in herring gulls (Larus argentatus) in Sweden and Iceland. Mutat. Res. 2010, 702, 24–31. [Google Scholar] [CrossRef]
- Bonisoli Alquati, A.; Voris, A.; Mousseau, T.A.; Møller, A.P.; Saino, N.; Wyatt, M.D. DNA damage in barn swallows Hirundo rustica from the Chernobyl region detected by use of the comet assay. Comp. Biochem. Phys. C 2010, 151, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Bonisoli-Alquati, A.; Koyama, K.; Tedeschi, D.J.; Kitamura, W.; Sukuzi, H.; Ostermiller, S.; Arai, E.; Møller, A.P.; Mousseau, T.A. Abundance and genetic damage of barn swallows from Fukushima. Sci. Rep. 2015, 5, 9432. [Google Scholar] [CrossRef] [PubMed]
- Baesse, C.Q.; Tolentino, V.C.; da Silva, A.M.; Silva, A.D.A.; Ferreira, G.Â.; Paniago, L.P.; Nepomuceno, J.C.; de Melo, C. Micronucleus as biomarker of genotoxicity in birds from Brazilian Cerrado. Ecotoxicol. Environ. Saf. 2015, 115, 223–228. [Google Scholar] [CrossRef] [PubMed]
- De Mas, E.; Benzal, J.; Merino, S.; Valera, F.; Palacios, M.J.; Cuervo, J.J.; Barbosa, A. Erythrocytic abnormalities in three Antarctic penguin species along the Antarctic Peninsula: Biomonitoring of genomic damage. Polar Biol. 2015, 38, 1067–1074. [Google Scholar] [CrossRef]
- Quero, A.A.M.; Ferré, D.M.; Zarco, A.; Cuervo, P.F.; Gorla, N.B.M. Erythrocyte micronucleus cytome assay of 17 wild bird species from the central Monte desert, Argentina. Environ. Sci. Pollut. Res. 2016, 23, 25224–25231. [Google Scholar] [CrossRef]
- Santos, C.S.; Brandão, R.; Monteiro, M.S.; Bastos, A.C.; Soares, A.M.; Loureiro, S. Assessment of DNA damage in Ardea cinerea and Ciconia ciconia: A 5-year study in Portuguese birds retrieved for rehabilitation. Ecotoxicol. Environ. Saf. 2017, 136, 104–110. [Google Scholar] [CrossRef]
- Souto, H.N.; de Campos Júnior, E.O.; Campos, C.F.; Rodrigues, T.S.; Pereira, B.B.; Morelli, S. Biomonitoring birds: The use of a micronuclei test as a tool to assess environmental pollutants on coffee farms in southeast Brazil. Environ. Sci. Pollut. Res. 2018, 25, 24084–24092. [Google Scholar] [CrossRef] [PubMed]
- Frixione, M.G.; D’Amico, V.; Adami, M.A.; Bertellotti, M. Urbanity as a source of genotoxicity in the synanthropic Kelp Gull (Larus dominicanus). Sci. Total Environ. 2022, 850, 157958. [Google Scholar] [CrossRef]
- El-Ghor, A.A.; Noshy, M.M.; Ashmaoui, H.M.; Eid, J.I.; Hassanane, M.S. Microsatellite instability at three microsatellite loci (D6mit3, D9mit2 and D15Mgh1) located in different common fragile sites of rats exposed to cadmium. Mutat. Res. 2010, 696, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Zienolddiny, S.; Svendsrud, D.H.; Ryberg, D.; Mikalsen, A.B.; Haugen, A. Nickel (II) induces microsatellite mutations in human lung cancer cell lines. Mutat. Res. 2000, 452, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Hirose, T.; Kondo, K.; Takahashi, Y.; Ishikura, H.; Fujino, H.; Tsuyuguchi, M.; Hashimoto, M.; Yokose, T.; Mukai, K.; Kodama, T.; et al. Frequent microsatellite instability in lung cancer from chromate-exposed workers. Mol. Carcinog. 2002, 33, 172–180. [Google Scholar] [CrossRef]
- Holmes, A.L.; Wise, S.S.; Wise, J.P., Sr. Carcinogenicity of hexavalent chromium. Indian J. Med. Res. 2008, 128, 353–372. [Google Scholar] [PubMed]
- El-Ghor, A.A.; Noshy, M.M.; Eid, J.I. Lead acetate and arsenic trioxide induce instability of microsatellites at three different fragile sites (6q21, 9q32-9q33 and 15p14) within the genome of the rat. Mutat. Res. 2011, 726, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, H.; Lopes, T.; Almeida, T.; Pereira, M.D.L.; Santos, C. Cadmium-induced genetic instability in mice testis. Hum. Exp. Toxicol. 2012, 31, 1228–1236. [Google Scholar] [CrossRef]
- Du, X.; Lan, T.; Yuan, B.; Chen, J.; Hu, J.; Ren, W.; Chen, Z. Cadmium-induced microsatellite instability in the kidneys and leukocytes of C57BL/6J mice. Environ. Toxicol. 2015, 30, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Miao, C.; He, Y.; Li, H.; Zhang, S.; Li, K.; Liu, H.; Li, W.; Zhao, J.; Xu, Y.; et al. The Influence of Heavy Metals on Gastric Tumorigenesis. J. Oncol. 2022, 2022, 6425133. [Google Scholar] [CrossRef]
- Wawrzyniak, J.; Kaliński, A.; Glądalski, M.; Bańbura, M.; Markowski, M.; Skwarska, J.; Zieliński, P.; Cyżewska, I.; Bańbura, J. Long-term variation in laying date and clutch size of the Great Tit Parus major in central Poland: A comparison between urban parkland and deciduous forest. Ardeola 2015, 62, 311–322. [Google Scholar] [CrossRef]
- Kaliński, A.; Bańbura, M.; Glądalski, M.; Markowski, M.; Skwarska, J.; Wawrzyniak, J.; Zieliński, P.; Cyżewska, I.; Bańbura, J. Long-term variation in hemoglobin concentration in nestling great tits Parus major. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2015, 185, 9–15. [Google Scholar] [CrossRef] [PubMed]
- TIBCO Software Inc. Statistica (Data Analysis Software System), Version 13. 2017. Available online: http://statistica.io (accessed on 1 September 2022).
- Markowski, M.; Kaliński, A.; Skwarska, J.; Wawrzyniak, J.; Bańbura, M.; Markowski, J.; Zieliński, P.; Bańbura, J. Avian feathers as bioindicators of the exposure to heavy metal contamination of food. Bull. Environ. Contam. Toxicol. 2013, 91, 302–305. [Google Scholar] [CrossRef] [PubMed]
- Eeva, T.; Rainio, M.; Berglund, Å.; Kanerva, M.; Stauffer, J.; Stöwe, M.; Ruuskanen, S. Experimental manipulation of dietary lead levels in great tit nestlings: Limited effects on growth, physiology and survival. Ecotoxicology 2014, 23, 914–928. [Google Scholar] [CrossRef] [PubMed]
- Saladin, V.; Bonfils, D.; Binz, T.; Richner, H. Isolation and characterization of 16 microsatellite loci in the Great Tit Parus major. Mol. Ecol. Notes 2003, 3, 520–522. [Google Scholar] [CrossRef]
- Van Oosterhout, C.; Hutchinson, W.F.; Wills, D.P.; Shipley, P. MICRO-CHECKER: Software for identifying and correcting genotyping errors in microsatellite data. Mol. Ecol. Res. 2004, 4, 535–538. [Google Scholar] [CrossRef]
- Eisler, R. Lead Hazards to Fish, Wildlife, and Invertebrates: A Synoptic Review; U.S. Fish and Wildlife Service Biological Report; Patuxent Wildlife Research Center: Laurel, MD, USA, 1988; Volume 85, pp. 1–14.
- Gidlow, D.A. Lead toxicity. Occup. Med. 2015, 65, 348–356. [Google Scholar] [CrossRef]
- Katavolos, P.; Staempfli, S.; Sears, W.; Gancz, A.Y.; Smith, D.A.; Bienzle, D. The effect of lead poisoning on hematologic and biochemical values in trumpeter swans and Canada geese. Vet. Clin. Pathol. 2007, 36, 341–347. [Google Scholar] [CrossRef]
- Flora, G.; Gupta, D.; Tiwari, A. Toxicity of lead: A review with recent updates. Interdiscip. Toxicol. 2012, 5, 47–58. [Google Scholar] [CrossRef]
- Chatelain, M.; Gasparini, J.; Frantz, A. Trace metals, melanin-based pigmentation and their interaction influence immune parameters in feral pigeons (Columba livia). Ecotoxicology 2016, 25, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Bauerová, P.; Vinklerová, J.; Hraníček, J.; Čorba, V.; Vojtek, L.; Svobodová, J.; Vinkler, M. Associations of urban environmental pollution with health-related physiological traits in a free-living bird species. Sci. Total Environ. 2017, 1, 1556–1565. [Google Scholar] [CrossRef] [PubMed]
- Perrault, J.R.; Stacy, N.I.; Lehner, A.F.; Poor, S.K.; Buchweitz, J.P.; Walsh, C.J. Toxic elements and associations with hematology, plasma biochemistry, and protein electrophoresis in nesting loggerhead sea turtles (Caretta caretta) from Casey Key, Florida. Environ. Pollut. 2017, 231, 1398–1411. [Google Scholar] [CrossRef]
- Lee, J.W.; Choi, H.; Hwang, U.K.; Kang, J.C.; Kang, Y.J.; Kim, K.I.; Kim, J.H. Toxic effects of lead exposure on bioaccumulation, oxidative stress, neurotoxicity, and immune responses in fish: A review. Environ. Toxicol. Pharmacol. 2019, 68, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Dunier, M. Water pollution and immunosuppression of freshwater fish. Ital. J. Zool. 1996, 63, 303–309. [Google Scholar] [CrossRef]
- Linder, G.; Grillitsch, B. Ecotoxicology of metals. In Ecotoxicology of Amphibians and Reptiles; Sparling, D.W., Linder, G., Bishop, C.A., Eds.; Society of Environmental Toxicology and Chemistry (SETAC): Pensacola, FL, USA, 2000; pp. 325–459. [Google Scholar]
- Perez-Coll, C.S.; Herkovits, J.; Salibian, A. Embryotoxicity of lead on Bufo arenarum. Bull. Environ. Contam. Toxicol. 1988, 41, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Sobotka, J.M.; Rahwan, R.G. Teratogenesis induced by shortand long-term exposure of Xenopus laevis progeny to lead. J. Toxicol. Environ. Health 1995, 44, 469–484. [Google Scholar] [CrossRef] [PubMed]
- Salice, C.J.; Suski, J.G.; Bazar, M.A.; Talent, L.G. Effects of inorganic lead on Western fence lizards (Sceloporus occidentalis). Environ. Pollut. 2009, 157, 3457–3464. [Google Scholar] [CrossRef]
- Arrieta, M.A.; Bruzzone, L.; Apartín, C.; Rosenberg, C.E.; Fink, N.E.; Salibián, A. Biosensors of inorganic lead exposure and effect in an adult amphibian. Arch. Environ. Contam. Toxicol. 2004, 46, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Wani, A.L.; Ara, A.; Usmani, J.A. Lead toxicity: A review. Interdiscip. Toxicol. 2015, 8, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Wei-Chun, M. Lead in Mammals. In Environmental Contaminants in Biota: Interpreting Tissue Concentrations, 2nd ed.; Beyer, W.N., Meador, J.P., Eds.; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar] [CrossRef]
- De Francisco, N.; Ruiz Troya, J.D.; Agüera, E.I. Lead and lead toxicity in domestic and free living birds. Avian Pathol. 2003, 32, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Ariza, M.E.; Williams, M.V. Lead and mercury mutagenesis: Type of mutation dependent upon metal concentration. J. Biochem. Mol. Toxicol. 1999, 13, 107–112. [Google Scholar] [CrossRef]
- Roy, N.K.; Rossman, T.G. Mutagenesis and comutagenesis by lead compounds. Mutat. Res. 1992, 298, 97–103. [Google Scholar] [CrossRef]

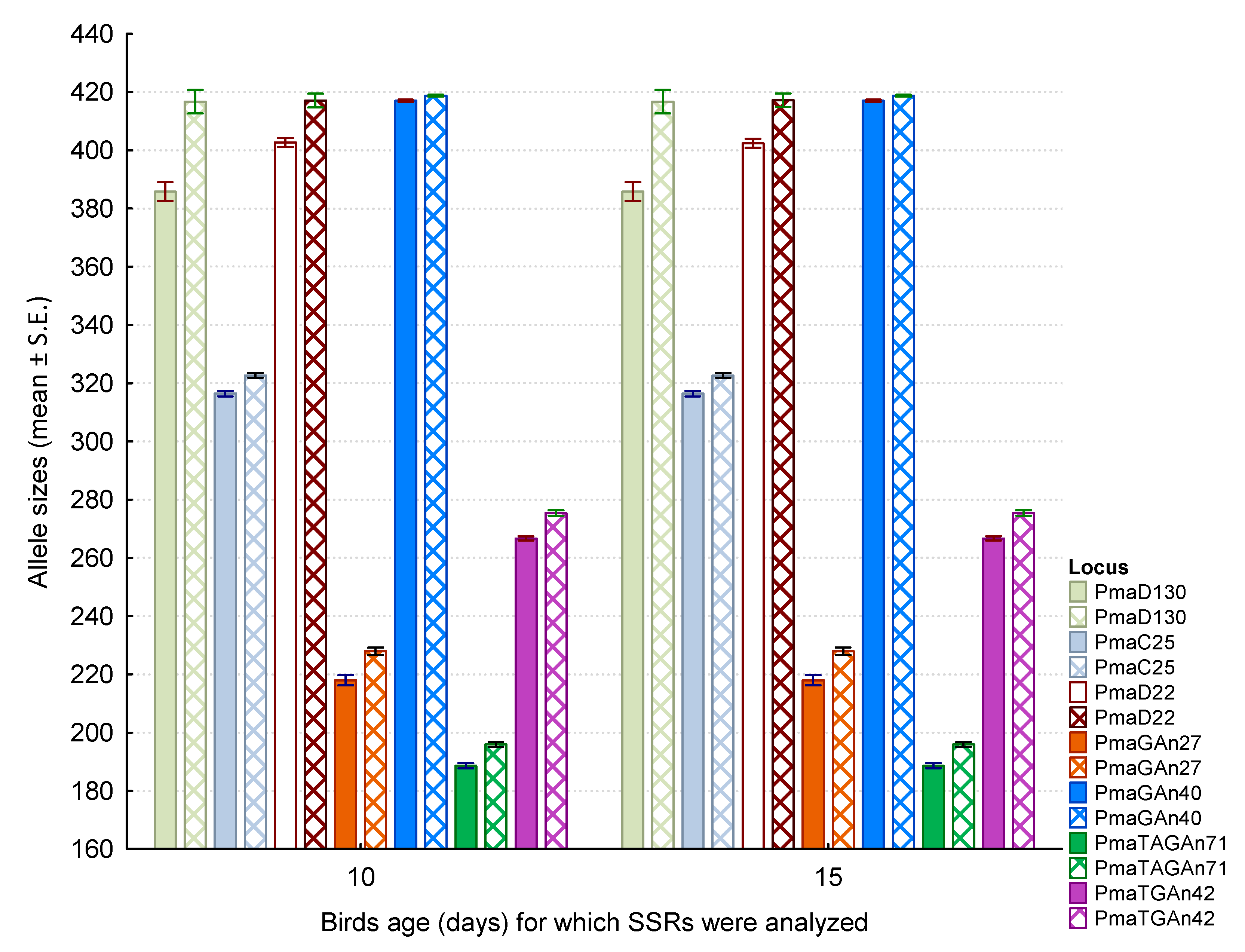
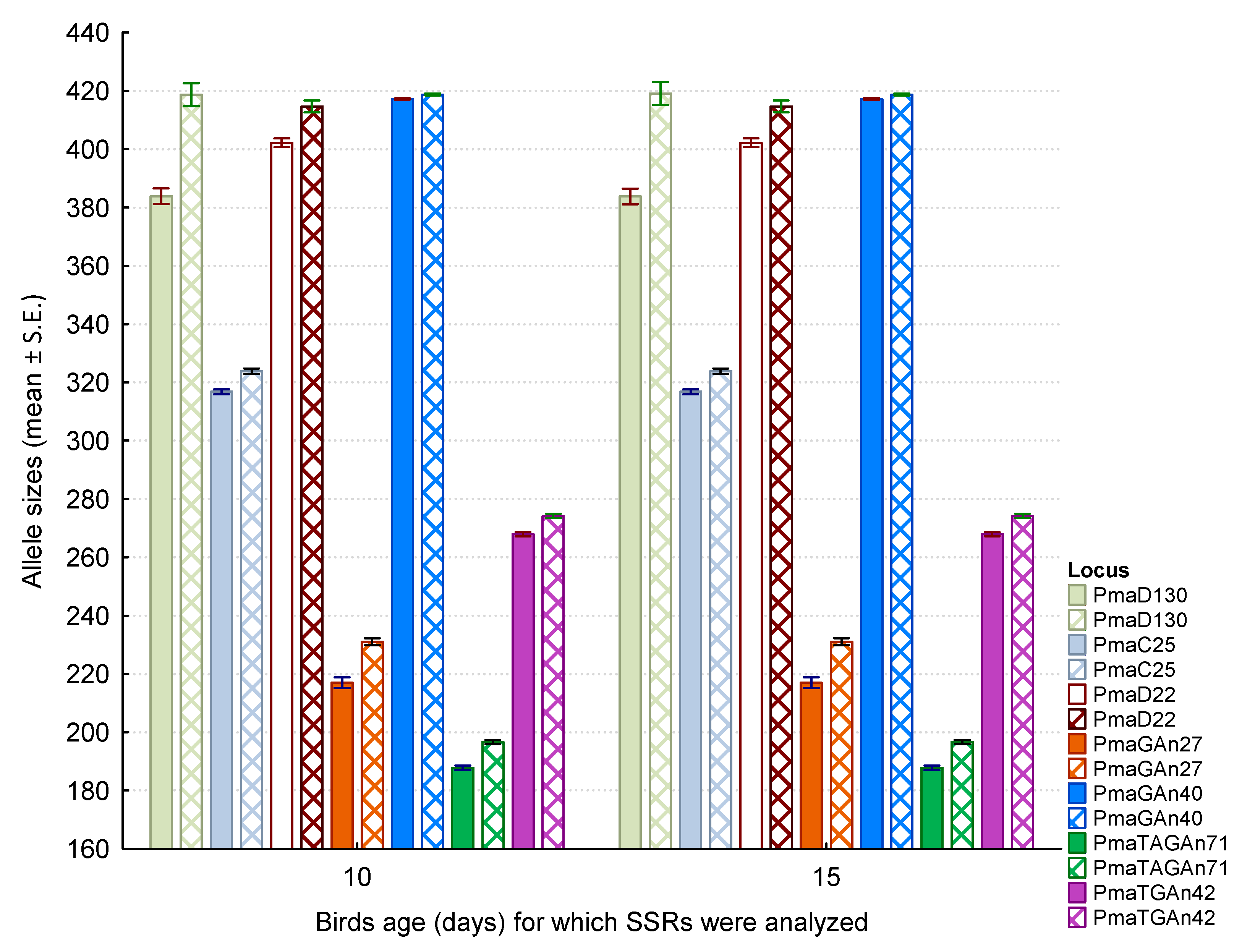
| Locus | N | Size Range (bp) | Motif of Cloned Allele | Primer Sequences |
|---|---|---|---|---|
| PmaD130 | 119 | 376–452 | (TAGA)17 |
F°:TGAGTGGAAAGATGCTGGC R°:CCCTATAAAAACCGAGGCTG |
| PmaC25 | 120 | 303–330 | (CAT)11 |
F°:CGTCCTGCTGTTTGTATTTCTG R°:CCATGAACCATTTTTAGGGTG |
| PmaD22 | 120 | 376–448 | (CTAT)15(CCAT)12 |
F°:GATCAGAGCTTGCCTCAACAC R°:TCTGGGCTGAAATACCTACCC |
| PmaGAn27 | 120 | 198–253 | (CAT)16 |
F°:TATAAACCACAGCCACACGC R°:CACAACCACAGAGGCATGAG |
| PmaGAn40 | 120 | 406–422 | (GA)10 |
F°:CGTTCCTCCTTTGCTTTCTG R°:AATGGCACAACACCTTCTCC |
| PmaTAGAn71 | 120 | 173–209 | (TAGG)6(TAGA)11 |
F°:TCAGCCTCCAAGGAAAACAG R°:GCATAAGCAACACCATGCAG |
| PmaTGAn42 | 120 | 260–292 | (TCCA)15 |
F°:ACTTCCACATGCCAGTTTCC R°:TGTTAAGGCAGAGAGGTGGG |
| Dose of Lead (μg/g of Body Mass) | |||
|---|---|---|---|
| Locus (Two Alleles) | Control | 15 | 30 |
| PmaD130 | Z = 0.013; p = 0.99 | Z = 0.007; p = 0.99 | Z = −0.007; p = 0.99 |
| PmaD130 | Z = -0.092; p = 0.93 | Z = 0.005; p = 1.00 | Z = −0.005; p = 1.00 |
| PmaC25 | Z = 0.005; p = 1.00 | Z = 0.005; p = 1.00 | Z = −0.005; p = 1.00 |
| PmaC25 | Z = 0.005; p = 1.00 | Z = 0.005; p = 1.00 | Z = −0.005; p = 1.00 |
| PmaD22 | Z = 0.005; p = 1.00 | Z = 0.005; p = 1.00 | Z = 0.226; p = 0.82 |
| PmaD22 | Z = 0.005; p = 1.00 | Z = 0.005; p = 1.00 | Z = 0.000; p = 1.00 |
| PmaGAn27 | Z = 0.005; p = 1.00 | Z = 0.000; p = 1.00 | Z = 0.005; p = 1.00 |
| PmaGAn27 | Z = 0.005; p = 1.00 | Z = −0.005; p = 1.00 | Z = 0.005; p = 1.00 |
| PmaGAn40 | Z = 0.006; p = 1.00 | Z = −0.006; p = 1.00 | Z = 0.006; p = 1.00 |
| PmaGAn40 | Z = 0.005; p = 1.00 | Z = −0.005; p = 1.00 | Z = 0.005; p = 1.00 |
| PmaTAGAn71 | Z = 0.005; p = 1.00 | Z = −0.005; p = 1.00 | Z = 0.005; p = 1.00 |
| PmaTAGAn71 | Z = 0.005; p = 1.00 | Z = −0.005; p = 1.00 | Z = 0.005; p = 1.00 |
| PmaTGAn42 | Z = 0.005; p = 1.00 | Z = −0.005; p = 1.00 | Z = 0.005; p = 1.00 |
| PmaTGAn42 | Z = 0.005; p = 1.00 | Z = −0.005; p = 1.00 | Z = 0.005; p = 1.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Markowski, M.; Kaliński, A.; Wawrzyniak, J.; Glądalski, M.; Skwarska, J.; Bańbura, J. Microsatellite Instability Assay as a Potential Approach to Evaluate Genotoxicity: Lead Exposure in a Nestling Passerine Bird at the Stage of Intensive Erythropoiesis. Animals 2023, 13, 1325. https://doi.org/10.3390/ani13081325
Markowski M, Kaliński A, Wawrzyniak J, Glądalski M, Skwarska J, Bańbura J. Microsatellite Instability Assay as a Potential Approach to Evaluate Genotoxicity: Lead Exposure in a Nestling Passerine Bird at the Stage of Intensive Erythropoiesis. Animals. 2023; 13(8):1325. https://doi.org/10.3390/ani13081325
Chicago/Turabian StyleMarkowski, Marcin, Adam Kaliński, Jarosław Wawrzyniak, Michał Glądalski, Joanna Skwarska, and Jerzy Bańbura. 2023. "Microsatellite Instability Assay as a Potential Approach to Evaluate Genotoxicity: Lead Exposure in a Nestling Passerine Bird at the Stage of Intensive Erythropoiesis" Animals 13, no. 8: 1325. https://doi.org/10.3390/ani13081325
APA StyleMarkowski, M., Kaliński, A., Wawrzyniak, J., Glądalski, M., Skwarska, J., & Bańbura, J. (2023). Microsatellite Instability Assay as a Potential Approach to Evaluate Genotoxicity: Lead Exposure in a Nestling Passerine Bird at the Stage of Intensive Erythropoiesis. Animals, 13(8), 1325. https://doi.org/10.3390/ani13081325





