High Frequency of Apodemus Mice Boosts Inverse Activity Pattern of Bank Voles, Clethrionomys glareolus, through Non-Aggressive Intraguild Competition
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Species Identification
2.2. Study Area
2.3. Camera Trap Study
2.3.1. Study Period
2.3.2. Type and Model
2.3.3. Modifications
2.3.4. Recordings
2.3.5. Definition of an Event
2.3.6. Bait
2.3.7. Layout
2.3.8. Spatiotemporal Independence
2.3.9. Camera Trap Placement
2.4. Data Treatment
2.5. Predictors
2.6. Statistical Analysis
3. Results
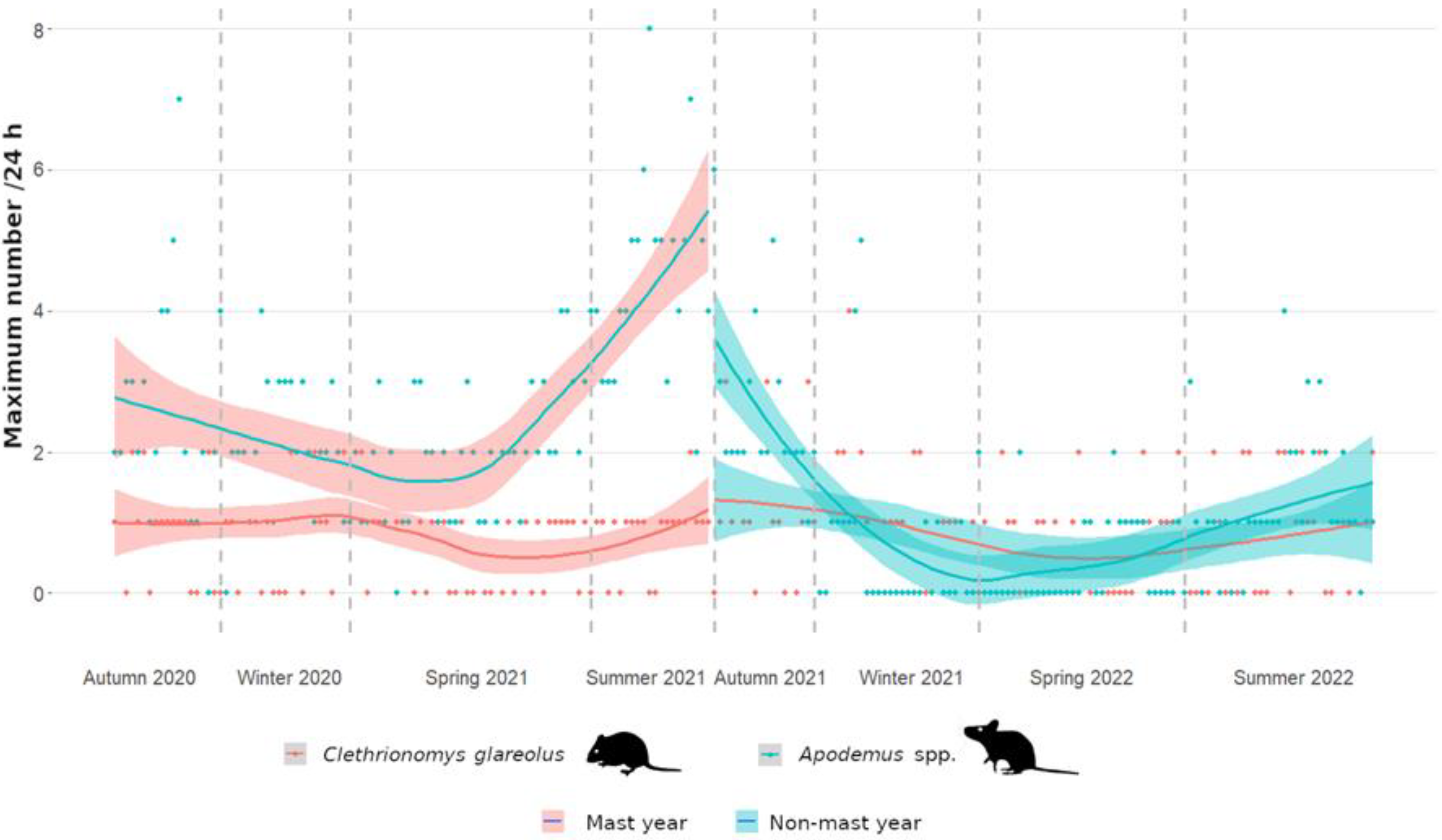
3.1. Frequency of Small Forest Rodents
3.2. Activity Patterns of Small Forest Rodents
3.3. Temporal Niche of Bank Voles
3.4. Intraguild Aggression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kryštufek, B.; Tesakov, A.S.; Lebedev, V.S.; Bannikova, A.A.; Abramson, N.I.; Shenbrot, G. Back to the future: The proper name for red-backed voles is Clethrionomys Tilesius and not Myodes Pallas. Mammalia 2020, 84, 214–217. [Google Scholar] [CrossRef]
- Vickery, W.L.; Bider, J.R. The influence of weather on rodent activity. J. Mammal. 1981, 62, 140–145. [Google Scholar] [CrossRef]
- Wróbel, A.; Bogdziewicz, M. It is raining mice and voles: Which weather conditions influence the activity of Apodemus flavicollis and Myodes glareolus? Eur. J. Wildl. Res. 2015, 61, 475–478. [Google Scholar] [CrossRef]
- Viviano, A.; Scarfò, M.; Mori, E. Temporal partitioning between forest-dwelling small rodents in a mediterranean deciduous woodland. Animals 2022, 12, 279. [Google Scholar] [CrossRef]
- Orrock, J.L.; Danielson, B.J. Temperature and cloud cover, but not predator urine, affect winter foraging of mice. Ethology 2009, 115, 641–648. [Google Scholar] [CrossRef]
- Hernández, M.C.; Navarro-Castilla, Á.; Wilsterman, K.; Bentley, G.E.; Barja, I. When food access is challenging: Evidence of wood mice ability to balance energy budget under predation risk and physiological stress reactions. Behav. Ecol. Sociobiol. 2019, 73, 145. [Google Scholar] [CrossRef]
- Butet, A.; Delettre, Y.R. Diet differentiation between European arvicoline and murine rodents. Acta Theriol. 2011, 56, 297–304. [Google Scholar] [CrossRef]
- Jędrzejewski, W.; Rychlik, L.; Jędrzejewska, B. Responses of bank voles to odours of seven species of predators: Experimental data and their relevance to natural predator-vole relationships. Oikos 1993, 68, 251–257. [Google Scholar] [CrossRef]
- Amori, G.; Castigliani, V.; Locasciulli, O.; Luiselli, L. Long-term density fluctuations and microhabitat use of sympatric Apodemus flavicollis and Myodes glareolus in central Italy. Community Ecol. 2015, 16, 196–205. [Google Scholar] [CrossRef]
- Pucek, Z.; Jędrzejewski, W.; Jędrzejewska, B.; Pucek, M. Rodent population dynamics in a primeval deciduous forest (Białowieża National Park) in relation to weather, seed crop, and predation. Acta Theriol. 1993, 38, 199–232. [Google Scholar] [CrossRef]
- Eccard, J.A.; Ylönen, H. Interspecific competition in small Rodents: From populations to individuals. Evol. Ecol. 2003, 17, 423–440. [Google Scholar] [CrossRef]
- Andreassen, H.P.; Sundell, J.; Ecke, F.; Halle, S.; Haapakoski, M.; Henttonen, H.; Huitu, O.; Jacob, J.; Johnsen, K.; Koskela, E.; et al. Population cycles and outbreaks of small rodents: Ten essential questions we still need to solve. Oecologia 2021, 195, 601–622. [Google Scholar] [CrossRef] [PubMed]
- Grant, P.R. Interspecific competition among rodents. Annu. Rev. Ecol. Syst. 1972, 3, 79–106. [Google Scholar] [CrossRef]
- Schoener, T.W. Field experiments on interspecific competition. Am. Nat. 1983, 122, 240–285. [Google Scholar] [CrossRef]
- Halle, S.; Stenseth, N.C. Chronoecology: New light through old windows—A conclusion. In Acitivity Patterns in Small Mammals. An Ecological Approach; Halle, S., Stenseth, N.C., Eds.; Springer: Berlin/Heidelberg, Germany, 2000; pp. 273–284. [Google Scholar]
- Schoener, T.W. Resource Partitioning in ecological communities: Research on how similar species divide resources helps reveal the natural regulation of species diversity. Science 1974, 185, 27–39. [Google Scholar] [CrossRef]
- Baláž, I.; Jakab, I.; Tulis, F.; Ambros, M. Spatial density of two sympatric species yellow-necked mouse Apodemus flavicollis and bank vole Clethrionomys glareolus in different environment. Folia Oecologica 2016, 43, 121–128. [Google Scholar]
- Hille, S.M.; Mortelliti, A. Microhabitat partitioning of Apodemus flavicollis and Myodes glareolus in the sub-montane Alps: A preliminary assessment. Hystrix 2011, 21, 157–163. [Google Scholar] [CrossRef]
- Buesching, C.D.; Newman, C.; Twell, R.; Macdonald, D.W. Reasons for arboreality in wood mice Apodemus sylvaticus and bank voles Myodes glareolus. Mammal. Biol. 2008, 73, 318–324. [Google Scholar] [CrossRef]
- Andrzejewski, R.; Olszewski, J. Social behaviour and interspecific relations in Apodemus flavicollis (Melchior, 1834) and Clethrionomys glareolus (Schreber, 1780). Acta Theriol. 1963, 10, 155–168. [Google Scholar] [CrossRef]
- Greenwood, P.J. Timing of activity of the bank vole Clethrionomys glareolus and the wood mouse Apodemus sylvaticus in a deciduous woodland. Oikos 1978, 31, 123–127. [Google Scholar] [CrossRef]
- Sozio, G.; Mortelliti, A. Empirical evaluation of the strength of interspecific competition in shaping small mammal communities in fragmented landscapes. Landsc. Ecol. 2016, 31, 775–789. [Google Scholar] [CrossRef]
- Park, O. Nocturnalism—The development of a problem. Ecol. Monogr. 1940, 10, 485–536. [Google Scholar] [CrossRef]
- Carothers, J.H.; Jaksic, F.M. Time as a niche difference: The role of interference competition. Oikos 1984, 42, 403–406. [Google Scholar] [CrossRef]
- Grüm, L.; Bujalska, G. Bank voles and yellow-necked mice: What are interrelations between them? Pol. J. Ecol. 2000, 48, 141–145. [Google Scholar]
- Casula, P.; Luiselli, L.; Amori, G. Which population density affects home ranges of co-occurring rodents? Basic Appl. Ecol. 2019, 34, 46–54. [Google Scholar] [CrossRef]
- Bartolommei, P.; Gasperini, S.; Bonacchi, A.; Manzo, E.; Cozzolino, R. Multiple Captures as indicator of social tolerance in a guild of terrestrial rodents. Mammal. Biol. 2018, 93, 169–172. [Google Scholar] [CrossRef]
- Niethammer, J.; Krapp, F. Handbuch der Säugetiere Europas: Sciuridae, Castoridae, Gliridae, Muridae; Akademische Verlagsgesellschaft: Wiesbaden, Germany, 1978. [Google Scholar]
- Niethammer, J.; Krapp, F. Handbuch der Säugetiere Europas: Cricetidae, Arvicolidae, Zapodidae, Spalacidae, Hystricidae, Capromyidae; Akademische Verlagsgesellschaft: Wiesbaden, Germany, 1982. [Google Scholar]
- Caravaggi, A.; Banks, P.B.; Burton, A.C.; Finlay, C.M.V.; Haswell, P.M.; Hayward, M.W.; Rowcliffe, M.J.; Wood, M.D. A Review of camera Trapping for conservation behaviour research. Remote Sens. Ecol. Conserv. 2017, 3, 109–122. [Google Scholar] [CrossRef]
- Rovero, F.; Zimmermann, F. Camera Trapping for Wildlife Research; Pelagic Publishing: Exeter, UK, 2016. [Google Scholar]
- Lashley, M.A.; Cove, M.V.; Chitwood, M.C.; Penido, G.; Gardner, B.; DePerno, C.S.; Moorman, C.E. Estimating wildlife activity curves: Comparison of methods and sample size. Sci. Rep. 2018, 8, 4173. [Google Scholar] [CrossRef]
- Caravaggi, A.; Gatta, M.; Vallely, M.-C.; Hogg, K.; Freeman, M.; Fadaei, E.; Dick, J.T.A.; Montgomery, W.I.; Reid, N.; Tosh, D.G. Seasonal and predator-prey effects on circadian activity of free-ranging mammals revealed by camera traps. PeerJ 2018, 6, e5827. [Google Scholar] [CrossRef]
- Nardotto, A. Living with the enemy: Activity rhythms of the red fox and some potential preys in an urban environment. Nat. Hist. Sci. 2022, 9, 63–66. [Google Scholar] [CrossRef]
- Wearn, O.R.; Glover-Kapfer, P. Snap happy: Camera traps are an effective sampling tool when compared with alternative methods. Royal Soc. Open Sci. 2019, 6, 181748. [Google Scholar] [CrossRef]
- Mills, C.A.; Godley, B.J.; Hodgson, D.J. Take only photographs, leave only footprints: Novel applications of non-invasive survey methods for rapid detection of small, arboreal animals. PLoS ONE 2016, 11, e0146142. [Google Scholar] [CrossRef]
- Ostfeld, R.S.; Keesing, F. Pulsed resources and community dynamics of consumers in terrestrial ecosystems. Trends Ecol. Evol. 2000, 15, 232–237. [Google Scholar] [CrossRef]
- Yang, L.H.; Bastow, J.L.; Spence, K.O.; Wright, A.N. What can we learn from resource pulses? Ecology 2008, 89, 621–634. [Google Scholar] [CrossRef]
- Yang, L.H.; Edwards, K.F.; Byrnes, J.E.; Bastow, J.L.; Wright, A.N.; Spence, K.O. A meta-analysis of resource pulse–consumer interactions. Ecol. Monogr. 2010, 80, 125–151. [Google Scholar] [CrossRef]
- Hansson, L.; Jędrzejewska, B.; Jędrzejewski, W. Regional differences in dynamics of bank vole populations in Europe. Pol. J. Ecol. 2000, 48, 163–177. [Google Scholar]
- Selva, N.; Hobson, K.A.; Cortés-Avizanda, A.; Zalewski, A.; Donázar, J.A. Mast pulses shape trophic interactions between fluctuating rodent populations in a primeval forest. PLoS ONE 2012, 7, e51267. [Google Scholar] [CrossRef] [PubMed]
- Mazurkiewicz, M. Population dynamics and demography of the bank vole in different tree stands. Acta Theriol. 1991, 36, 207–227. [Google Scholar] [CrossRef]
- Zwander, V.H.; Aigner, S.; Koll, H. Der Pollenflug in Kärnten im Jahr 2020. Carinthia II 2021, 211, 163–177. [Google Scholar]
- Geburek, T.; Hiess, K.; Litschauer, R.; Milasowszky, N. Temporal pollen pattern in temperate trees: Expedience or fate? Oikos 2012, 121, 1603–1612. [Google Scholar] [CrossRef]
- Kasprzyk, I.; Ortyl, B.; Dulska-Jeż, A. Relationships among weather parameters, airborne pollen and seed crops of Fagus and Quercus in Poland. Agric. For. Meteorol. 2014, 197, 111–122. [Google Scholar] [CrossRef]
- Reil, D.; Imholt, C.; Eccard, J.A.; Jacob, J. Beech fructification and bank vole population dynamics—Combined analyses of promoters of human Puumala virus infections in Germany. PLoS ONE 2015, 10, e0134124. [Google Scholar] [CrossRef] [PubMed]
- Ascari, L.; Siniscalco, C.; Palestini, G.; Lisperguer, M.J.; Suarez Huerta, E.; De Gregorio, T.; Bregaglio, S. Relationships between yield and pollen concentrations in Chilean hazelnut orchards. Eur. J. Agron. 2020, 115, 126036. [Google Scholar] [CrossRef]
- Lanszki, J.; Zalewski, A.; Horváth, G. Comparison of red fox Vulpes vulpes and pine marten Martes martes food habits in a deciduous forest in Hungary. Wildlife Biol. 2007, 13, 258–271. [Google Scholar] [CrossRef]
- Luka, V.; Riegert, J. Apodemus mice as the main prey that determines reproductive output of tawny owl (Strix aluco) in central Europe. Popul. Ecol. 2018, 60, 237–249. [Google Scholar] [CrossRef]
- Jensen, T.S. Seed-seed predator interactions of European beech, Fagus silvatica and forest rodents, Clethrionomys glareolus and Apodemus flavicollis. Oikos 1985, 44, 149–156. [Google Scholar] [CrossRef]
- Nopp-Mayr, U.; Kempter, I.; Muralt, G.; Gratzer, G. Seed survival on experimental dishes in a central European old-growth mixed-species forest—Effects of predator guilds, tree masting and small mammal population dynamics. Oikos 2012, 121, 337–346. [Google Scholar] [CrossRef]
- Zwolak, R.; Crone, E.E. Quantifying the outcome of plant-granivore interactions. Oikos 2012, 121, 20–27. [Google Scholar] [CrossRef]
- Imholt, C.; Reil, D.; Plašil, P.; Rödiger, K.; Jacob, J. Long-term population patterns of rodents and associated damage in German forestry. Pest Manag. Sci. 2017, 73, 332–340. [Google Scholar] [CrossRef]
- Suchomel, J.; Šipoš, J.; Ouředníčková, J.; Skalský, M.; Heroldová, M. Bark gnawing by rodents in orchards during the growing season—Can we detect relation with forest damages? Agronomy 2022, 12, 251. [Google Scholar] [CrossRef]
- Heyman, P.; Mele, R.V.; Smajlovic, L.; Dobly, A.; Cochez, C.; Vandenvelde, C. Association between habitat and prevalence of Hantavirus infections in bank voles (Myodes glareolus) and wood mice (Apodemus sylvaticus). Vector Borne Zoonotic Dis. 2009, 9, 141–146. [Google Scholar] [CrossRef]
- Zöldi, V.; Papp, T.; Reiczigel, J.; Egyed, L. Bank voles show high seropositivity rates in a natural TBEV focus in Hungary. Infect. Dis. 2015, 47, 178–181. [Google Scholar] [CrossRef]
- Schmitt, M. Das Apodemus-Problem. Eulen Rundblick 2021, 71, 127–129. [Google Scholar]
- Dubey, S.; Michaux, J.; Brünner, H.; Hutterer, R.; Vogel, P. False phylogenies on wood mice due to cryptic cytochrome-b pseudogene. Mol. Phylogenet. Evol. 2009, 50, 633–641. [Google Scholar] [CrossRef]
- Groenenberg, D.S.J.; Dekker, R.W.R.J. A mouse’s tail: How to settle an insurance dispute. Forensic Sci. Int. 2011, 207, e24–e27. [Google Scholar] [CrossRef]
- Martin Cerezo, M.L.; Kucka, M.; Zub, K.; Chan, Y.F.; Bryk, J. Population structure of Apodemus flavicollis and comparison to Apodemus sylvaticus in northern Poland based on RAD-Seq. BMC Genom. 2020, 21, 241. [Google Scholar] [CrossRef] [PubMed]
- Ancillotto, L.; Mori, E.; Sozio, G.; Solano, E.; Bertolino, S.; Russo, D. A novel approach to field identification of cryptic Apodemus wood mice: Calls differ more than morphology. Mamm. Rev. 2017, 47, 6–10. [Google Scholar] [CrossRef]
- Gasperini, S.; Mortelliti, A.; Bartolommei, P.; Bonacchi, A.; Manzo, E.; Cozzolino, R. Effects of forest management on density and survival in three forest rodent species. For. Ecol. Manag. 2016, 382, 151–160. [Google Scholar] [CrossRef]
- Spitzenberger, F. Die Säugetierfauna Österreichs; Grüne Reihe des Bundesministeriums für Land- und Forstwirtschaft, Umwelt und Wasserwirtschaft: Graz, Austria, 2001. [Google Scholar]
- Willner, W.; Grabherr, G. Die Wälder und Gebüsche Österreichs: Ein Bestimmungswerk mit Tabellen; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Soné, K.; Kohno, A. Acorn hoarding by the field Mouse, Apodemus speciosus Temminck (Rodentia: Muridae). J. For. Res. 1999, 4, 167–175. [Google Scholar] [CrossRef]
- Meek, P.D.; Ballard, G.; Claridge, A.; Kays, R.; Moseby, K.; O’Brien, T.; O’Connell, A.; Sanderson, J.; Swann, D.E.; Tobler, M.; et al. Recommended guiding principles for reporting on camera trapping research. Biodivers. Conserv. 2014, 23, 2321–2343. [Google Scholar] [CrossRef]
- Rooney, S.M.; Wolfe, A.; Hayden, T.J. Autocorrelated data in telemetry studies: Time to independence and the problem of behavioural effects. Mamm. Rev. 1998, 28, 89–98. [Google Scholar] [CrossRef]
- Korn, H. Changes in home range size during growth and maturation of the wood mouse (Apodemus syIvaticus) and the bank vole (Clethrionomys glareolus). Oecologia 1986, 68, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Mori, E.; Sangiovanni, G.; Corlatti, L. Gimme Shelter: The effect of rocks and moonlight on occupancy and activity pattern of an endangered rodent, the garden dormouse Eliomys quercinus. Behav. Process. 2020, 170, 103999. [Google Scholar] [CrossRef] [PubMed]
- Lešo, P.; Lešová, A.; Kropil, R.; Kaňuch, P. Response of the dominant rodent species to close-to-nature logging practices in a temperate mixed forest. Ann. For. Res. 2016, 59, 259–268. [Google Scholar] [CrossRef]
- Bowman, J.C.; Sleep, D.; Forbes, G.J.; Edwards, M. The association of small mammals with coarse woody debris at log and stand scales. For. Ecol. Manag. 2000, 129, 119–124. [Google Scholar] [CrossRef]
- Martinez, R.; Morato, S. Thigmotaxis and exploration in adult and pup rats. Rev. Etol. 2004, 6, 49–54. [Google Scholar]
- Fitak, R.R.; Johnsen, S. Bringing the analysis of animal orientation data full circle: Model-based approaches with maximum likelihood. J. Exp. Biol. 2017, 220, 3878–3882. [Google Scholar] [CrossRef]
- Landler, L.; Ruxton, G.D.; Malkemper, E.P. The Hermans–Rasson test as a powerful alternative to the Rayleigh test for circular statistics in biology. BMC Ecol. 2019, 19, 30. [Google Scholar] [CrossRef]
- Ridout, M.S.; Linkie, M. Estimating Overlap of Daily Activity Patterns from Camera Trap Data. J. Agric. Biol. Environ. Stat. 2009, 14, 322–337. [Google Scholar] [CrossRef]
- Meredith, M.; Ridout, M. Overview of the Overlap Package. 2021. Available online: https://cran.r-project.org/web/packages/overlap/vignettes/overlap.pdf (accessed on 14 December 2022).
- Mazza, G.; Marraccini, D.; Mori, E.; Priori, S.; Marianelli, L.; Roversi, P.F.; Gargani, E. Assessment of color response and activity rhythms of the invasive black planthopper Ricania speculum (Walker, 1851) using sticky traps. Bull. Entomol. Res. 2020, 110, 480–486. [Google Scholar] [CrossRef]
- Finke, D.L.; Snyder, W.E. Niche partitioning increases resource exploitation by diverse communities. Science 2008, 321, 1488–1490. [Google Scholar] [CrossRef] [PubMed]
- Levine, S.H. Competitive interactions in ecosystems. Am. Nat. 1976, 110, 903–910. [Google Scholar] [CrossRef]
- Savić, I.; Todorović, M.; Mikeš, M. Teriološka istraživanja u stacionarnim šumskim ekosistemima i agrobiocenozama. Ekologija 1976, 11, 167–179. [Google Scholar]
- Gurnell, J. Woodland rodents communities. In The Ecology of Woodland Rodents; Flowerdew, J.R., Gurnell, J., Gipps, J.H.W., Eds.; Symposia of the Zoological Society of London 55; Oxford University Press: Oxford, UK, 1985; pp. 377–411. [Google Scholar]
- Brown, L.E. Field experiments on the activity of the small mammals, Apodemus, Clethrionomys and Microtus. Proc. Zool. Soc. Lond. 1956, 126, 549–564. [Google Scholar] [CrossRef]
- Wójcik, J.M.; Wołk, K. The daily activity rhythm of two competitive rodents: Clethrionomys glareolus and Apodemus flavicollis. Acta Theriol. 1985, 30, 241–258. [Google Scholar] [CrossRef]
- Kikkawa, J. Movement, activity and distribution of the small rodents Clethrionomys glareolus and Apodemus sylvaticus in woodland. J. Anim. Ecol. 1964, 33, 259–299. [Google Scholar] [CrossRef]
- Bergstedt, B. Distribution, Reproduction, growth and dynamics of the rodent species Clethrionomys glareolus (Schreber), Apodemus flavicollis (Melchior) and Apodemus sylvaticus (Linné) in southern Sweden. Oikos 1965, 16, 132–160. [Google Scholar] [CrossRef]
- Miller, R.S.; Elton, C. Activity rhythms in the wood mouse, Apodemus sylvaticus and the bank vole, Clethrionomys glareolus. Proc. Zool. Soc. Lond. 1955, 125, 505–519. [Google Scholar] [CrossRef]
- Grodzinski, W. Seasonal changes in the circadian activity of small rodents. Ekol. Polska Ser. B 1963, 9, 3–17. [Google Scholar]
- Ashby, K.R. Patterns of daily activity in mammals. Mamm. Rev. 1972, 1, 171–185. [Google Scholar] [CrossRef]
- Dell’omo, G.; Shore, R.F.; Lipp, H.-P. An automated system, based on microchips, for monitoring individual activity in wild small mammals. J. Exp. Zool. 1998, 280, 97–99. [Google Scholar] [CrossRef]
- Yoccoz, N.G.; Mesnager, S.; Mesnager, S. Are alpine bank voles larger and more sexually dimorphic because adults survive better? Oikos 1998, 82, 85–98. [Google Scholar] [CrossRef]
- Connell, J.H. On the prevalence and relative importance of interspecific competition: Evidence from field experiments. Am. Nat. 1983, 122, 661–696. [Google Scholar] [CrossRef]
- Molsher, R.L.; Gifford, E.J.; McIlroy, J.C. Temporal, spatial and individual variation in the diet of red foxes (Vulpes vulpes) in central New South Wales. Wildl. Res. 2000, 27, 593–601. [Google Scholar] [CrossRef]
- Orrock, J.L. Rodent foraging is affected by indirect, but not by direct, cues of predation risk. Behav. Ecol. 2004, 15, 433–437. [Google Scholar] [CrossRef]
- Kołakowski, M.; Jancewicz, E.; Kielan, E. Czasowa i przestrzenna aktywność nornicy rudej Myodes (Clethrionomys) glareolus i myszy leśnej Apodemus flavicollis w siedlisku grądowym Puszczy Białowieskiej. Sylwan 2018, 162, 1029–1037. [Google Scholar]
- Montgomery, W.I. Seasonal variation in numbers of Apodemus sylvaticus, A. flavicollis and Clethrionomys glareolus. J. Zool. 1979, 188, 283–286. [Google Scholar] [CrossRef]
- Montgomery, W.I. The use of arboreal runways by the woodland rodents, Apodemus sylvaticus (L.), A. flavicollis (Melchior) and Clethrionomys glareolus (Schreber). Mamm. Rev. 1980, 10, 189–195. [Google Scholar] [CrossRef]
- Fasola, M.; Canova, L. Asymmetrical competition between the bank Vole and the wood Mouse, a removal experiment. Acta Theriol. 2000, 45, 353–365. [Google Scholar] [CrossRef]
- Apfelbach, R.; Blanchard, C.D.; Blanchard, R.J.; Hayes, R.A.; McGregor, I.S. The effects of predator odors in mammalian prey species: A review of field and laboratory studies. Neurosci. Biobehav. Rev. 2005, 29, 1123–1144. [Google Scholar] [CrossRef]
- Navarro-Castilla, Á.; Barja, I. Does predation risk, through moon phase and predator cues, modulate food intake, antipredatory and physiological responses in wood mice (Apodemus sylvaticus)? Behav. Ecol. Sociobiol. 2014, 68, 1505–1512. [Google Scholar] [CrossRef]
- Navarro-Castilla, Á.; Barja, I.; Díaz, M. Foraging, feeding, and physiological stress responses of wild wood mice to increased illumination and common genet cues. Curr. Zool. 2018, 64, 409–417. [Google Scholar] [CrossRef]
- Randler, C.; Kalb, J. Predator avoidance behavior of nocturnal and diurnal rodents. Behav. Process. 2020, 179, 10421. [Google Scholar] [CrossRef] [PubMed]
- Gasperini, S.; Bonacchi, A.; Bartolommei, P.; Manzo, E.; Cozzolino, R. Seasonal cravings: Plant food preferences of syntopic small mammals. Ethol. Ecol. Evol. 2018, 30, 12–25. [Google Scholar] [CrossRef]
- Canova, L. Resource partitioning between the bank vole Clethrionomys glareolus and the wood mouse Apodemus sylvaticus in woodland habitats. Ital. J. Zool. 1993, 60, 193–198. [Google Scholar] [CrossRef]
- Daan, S.; Aschoff, J. Circadian contributions to survival. In Vertebrate Circadian Systems; Aschoff, J., Daan, S., Groos, G.A., Eds.; Springer: Berlin/Heidelberg, Germany, 1982; pp. 305–321. [Google Scholar]
- Halle, S. Diel pattern of predation risk in microtine rodents. Oikos 1993, 68, 510–518. [Google Scholar] [CrossRef]
| Estimate | Standard Error | Z-Score | p-Values | Odds Ratio | 2.50% | 97.50% | |
|---|---|---|---|---|---|---|---|
| (Intercept) | 0.186 | 0.163 | 1.139 | 0.255 | 1.205 | 0.874 | 1.660 |
| Non-mast | 0.845 | 0.132 | 6.426 | <0.001 | 2.329 | 1.801 | 3.017 |
| Lunar Illumination | −0.003 | 0.001 | −1.949 | 0.051 | 0.997 | 0.994 | 1.000 |
| TemperatureMin | 0.002 | 0.008 | 0.217 | 0.828 | 1.002 | 0.987 | 1.017 |
| PrecipitationNight | −0.07 | 0.020 | −3.551 | <0.001 | 0.932 | 0.896 | 0.969 |
| Predators | −0.219 | 0.273 | −0.802 | 0.423 | 0.803 | 0.470 | 1.374 |
| Cover | −0.061 | 0.049 | −1.243 | 0.214 | 0.940 | 0.853 | 1.036 |
| Apodemus spp. | −0.009 | 0.005 | −1.884 | 0.060 | 0.992 | 0.983 | 1.000 |
| Nagelkerkes R2 = 0.083 | |||||||
| No Aggression | Avoidance | Chase | Total | |
|---|---|---|---|---|
| Mast Year | 74 (43.0%) | 77 (44.8%) | 21 (12.2%) | 172 (49.1%) |
| Non-mast Year | 52 (29.2%) | 99 (55.6%) | 27 (15.1%) | 178 (50.9%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Probst, R.; Probst, R. High Frequency of Apodemus Mice Boosts Inverse Activity Pattern of Bank Voles, Clethrionomys glareolus, through Non-Aggressive Intraguild Competition. Animals 2023, 13, 981. https://doi.org/10.3390/ani13060981
Probst R, Probst R. High Frequency of Apodemus Mice Boosts Inverse Activity Pattern of Bank Voles, Clethrionomys glareolus, through Non-Aggressive Intraguild Competition. Animals. 2023; 13(6):981. https://doi.org/10.3390/ani13060981
Chicago/Turabian StyleProbst, Remo, and Renate Probst. 2023. "High Frequency of Apodemus Mice Boosts Inverse Activity Pattern of Bank Voles, Clethrionomys glareolus, through Non-Aggressive Intraguild Competition" Animals 13, no. 6: 981. https://doi.org/10.3390/ani13060981
APA StyleProbst, R., & Probst, R. (2023). High Frequency of Apodemus Mice Boosts Inverse Activity Pattern of Bank Voles, Clethrionomys glareolus, through Non-Aggressive Intraguild Competition. Animals, 13(6), 981. https://doi.org/10.3390/ani13060981





