Simple Summary
It is essential to study animal models of human disease to understand the progression of disease, the mechanisms involved and their interventional testing on a pre-clinical basis. We proposed to observe a lipopolysaccharide (LPS)-induced effective model of mammary gland inflammation. Additionally, the nutraceutical role of resveratrol, a phenolic compound, against the negative effects of lipopolysaccharides (LPS) in mammary tissues caused by oxidative damage and inflammation has not been fully studied. In our experiments, we found that LPS significantly enhances oxidative stress by repressing the expression of oxidative-related factors and stimulating the secretion of inflammatory cytokines. Moreover, LPS decreases the body weight, water and feed intake and activates NF-κB, Jnk, Erk, and Nrf2. The relative protein intensity of P65 and pP65 were increased by LPS. However, resveratrol administration remarkably decreases the oxidative response as well as inflammatory genes and plays a vital role in protection of the mammary glands from negative alterations in female mice.
Abstract
The aim of this study is to evaluate the defensive role of resveratrol, which is antagonistic to the oxidative stress and inflammation that is prompted by LPS in mammary tissue of female mice. Thirty adult mice were distributed into three groups (n = 10) control (CON), lipopolysaccharides at 2.5 mg/kg (LPS), and lipopolysaccharides at 2.5 mg/kg with 2 mg/kg of resveratrol (RES + LPS). The treatments were applied for 15 consecutive days. Spectrophotometry was used to quantify ROS in the blood, and proinflammatory cytokines concentrations were determined through radioimmunoassay. NF-κB, Jnk, IL-1β, Erk, IL-6, Nrf2 and TNF-α were quantified by RT-qPCR, and Western blots were used to quantifyP65 and pP65 protein intensities. MDA production was considerably increased, and the activity of T-AOC declined in the LPS treatment in comparison with the CON group but was significantly reversed in the RES + LPS group. Proinflammatory cytokines production and the genes responsible for inflammation and oxidative stress also showed higher mRNA and pP65 protein intensity in the LPS group, while Nrf2 showed a remarkable decline in mRNA expression in the LPS versus the CON group. All these mRNA intensities were reversed in the RES + LPS group. There were no remarkable changes in P65 protein intensity observed between the CON, LPS, and RES + LPS groups. In conclusion, resveratrol acts as a protective agent to modulate cellular inflammation and oxidative stress caused by LPS in mammary tissue of female mice.
1. Introduction
Lipopolysaccharides (LPS) are important molecules in a large, unique class of macromolecules. LPS are involved in the interaction of the cell with the environment. As a result, bacteria coming into contact with the immune system stimulate the production of certain antibodies that are primarily directed towards certain lipopolysaccharide structural components. Consequently, the primary surface antigens of Gram-negative bacteria are lipopolysaccharides [1]. A pathogen-associated molecular pattern (PAMP) based on circulating LPS can activate subsequently trigger a local or systemic inflammatory responses [2]. LPS is capable of triggering inflammation by activating cells other than immune cells. Such inflammatory cytokines can be closely monitored in an immune system response. The inflammatory pathways are the most essential part of innate immunity and possess the capability to trigger the complement system by the way of ROS pathways through the induction of endotoxins such as LPS [2]. The absence of LPS production by any given organism has long-term effects on the way other parts of the cell membrane are assembled [3]. Preeminent intensities of ROS activate inflammatory responses, trigger the NF-κB pathway, pro-inflammatory cytokines up-regulation and increase the production of ROS [4]. Gram-negative bacteria are an effective stimulator of inflammation at the outer membrane and have been frequently used as a hygienic sepsis model for lipopolysaccharide [5,6,7].
To inhibit ROS-induced injury, there is a large involvement of mammalian cells in the defense system of antioxidants. An enzyme called glutathione peroxidase (GPX) uses glutathione to neutralize dangerous lipid peroxides such as hydrogen peroxide [8]. Due to over production of antioxidant enzymes, oxidative stress occurs. Oxidative stress is the primary cause of several infectious diseases such as mastitis, pneumonia and enteritis in domestic animals, and earlier reports revealed that besides oxidative stress, inflammatory disease also occurs due to increased levels of nitric oxide (NO), ROS and Malon-dialdehyde (MDA) in serum [9,10]. In addition, oxidative stress is crucial for controlling metabolic processes in some organs and aids in maintaining or increasing domestic animal production [11]. In addition, dairy cows are under metabolic stress during lactation and pregnancy [12,13,14]. It is also thought that oxidative stress has a large impact on how productive farm animals are and how particular organs regulate their metabolism [15,16,17,18].
Most of the therapeutic compounds such as statins are well recognized for their roles in comprising immuno-modulatory and anti-inflammatory effects in addition to their innovative roles in disease treatment [19]. During stress conditions, herbal derivative compounds such as resveratrol (3, 5, 40-trihydroxy-trans-stilbene) have been shown to possess remedial effects [20]. Resveratrol has received attention because it possesses anti-inflammatory, anti-platelet aggregation, antioxidant, anti-atherogenic, anti-tumor and anti-aging activities [21]. Previously, resveratrol supplementation was found to be an effective for the treatment and prophylaxis of intestinal inflammation [22]. Previously, different compounds, such as sodium butyrate, have been shown to inhibit NF-κB signaling, which leads to an overall decrease in inflammation [23]. Resveratrol has been used to treat various diseases such as experimental colitis [24], pancreatitis [25] and arthritis [26]. The nutraceutical role of resveratrol, a phenolic compound, in combating the negative effects of LPS in mammary tissues caused by oxidative damage and inflammation have not been fully studied. Therefore, we propose to explore the key role of resveratrol in mitigating the harmful effects of lipopolysaccharides, such as oxidative damage, inflammation, and prompting the NF-κB/Nrf2 pathways, in the mammary tissue of a female mice model.
2. Materials and Methods
2.1. Ethical Approval
All experiments were conducted with the approval of The Institutional Animal Care and Use Committee (IACUC) which reviewed the experimental methods used in this research according to Department of Preventive Veterinary Medicine, Faculty of Veterinary and Animal Sciences, Ziauddin University Karachi.
2.2. Experimental Plan
In total, 30 adult (female) mice were purchased from the local market in Karachi, Pakistan. The mice were initially weighed (20–25 g) and equally distributed into three groups (n = 10). The first group was kept as a Control (CON) with no treatment. The second group was given lipopolysaccharides (2.5 mg/kg) LPS, and the third group was supplemented (2.5 mg/kg) lipopolysaccharides with resveratrol (2 mg/kg) RES+LPS. Standard balanced rodent feed in pellet form was provided to all mice (Jiangsu Synergistic Pharmaceutical Bioengineering Co., Limited, Nanjing, China). Bottles of water were replenished to maintain appropriate cleanliness and procedures. All the mice were allowed to adjust to the normal environmental conditions up to one week before the experiment was conducted. The feed intake, respiratory rate, and rectal temperature were observed daily throughout the trial. The mice were sacrificed for sampling on day 15.
Chemicals
Lipopolysaccharide (LPS, 0111: B4) and resveratrol were purchased from (Sigma Chemical Co., St. Louis, MO, USA). To investigate LPS-induced inflammation in mice, 2.5 mg/kg LPS was injected (intraperitoneal route) [27], and 2.0 mg/kg resveratrol was sequentially directed through oral gavage up to 15 days [28].
2.3. Determination of Body Weight, Feed and Water Intake in Each Group of Mice
Body weight (g), feed consumption (g) and the drinking of water (mL) were monitored continuously during the whole experimental period [29].
2.4. Biochemical Analysis of Oxidative and Antioxidative Biomarkersin Mammary Tissue
The antioxidant assays from mammary tissue were determined using commercially available assay kits for MDA, T-AOC, CAT, GPX and SOD, which were purchased from the Nanjing Jian Cheng Institute of Bioengineering. The analysis was also carried out in accordance with a previously published publication [18]. Briefly, 50 mg of frozen mammary tissue was homogenized for 30 s on ice at rpm in 10 mL of homogenization buffer consisting of 0.9% cool physiological saline. After subsequent centrifugation the supernatant was further diluted to different concentrations using physiological saline (0.9%), and the samples were stored at −20 °C for the analysis of various antioxidants as outlined below.
2.4.1. Glutathione Peroxidase (GPX)
Glutathione peroxidase was analyzed spectrophotometrically (UV3600, Daojin Corp., Kyoto, Japan) at 412 nm using a commercially available kit (Nanjing Jiancheng Bioengineering Institute commercial kit, Nanjing, Jiangsu, China). To determine the decrease in GSH, the GSH’s rate of oxidation was measured within a set amount of time.
2.4.2. Superoxide Dismutase (SOD)
Superoxide dismutase was measured in the mammary tissue homogenate. A reactive system containing xanthine and xanthine oxidase makes up the SOD activity. Superoxide an ions oxidise hydroxylamine to produce nitrite at 550 nm, which was determined spectrophotometrically (UV3600, Daojin Corp., Kyoto, Japan). A sample’s ability to block the nitro blue tetrazolium reduction by 50% is considered to be one unit of SOD.
2.4.3. Catalase (CAT)
Using a spectrophotometer (UV3600, Daojin Corp., Kyoto, Japan), catalase was detected in the breast tissue at 405 nm. According to a previous study by Wang et al. [30], the catalase activity often depends on the drop in H2O2 concentration in 15s. The breakdown of one MOL of H2O2 in one second constitutes one unit of catalase activity.
2.4.4. Malondialdehyde (MDA)
Using commercially available kits, malondialdehyde concentrations were measured spectrophotometrically at 532 nm (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). MDA activity was measured by a previously described technique, the thiobarbituric acid reaction method described by Chen et al. [31].
2.4.5. Determination of Total Antioxidants (T-AOC)
Total antioxidant concentration (T-AOC) was measured using spectrophotometry. The reduction acts together with phenanthrene to produce a colored molecule that can be seen at 520 nm. The optical density is raised by 0.01 units of T-AOC per milligram of protein.
2.5. Radioimmunoassay
Plasma samples of mice were separated from blood, frozen and stored at −20 °C. IL-1, IL-6, and TNF-a concentrations were evaluated using radioimmunoassay kits according to manufacturer’s instructions (Beijing North Institute of Biological Sciences, Beijing, China).
2.6. Real-Time (qRT-PCR)
Total RNA Isolation and Quantitative Real-Time PCR
Each sample was homogenized with Tryzol for 15s and analyzed by absorption at 260 nm. The RNA was quantified by agarose gel electrophoresis and by nano-drop with spectrophotometer (Eppendorf Biotechnology, Hamburg, Germany). Samples with a ratio of A260-A280 and in a range of 1.8 to 2.1 were used in these experiments. First-strand cDNA was primed with a commercial kit, the Prime Script RT Master Mix Perfect Real Time Kit (Takara Co., Otsu, Japan) according to the manufacturer guidelines.
To quantify NF-κB, IL-1β, Jnk, IL-6, Erk, TNF-α and Nrf2 transcripts, the primer sequence for the specified genes and β-actin as a reference gene were derived from a previous study [31,32,33,34]. All the analyses were conducted in triplicate, and a gel electrophoresis was performed to confirm the particular PCR products on a 3.0% agarose gel. A detailed procedure for qRT-PCR was followed as described in previous research [2].
2.7. Western Blot Analysis
Total protein was isolated from 100 mg of glandular tissue sample and quantified by the Bradford protein assay (Bio-Rad, Hercules, CA, USA). Centrifugation at 14,000 rpm for 15 min was used to create the supernatant. A bicinchoninic acid (BCA) protein assay kit was used to measure the amount of protein in the sample (Beyotime, Shanghai, China). A 30 g sample of protein was electrophoretically separated on a 10% Bis-Tris Nu-PAGE gel before being transferred to PVDF membranes. The gel was transferred into the nitrocellulose membranes by means of a 100 V wet transfer device for 60 min after the electrophoresis process. The membrane blocking was performed for 2h in 10% bovine serum albumin (BSA) or 5–10% skimmed milk diluted in 1*TBST, followed by primary antibodies incubation (1:1000 dilution) at 4 °C for 12 to 14 h. The membranes were then washed thrice with TBST and incubated with a secondary antibody (1: dilution anti-rabbit or anti-goat IgG) conjugate-(HRP) at room temperature for 2 h. The washing step was repeated thrice, and the proteins were detected by chemiluminescence [35].
2.8. Statistical Analysis
One-way ANOVA was utilized for grouped analysis. Deviations were considered significant when p < 0.05. The visualization was created using Graph Pad 8.0 software (CA, USA). * p < 0.05, ** p < 0.005 LPS vs. CON.
3. Results
3.1. Comparison of Anthropometric Parameters
The mice body weight in LPS was markedly different from CON and RES groups, as described in Figure 1A. Variation in the food and water intake was observed in the LPS and RES groups in contrast to the CON group throughout the experimental period of 15 days (Figure 1B,C).
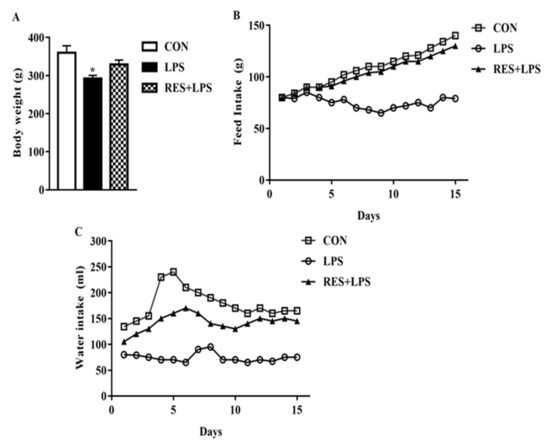
Figure 1.
(A) Body weight, (B) feed intake, and (C) water intake of Control (CON), Lipopolysaccharide (LPS) and Resveratrol (RES) groups. Levels were significantly decreased in the LPS-fed group compared with the CON and RES+LPS groups. No significant difference was observed for the CON vs. LPS+RES group comparison. * p < 0.05, LPS vs. CON.
3.2. MDA Production and T-AOC Capacity in the Plasma Samples of Female Mice
Malondialdehyde (MDA) level increased dramatically (p < 0.002; Figure 1A), whereas total antioxidant capacity (T-AOC) substantially declined (p < 0.04; Figure 1B) in the mammary tissue samples of mice in comparison with the CON group. Ultimately, these changes were totally reversed by resveratrol, as observed in the RES group in contrast to LPS. No significant changes were observed between the CON or RES groups (Figure 2).
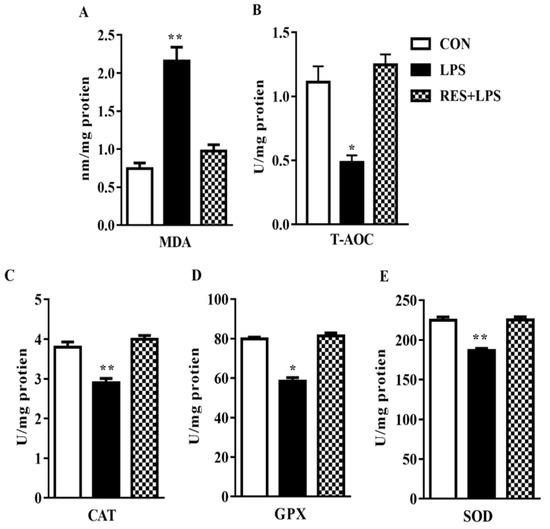
Figure 2.
The effect of resveratrol on antioxidant enzyme activity and total antioxidant capacity (A) Malondialdehyde (MDA) (B), Total Antioxidants (T-AOC) (C), Catalase (CAT) (D), Glutathione Peroxidase (GPX) and (E) Superoxide Dismutase (SOD) enzyme activities in the mammary tissue of female mice. * p < 0.05 significant and ** p < 0.005 highly significant.
3.3. Proinflammatory Cytokines Concentration in the Plasma Samples
The concentrations of proinflammatory cytokines in plasma samples were significantly increased in the LPS group in comparison with the CON group. However, these increased levels were reversed by resveratrol, as can be observed in the RES group vs LPS comparison (Figure 3).
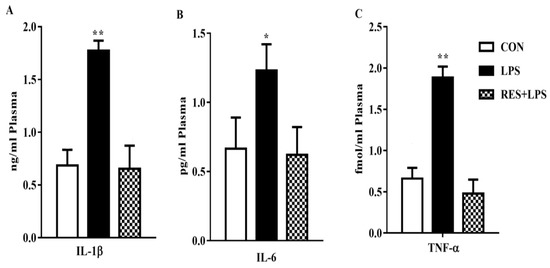
Figure 3.
Concentration of proinflammatory cytokines in mammary tissue of female mice: (A) IL-1β, (B) IL-6, and (C) TNF-α. * p < 0.05 significant and ** p < 0.005 highly significant.
3.4. The mRNA Levels of Proinflammatory Cytokines in Mammary Tissue of Female Mice
The mRNA expressions in mammary tissues were substantially upregulated by LPS in comparison with the CON group. Meanwhile, these mRNA levels were reversed by resveratrol, as observed in the comparison between the RES and LPS groups (Figure 4).
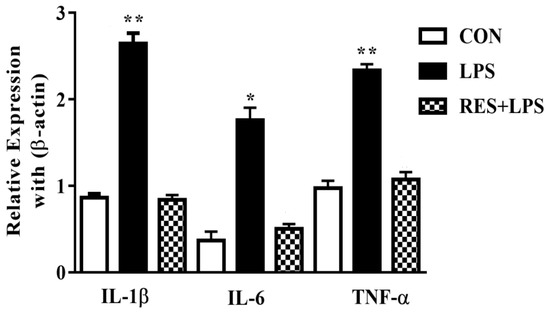
Figure 4.
The mRNA expressions of proinflammatory cytokines (IL-1β, IL-6 and TNF-α) in mammary tissue of female mice. * p < 0.05 significant and ** p < 0.005 highly significant.
3.5. The mRNAs Expression of NF-κB, JNK, ERK and Nrf2 in Mammary Tissue of Female Mice
The mRNA levels of adaptor/inflammatory molecules were remarkably increased by LPS in comparison with the CON and RES groups. However, the Nrf2 mRNA expressions (p < 0.002) were completely opposite in the LPS group compared with the CON and RES+LPS groups. The expressions of NF-κB and Nrf2 were dramatically reversed by resveratrol, as observed in RES in comparison with the LPS group. No significant differences were observed between the RES and CON groups (Figure 5).
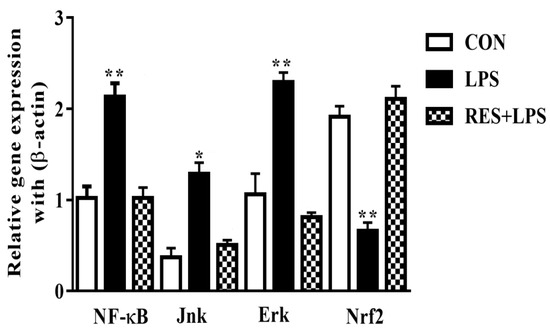
Figure 5.
The mRNA expressions of NF-κB, Jnk, Erk, and Nrf2 in mammary tissue of female mice. NF-κB, JNK, and ERK levels were significantly different in the LPS group, while the Nrf2 mRNA expressions were significantly different in the LPS group in contrast to the CON and LPS+RES groups. * p < 0.05 significant and ** p < 0.005 highly significant.
3.6. The Protein Expression of NF-κB (P65 and PP65) in Mammary Tissue
The pP65 (p < 0.003) protein intensity was remarkably increased in the LPS in comparison with the CON groups. Additionally, this intensity of P65 (p < 0.6) was significantly reduced in the RES group. However, the P65 intensity remained similar, and no remarkable changes were found in any of the groups (Figure 6A,B).
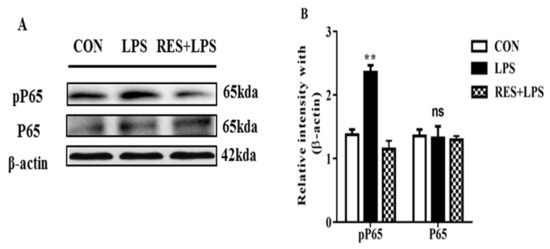
Figure 6.
(A) Immunoblots and (B) quantification of pP65 and P65 in LPS in comparison with CON and RES+LPS groups. ns: not significant and ** p < 0.005 highly significant.
4. Discussion
Lipopolysaccharide (LPS), a substance found in the outer membranes of Gram-negative bacteria, triggers several significant cellular reactions that are vital to the pathophysiology of inflammatory responses. An immediate inflammatory response to pathogens may result from LPS. As bacterial LPS causes a variety of cell types to generate inflammatory cytokines, including IL 6 and IL 1, it has been widely employed to create an inflammatory model. [31]. It has been observed that ROS plays a dynamic role in mastitis and its pathophysiology [36], in lung injury, and in cancers [37]. Pathogenic bacterial infections activate immune responses in host cells during mastitis, which leads to ROS production [38]. As reported previously, disproportionate amounts of ROS may cause cellular damage because of lipid oxidation [39]. Detection of MDA in plasma as an effect of oxidative lipid damage can provide comprehensive evidence about lipid oxidation, and higher levels of MDA production parallel to an imbalance of oxidants and antioxidants [40]. Consistent with a previous study, we also observed higher MDA production and T-AOC subordination in the LPS group [41]. It is clear that the augmented Nrf2 and its interrelated antioxidant levels could be linked to an adaptive response in the body prompted by LPS. Nrf2 is an important transcription factor as it sets the expression of several genes such as detoxification and antioxidant genes. When activated, it can increase the production of a variety of antioxidant enzymes and proteins, including glutathione, catalase, and superoxide dismutase, which can help protect cells from oxidative stress and inflammation. The innate immune system is highly boosted by LPS, which is detected by TLR4 and causes the immune system to respond. Auxiliary molecules such as LPS binding protein (LBP) and differentiation group 14 contribute to the recognition of LPS TLR4 (CD14) [42].The primary transcription factor which controls the production of cytokine genes is NF-κB.NF-κB is frequently present in cytoplasm with the inhibitor κB in an inactive form. It is released from NF-B-IκB after it has been phosphorylated [43].
In response to LPS being present in the blood, the immune system can trigger an immune response. This response may involve activation of immune cells which recognize and respond to LPS via specific receptors called toll receptors (TLRs) [44].A higher level of ROS causes release of NF-κB and increases cytokine concentration to initiate inflammatory responses [4,45]. Here, we found that NF-κB, JNK, ERK and pro-inflammatory cytokine mRNA levels were remarkably increased in the LPS group, an effect which was predominantly reversed by resveratrol, as indicated by the RES group data. As shown previously, during the inflammatory process, various transcriptional molecules, e.g., NF-κB, AP-1 and MAPK, are strongly regulated in a redox-dependent manner [46,47]. TNF is the first endogenous mediator of an inflammatory reaction and is essential for the acute phase response of inflammation as successive inflammatory reactions drive mammary epithelial cells to produce more TNF- and IL-1 [45]. These inflammatory factors can serve as indicators of the degree of inflammation. The amount of proinflammatory cytokines was remarkably higher in the LPS group, and these changes were reversed by resveratrol in the RES group. This contrasts with the view that resveratrol supplementation is a prospective approach against intestinal inflammation therapy because it acts as an anti-allergic, antioxidant and anti-inflammatory agent [21,22,28]. The increased number of cells such neutrophils, macrophages, and differentiated cells are some of the significant factors that contribute to the copious production of free radicals in mastitis milk [48]. Increased levels of TNF-, IL-1b, IL-6, and (NO) nitric oxide are another contributing cause [49]. Reactive nitrogen intermediates (RNI), which are involved in the complex process of inflammation, are activated by cytokines [50].
To maintain tissue integrity, Nrf2 dynamically defends cellular antioxidants against ROS [51]. In the regulation of target regions, Nrf2 binds with antioxidant response element (ARE) due to oxidative damage and activates several cytoprotective genes and antioxidants [52]. Decreases in Nrf2 and the mRNA concentrations of other antioxidants in the LPS group indicate suppression of the antioxidant system. Our results revealed that the downregulation of Nrf2 is because of higher amounts of oxidative stress, as Nrf2 protects cell and tissue damage, whereas a previous report found that P38 and JNK yield stress cause inflammation [43]. Moreover, Zhang et al. also revealed that stimulation of signaling pathways such as MAPK is associated with inflammatory effects and stress on the mammary glands of cows. When activated, MAPK can lead to increased cell proliferation and differentiation, which can be beneficial for mammary gland development and function [53]. It has been also reported that NF-κB is an LPS-mediated pro-inflammatory cytokines activator [54,55]. We also investigated the protein intensity of NF-κB. The protein density of pP65 was remarkably higher in LPS compared with the CON group. Additionally, the density of PP65 was significantly decreased in the RES group. All the groups showed no remarkable changes in P65 density. In this research, we have uncovered the defensive role of resveratrol in combating the adverse influences of LPS, such as the induction of oxidative damage and inflammation in the mammary tissue of female mice.
5. Conclusions
Taken together, our results reveal that resveratrol acts as a protective agent to modulate LPS-induced cellular injury and oxidative stress responses in the mammary tissue of female mice. This protection mainly occurs by combating the NF-κB and MAPK mechanisms, by Nrf2 signaling activation, thereby decreasing the proinflammatory cytokine production and improving the antioxidant levels. Importantly, uncovering the detailed mechanisms of action of resveratrol in the LPS-induced inflammatory animal model is essential in the future.
Author Contributions
M.U.U.H.M. and N.H. contributed to the conceptualization, data curation and formal analysis. Funding acquisition and methodology were supported by M.K., Z.u.A. and A.H.A. Project administration and data resources were contributed by R.S., R.A.A.-E., M.S.M. and N.A.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia, under the Supporting Researchers Project Number (PNURSP2023R249), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia. In addition, the authors thank Taif, and Tabuk Universities for their scientific contributions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lüderitz, O.; Galanos, C.; Rietschel, E. Lipopolysaccharides of gram-negative bacteria. In Current Topics in Membranes and Transport; Elsevier: Amsterdam, The Netherlands, 1982; pp. 79–151. [Google Scholar]
- Dai, H.; Liu, X.; Yan, J.; Aabdin, Z.U.; Bilal, M.S.; Shen, X. Sodium Butyrate Ameliorates High-Concentrate Diet-Induced Inflammation in the Rumen Epithelium of Dairy Goats. Can. J. Agric. Food Chem. 2017, 65, 596–604. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Meredith, T.C.; Kahne, D. On the essentiality of lipopolysaccharide to Gram-negative bacteria. Curr. Opin. Microbiol. 2013, 16, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Youn, G.S.; Lee, K.W.; Choi, S.Y.; Park, J. Overexpression of HDAC6 induces pro-inflammatory responses by regulating ROS-MAPK-NF-κB/AP-1 signaling pathways in macrophages. Free Radic. Biol. Med. 2016, 97, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.S.; Abaker, J.A.; Aabdin, Z.U.; Xu, T.; Dai, H.; Zhang, K.; Liu, X.; Shen, X. Lipopolysaccharide derived from the digestive tract triggers an inflammatory response in the uterus of mid-lactating dairy cows during SARA. BMC Vet. Res. 2016, 12, 284. [Google Scholar] [CrossRef]
- Roy, A.C.; Chang, G.; Ma, N.; Wang, Y.; Roy, S.; Liu, J.; Aabdin, Z.U.; Shen, X. Sodium butyrate suppresses NOD1-mediated inflammatory molecules expressed in bovine hepatocytes during iE-DAP and LPS treatment. J. Cell Physiol. 2019, 234, 19602–19620. [Google Scholar] [CrossRef]
- Cronin, J.G.; Turner, M.; Goetze, L.; Bryant, C.E.; Sheldon, I.M. Toll-like receptor 4 and MYD88-dependent signaling mechanisms of the innate immune system are essential for the response to lipopolysaccharide by epithelial and stromal cells of the bovine endometrium. Biol. Reprod. 2012, 86, 51. [Google Scholar] [CrossRef]
- Handy, D.E.; Loscalzo, J. Redox regulation of mitochondrial function. Antioxid. Redox Signal. 2012, 16, 1323–1367. [Google Scholar] [CrossRef]
- Liu, L.L. Oxidative Damage and Mechanism in Dairy Goats with Mastitis; Northeast Agricultural University: Harbin, China, 2007. [Google Scholar]
- Yin, B.-S.; Li, J.J.; Hu, S.H.; Cui, Y.Z.; Song, T. Study on Relationship between Free Radical Oxidative Damage and Cow Mastitis. Prog. Vet. Med. 2011, 10, 14. [Google Scholar]
- Grant, C.M. Metabolic reconfiguration is a regulated response to oxidative stress. J. Biol. 2008, 7, 1. [Google Scholar] [CrossRef]
- Drackley, J.K. Biology of dairy cows during the transition period: The final frontier? J. Dairy Sci. 1999, 82, 2259–2273. [Google Scholar] [CrossRef]
- Swaisgood, H.E. Enzymes indigenous to bovine milk. In Handbook of Milk Composition; Academic Press: New York, NY, USA, 1995; pp. 472–476. [Google Scholar]
- Abd Ellah, M. Role of free radicals and antioxidants in mastitis. J. Adv. Vet. Res. 2013, 3, 1–7. [Google Scholar]
- Braun, S.; Hanselmann, C.; Gassmann, M.G.; Keller, U.A.D.; Berclaz, C.B.; Chen, K.; Ken, Y.W.; Werner, S. Nrf2 transcription factor, a novel target of keratinocyte growth factor action which regulates gene expression and inflammation in the healing skin wound. Mol. Cell. Biol. 2002, 22, 5492–5505. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-L.; Dodd, G.; Thomas, S.; Zhang, X.; Wasserman, M.A.; Rovin, B.H.; Kunsch, C. Activation of Nrf2/ARE pathway protects endothelial cells from oxidant injury and inhibits inflammatory gene expression. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, H1862–H1870. [Google Scholar] [CrossRef] [PubMed]
- Arisawa, T.; Tahara, T.; Shibata, T.; Nagasaka, M.; Nakamura, M.; Kamiya, Y.; Fujita, H.; Hasegawa, S.; Takagi, T.; Wang, F.U.; et al. The relationship between Helicobacter pylori infection and promoter polymorphism of the Nrf2 gene in chronic gastritis. Int. J. Mol. Med. 2007, 19, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Abaker, J.A.; Bilal, M.S.; Dai, H.; Shen, X. Sodium butyrate improves antioxidant stability in sub-acute ruminal acidosis in dairy goats. BMC Vet. Res. 2018, 14, 275. [Google Scholar] [CrossRef]
- Kobashigawa, J.A.; Katznelson, S.; Laks, H.; Johnson, J.A.; Yeatman, L.; Wang, X.M.; Chia, D.; Terasaki, P.I.; Sabad, A.; Cogert, G.E. Effect of pravastatin on outcomes after cardiac transplantation. N. Engl. J. Med. 1995, 333, 621–627. [Google Scholar] [CrossRef]
- Cai, W.; Zhang, L.; Song, Y.; Zhang, B.; Cui, X.; Hu, G.; Fang, J. 3,4,4′-Trihydroxy-trans-stilbene, an analogue of resveratrol, is a potent antioxidant and cytotoxic agent. Free Radic. Res. 2011, 45, 1379–1387. [Google Scholar] [CrossRef]
- Baur, J.A.; Pearson, K.J.; Price, N.L.; Jamieson, H.A.; Lerin, C.; Kalra, A.; Prabhu, V.V.; Allard, J.S.; Lluch, G.L.; Lewis, K.; et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature 2006, 444, 337–342. [Google Scholar] [CrossRef]
- Bereswill, S.; Muñoz, M.; Fischer, A.; Plickert, R.; Haag, L.M.; Otto, B.; Kühl, A.A.; Loddenkemper, C.; Göbel, U.B.; Heimesaat, M.M. Anti-inflammatory effects of resveratrol, curcumin and simvastatin in acute small intestinal inflammation. PLoS ONE 2010, 5, e15099. [Google Scholar] [CrossRef]
- Aabdin, U.Z.; Bilal, M.S.; Dai, H.; Abaker, J.A.; Liu, X.; Benazir, S.; Yan, J.; Shen, X. NOD1/NF-kappaB signaling pathway inhibited by sodium butyrate in the mammary gland of lactating goats during sub-acute ruminal acidosis. Microb. Pathog. 2018, 122, 58–62. [Google Scholar] [CrossRef]
- Larrosa, M.; Yañéz-Gascón, M.J.; Selma, M.V.; González-Sarrías, A.; Toti, S.; Cerón, J.J.; Tomás-Barberán, F.; Dolara, P.; Espín, J.C. Effect of a low dose of dietary resveratrol on colon microbiota, inflammation and tissue damage in a DSS-induced colitis rat model. J. Agric. Food Chem. 2009, 57, 2211–2220. [Google Scholar] [CrossRef]
- Ma, Z.H.; Ma, Q.Y.; Wang, L.C.; Sha, H.C.; Wu, S.L.; Zhang, M. Effect of resveratrol on peritoneal macrophages in rats with severe acute pancreatitis. Inflamm. Res. 2005, 54, 522–527. [Google Scholar] [CrossRef] [PubMed]
- Elmali, N.; Baysal, O.; Harma, A.; Esenkaya, I.; Mizrak, B. Effects of resveratrol in inflammatory arthritis. Inflammation 2007, 30, 1–6. [Google Scholar] [CrossRef]
- Jarlhelt, I.; Genster, N.; Kirketerp-Møller, N.; Skjoedt, M.O.; Garred, P. The ficolin response to LPS challenge in mice. Mol. Immunol. 2019, 108, 121–127. [Google Scholar] [CrossRef]
- Mustafa, S.; Wei, Q.; Ennab, W.; Lv, Z.; Nazar, K.; Siyal, F.A.; Rodeni, S.; Kavita, N.M.X.; Shi, F. Resveratrol ameliorates testicular histopathology of mice exposed to restraint stress. Animals 2019, 9, 743. [Google Scholar] [CrossRef] [PubMed]
- Mehfooz, A.; Wei, Q.; Zheng, K.; Fadlalla, M.B.; Maltasic, G.; Shi, F. Protective roles of Rutin against restraint stress on spermatogenesis in testes of adult mice. Tissue Cell 2018, 50, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, L.J.; Zhu, F.X.; Zhu, J.Y.; Chen, X.D.; Zou, L.; Saito, M. In vitro and in vivo studies on the antioxidant activities of the aqueous extracts of Douchi (a traditional Chinese salt-fermented soybean food). Food Chem. 2008, 107, 1421–1428. [Google Scholar] [CrossRef]
- Chen, H.; Ma, N.; Song, X.; Wei, G.; Zhang, H.; Liu, J.; Shen, X.; Zhuge, X.; Chang, G. Protective Effects of N-Acetylcysteine on Lipopolysaccharide-Induced Respiratory Inflammation and Oxidative Stress. Antioxidants 2022, 11, 879. [Google Scholar] [CrossRef]
- Huang, J.; Liu, J.; Chang, G.; Wang, Y.; Ma, N.; Roy, A.C.; Shen, X. Glutamine Supplementation Attenuates the Inflammation Caused by LPS-Induced Acute Lung Injury in Mice by Regulating the TLR4/MAPK Signaling Pathway. Inflammation 2021, 44, 2180–2192. [Google Scholar] [CrossRef]
- Lu, H.; Lei, X.; Zhang, Q. Moderate activation of IKK2-NF-kB in unstressed adult mouse liver induces cytoprotective genes and lipogenesis without apparent signs of inflammation or fibrosis. BMC Gastroenterol. 2015, 15, 94. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Kwon, D.J.; Ju, S.M.; Youn, G.S.; Choi, S.Y.; Park, J. Suppression of iNOS and COX-2 expression by flavokawain A via blockade of NF-κB and AP-1 activation in RAW 264.7 macrophages. Food Chem. Toxicol. 2013, 58, 479–486. [Google Scholar] [CrossRef]
- Turk, R.; Piras, C.; Kovačić, M.; Samardžija, M.; Ahmed, H.; De-Canio, M.; Urbani, A.; Meštrić, Z.F.; Soggiu, A.; Bonizzi, L.; et al. Proteomics of inflammatory and oxidative stress response in cows with subclinical and clinical mastitis. J. Proteom. 2012, 75, 4412–4428. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Deng, S.; Yu, K.; Jiang, W.; Li, Y.; Wang, S.; Deng, Z.; Yao, Y.; Zhang, B.; Liu, G.; Liu, Y.; et al. Over-expression of Toll-like receptor 2 up-regulates heme oxygenase-1 expression and decreases oxidative injury in dairy goats. J. Anim. Sci. Biotechnol. 2017, 8, 3. [Google Scholar] [CrossRef]
- Min, B.; Ahn, D. Mechanism of lipid peroxidation in meat and meat products—A review. Food Sci. Biotechnol. 2005, 14, 152–163. [Google Scholar]
- Bouwstra, R.J.; Nielen, M.; Stegeman, J.A.; Dobbelaar, P.; Newbold, J.R.; Jansen, E.H.; Van-Werven, T. Vitamin E supplementation during the dry period in dairy cattle. Part I: Adverse effect on incidence of mastitis postpartum in a double-blind randomized field trial. J. Dairy Sci. 2010, 93, 5684–5695. [Google Scholar] [CrossRef]
- Abaker, J.; Xu, T.; Jin, D.; Chang, G.; Zhang, K.; Shen, X. Lipopolysaccharide derived from the digestive tract provokes oxidative stress in the liver of dairy cows fed a high-grain diet. J. Dairy Sci. 2017, 100, 666–678. [Google Scholar] [CrossRef]
- Sohn, M.-J.; Hur, G.M.; Byun, H.S.; Kim, W.G. Cyclo (dehydrohistidyl-l-tryptophyl) inhibits nitric oxide production by preventing the dimerization of inducible nitric oxide synthase. Biochem. Pharmacol. 2008, 75, 923–930. [Google Scholar] [CrossRef]
- Zhang, K.; Chang, G.; Xu, T.; Xu, L.; Guo, J.; Jin, D.; Shen, X. Lipopolysaccharide derived from the digestive tract activates inflammatory gene expression and inhibits casein synthesis in the mammary glands of lactating dairy cows. Oncotarget 2016, 7, 9652–9665. [Google Scholar] [CrossRef]
- Dong, G.; Liu, S.; Wu, Y.; Lei, C.; Zhou, J.; Zhang, S. Diet-induced bacterial immunogens in the gastrointestinal tract of dairy cows: Impacts on immunity and metabolism. Acta Vet. Scand. 2011, 53, 48. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, L.; Wei, Z.; Zhang, X.; Wang, Y.; Li, F.; Fu, Y.; Liu, B. Inhibition of histone deacetylase reduces lipopolysaccharide-induced-inflammation in primary mammary epithelial cells by regulating ROS-NF-κB signaling pathways. Int. Immunopharmacol. 2018, 56, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Gwinn, M.R.; Vallyathan, V. Respiratory burst: Role in signal transduction in alveolar macrophages. J. Toxicol. Environ. Health B Crit. Rev. 2006, 9, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Brady, S.T. Post-translational modifications of tubulin: Pathways to functional diversity of microtubules. Trends Cell Biol. 2015, 25, 125–136. [Google Scholar] [CrossRef]
- Knaapen, A.M.; Seiler, F.; Schilderman, P.A.; Nehls, P.; Bruch, J.; Schins, R.P.; Borm, P.J. Neutrophils cause oxidative DNA damage in alveolar epithelial cells. Free. Radic. Biol. Med. 1999, 27, 234–240. [Google Scholar] [CrossRef]
- Notebaert, S.; Demon, D.; Vanden-Berghe, T.; Vandenabeele, P.; Meyer, E. Inflammatory mediators in Escherichia coli-induced mastitis in mice. Comp. Immunol. Microbiol. Infect. Dis. 2008, 31, 551–565. [Google Scholar] [CrossRef]
- MacMicking, J.; Xie, Q.-W.; Nathan, C. Nitric oxide and macrophage function. Annu. Rev. Immunol. 1997, 15, 323–350. [Google Scholar] [CrossRef]
- Kansanen, E.; Kuosmanen, S.M.; Leinonen, H.; Levonen, A.L. The Keap1-Nrf2 pathway: Mechanisms of activation and dysregulation in cancer. Redox Biol. 2013, 1, 45–49. [Google Scholar] [CrossRef]
- Gessner, D.K.; Fiesel, A.; Most, E.; Dinges, J.; Wen, G.; Ringseis, R.; Eder, K. Supplementation of a grape seed and grape marc meal extract decreases activities of the oxidative stress-responsive transcription factors NF-κB and Nrf2 in the duodenal mucosa of pigs. Acta Vet. Scand. 2013, 55, 18. [Google Scholar] [CrossRef]
- Ki, Y.W.; Park, J.H.; Lee, J.E.; Shin, I.C.; Koh, H.C. JNK and p38 MAPK regulate oxidative stress and the inflammatory response in chlorpyrifos-induced apoptosis. Toxicol. Lett. 2013, 218, 235–245. [Google Scholar] [CrossRef]
- He, X.; Wei, Z.; Zhou, E.; Chen, L.; Kou, J.; Wang, J.; Yang, Z. Baicalein attenuates inflammatory responses by suppressing TLR4 mediated NF-κB and MAPK signaling pathways in LPS-induced mastitis in mice. Int. Immunopharmacol. 2015, 28, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Zhou, E.; Wei, Z.; Liang, D.; Wang, W.; Wang, T.; Guo, M.; Zhang, N.; Yang, Z. Glycyrrhizin inhibits the inflammatory response in mouse mammary epithelial cells and a mouse mastitis model. FEBS J. 2014, 281, 2543–2557. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).