Expression of IkappaB Family in the Ovine Liver during Early Pregnancy
Abstract
Simple Summary
Abstract
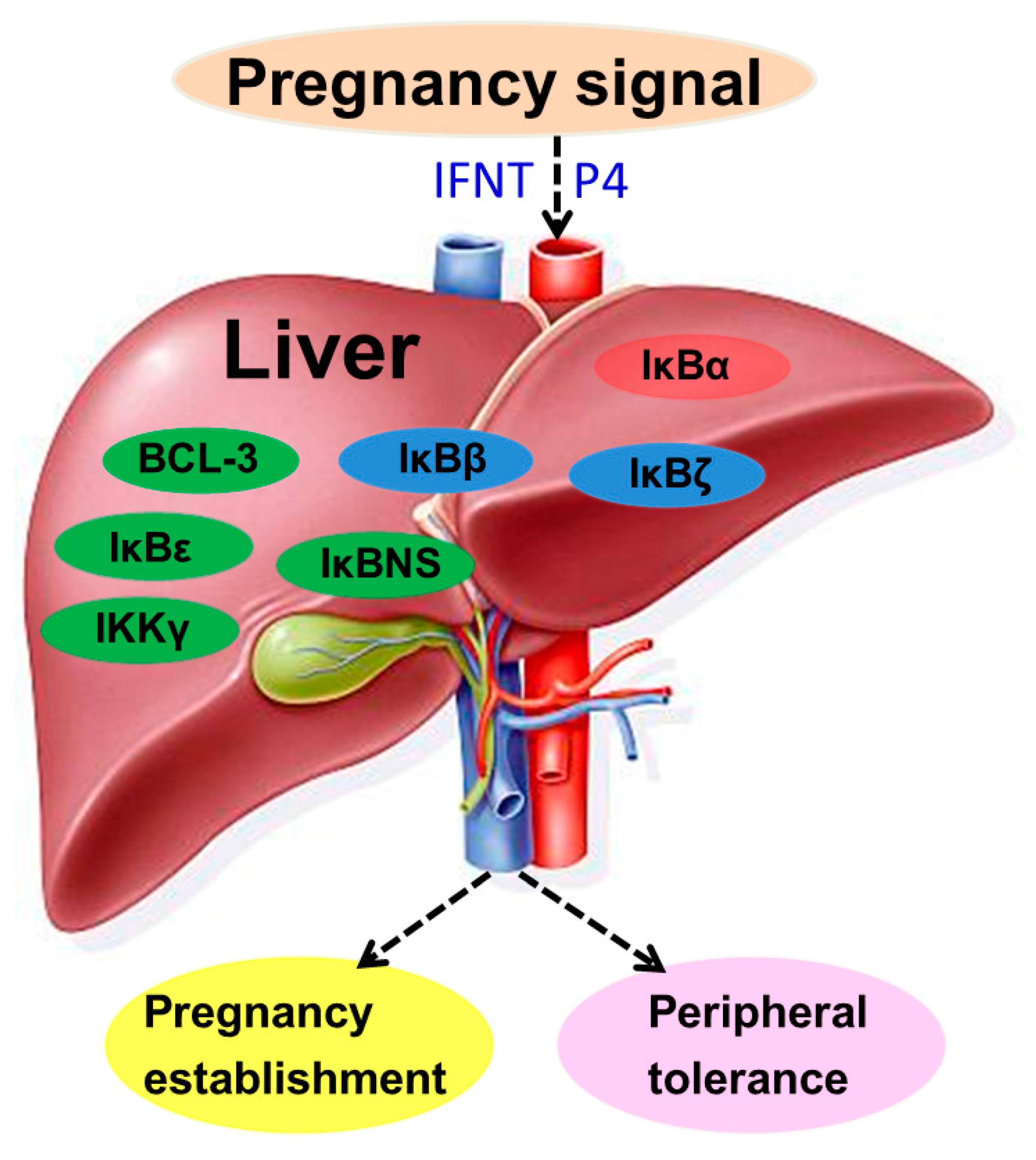
1. Introduction
2. Materials and Methods
2.1. Animal Tissue Collection
2.2. RT-qPCR Assay
2.3. Western Blot
2.4. Immunohistochemistry Analysis
2.5. Statistical Analysis
3. Results
3.1. Gene Expression of IκB Family in the Liver
3.2. Protein Expression of IκB Family in the Livers
3.3. Immunohistochemistry for IκBβ and IKKγ Proteins in the Livers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abu-Raya, B.; Michalski, C.; Sadarangani, M.; Lavoie, P.M. Maternal immunological adaptation during normal pregnancy. Front. Immunol. 2020, 11, 575197. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Wang, L.; Zhao, S.; Liu, H.; Chen, S.; Wu, L.; Liu, L.; Ding, J.; Yang, H.; Maxwell, A.; et al. Pregnancy induces an immunological memory characterized by maternal immune alterations through specific genes methylation. Front. Immunol. 2021, 12, 686676. [Google Scholar] [CrossRef] [PubMed]
- Quirke, L.D.; Maclean, P.H.; Haack, N.A.; Edwards, S.J.; Heiser, A.; Juengel, J.L. Characterization of local and peripheral immune system in pregnant and nonpregnant ewes. J. Anim. Sci. 2021, 99, skab208. [Google Scholar] [CrossRef] [PubMed]
- Rocha, C.C.; da Silveira, J.C.; Forde, N.; Binelli, M.; Pugliesi, G. Conceptus-modulated innate immune function during early pregnancy in ruminants: A review. Anim. Reprod. 2021, 18, e20200048. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Liu, B.; Yan, X.; Zhang, L.; Gao, F.; Liu, Z. Expression of ISG15 in bone marrow during early pregnancy in ewes. Kafkas Univ. Vet. Fak. Derg. 2017, 23, 767–772. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Mi, H.; Yan, J.K.; Yan, X.X.; Yang, L. Pregnancy-associated changes in expression of progesterone receptor and progesterone-induced blocking factor genes in bone marrow of ewes. Anim. Reprod. Sci. 2017, 186, 77–84. [Google Scholar] [CrossRef]
- Zhang, L.; Xue, J.; Wang, Q.; Lv, W.; Mi, H.; Liu, Y.; Yang, L. Changes in expression of ISG15, progesterone receptor and progesterone-induced blocking factor in ovine thymus during early pregnancy. Theriogenology 2018, 121, 153–159. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, Z.; Wang, Y.; Li, N.; Cao, N.; Yang, L. Changes in expression of interferon-stimulated genes and ubiquitin activating enzyme E1-like in ovine thymus during early pregnancy. Anim. Reprod. 2020, 17, e20190134. [Google Scholar] [CrossRef]
- Yang, L.; Liu, Y.; Lv, W.; Wang, P.; Wang, B.; Xue, J.; Zhang, L. Expression of interferon-stimulated gene 15-kDa protein, cyclooxygenase (COX) 1, COX-2, aldo-keto reductase family 1, member B1, and prostaglandin E synthase in the spleen during early pregnancy in sheep. Anim. Sci. J. 2018, 89, 1540–1548. [Google Scholar] [CrossRef]
- Yang, L.; Guo, R.; Yao, X.; Yan, J.; Bai, Y.; Zhang, L. Expression of progesterone receptor and progesterone-induced blocking factor in the spleen during early pregnancy in ewes. Livest. Sci. 2018, 209, 14–19. [Google Scholar] [CrossRef]
- Wang, Y.; Han, X.; Zhang, L.; Cao, N.; Cao, L.; Yang, L. Early pregnancy induces expression of STAT1, OAS1 and CXCL10 in ovine spleen. Animals 2019, 9, 882. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, Q.; Liu, Y.; Zhang, L.; Lv, W.; Liu, B. Expression profiles of interferon-stimulated gene 15 and prostaglandin synthases in the ovine lymph nodes during early pregnancy. Mol. Reprod. Dev. 2019, 86, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Cao, L.; Yang, F.; Han, X.; Wang, Y.; Cao, N.; Yang, L. Relative abundance of interferon-stimulated genes STAT1, OAS1, CXCL10 and MX1 in ovine lymph nodes during early pregnancy. Anim. Reprod. Sci. 2020, 214, 106285. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zang, S.; Bai, Y.; Yao, X.; Zhang, L. Effect of early pregnancy on the expression of progesterone receptor and progesterone-induced blocking factor in ovine lymph node. Theriogenology 2017, 93, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Tian, Z. Liver-mediated adaptive immune tolerance. Front. Immunol. 2019, 10, 2525. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, A.Q.; Vesco, K.K.; Purnell, J.Q.; Francisco, M.; Goddard, E.; Guan, X.; DeBarber, A.; Leo, M.C.; Baetscher, E.; Rooney, W.; et al. Pregnancy and weaning regulate human maternal liver size and function. Proc. Natl. Acad. Sci. USA 2021, 118, e2107269118. [Google Scholar] [CrossRef]
- Garczyńska, K.; Tzschätzsch, H.; Kühl, A.A.; Morr, A.S.; Lilaj, L.; Häckel, A.; Schellenberger, E.; Berndt, N.; Holzhütter, H.G.; Braun, J.; et al. Changes in liver mechanical properties and water diffusivity during normal pregnancy are driven by cellular hypertrophy. Front. Physiol. 2020, 11, 605205. [Google Scholar] [CrossRef]
- Rosato, R.; Lindenbergh-Kortleve, D.; Neck, J.; Drop, S.; Jahn, G. Effect of chronic thyroxine treatment on IGF-I, IGF-II and IGF-binding protein expression in mammary gland and liver during pregnancy and early lactation in rats. Eur. J. Endocrinol. 2002, 146, 729–739. [Google Scholar] [CrossRef]
- Yang, L.; Han, X.; Zhang, L.; Li, N.; Zhao, Z.; Bai, J. Changes in expression of prostaglandin synthase in ovine liver during early pregnancy. Can. J. Anim. Sci. 2020, 100, 432–439. [Google Scholar] [CrossRef]
- Yang, L.; Bai, J.; Zhao, Z.; Li, N.; Wang, Y.; Zhang, L. Differential expression of T helper cytokines in the liver during early pregnancy in sheep. Anim. Reprod. 2019, 16, 332–339. [Google Scholar] [CrossRef]
- Bai, J.; Zhang, L.; Zhao, Z.; Li, N.; Wang, B.; Yang, L. Expression of melatonin receptors and CD4 in the ovine thymus, lymph node, spleen and liver during early pregnancy. Immunology 2020, 160, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Cao, N.; Cao, L.; Gao, M.; Wang, H.; Zhang, L.; Yang, L. Changes in mRNA and protein levels of gonadotropin releasing hormone and receptor in ovine thymus, lymph node, spleen, and liver during early pregnancy. Domest. Anim. Endocrinol. 2021, 76, 106607. [Google Scholar] [CrossRef] [PubMed]
- Feng, P.; Wu, J.; Ren, Y.; Zhang, L.; Cao, J.; Yang, L. Early pregnancy regulates the expression of prolactin and its receptor in the thymus, the liver, the spleen and lymph nodes in sheep. Domest. Anim. Endocrinol. 2022, 81, 106731. [Google Scholar] [CrossRef]
- Gao, M.; Cai, C.; Han, X.; Wang, L.; Zhang, W.; Zhang, L.; Yang, L. The early stage of pregnancy modulates toll-like receptor signaling in the ovine liver. J. Appl. Anim. Res. 2021, 49, 374–381. [Google Scholar] [CrossRef]
- Feng, P.; Yang, G.; Zhang, W.; Zhang, L.; Wu, J.; Yang, L. Early pregnancy regulates expression of complement components in ovine liver. Anim. Sci. J. 2021, 92, e13660. [Google Scholar] [CrossRef]
- Fang, S.; Zhang, T.; Qiao, H.; Hao, S.; Zhang, L.; Yang, L. Expression of nuclear factor kappa B components in the ovine maternal liver in early pregnancy periods. Anim. Sci. J. 2022, 93, e13724. [Google Scholar] [CrossRef] [PubMed]
- Hao, S.; Fang, H.; Fang, S.; Zhang, T.; Zhang, L.; Yang, L. Changes in nuclear factor kappa B components expression in the ovine spleen during early pregnancy. J. Anim. Feed. Sci. 2022, 31, 3–11. [Google Scholar] [CrossRef]
- Yang, L.; Cai, C.; Fang, S.; Hao, S.; Zhang, T.; Zhang, L. Changes in expression of nuclear factor kappa B subunits in the ovine thymus during early pregnancy. Sci. Rep. 2022, 12, 17683. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, T.; Yang, Z.; Cai, C.; Hao, S.; Yang, L. Expression of nuclear factor kappa B in ovine maternal inguinal lymph nodes during early pregnancy. BMC Vet. Res. 2022, 18, 266. [Google Scholar] [CrossRef]
- Yamauchi, S.; Ito, H.; Miyajima, A. IkappaBeta, a nuclear IkappaB protein, positively regulates the NF-kappaB-mediated expression of proinflammatory cytokines. Proc. Natl. Acad. Sci. USA 2010, 107, 11924–11929. [Google Scholar] [CrossRef]
- Sierra-Mondragón, E.; Gómez-Chávez, F.; Murrieta-Coxca, M.; Vázquez-Sánchez, E.A.; Martínez-Torres, I.; Cancino-Díaz, M.E.; Rojas-Espinosa, O.; Cancino-Díaz, J.C.; Reyes-Sánchez, J.L.; Rodríguez-Muñóz, R.; et al. Low expression of IL-6 and TNF-α correlates with the presence of the nuclear regulators of NF-κB, IκBNS and BCL-3, in the uterus of mice. Mol. Immunol. 2015, 68, 333–340. [Google Scholar] [CrossRef] [PubMed]
- McCracken, S.A.; Drury, C.L.; Lee, H.S.; Morris, J.M. Pregnancy is associated with suppression of the nuclear factor kappaB/IkappaB activation pathway in peripheral blood mononuclear cells. J. Reprod. Immunol. 2003, 58, 27–47. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; MacIntyre, D.A.; Firmino Da Silva, M.; Blanks, A.M.; Lee, Y.S.; Thornton, S.; Bennett, P.R.; Terzidou, V. Oxytocin activates NF-κB-mediated inflammatory pathways in human gestational tissues. Mol. Cell Endocrinol. 2015, 403, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Palmer, S.; Chen, Y.H. Bcl-3, a multifaceted modulator of NF-kappaB-mediated gene transcription. Immunol. Res. 2008, 42, 210–218. [Google Scholar] [CrossRef]
- Gómez-Chávez, F.; Correa, D.; Navarrete-Meneses, P.; Cancino-Diaz, J.C.; Cancino-Diaz, M.E.; Rodríguez-Martínez, S. NF-κB and its regulators during pregnancy. Front. Immunol. 2021, 12, 679106. [Google Scholar] [CrossRef]
- Muggia, A.; Teesalu, T.; Neri, A.; Blasi, F.; Talarico, D. Trophoblast giant cells express NF-kappa B2 during early mouse development. Dev. Genet. 1999, 25, 23–30. [Google Scholar] [CrossRef]
- Hoffmann, A.; Levchenko, A.; Scott, M.L.; Baltimore, D. The IkappaB-NF-kappaB signaling module: Temporal control and selective gene activation. Science 2002, 298, 1241–1245. [Google Scholar] [CrossRef]
- Mendelson, C.R.; Gao, L.; Montalbano, A.P. Multifactorial regulation of myometrial contractility during pregnancy and parturition. Front. Endocrinol. 2019, 10, 714. [Google Scholar] [CrossRef]
- Zhang, L.; Zhuang, C.; Zhao, Z.; Li, N.; Bai, J.; Yang, L. Effect of early pregnancy on the expression of progesterone receptor and progesterone-induced blocking factor 1 in ovine liver. Czech J. Anim. Sci. 2019, 64, 317–323. [Google Scholar] [CrossRef]
- Zhang, H.; Chu, X.; Huang, Y.; Li, G.; Wang, Y.; Li, Y.; Sun, C. Maternal vitamin D deficiency during pregnancy results in insulin resistance in rat offspring, which is associated with inflammation and Iκbα methylation. Diabetologia 2014, 57, 2165–2172. [Google Scholar] [CrossRef] [PubMed]
- Sheller-Miller, S.; Radnaa, E.; Yoo, J.K.; Kim, E.; Choi, K.; Kim, Y.; Kim, Y.N.; Richardson, L.; Choi, C.; Menon, R. Exosomal delivery of NF-κB inhibitor delays LPS-induced preterm birth and modulates fetal immune cell profile in mouse models. Sci. Adv. 2021, 7, eabd3865. [Google Scholar] [CrossRef] [PubMed]
- McKenna, S.; Wright, C.J. Inhibiting IκBβ-NFκB signaling attenuates the expression of select pro-inflammatory genes. J. Cell. Sci. 2015, 128, 2143–2155. [Google Scholar] [CrossRef] [PubMed]
- Shoji, S.; Hanada, K.; Takahashi, M.; Watanabe, K.; Yonemochi, M.; Tomabechi, Y.; Shirouzu, M. The NF-κB regulator IκBβ exhibits different molecular interactivity and phosphorylation status from IκBα in an IKK2-catalysed reaction. FEBS Lett. 2020, 594, 1532–1549. [Google Scholar] [CrossRef] [PubMed]
- Sakowicz, A.; Bralewska, M.; Pietrucha, T.; Habrowska-Górczyńska, D.E.; Piastowska-Ciesielska, A.W.; Gach, A.; Rybak-Krzyszkowska, M.; Witas, P.J.; Huras, H.; Grzesiak, M.; et al. Canonical, non-canonical and atypical pathways of nuclear factor кb activation in preeclampsia. Int. J. Mol. Sci. 2020, 21, 5574. [Google Scholar] [CrossRef]
- Yang, L.; Li, N.; Zhang, L.; Bai, J.; Zhao, Z.; Wang, Y. Effects of early pregnancy on expression of interferon-stimulated gene 15, STAT1, OAS1, MX1, and IP-10 in ovine liver. Anim. Sci. J. 2020, 91, e13378. [Google Scholar] [CrossRef]
- Tam, W.F.; Sen, R. IkappaB family members function by different mechanisms. J. Biol. Chem. 2001, 276, 7701–7704. [Google Scholar] [CrossRef]
- Alves, B.N.; Tsui, R.; Almaden, J.; Shokhirev, M.N.; Davis-Turak, J.; Fujimoto, J.; Birnbaum, H.; Ponomarenko, J.; Hoffmann, A. IκBε is a key regulator of B cell expansion by providing negative feedback on cRel and RelA in a stimulus-specific manner. J. Immunol. 2014, 192, 3121–3132. [Google Scholar] [CrossRef]
- Ramsey, K.M.; Chen, W.; Marion, J.D.; Bergqvist, S.; Komives, E.A. Exclusivity and Compensation in NFκB Dimer Distributions and IκB Inhibition. Biochemistry 2019, 58, 2555–2563. [Google Scholar] [CrossRef]
- Gieling, R.G.; Elsharkawy, A.M.; Caamaño, J.H.; Cowie, D.E.; Wright, M.C.; Ebrahimkhani, M.R.; Burt, A.D.; Mann, J.; Raychaudhuri, P.; Liou, H.C.; et al. The c-Rel subunit of nuclear factor-kappaB regulates murine liver inflammation, wound-healing, and hepatocyte proliferation. Hepatology 2010, 51, 922–931. [Google Scholar] [CrossRef]
- Blanchett, S.; Boal-Carvalho, I.; Layzell, S.; Seddon, B. NF-κB and extrinsic cell death pathways—Entwined do-or-die decisions for T cells. Trends Immunol. 2021, 42, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Sakowicz, A.; Hejduk, P.; Pietrucha, T.; Nowakowska, M.; Płuciennik, E.; Pospiech, K.; Gach, A.; Rybak-Krzyszkowska, M.; Sakowicz, B.; Kaminski, M.; et al. Finding NEMO in preeclampsia. Am. J. Obstet. Gynecol. 2016, 214, e1–e538. [Google Scholar] [CrossRef] [PubMed]
- Sakowicz, A.; Lisowska, M.; Biesiada, L.; Płuciennik, E.; Gach, A.; Rybak-Krzyszkowska, M.; Huras, H.; Sakowicz, B.; Romanowicz, H.; Piastowska-Ciesielska, A.W.; et al. Placental expression of NEMO protein in normal pregnancy and preeclampsia. Dis. Markers 2019, 2019, 8418379. [Google Scholar] [CrossRef]
- Huh, J.Y.; Saltiel, A.R. Roles of IκB kinases and TANK-binding kinase 1 in hepatic lipid metabolism and nonalcoholic fatty liver disease. Exp. Mol. Med. 2021, 53, 1697–1705. [Google Scholar] [CrossRef] [PubMed]
- Arra, M.; Swarnkar, G.; Alippe, Y.; Mbalaviele, G.; Abu-Amer, Y. IκB-ζ signaling promotes chondrocyte inflammatory phenotype, senescence, and erosive joint pathology. Bone Res. 2022, 10, 12. [Google Scholar] [CrossRef]
- He, Y.; Feng, D.; Hwang, S.; Mackowiak, B.; Wang, X.; Xiang, X.; Rodrigues, R.M.; Fu, Y.; Ma, J.; Ren, T.; et al. Interleukin-20 exacerbates acute hepatitis and bacterial infection by downregulating IκBζ target genes in hepatocytes. J. Hepatol. 2021, 75, 163–176. [Google Scholar] [CrossRef]
- Ishikawa, H.; Hayakawa, M.; Baatartsogt, N.; Kakizawa, N.; Ohto-Ozaki, H.; Maruyama, T.; Miura, K.; Suzuki, K.; Rikiyama, T.; Ohmori, T. IκBζ regulates the development of nonalcoholic fatty liver disease through the attenuation of hepatic steatosis in mice. Sci. Rep. 2022, 12, 11634. [Google Scholar] [CrossRef]
- Gómez-Chávez, F.; Castro-Leyva, V.; Espejel-Núñez, A.; Zamora-Mendoza, R.G.; Rosas-Vargas, H.; Cancino-Díaz, J.C.; Cancino-Díaz, M.E.; Estrada-Gutierrez, G.; Rodríguez-Martínez, S. Galectin-1 reduced the effect of LPS on the IL-6 production in decidual cells by inhibiting LPS on the stimulation of IκBζ. J. Reprod. Immunol. 2015, 112, 46–52. [Google Scholar] [CrossRef]
- Hosokawa, J.; Suzuki, K.; Meguro, K.; Tanaka, S.; Maezawa, Y.; Suto, A.; Fujimura, L.; Sakamoto, A.; Clevers, H.; Ohara, O.; et al. IκBNS enhances follicular helper T-cell differentiation and function downstream of ASCl2. J. Allergy Clin. Immunol. 2017, 140, 288–291.e8. [Google Scholar] [CrossRef]
- Erikson, E.; Ádori, M.; Khoenkhoen, S.; Zhang, J.; Rorbach, J.; Castro Dopico, X.; Karlsson Hedestam, G. Impaired plasma cell differentiation associates with increased oxidative metabolism in IκBNS-deficient B cells. Cell Immunol. 2022, 375, 104516. [Google Scholar] [CrossRef]
- Frentzel, S.; Katsoulis-Dimitriou, K.; Jeron, A.; Schmitz, I.; Bruder, D. Essential role of IκBNS for in vivo CD4+ T-cell activation, proliferation, and Th1-cell differentiation during Listeria monocytogenes infection in mice. Eur. J. Immunol. 2019, 49, 1391–1398. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Chávez, F.; López-Portales, Ó.H.; Baeza-Martínez, D.A.; Cancino-Díaz, J.C.; Murrieta-Coxca, J.M.; Cancino-Díaz, M.E.; Pérez-Tapia, S.M.; Rodríguez-Martínez, S. IκBNS and IL-6 expression is differentially established in the uterus of pregnant healthy and infected mice. Heliyon 2020, 6, e04122. [Google Scholar] [CrossRef] [PubMed]



| Gene | Primer | Sequence | Size (bp) | Accession Numbers |
|---|---|---|---|---|
| BCL-3 | Forward | GCGACCAGAGGCAATTTACTACCAG | 98 | XM_027978453.2 |
| Reverse | GAGGTGTAGGCAAGTTCAGCAGAG | |||
| NFKBIA | Forward | AGGACGAGGAGTATGAGCAGATGG | 130 | NM_001166184.1 |
| Reverse | GCCAAGTGCAGGAACGAGTCTC | |||
| NFKBIB | Forward | CCCCAAGACCTACCTCGCTCAG | 119 | XM_027978262.2 |
| Reverse | TCCAGTCCTCTTCACTCTCATCCTC | |||
| NFKBIE | Forward | GCACTCACGTACATTTCCGAGGAC | 97 | XM_042236979.1 |
| Reverse | GCAGCAGAGCCAGGCAATACAG | |||
| IKBKG | Forward | GGGCAACCAGAGGGAGGAGAAG | 146 | XM_027963334.2 |
| Reverse | GGCATGTCTTCAGGCGTTCCAC | |||
| NFKBIZ | Forward | GCAAAGGCGTACAATGGAAACACC | 137 | NM_001306117.1 |
| Reverse | GGCTGCTCGTTCTCCAAGTTCC | |||
| NFKBID | Forward | ACATTCGTGAGCATAAGGGCAAGAC | 114 | XM_027977435.2 |
| Reverse | GATGGTCAGTGGCATTGGGTTCC | |||
| GAPDH | Forward | GGGTCATCATCTCTGCACCT | 176 | NM_001190390.1 |
| Reverse | GGTCATAAGTCCCTCCACGA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, C.; Ren, Y.; Cao, J.; Fang, S.; Zhang, L.; Yang, L. Expression of IkappaB Family in the Ovine Liver during Early Pregnancy. Animals 2023, 13, 1057. https://doi.org/10.3390/ani13061057
Cai C, Ren Y, Cao J, Fang S, Zhang L, Yang L. Expression of IkappaB Family in the Ovine Liver during Early Pregnancy. Animals. 2023; 13(6):1057. https://doi.org/10.3390/ani13061057
Chicago/Turabian StyleCai, Chunjiang, Ying Ren, Jianhua Cao, Shengya Fang, Leying Zhang, and Ling Yang. 2023. "Expression of IkappaB Family in the Ovine Liver during Early Pregnancy" Animals 13, no. 6: 1057. https://doi.org/10.3390/ani13061057
APA StyleCai, C., Ren, Y., Cao, J., Fang, S., Zhang, L., & Yang, L. (2023). Expression of IkappaB Family in the Ovine Liver during Early Pregnancy. Animals, 13(6), 1057. https://doi.org/10.3390/ani13061057





