Effect of Fermented Rapeseed Meal in Feeds for Growing Piglets on Bone Morphological Traits, Mechanical Properties, and Bone Metabolism
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Fermented Rapeseed Meal (FRSM)
2.2. Animals and Study Design
2.3. Sample Collection
2.4. Bone Analysis
2.5. Bone Turnover Markers
2.6. Statistical Analysis
3. Results
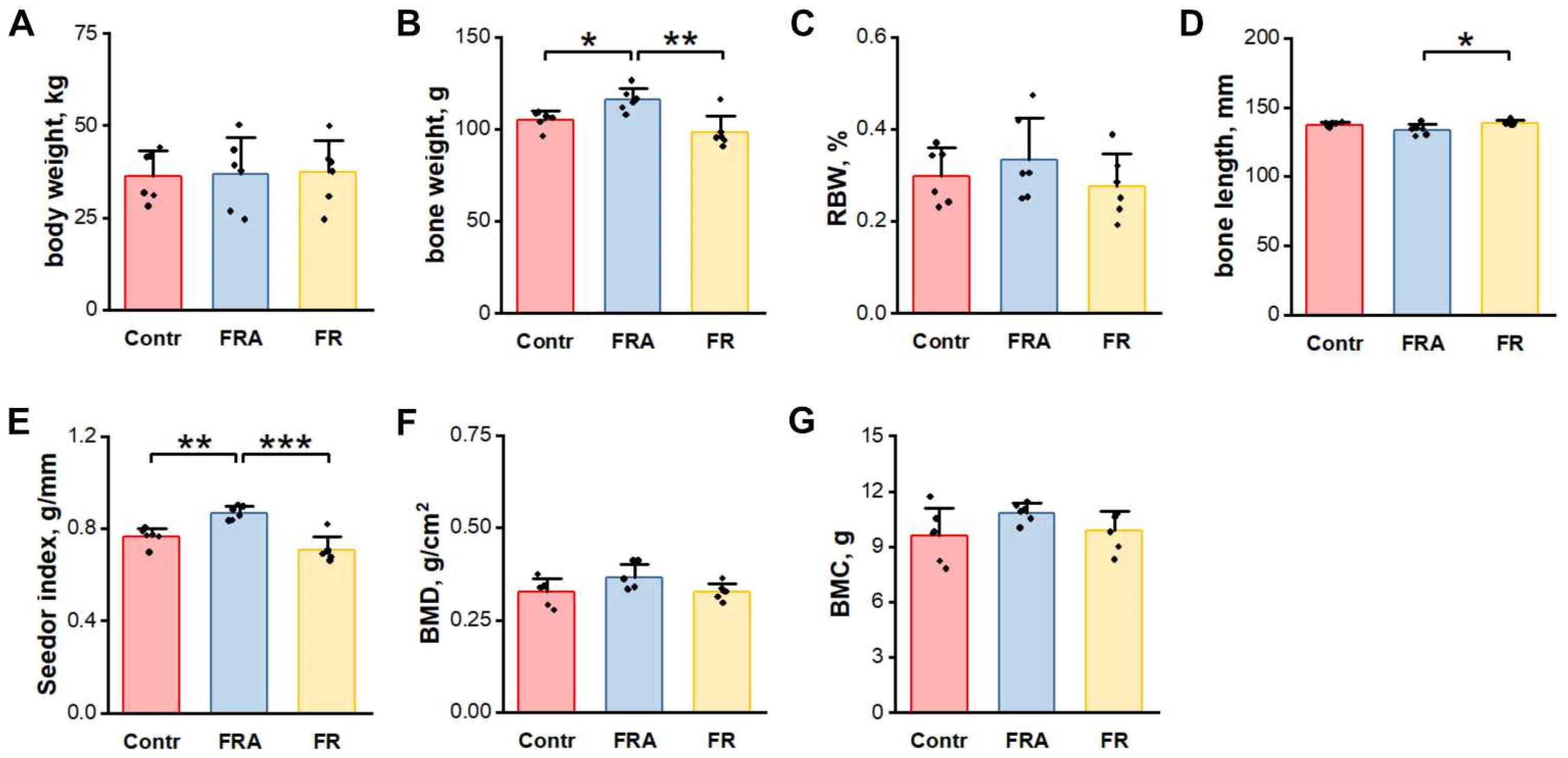
3.1. Body Weight
3.2. Basic Femur Properties
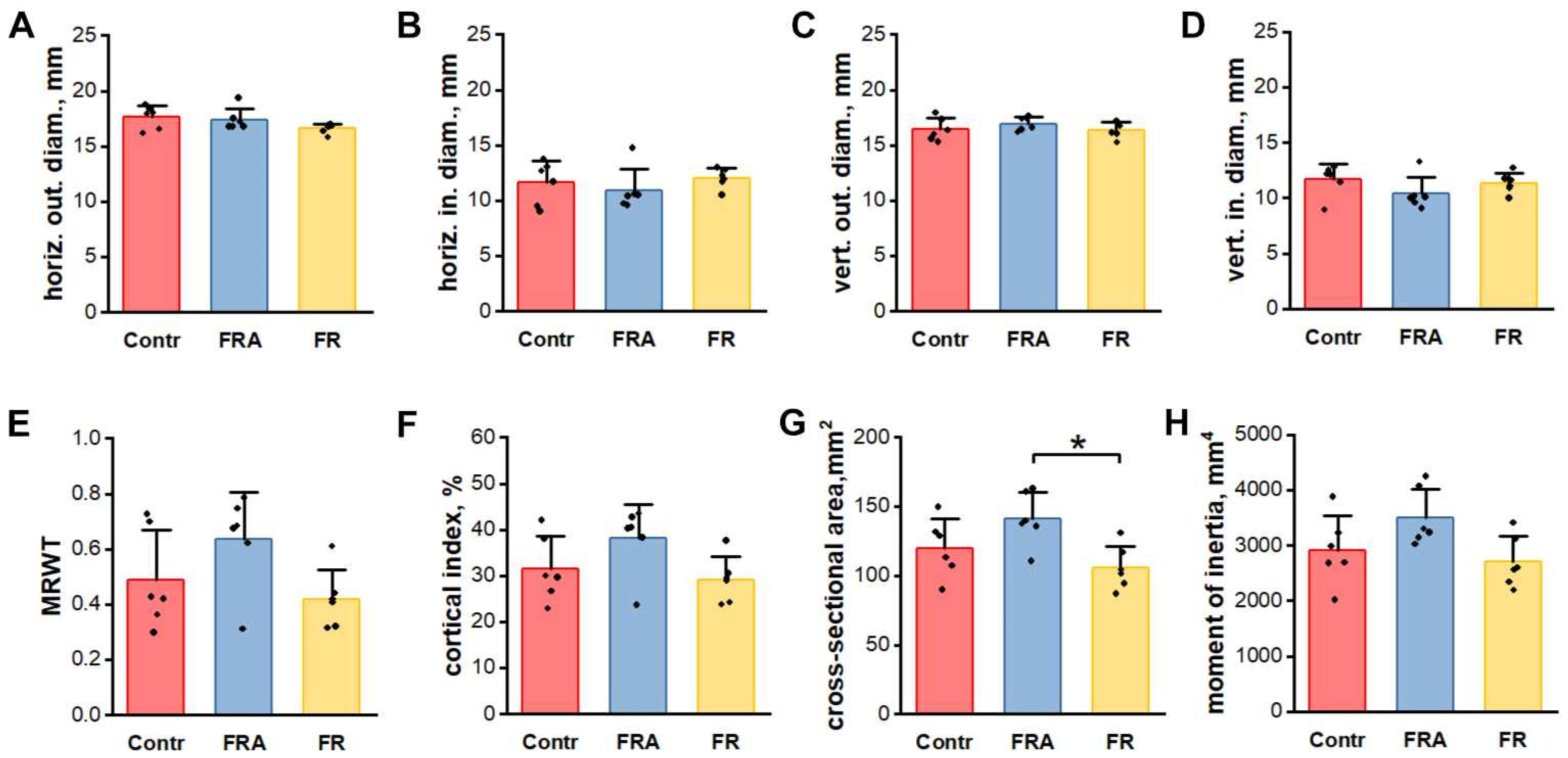
3.3. Geometrical Properties of Femoral Mid-Diaphysis
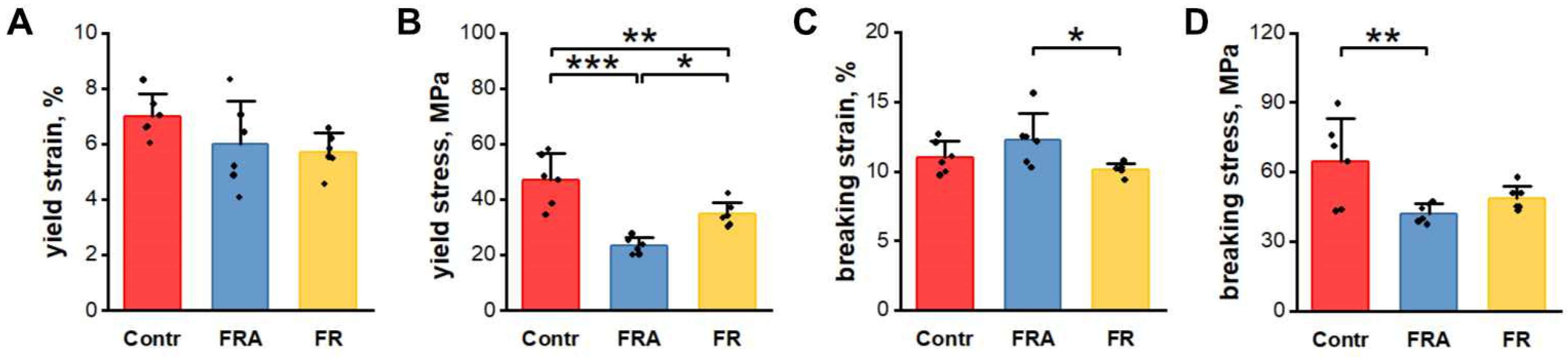
3.4. Mechanical Properties of Femurs
3.5. Bone Material Properties
3.6. Bone Turnover Markers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gołębiewska, K.; Fraś, A.; Gołębiewski, D. Rapeseed Meal as a Feed Component in Monogastric Animal Nutrition—A Review. Ann. Anim. Sci. 2022, 22, 1163–1183. [Google Scholar] [CrossRef]
- Ates, A.M.; Bukowski, M. Oil Crops Outlook: September 2022. Available online: https://www.ers.usda.gov/publications/pub-details/?pubid=104713 (accessed on 3 March 2023).
- Bell, J.M. Nutrients and Toxicants in Rapeseed Meal: A Review. J. Anim. Sci. 1984, 58, 996–1010. [Google Scholar] [CrossRef]
- Cheng, H.; Liu, X.; Xiao, Q.; Zhang, F.; Liu, N.; Tang, L.; Wang, J.; Ma, X.; Tan, B.; Chen, J.; et al. Rapeseed Meal and Its Application in Pig Diet: A Review. Agriculture 2022, 12, 849. [Google Scholar] [CrossRef]
- Schöne, F.; Tischendorf, F.; Leiterer, M.; Hartung, H.; Bargholz, J. Effects of Rapeseed-press Cake Glucosinolates and Iodine on the Performance, the Thyroid Gland and the Liver Vitamin a Status of Pigs. Arch. Tierernaehrung 2001, 55, 333–350. [Google Scholar] [CrossRef]
- Rodrigues, I.M.; Carvalho, M.G.V.S.; Rocha, J.M.S. Increasing the Protein Content of Rapeseed Meal by Enzymatic Hydrolysis of Carbohydrates. Bioresources 2014, 9, 2010–2025. [Google Scholar] [CrossRef]
- Kracht, W.; Dänicke, S.; Kluge, H.; Keller, K.; Matzke, W.; Hennig, U.; Schumann, W. Effect of Dehulling of Rapeseed on Feed Value and Nutrient Digestibility of Rape Products in Pigs. Arch. Anim. Nutr. 2004, 58, 389–404. [Google Scholar] [CrossRef]
- Grela, E.R.; Czech, A.; Kiesz, M.; Wlazło, Ł.; Nowakowicz-Dębek, B. A Fermented Rapeseed Meal Additive: Effects on Production Performance, Nutrient Digestibility, Colostrum Immunoglobulin Content and Microbial Flora in Sows. Anim. Nutr. 2019, 5, 373–379. [Google Scholar] [CrossRef]
- Zhao, Y.-S.; Eweys, A.S.; Zhang, J.-Y.; Zhu, Y.; Bai, J.; Darwesh, O.M.; Zhang, H.-B.; Xiao, X. Fermentation Affects the Antioxidant Activity of Plant-Based Food Material through the Release and Production of Bioactive Components. Antioxidants 2021, 10, 2004. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, Y.; Li, A.; Wang, Z.; Zhang, X.; Yun, T.; Qiu, L.; Yin, Y. Effects of Fermented Rapeseed Meal on Antioxidant Functions, Serum Biochemical Parameters and Intestinal Morphology in Broilers. Food Agric. Immunol. 2016, 27, 182–193. [Google Scholar] [CrossRef]
- Czech, A.; Grela, E.R.; Kiesz, M. Dietary Fermented Rapeseed or/and Soybean Meal Additives on Performance and Intestinal Health of Piglets. Sci. Rep. 2021, 11, 16952. [Google Scholar] [CrossRef] [PubMed]
- Szmigiel, I.; Konkol, D.; Korczyński, M.; Łukaszewicz, M.; Krasowska, A. Changes in the Microbial Composition of the Cecum and Histomorphometric Analysis of Its Epithelium in Broilers Fed with Feed Mixture Containing Fermented Rapeseed Meal. Microorganisms 2021, 9, 360. [Google Scholar] [CrossRef]
- Wu, Z.; Chen, J.; Ahmed Pirzado, S.; Haile, T.H.; Cai, H.; Liu, G. The Effect of Fermented and Raw Rapeseed Meal on the Growth Performance, Immune Status and Intestinal Morphology of Broiler Chickens. J. Anim. Physiol. Anim. Nutr. 2022, 106, 296–307. [Google Scholar] [CrossRef]
- Czech, A.; Nowakowicz-Debek, B.; Łukaszewicz, M.; Florek, M.; Ossowski, M.; Wlazło, Ł. Effect of Fermented Rapeseed Meal in the Mixture for Growing Pigs on the Gastrointestinal Tract, Antioxidant Status, and Immune Response. Sci. Rep. 2022, 12, 15764. [Google Scholar] [CrossRef] [PubMed]
- Czech, A.; Stępniowska, A.; Kiesz, M. Effect of Fermented Rapeseed Meal as a Feed Component on the Redox and Immune System of Pregnant Sows and Their Offspring. Ann. Anim. Sci. 2022, 22, 201–219. [Google Scholar] [CrossRef]
- Palacios, C. The Role of Nutrients in Bone Health, from A to Z. Crit. Rev. Food Sci. Nutr. 2006, 46, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Sobol, M.; Skiba, G.; Raj, S.; Kowalczyk, P.; Kramkowski, K.; Świątkiewicz, M.; Grela, E.R. Chemical Body Composition and Bone Growth of Young Pigs as Affected by Deficiency, Adequate and Excess of Dietary Phosphorus Supply. Ann. Anim. Sci. 2022, 22, 1363–1372. [Google Scholar] [CrossRef]
- Heaney, R.P.; Layman, D.K. Amount and Type of Protein Influences Bone Health. Am. J. Clin. Nutr. 2008, 87, 1567S–1570S. [Google Scholar] [CrossRef]
- Bonjour, J.-P. Protein Intake and Bone Health. Int. J. Vitam. Nutr. Res. 2011, 81, 134–142. [Google Scholar] [CrossRef]
- Morgan, E.F.; Unnikrisnan, G.U.; Hussein, A.I. Bone Mechanical Properties in Healthy and Diseased States. Annu. Rev. Biomed. Eng. 2018, 20, 119–143. [Google Scholar] [CrossRef] [PubMed]
- Szmigiel, I.; Kwiatkowska, D.; Łukaszewicz, M.; Krasowska, A. Xylan Decomposition in Plant Cell Walls as an Inducer of Surfactin Synthesis by Bacillus subtilis. Biomolecules 2021, 11, 239. [Google Scholar] [CrossRef]
- National Research Council. Nutrient Requirements of Swine, 11th ed.; The National Academies Press: Washington, DC, USA, 2012; ISBN 978-0-309-48903-4. [Google Scholar]
- Skomorucha, I.; Sosnówka-Czajka, E. A Comparison of Morphometric Indices, Mineralization Level of Long Bones and Selected Blood Parameters in Hens of Three Breeds. Ann. Anim. Sci. 2021, 21, 869–885. [Google Scholar] [CrossRef]
- Muszyński, S.; Kwiecień, M.; Tomaszewska, E.; Świetlicka, I.; Dobrowolski, P.; Kasperek, K.; Jeżewska-Witkowska, G. Effect of Caponization on Performance and Quality Characteristics of Long Bones in Polbar Chickens. Poult. Sci. 2017, 96, 491–500. [Google Scholar] [CrossRef]
- Czech, A.; Wlazło, Ł.; Łukaszewicz, M.; Florek, M.; Nowakowicz-Dębek, B. Fermented Rapeseed Meal Enhances the Digestibility of Protein and Macro- and Microminerals and Improves the Performance of Weaner Pigs. Department of Animal Hygiene and Environmental Hazards, Faculty of Animal Sciences and Bioeconomy, University of Life Sciences in Lublin: Lublin, Poland, 2023; to be submitted. [Google Scholar]
- Mejicanos, G.; Sanjayan, N.; Kim, I.H.; Nyachoti, C.M. Recent Advances in Canola Meal Utilization in Swine Nutrition. J. Anim. Sci. Technol. 2016, 58, 7. [Google Scholar] [CrossRef]
- El-Batal, A.I.; Abdel Karem, H. Phytase Production and Phytic Acid Reduction in Rapeseed Meal by Aspergillus Niger during Solid State Fermentation. Food Res. Int. 2001, 34, 715–720. [Google Scholar] [CrossRef]
- Pal Vig, A.; Walia, A. Beneficial Effects of Rhizopus Oligosporus Fermentation on Reduction of Glucosinolates, Fibre and Phytic Acid in Rapeseed (Brassica Napus) Meal. Bioresour. Technol. 2001, 78, 309–312. [Google Scholar] [CrossRef] [PubMed]
- Rozan, P.; Villaum, C.; Bau, H.M.; Schwertz, A.; Nicolas, J.P.; Mejean, L. Detoxication of Rapeseed Meal by Rhizopus Oligosporus Sp-T3: A First Step towards Rapeseed Protein Concentrate. Int. J. Food Sci. Technol. 1996, 31, 85–90. [Google Scholar] [CrossRef]
- Hill, R. A Review of the ‘Toxic’ Effects of Rapeseed Meals with Observations on Meal from Improved Varieties. Br. Vet. J. 1979, 135, 3–16. [Google Scholar] [CrossRef]
- Rakariyatham, N.; Sakorn, P. Biodegradation of Glucosinolates in Brown Mustard Seed Meal (Brassica Juncea) by Aspergillus Sp. NR-4201 in Liquid and Solid-State Cultures. Biodegradation 2002, 13, 395–399. [Google Scholar] [CrossRef]
- Hong, K.-J.; Lee, C.-H.; Kim, S.W. Aspergillus Oryzae GB-107 Fermentation Improves Nutritional Quality of Food Soybeans and Feed Soybean Meals. J. Med. Food 2004, 7, 430–435. [Google Scholar] [CrossRef]
- Hölker, U.; Höfer, M.; Lenz, J. Biotechnological Advantages of Laboratory-Scale Solid-State Fermentation with Fungi. Appl. Microbiol. Biotechnol. 2004, 64, 175–186. [Google Scholar] [CrossRef]
- Ouoba, L.I.I.; Rechinger, K.B.; Barkholt, V.; Diawara, B.; Traore, A.S.; Jakobsen, M. Degradation of Proteins during the Fermentation of African Locust Bean (Parkia biglobosa) by Strains of Bacillus subtilis and Bacillus Pumilus for Production of Soumbala. J. Appl. Microbiol. 2003, 94, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.B.; Wang, Z.; Xu, S.Y. Downstream Processes for Aqueous Enzymatic Extraction of Rapeseed Oil and Protein Hydrolysates. J. Am. Oil Chem. Soc. 2007, 84, 693–700. [Google Scholar] [CrossRef]
- Yun, H.M.; Lei, X.J.; Lee, S.I.; Kim, I.H. Rapeseed Meal and Canola Meal Can Partially Replace Soybean Meal as a Protein Source in Finishing Pigs. J. Appl. Anim. Res. 2018, 46, 195–199. [Google Scholar] [CrossRef]
- Czech, A.; Grela, E.R.; Nowakowicz-Dębek, B.; Wlazło, Ł. The Effects of a Fermented Rapeseed Meal or/and Soybean Meal Additive on the Blood Lipid Profile and Immune Parameters of Piglets and on Minerals in Their Blood and Bone. PLoS ONE 2021, 16, e0253744. [Google Scholar] [CrossRef] [PubMed]
- Landero, J.L.; Beltranena, E.; Cervantes, M.; Morales, A.; Zijlstra, R.T. The Effect of Feeding Solvent-Extracted Canola Meal on Growth Performance and Diet Nutrient Digestibility in Weaned Pigs. Anim. Feed Sci. Technol. 2011, 170, 136–140. [Google Scholar] [CrossRef]
- Landero, J.L.; Wang, L.F.; Beltranena, E.; Bench, C.J.; Zijlstra, R.T. Feed Preference of Weaned Pigs Fed Diets Containing Soybean Meal, Brassica Napus Canola Meal, or Brassica Juncea Canola Meal. J. Anim. Sci. 2018, 96, 600–611. [Google Scholar] [CrossRef]
- Sanjayan, N.; Heo, J.M.; Nyachoti, C.M. Nutrient Digestibility and Growth Performance of Pigs Fed Diets with Different Levels of Canola Meal from Brassica Napus Black and Brassica Juncea Yellow1. J. Anim. Sci. 2014, 92, 3895–3905. [Google Scholar] [CrossRef]
- Hong, J.; Ndou, S.P.; Adams, S.; Scaria, J.; Woyengo, T.A. Canola Meal in Nursery Pig Diets: Growth Performance and Gut Health. J. Anim. Sci. 2020, 98, skaa338. [Google Scholar] [CrossRef]
- Parr, C.K.; Liu, Y.; Parsons, C.M.; Stein, H.H. Effects of High-Protein or Conventional Canola Meal on Growth Performance, Organ Weights, Bone Ash, and Blood Characteristics of Weanling Pigs. J. Anim. Sci. 2015, 93, 2165–2173. [Google Scholar] [CrossRef]
- Tomaszewska, E.; Muszyński, S.; Dobrowolski, P.; Kamiński, D.; Czech, A.; Grela, E.R.; Wiącek, D.; Tomczyk-Warunek, A. Dried Fermented Post-Extraction Rapeseed Meal given to Sows as an Alternative Protein Source for Soybean Meal during Pregnancy Improves Bone Development of Their Offspring. Livest. Sci. 2019, 224, 60–68. [Google Scholar] [CrossRef]
- Śliwa, E.; Radzki, R.P.; Puzio, I. Osteochondrosis and Tibial Dyschondroplasia in Chickens, Pigs and Foals. Med. Weter. 1996, 52, 156–158. [Google Scholar]
- Upadhaya, S.D.; Kim, I.H. Importance of Micronutrients in Bone Health of Monogastric Animals and Techniques to Improve the Bioavailability of Micronutrient Supplements—A Review. Asian-Australas. J. Anim. Sci. 2020, 33, 1885–1895. [Google Scholar] [CrossRef]
- van Grevenhof, E.M.; Heuven, H.C.M.; van Weeren, P.R.; Bijma, P. The Relationship between Growth and Osteochondrosis in Specific Joints in Pigs. Livest. Sci. 2012, 143, 85–90. [Google Scholar] [CrossRef]
- Gerlinger, C.; Oster, M.; Reyer, H.; Polley, C.; Vollmar, B.; Muráni, E.; Wimmers, K.; Wolf, P. Effects of Excessive or Restricted Phosphorus and Calcium Intake during Early Life on Markers of Bone Architecture and Composition in Pigs. J. Anim. Physiol. Anim. Nutr. 2021, 105, 52–62. [Google Scholar] [CrossRef]
- Lorenzett, M.P.; Cecco, B.S.; Bianchi, M.V.; Cruz, R.A.S.; Linhares, D.C.L.; Guedes, R.M.C.; Driemeier, D.; Pavarini, S.P. Osteoporosis in Swine. Pesqui. Veterinária Bras. 2022, 42, e07068. [Google Scholar] [CrossRef]
- Crenshaw, T.D.; Rortvedt-Amundson, L.A. Nutritionally Induced Cellular Signals That Affect Skeletal Integrity in Swine. In Proceedings of the 23rd International Pig Veterinary Congress (IPVS), Cancun, Mexico, 8 June 2014; Volume 1, pp. 75–83. Available online: https://en.engormix.com/pig-industry/articles/nutritionally-induced-cellular-signals-t36231.htm (accessed on 3 March 2023).
- Miesorski, M.; Gerlinger, C.; Borgelt, L.; Lieboldt, M.A.; Oster, M.; Wimmers, K.; Wolf, P. Bone Mineralization as Diagnostic Parameter for the Assessment of Dietary Phosphorous Supply in Pigs—Are There Differences between Bones. In Proceedings of the 22nd Congress of the European Society of Veterinary and Comparative Nutrition, Munich, Germany, 6 September 2018; p. 183. [Google Scholar]
- Hart, N.H.; Nimphius, S.; Rantalainen, T.; Ireland, A.; Siafarikas, A.; Newton, R.U. Mechanical Basis of Bone Strength: Influence of Bone Material, Bone Structure and Muscle Action. J. Musculoskelet. Neuronal Interact. 2017, 17, 114–139. [Google Scholar]
- Lieberman, D.E.; Polk, J.D.; Demes, B. Predicting Long Bone Loading from Cross-Sectional Geometry. Am. J. Phys. Anthropol. 2004, 123, 156–171. [Google Scholar] [CrossRef]
- Fang, Z.F.; Peng, J.; Tang, T.J.; Liu, Z.L.; Dai, J.J.; Jin, L.Z. Xylanase Supplementation Improved Digestibility and Performance of Growing Pigs Fed Chinese Double-Low Rapeseed Meal Inclusion Diets: In Vitro and In Vivo Studies. Asian-Australas. J. Anim. Sci. 2007, 20, 1721–1728. [Google Scholar] [CrossRef]
- Stover, K.K.; Weinreich, D.M.; Roberts, T.J.; Brainerd, E.L. Patterns of Musculoskeletal Growth and Dimensional Changes Associated with Selection and Developmental Plasticity in Domestic and Wild Strain Turkeys. Ecol. Evol. 2018, 8, 3229–3239. [Google Scholar] [CrossRef] [PubMed]
- Muszyński, S.; Arczewska, M.; Świątkiewicz, S.; Arczewska-Włosek, A.; Dobrowolski, P.; Świetlicka, I.; Hułas-Stasiak, M.; Blicharski, T.; Donaldson, J.; Schwarz, T.; et al. The Effect of Dietary Rye Inclusion and Xylanase Supplementation on Structural Organization of Bone Constitutive Phases in Laying Hens Fed a Wheat-Corn Diet. Animals 2020, 10, 2010. [Google Scholar] [CrossRef]
- Sørensen, K.U.; Shiguetomi-Medina, J.M.; Poulsen, H.D. Mineralisation of Tubular Bones Is Affected Differently by Low Phosphorus Supply in Growing-finishing Pigs. J. Sci. Food Agric. 2019, 99, 3628–3634. [Google Scholar] [CrossRef] [PubMed]
- Pointillart, A.; Coxam, V.; Sève, B.; Colin, C.; Lacroix, C.H. Availability of Calcium from Skim Milk, Calcium Sulfate and Calcium Carbonate for Bone Mineralization in Pigs. Reprod. Nutr. Dev. 2002, 40, 49–61. [Google Scholar] [CrossRef]
- Crenshaw, T.D.; Peo, E.R., Jr.; Lewis, A.J.; Moser, B.D.; Olson, D. Influence of Age, Sex and Calcium and Phosphorus Levels on the Mechanical Properties of Various Bones in Swine. J. Anim. Sci. 1981, 52, 1319–1329. [Google Scholar] [CrossRef]
- van der Meulen, M.C.H.; Jepsen, K.J.; Mikić, B. Understanding Bone Strength: Size Isn’t Everything. Bone 2001, 29, 101–104. [Google Scholar] [CrossRef]
- Ferretti, J.L.; Capozza, R.F.; Mondelo, N.; Zanchetta, J.R. Interrelationships between Densitometric, Geometric, and Mechanical Properties of Rat Femora: Inferences Concerning Mechanical Regulation of Bone Modeling. J. Bone Miner. Res. 2009, 8, 1389–1396. [Google Scholar] [CrossRef]
- McPherson, R.; Pincus, M. Henry’s Clinical Diagnosis and Management by Laboratory Methods, 24th ed.; Elsevier: Philadelphia, PA, USA, 2021; ISBN 978-0-323-67320-4. [Google Scholar]
- Yamaguchi, M. Role of Zinc in Bone Metabolism and Preventive Effect on Bone Disorder. Biomed Res Trace Elements 2007, 18, 346–366. [Google Scholar] [CrossRef]
- Ma, Z.J.; Yamaguchi, M. Alternation in Bone Components with Increasing Age of Newborn Rats: Role of Zinc in Bone Growth. J. Bone Miner. Metab. 2000, 18, 264–270. [Google Scholar] [CrossRef]
- Hadley, K.B.; Newman, S.M.; Hunt, J.R. Dietary Zinc Reduces Osteoclast Resorption Activities and Increases Markers of Osteoblast Differentiation, Matrix Maturation, and Mineralization in the Long Bones of Growing Rats. J. Nutr. Biochem. 2010, 21, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Dallas, S.L.; Prideaux, M.; Bonewald, L.F. The Osteocyte: An Endocrine Cell … and More. Endocr. Rev. 2013, 34, 658–690. [Google Scholar] [CrossRef]
- Neve, A.; Corrado, A.; Cantatore, F.P. Osteoblast Physiology in Normal and Pathological Conditions. Cell Tissue Res. 2011, 343, 289–302. [Google Scholar] [CrossRef] [PubMed]
- Skiba, G.; Raj, S.; Sobol, M.; Kowalczyk, P.; Grela, E.R. Role of Polyphenols in the Metabolism of the Skeletal System in Humans and Animals—A Review. Ann. Anim. Sci. 2021, 21, 1275–1300. [Google Scholar] [CrossRef]
- Tomaszewska, E.; Dobrowolski, P.; Bieńko, M.; Prost, Ł.; Szymańczyk, S.; Zdybel, A. Effects of 2-Oxoglutaric Acid on Bone Morphometry, Densitometry, Mechanics, and Immunohistochemistry in 9-Month-Old Boars with Prenatal Dexamethasone-Induced Osteopenia. Connect. Tissue Res. 2015, 56, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Tomaszewska, E.; Donaldson, J.; Kosiński, J.; Dobrowolski, P.; Tomczyk-Warunek, A.; Hułas-Stasiak, M.; Lamorski, K.; Laskowska-Woźniak, D.; Muszyński, S.; Blicharski, R.; et al. β-Hydroxy-β-Methylbutyrate (HMB) Supplementation Prevents Bone Loss during Pregnancy—Novel Evidence from a Spiny Mouse (Acomys Cahirinus) Model. Int. J. Mol. Sci. 2021, 22, 3047. [Google Scholar] [CrossRef] [PubMed]
- Blicharski, T.; Tomaszewska, E.; Dobrowolski, P.; Hułas-Stasiak, M.; Muszyński, S. A Metabolite of Leucine (β-Hydroxy-β-Methylbutyrate) given to Sows during Pregnancy Alters Bone Development of Their Newborn Offspring by Hormonal Modulation. PLoS ONE 2017, 12, e0179693. [Google Scholar] [CrossRef]
- Tomaszewska, E.; Muszyński, S.; Dobrowolski, P.; Wiącek, D.; Tomczyk-Warunek, A.; Świetlicka, I.; Pierzynowski, S.G. Maternal HMB Treatment Affects Bone and Hyaline Cartilage Development in Their Weaned Piglets via the Leptin/Osteoprotegerin System. J. Anim. Physiol. Anim. Nutr. 2019, 103, 626–643. [Google Scholar] [CrossRef] [PubMed]
- Hofbauer, L.C.; Kühne, C.A.; Viereck, V. The OPG/RANKL/RANK System in Metabolic Bone Diseases. J. Musculoskelet. Neuronal Interact. 2004, 4, 268–275. [Google Scholar]
- Skugor, A.; Kjos, N.P.; Sundaram, A.Y.M.; Mydland, L.T.; Ånestad, R.; Tauson, A.-H.; Øverland, M. Effects of Long-Term Feeding of Rapeseed Meal on Skeletal Muscle Transcriptome, Production Efficiency and Meat Quality Traits in Norwegian Landrace Growing-Finishing Pigs. PLoS ONE 2019, 14, e0220441. [Google Scholar] [CrossRef] [PubMed]
- Wlazło, Ł.; Nowakowic-Dębek, B.; Ossowski, M.; Łukaszewicz, M.; Czech, A. Effect of Fermented Rapeseed Meal in Diets for Piglets on Blood Biochemical Parameters and the Microbial Composition of the Feed and Faeces. Animals 2022, 12, 2972. [Google Scholar] [CrossRef]


| Ingredient, % | Group | ||
|---|---|---|---|
| Contr | FRA | FR | |
| Wheat | 60.48 | 58.33 | 58.8 |
| Barley | 20 | 20 | 20 |
| Soybean meal, 46.5% CP | 9.24 | 3.46 | 3.32 |
| Fermented rapeseed meal (FRSM) | 0 | 8 | 8 |
| Fish meal, 65% | 4 | 4 | 4 |
| Soybean oil | 2.1 | 2.13 | 2.13 |
| Chalk 0.95 0.87 0.87 | 0.95 | 0.87 | 0.87 |
| L-Lysine_HCl, 78% | 0.82 | 0.91 | 0.91 |
| L-Threonine | 0.37 | 0.4 | 0.4 |
| DL-Methionine | 0.26 | 0.25 | 0.25 |
| Sodium chloride | 0.43 | 0.39 | 0.39 |
| Calcium monophosphate | 0.52 | 0.43 | 0.43 |
| Premix 1 | 0.5 | 0.5 | 0.5 |
| Feed additive 2 | 0.33 | 0.33 | 0 |
| Item | Group | ||
|---|---|---|---|
| Contr | FRA | FR | |
| ME, MJ/kg | 13.48 | 13.51 | 13.50 |
| Crude protein | 170.9 | 170.2 | 169.9 |
| Crude fat | 37.65 | 39.05 | 40.45 |
| Crude fibre | 27.14 | 29.64 | 30.14 |
| Crude ash | 51.83 | 56.85 | 51.27 |
| Ca | 7.07 | 7.00 | 7.14 |
| P | 5.47 | 5.54 | 5.43 |
| Phytin P | 2.47 | 2.08 | 2.04 |
| Fe | 0.204 | 0.209 | 0.205 |
| Cu | 0.015 | 0.016 | 0.015 |
| Zn | 0.167 | 0.169 | 0.040 |
| Lactic acid | 0.885 | 5.28 | 5.00 |
| Tannins | 7.13 | 3.22 | 4.98 |
| Glucosinolates, mmol/kg | 11.40 | 0.101 | 0.987 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muszyński, S.; Dajnowska, A.; Arciszewski, M.B.; Rudyk, H.; Śliwa, J.; Krakowiak, D.; Piech, M.; Nowakowicz-Dębek, B.; Czech, A. Effect of Fermented Rapeseed Meal in Feeds for Growing Piglets on Bone Morphological Traits, Mechanical Properties, and Bone Metabolism. Animals 2023, 13, 1080. https://doi.org/10.3390/ani13061080
Muszyński S, Dajnowska A, Arciszewski MB, Rudyk H, Śliwa J, Krakowiak D, Piech M, Nowakowicz-Dębek B, Czech A. Effect of Fermented Rapeseed Meal in Feeds for Growing Piglets on Bone Morphological Traits, Mechanical Properties, and Bone Metabolism. Animals. 2023; 13(6):1080. https://doi.org/10.3390/ani13061080
Chicago/Turabian StyleMuszyński, Siemowit, Aleksandra Dajnowska, Marcin B. Arciszewski, Halyna Rudyk, Jadwiga Śliwa, Dominika Krakowiak, Małgorzata Piech, Bożena Nowakowicz-Dębek, and Anna Czech. 2023. "Effect of Fermented Rapeseed Meal in Feeds for Growing Piglets on Bone Morphological Traits, Mechanical Properties, and Bone Metabolism" Animals 13, no. 6: 1080. https://doi.org/10.3390/ani13061080
APA StyleMuszyński, S., Dajnowska, A., Arciszewski, M. B., Rudyk, H., Śliwa, J., Krakowiak, D., Piech, M., Nowakowicz-Dębek, B., & Czech, A. (2023). Effect of Fermented Rapeseed Meal in Feeds for Growing Piglets on Bone Morphological Traits, Mechanical Properties, and Bone Metabolism. Animals, 13(6), 1080. https://doi.org/10.3390/ani13061080






