Anatomical Description of Rhinoceros Iguana (Cyclura cornuta cornuta) Head by Computed Tomography, Magnetic Resonance Imaging and Gross-Sections
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Anatomic Evaluation
2.3. CT Technique
2.4. MRI Technique
3. Results
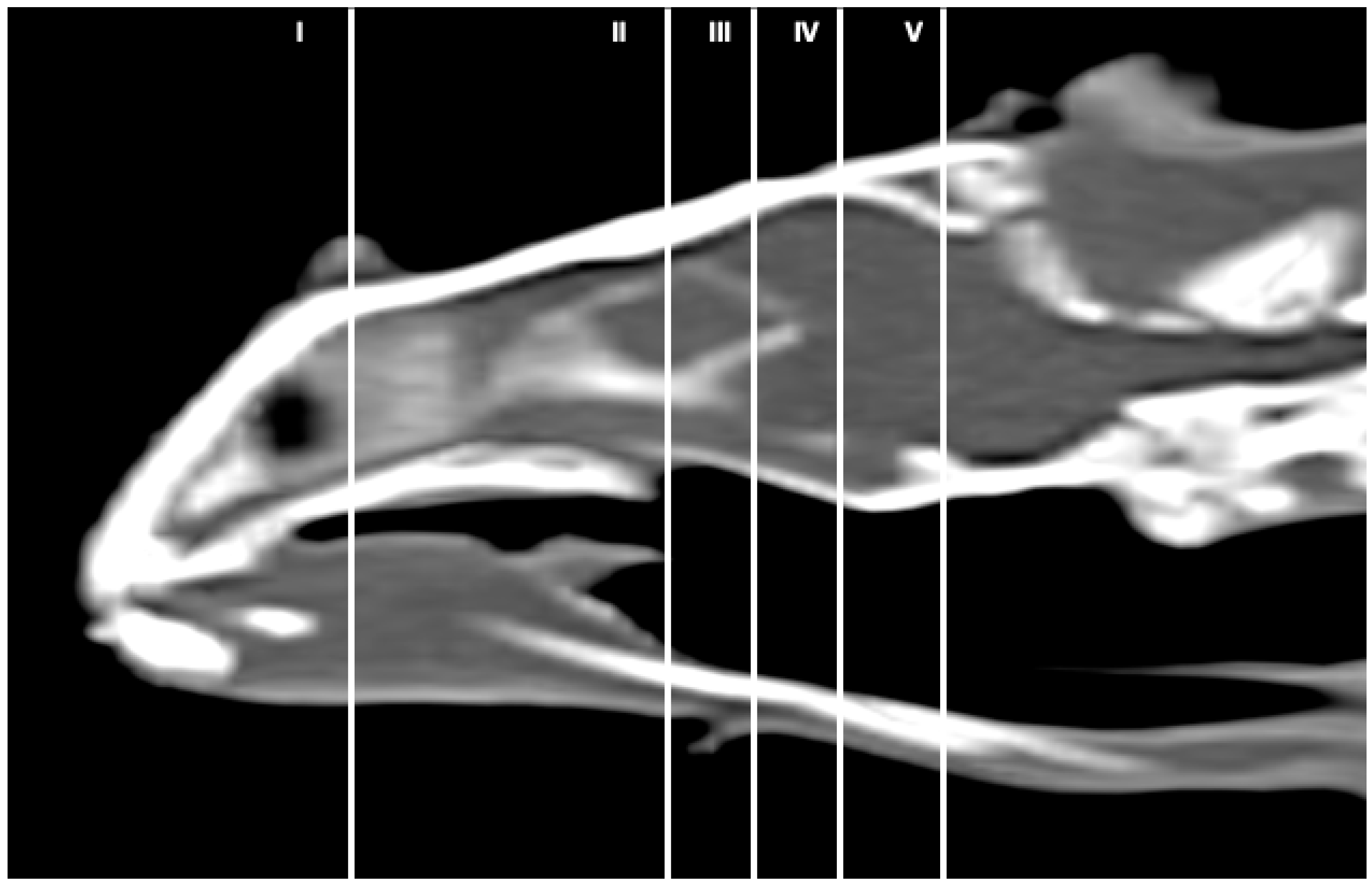
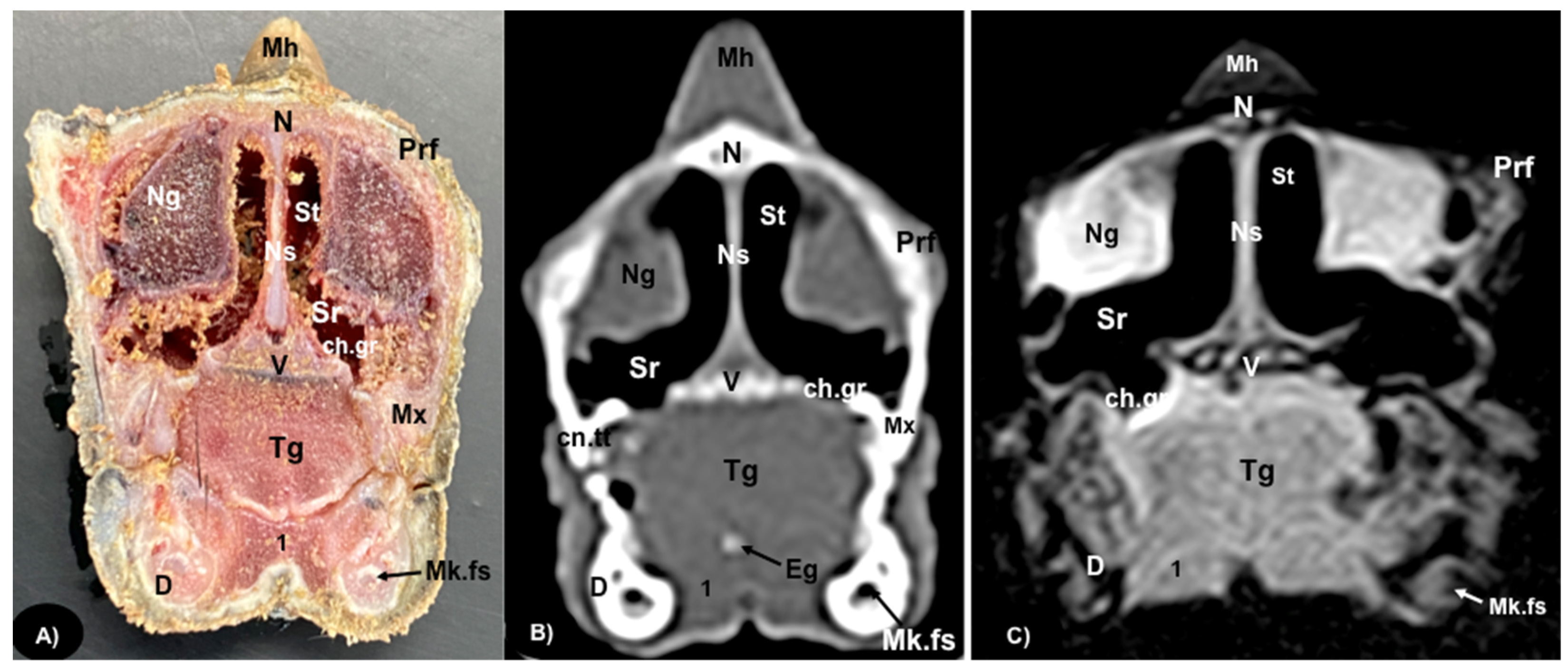
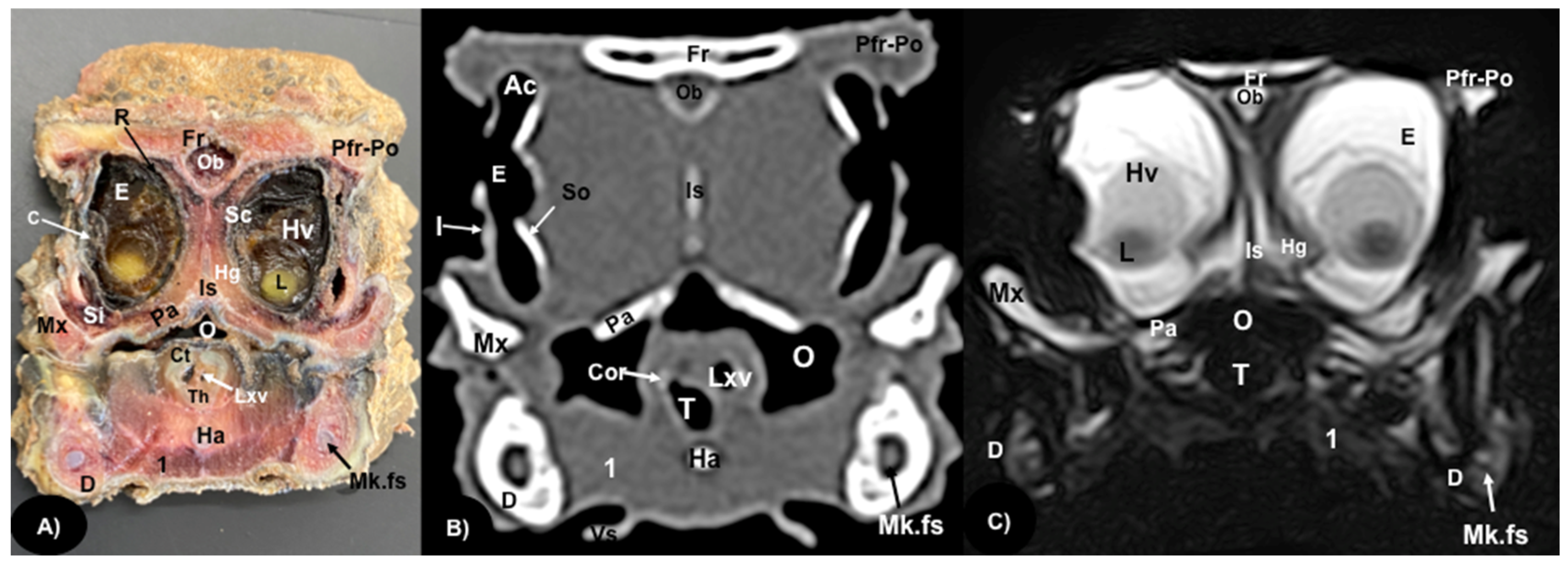
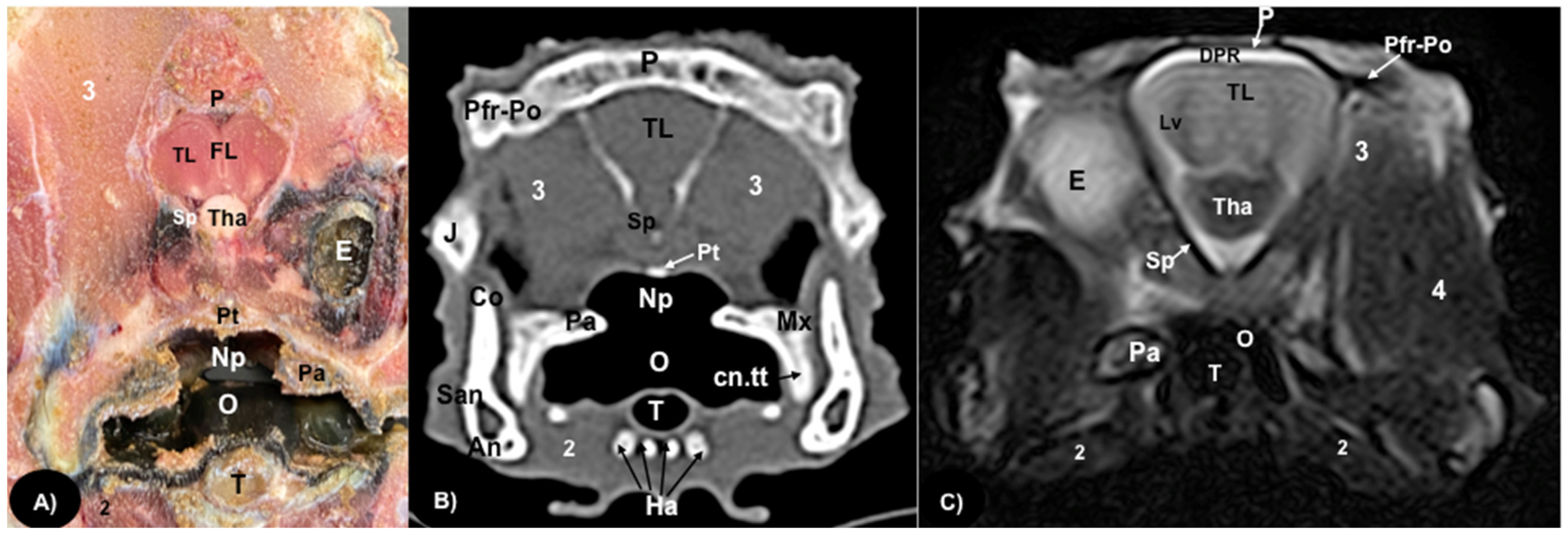
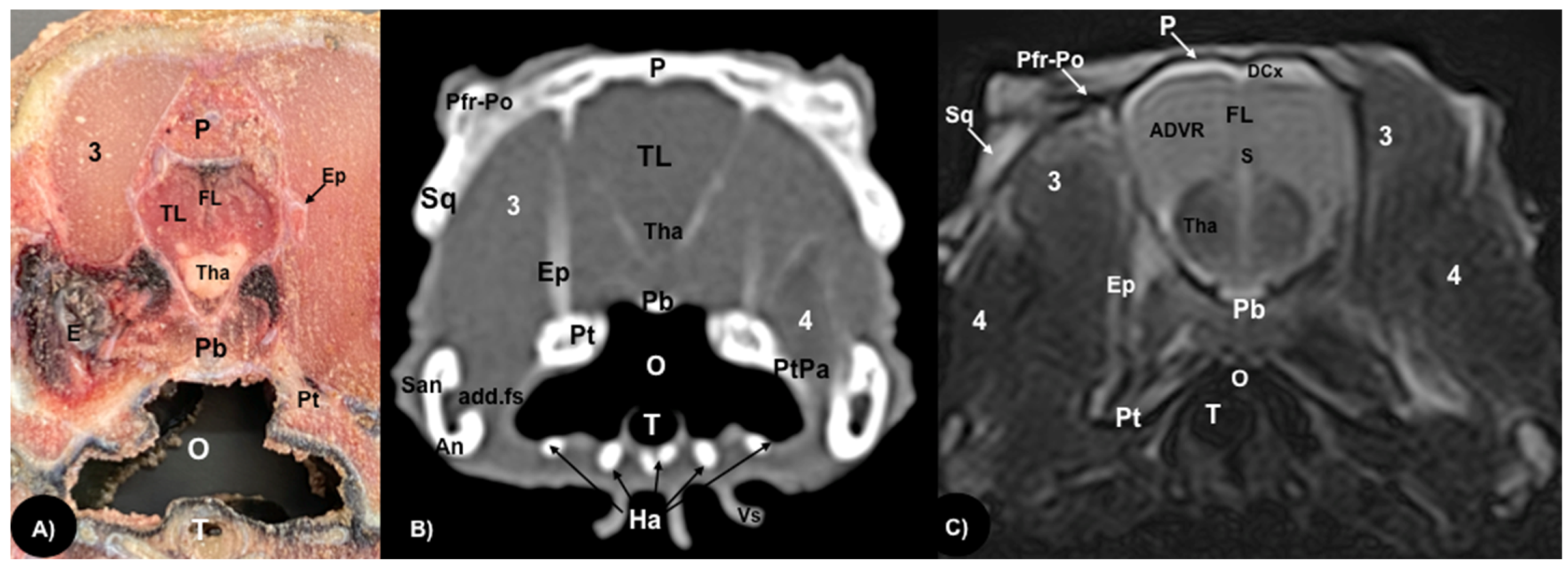
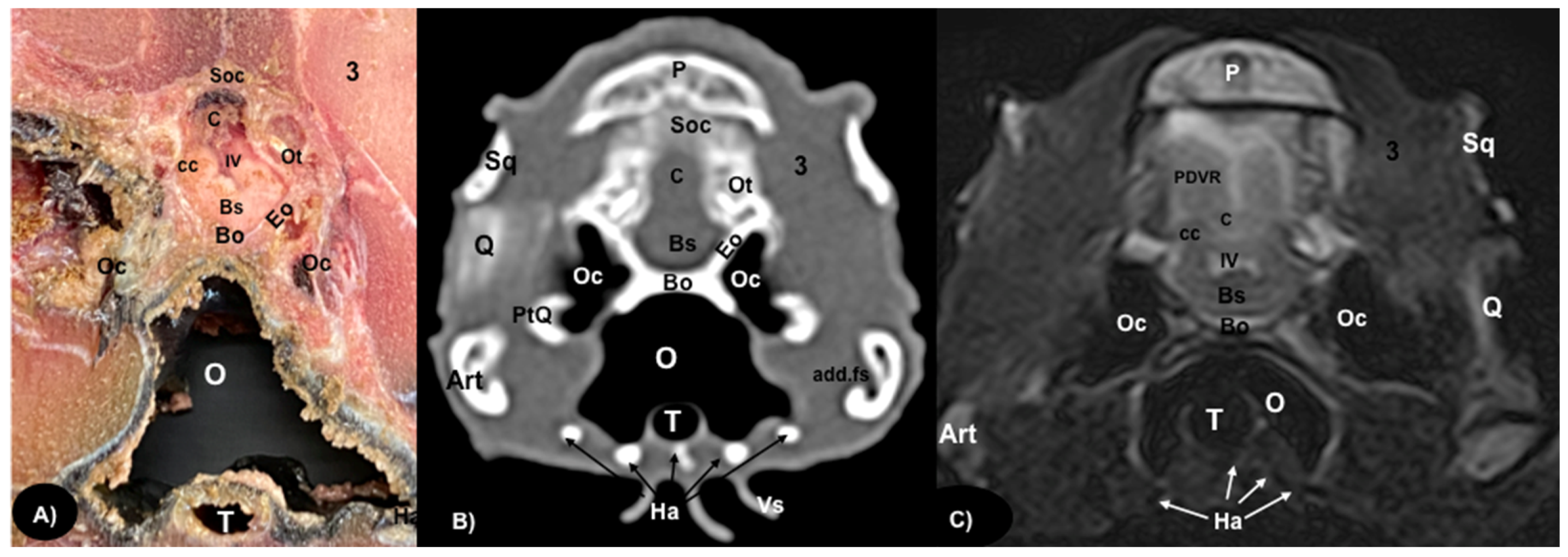
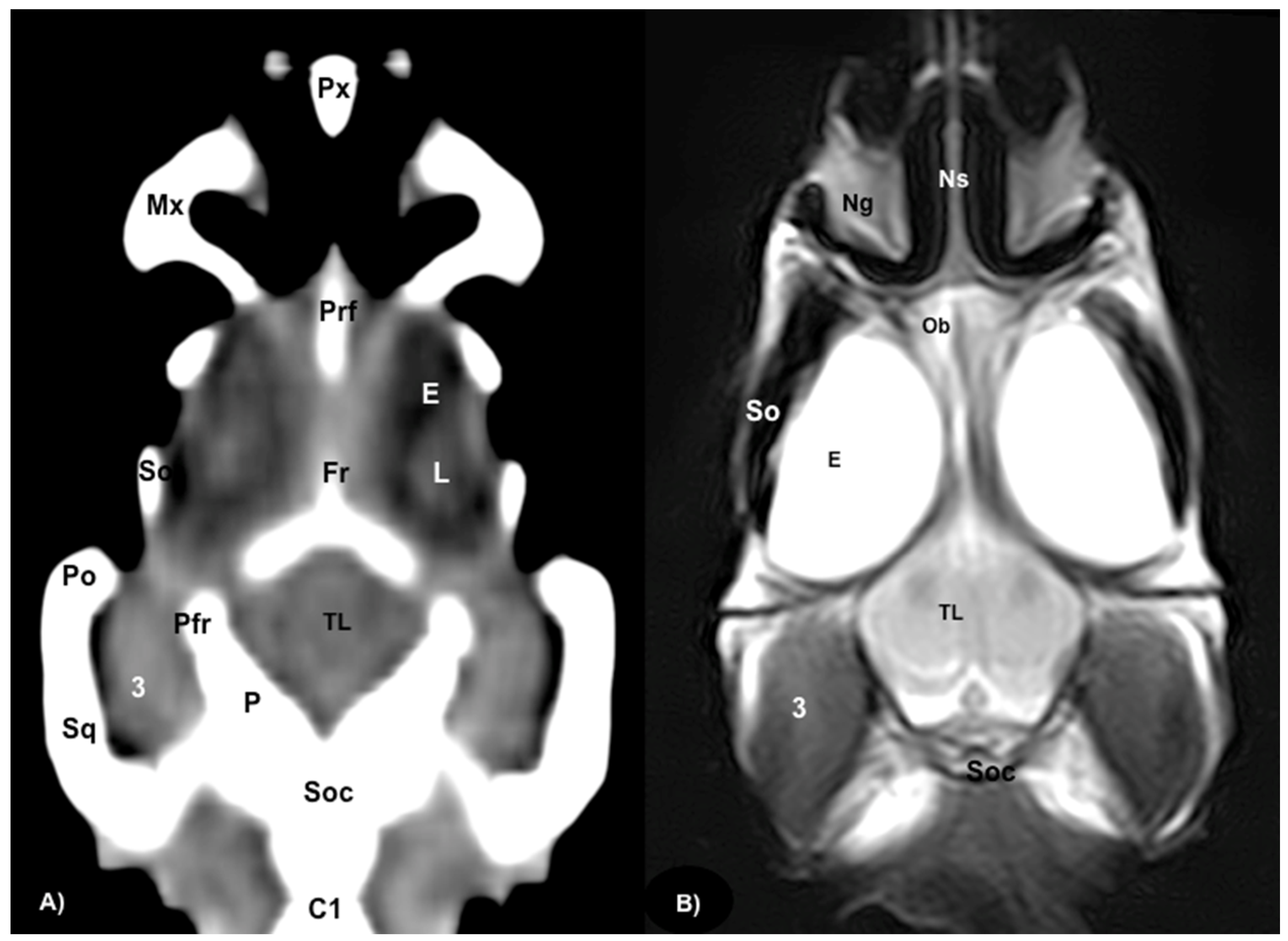
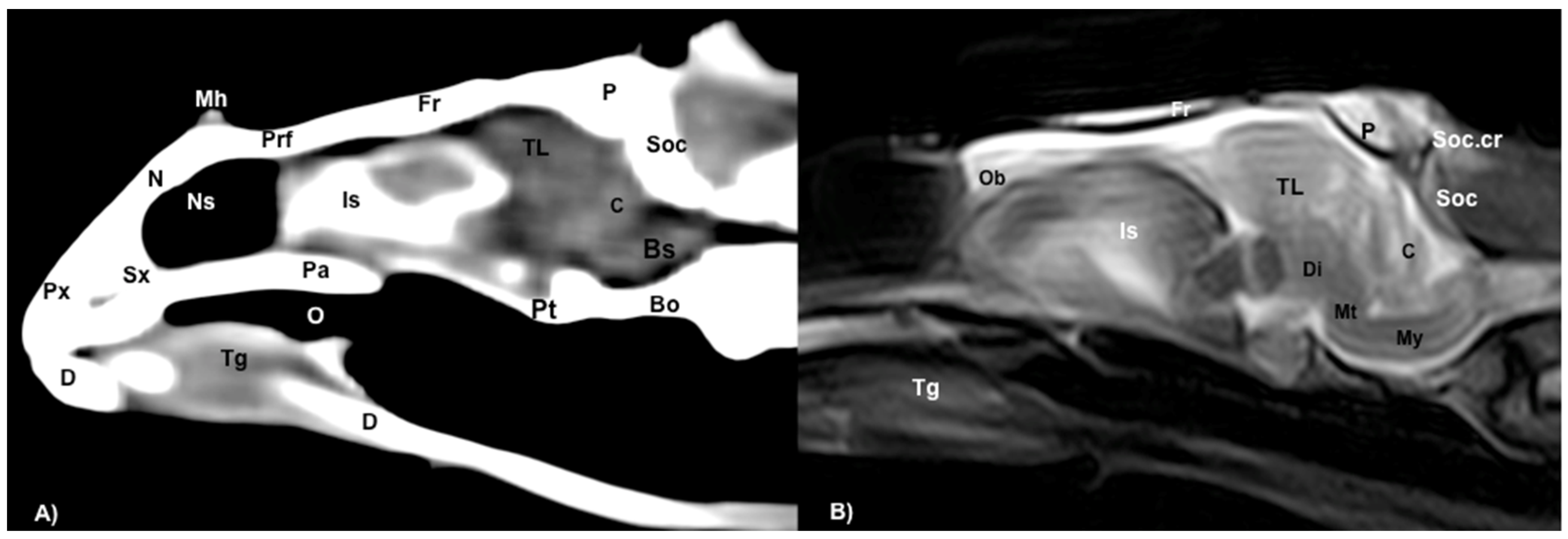
3.1. Anatomical Sections
3.2. Computed Tomography (CT)
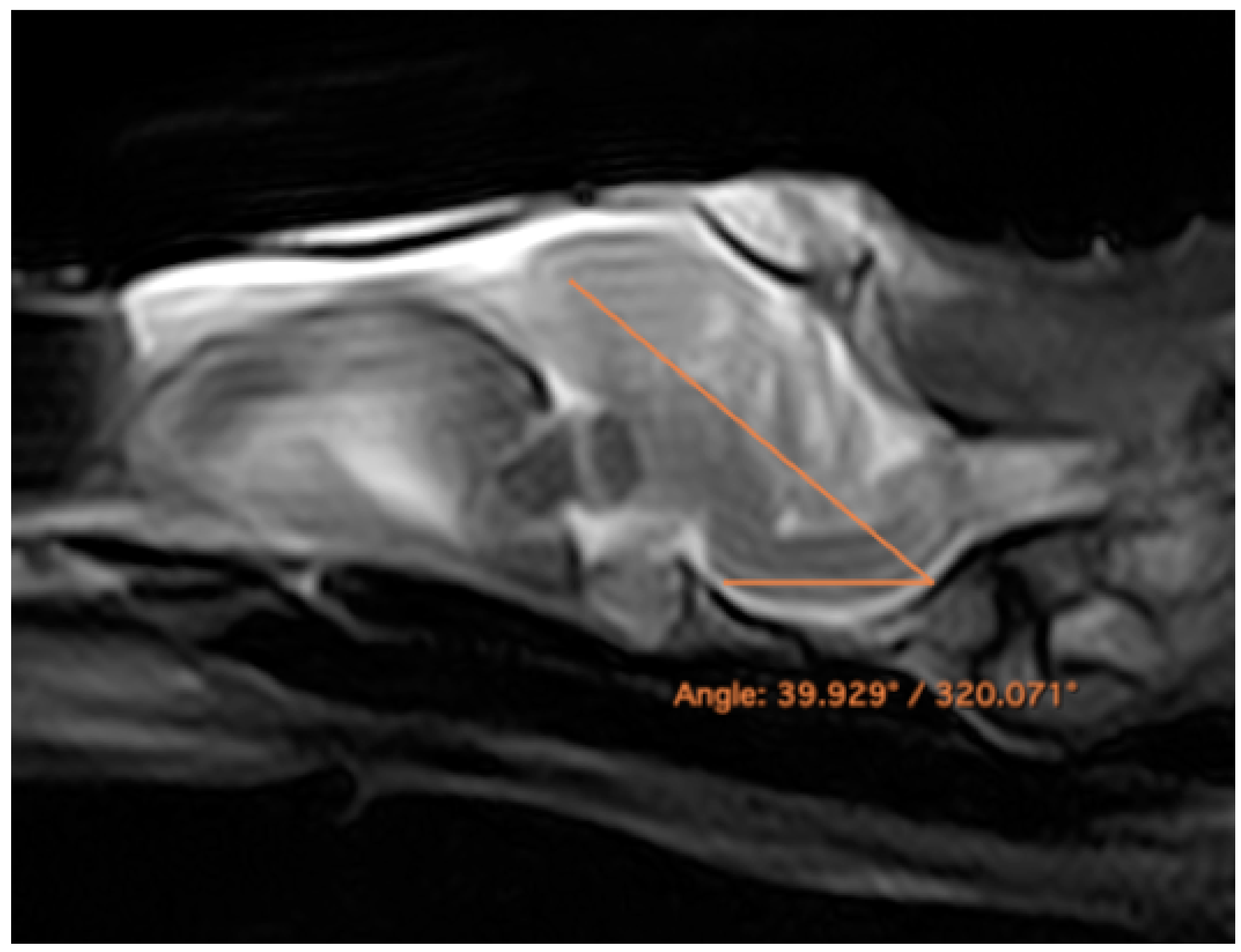
3.3. Magnetic Resonance Imaging (MRI)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alberts, A.C. West Indian Iguanas. Status Survey and Conservation Action Plan; IUCN: Gland, Switzerland; Cambridge, UK, 2000; ISBN 2831704561. [Google Scholar]
- Glor, R.E.; Powell, R.; Parmerlee, J.S., Jr. Cyclura Cornuta. Cat. Am. Amphib. Reptil. 2000, 8235, 1–6. [Google Scholar]
- Egnatios-Beene, J. Cyclura Cornuta. Available online: https://animaldiversity.org/accounts/Cyclura_cornuta/ (accessed on 13 February 2023).
- Banzato, T.; Russo, E.; Di Toma, A.; Palmisano, G.; Zotti, A. Evaluation of Radiographic, Computed Tomographic, and Cadaveric Anatomy of the Head of Boa Constrictors. Am. J. Vet. Res. 2011, 72, 1–8. [Google Scholar] [CrossRef]
- Lauridsen, H.; Hansen, K.; Wang, T.; Agger, P.; Andersen, J.L.; Knudsen, P.S.; Rasmussen, A.S.; Uhrenholt, L.; Pedersen, M. Inside out: Modern Imaging Techniques to Reveal Animal Anatomy. PLoS One 2011, 6, e17879. [Google Scholar] [CrossRef] [PubMed]
- Knotek, Z. Reptile Medicine and Surgery in Clinical Practice. Reptil. Med. Surg. 2006, 471–489. [Google Scholar] [CrossRef]
- Urbanová, D.; Halán, M. The Use of Ultrasonography in Diagnostic Imaging of Reptiles. Folia Vet. 2016, 60, 51–57. [Google Scholar] [CrossRef]
- Bannas, P.; Adam, G.; Derlin, T. Modern Imaging Techniques in Patients with Multiple Myeloma. RoFo 2013, 185, 26–33. [Google Scholar]
- Behroozi, M.; Billings, B.K.; Helluy, X.; Manger, P.R.; Güntürkün, O.; Ströckens, F. Functional MRI in the Nile Crocodile: A New Avenue for Evolutionary Neurobiology. Proc. R. Soc. B Biol. Sci. 2018, 285, 20180178. [Google Scholar] [CrossRef] [PubMed]
- Knipe, M.F. Principles of Neurological Imaging of Exotic Animal Species. Vet. Clin. North Am.—Exot. Anim. Pract. 2007, 10, 893–907. [Google Scholar] [CrossRef]
- Banzato, T.; Hellebuyck, T.; Van Caelenberg, A.; Saunders, J.H.; Zotti, A. A Review of Diagnostic Imaging of Snakes and Lizards. Vet. Rec. 2013, 173, 43–49. [Google Scholar] [CrossRef]
- Pérez, S.; Encinoso, M.; Morales, M.; Arencibia, A.; Suárez-Bonnet, A.; González-Rodríguez, E.; Jaber, J.R. Comparative Evaluation of the Komodo Dragon (Varanus Komodoensis) and the Green Iguana (Iguana Iguana) Skull by Three-Dimensional Computed Tomographic Reconstruction. Slov. Vet. Res. 2021, 58, 111–116. [Google Scholar] [CrossRef]
- Hernández Morales, C.; Peloso, P.L.V.; Bolívar García, W.; Daza, J.D. Skull Morphology of the Lizard Ptychoglossus Vallensis (Squamata: Alopoglossidae) with Comments on the Variation Within Gymnophthalmoidea. Anat. Rec. 2019, 302, 1074–1092. [Google Scholar] [CrossRef]
- Sterli, J.; Müller, J.; Anquetin, J.; Hilger, A. The Parabasisphenoid Complex in Mesozoic Turtles and the Evolution of the Testudinate Basicranium. Can. J. Earth Sci. 2010, 47, 1337–1346. [Google Scholar] [CrossRef]
- Jaber, J.R.; González-Rodríguez, E.; Arencibia, A.; Deniz, S.; Carrascosa, C.; Encinoso, M. Anatomical Description of Loggerhead Turtle (Caretta Caretta) and Green Iguana (Iguana Iguana) Skull by Three-Dimensional Computed Tomography Reconstruction and Maximum Intensity Projection Images. Animals 2023, 13, 621. [Google Scholar] [CrossRef]
- Arencibia, A.; Melián, A.; Orós, J. Anatomic Interactive Atlas of the Loggerhead Sea Turtle (Caretta Caretta) Head. Animals 2021, 11, 198. [Google Scholar] [CrossRef] [PubMed]
- Arencibia, A.; Rivero, M.A.; De Miguel, I.; Contreras, S.; Cabrero, A.; Orós, J. Computed Tomographic Anatomy of the Head of the Loggerhead Sea Turtle (Caretta Caretta). Res. Vet. Sci. 2006, 81, 165–169. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Kitayama, C.; Tanaka, S.; Kondo, S.; Miyazaki, A.; Okamoto, K.; Yanagawa, M.; Kondoh, D. Computed Tomographic Analysis of Internal Structures within the Nasal Cavities of Green, Loggerhead and Leatherback Sea Turtles. Anat. Rec. 2021, 304, 584–590. [Google Scholar] [CrossRef]
- Pérez, S.; Encinoso, M.; Corbera, J.A.; Morales, M.; Arencibia, A.; González-rodríguez, E.; Déniz, S.; Melián, C.; Suárez-bonnet, A.; Jaber, J.R. Cranial Structure of Varanus Komodoensis as Revealed by Computed-tomographic Imaging. Animals 2021, 11, 1078. [Google Scholar] [CrossRef]
- Moreno, K.; Wroe, S.; Clausen, P.; McHenry, C.; D’Amore, D.C.; Rayfield, E.J.; Cunningham, E. Cranial Performance in the Komodo Dragon (Varanus Komodoensis) as Revealed by High-Resolution 3-D Finite Element Analysis. J. Anat. 2008, 212, 736–746. [Google Scholar] [CrossRef]
- Banzato, T.; Selleri, P.; Veladiano, I.A.; Zotti, A. Comparative Evaluation of the Cadaveric and Computed Tomographic Features of the Coelomic Cavity in the Green Iguana (Iguana Iguana), Black and White Tegu (Tupinambis Merianae) and Bearded Dragon (Pogona Vitticeps). J. Vet. Med. Ser. C Anat. Histol. Embryol. 2013, 42, 453–460. [Google Scholar] [CrossRef]
- Banzato, T.; Selleri, P.; Veladiano, I.A.; Martin, A.; Zanetti, E.; Zotti, A. Comparative Evaluation of the Cadaveric, Radiographic and Computed Tomographic Anatomy of the Heads of Green Iguana (Iguana Iguana), Common Tegu (Tupinambis Merianae) and Bearded Dragon (Pogona Vitticeps). BMC Vet. Res. 2012, 8, 1. [Google Scholar] [CrossRef]
- Pasachnik, S.A.; Colosimo, G.; Carreras-De León, R.; Gerber, G. Genetic Structure of Rhinoceros Rock Iguanas, Cyclura Cornuta, in the Dominican Republic, with Insights into the Impact of Captive Facilities and the Taxonomic Status of Cyclura on Mona Island. Conserv. Genet. 2020, 21, 837–851. [Google Scholar] [CrossRef]
- Reynolds, R.G.; Miller, A.H.; Pasachnik, S.A.; Knapp, C.R.; Welch, M.E.; Colosimo, G.; Gerber, G.P.; Drawert, B.; Iverson, J.B. Phylogenomics and Historical Biogeography of West Indian Rock Iguanas (Genus Cyclura). Mol. Phylogenet. Evol. 2022, 174, 107548. [Google Scholar] [CrossRef] [PubMed]
- Reece, R.L.; Dickson, D.B.; Butler, R. An Osteopetrosis-like Condition in a Juvenile Rhinocerus Iguana (Cyclura Cornuta). Aust. Vet. J. 1986, 63, 343–344. [Google Scholar] [CrossRef]
- Evans, S.E. The Skull of Lizards and Tuatara. Biol. Reptil. 2008, 20, 2–227. [Google Scholar]
- Arencibia, A.; Hidalgo, M.R.; Vázquez, J.M.; Contreras, S.; Ramírez, G.; Orós, J. Sectional Anatomic and Magnetic Resonance Imaging Features of the Head of Juvenile Loggerhead Sea Turtles (Caretta Caretta). Am. J. Vet. Res. 2012, 73, 1119–1127. [Google Scholar] [CrossRef] [PubMed]
- Spadola, F.; Barillaro, G.; Morici, M.; Nocera, A.; Knotek, Z. The Practical Use of Computed Tomography in Evaluation of Shell Lesions in Six Loggerhead Turtles (Caretta Caretta). Vet. Med. 2016, 61, 394–398. [Google Scholar] [CrossRef]
- Christman, J.; Devau, M.; Wilson-Robles, H.; Hoppes, S.; Rech, R.; Russell, K.E.; Heatley, J.J. Oncology of Reptiles: Diseases, Diagnosis, and Treatment. Vet. Clin. North Am.—Exot. Anim. Pract. 2017, 20, 87–110. [Google Scholar] [CrossRef]
- Avens, L.; Taylor, J.C.; Goshe, L.R.; Jones, T.T.; Hastings, M. Use of Skeletochronological Analysis to Estimate the Age of Leatherback Sea Turtles Dermochelys Coriacea in the Western North Atlantic. Endanger. Species Res. 2009, 8, 165–177. [Google Scholar] [CrossRef]
- De Queiroz, K. The Scleral Ossicles of Sceloporine Iguanids: A Reexamination with Comments on Their Phylogenetic Significance. Herpetologica 2023, 38, 302–311. [Google Scholar]
- Hoops, D.; Desfilis, E.; Ullmann, J.F.P.; Janke, A.L.; Stait-Gardner, T.; Devenyi, G.A.; Price, W.S.; Medina, L.; Whiting, M.J.; Keogh, J.S. A 3D MRI-Based Atlas of a Lizard Brain; Wiley Periodicals, Inc: Hoboken, NJ, USA, 2018; Volume 526, ISBN 3415253201. [Google Scholar]
- Getty, R. The Anatomy of the Domestic Animals, 5th ed.; Macmillan Company of India Limited: Mumbai, India, 2004. [Google Scholar]
- du Plessis, A.; Broeckhoven, C.; Guelpa, A.; le Roux, S.G. Laboratory X-Ray Micro-Computed Tomography: A User Guideline for Biological Samples. Gigascience 2017, 6, 1–11. [Google Scholar] [CrossRef]
- Broeckhoven, C.; Du Plessis, A. X-Ray Microtomography in Herpetological Research: A Review. Amphib. Reptil. 2018, 39, 377–401. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González Rodríguez, E.; Encinoso Quintana, M.; Morales Bordon, D.; Garcés, J.G.; Artiles Nuez, H.; Jaber, J.R. Anatomical Description of Rhinoceros Iguana (Cyclura cornuta cornuta) Head by Computed Tomography, Magnetic Resonance Imaging and Gross-Sections. Animals 2023, 13, 955. https://doi.org/10.3390/ani13060955
González Rodríguez E, Encinoso Quintana M, Morales Bordon D, Garcés JG, Artiles Nuez H, Jaber JR. Anatomical Description of Rhinoceros Iguana (Cyclura cornuta cornuta) Head by Computed Tomography, Magnetic Resonance Imaging and Gross-Sections. Animals. 2023; 13(6):955. https://doi.org/10.3390/ani13060955
Chicago/Turabian StyleGonzález Rodríguez, Eligia, Mario Encinoso Quintana, Daniel Morales Bordon, José Guerra Garcés, Himar Artiles Nuez, and José Raduan Jaber. 2023. "Anatomical Description of Rhinoceros Iguana (Cyclura cornuta cornuta) Head by Computed Tomography, Magnetic Resonance Imaging and Gross-Sections" Animals 13, no. 6: 955. https://doi.org/10.3390/ani13060955
APA StyleGonzález Rodríguez, E., Encinoso Quintana, M., Morales Bordon, D., Garcés, J. G., Artiles Nuez, H., & Jaber, J. R. (2023). Anatomical Description of Rhinoceros Iguana (Cyclura cornuta cornuta) Head by Computed Tomography, Magnetic Resonance Imaging and Gross-Sections. Animals, 13(6), 955. https://doi.org/10.3390/ani13060955






