The Oral Inactivated Porcine Epidemic Diarrhea Virus Presenting in the Intestine Induces Mucosal Immunity in Mice with Alginate–Chitosan Microcapsules
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell and Virus
2.2. The Production of Microcapsules
2.3. Analysis of Encapsulation Efficiency and Protein Content
2.4. In Vitro Release Experiment of Microcapsules in Different Saline Solutions, pH Values, and Room Temperature Storage Tolerances
2.5. Immunization of Microcapsules in SPF Mice
2.6. Enzyme-Linked Immunosorbent Assay (ELISA)
2.7. PEDV Neutralization Assay
2.8. T-Cell Proliferation
2.9. Flow Cytometry and Cell Sorting
2.10. Cytokine Assays
2.11. Statistical Analysis
2.12. Ethical Statement
3. Results
3.1. The Excellent Storage Tolerances of Microcapsules in Different Saline Solutions, pH Values, and Room Temperature
3.2. The Microcapsules Stimulate the Specific Humoral and Mucosal Immunity
3.3. The Specific IgG and IgA Neutralized PEDV
3.4. PEDV Microcapsules Stimulated Immune Memory
3.5. Microcapsules Enhanced the B Cell Differentiation
3.6. Microcapsules Inhibit the Inflammation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jung, K.; Saif, L.J. Porcine epidemic diarrhea virus infection: Etiology, epidemiology, pathogenesis and immunoprophylaxis. Vet. J. 2015, 204, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Langel, S.N.; Paim, F.C.; Lager, K.M.; Vlasova, A.N.; Saif, L.J. Lactogenic immunity and vaccines for porcine epidemic diarrhea virus (PEDV): Historical and current concepts. Virus Res. 2016, 226, 93–107. [Google Scholar] [CrossRef]
- Scruggs, A.K.; Cioffi, E.A.; Cioffi, D.L.; King, J.A.; Bauer, N.N. Lectin-Based Characterization of Vascular Cell Microparticle Glycocalyx. PLoS ONE 2015, 10, e0135533. [Google Scholar] [CrossRef]
- Jhunjhunwala, S.; Raimondi, G.; Thomson, A.W.; Little, S.R. Delivery of rapamycin to dendritic cells using degradable microparticles. J. Control. Release 2009, 133, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Hou, X.; Tang, L.; Jiang, Y.; Ma, G.; Li, Y. A phase trial of the oral Lactobacillus casei vaccine polarizes Th2 cell immunity against transmissible gastroenteritis coronavirus infection. Appl. Microbiol. Biotechnol. 2016, 100, 7457–7469. [Google Scholar] [CrossRef]
- Jiang, X.; Yu, M.; Qiao, X.; Liu, M.; Tang, L.; Jiang, Y.; Cui, W.; Li, Y. Up-regulation of MDP and tuftsin gene expression in Th1 and Th17 cells as an adjuvant for an oral Lactobacillus casei vaccine against anti-transmissible gastroenteritis virus. Appl. Microbiol. Biotechnol. 2014, 98, 8301–8312. [Google Scholar] [CrossRef]
- Zhang, H.; Ma, W.; Sun, Z.; Zhu, C.; Werid, G.M.; Ibrahim, Y.M.; Zhang, W.; Pan, Y.; Shi, D.; Chen, H.; et al. Abundance of Lactobacillus in porcine gut microbiota is closely related to immune response following PRRSV immunization. Vet. Microbiol. 2021, 259, 109134. [Google Scholar] [CrossRef]
- Moser, C.A.; Speaker, T.J.; Offit, P.A. Effect of water-based microencapsulation on protection against EDIM rotavirus challenge in mice. J. Virol. 1998, 72, 3859–3862. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.M.; Helm, E.T.; Groeltz-Thrush, J.M.; Gabler, N.K.; Burrough, E.R. Epithelial-mesenchymal transition of absorptive enterocytes and depletion of Peyer’s patch M cells after PEDV infection. Virology 2021, 552, 43–51. [Google Scholar] [CrossRef]
- Wang, Z.; Jiyuan, Y.; Su, C.; Xinyuan, Q.; Lijie, T.; Yijing, L. Development of an antigen capture enzyme-linked immunosorbent assay for virus detection based on porcine epidemic diarrhea virus monoclonal antibodies. Viral. Immunol. 2015, 28, 184–189. [Google Scholar] [CrossRef]
- Goldring, J.P.D. Measuring Protein Concentration with Absorbance, Lowry, Bradford Coomassie Blue, or the Smith Bicinchoninic Acid Assay Before Electrophoresis. Methods Mol. Biol. 2019, 1855, 31–39. [Google Scholar] [CrossRef]
- Dai, C.; Wang, B.; Zhao, H.; Li, B. Factors affecting protein release from microcapsule prepared by liposome in alginate. Colloids Surf. B Biointerfaces 2005, 42, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Oviedo, M.J.; Quester, K.; Hirata, G.A.; Vazquez-Duhalt, R. Determination of conjugated protein on nanoparticles by an adaptation of the Coomassie blue dye method. MethodsX 2019, 6, 2134–2140. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Xia, S.; He, X.; Ma, H.; Feng, Y.; Liu, Z.; Wang, W.; Tian, M.; Chen, H.; Peng, F.; et al. Targeting peptide-enhanced antibody and CD11c(+) dendritic cells to inclusion bodies expressing protective antigen against ETEC in mice. FASEB J. 2019, 33, 2836–2847. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Pan, Y.; Xu, Y.; Zhang, W.; Zhang, L.; Li, X.; Tian, Z.; Chen, H.; Wang, Y. Unveiling the long non-coding RNA profile of porcine reproductive and respiratory syndrome virus-infected porcine alveolar macrophages. BMC Genomics 2021, 22, 177. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zeng, X.; Zhang, X.; Liu, H.; Xing, H. Ammonia exposure induces oxidative stress and inflammation by destroying the microtubule structures and the balance of solute carriers in the trachea of pigs. Ecotoxicol. Environ. Saf. 2021, 212, 111974. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Xu, J.; Guo, L.; Guo, T.; Zhang, L.; Feng, L.; Chen, H.; Wang, Y. Porcine Epidemic Diarrhea Virus-Induced Epidermal Growth Factor Receptor Activation Impairs the Antiviral Activity of Type I Interferon. J. Virol. 2018, 92. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Gu, S.; Liu, D.; Zhao, L.; Xia, S.; He, X.; Chen, H.; Ge, J. Lactobacillus brevis 23017 Relieves Mercury Toxicity in the Colon by Modulation of Oxidative Stress and Inflammation Through the Interplay of MAPK and NF-kappaB Signaling Cascades. Front. Microbiol. 2018, 9, 2425. [Google Scholar] [CrossRef]
- Su, M.; Li, C.; Qi, S.; Yang, D.; Jiang, N.; Yin, B.; Guo, D.; Kong, F.; Yuan, D.; Feng, L.; et al. A molecular epidemiological investigation of PEDV in China: Characterization of co-infection and genetic diversity of S1-based genes. Transbound. Emerg. Dis. 2020, 67, 1129–1140. [Google Scholar] [CrossRef]
- Ibrahim, Y.M.; Zhang, W.; Werid, G.M.; Zhang, H.; Pan, Y.; Zhang, L.; Xu, Y.; Li, C.; Chen, H.; Wang, Y. Characterization of parainfluenza virus 5 from diarrheic piglet highlights its zoonotic potential. Transbound. Emerg. Dis. 2022, 69, e1510–e1525. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, L.; Pan, Y.; Gao, J.; Xu, Y.; Li, X.; Tian, Z.; Chen, H.; Wang, Y. Downregulation of miR-218 by porcine reproductive and respiratory syndrome virus facilitates viral replication via inhibition of type I interferon responses. J. Biol. Chem. 2021, 296, 100683. [Google Scholar] [CrossRef]
- Furutani, A.; Sekiguchi, S.; Sueyoshi, M.; Sasaki, Y. Effect of intervention practices to control the porcine epidemic diarrhea (PED) outbreak during the first epidemic year (2013–2014) on time to absence of clinical signs and the number of dead piglets per sow in Japan. Prev. Vet. Med. 2019, 169, 104710. [Google Scholar] [CrossRef]
- VanCott, J.L.; Brim, T.A.; Simkins, R.A.; Saif, L.J. Isotype-specific antibody-secreting cells to transmissible gastroenteritis virus and porcine respiratory coronavirus in gut- and bronchus-associated lymphoid tissues of suckling pigs. J. Immunol. 1993, 150, 3990–4000. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.B.; Yang, W.T.; Shi, C.W.; Feng, B.; Huang, K.Y.; Zhao, G.X.; Li, Q.Y.; Xie, J.; Huang, H.B.; Jiang, Y.L.; et al. Immune responses induced by recombinant Lactobacillus plantarum expressing the spike protein derived from transmissible gastroenteritis virus in piglets. Appl. Microbiol. Biotechnol. 2018, 102, 8403–8417. [Google Scholar] [CrossRef] [PubMed]
- Langel, S.N.; Wang, Q.; Vlasova, A.N.; Saif, L.J. Host Factors Affecting Generation of Immunity Against Porcine Epidemic Diarrhea Virus in Pregnant and Lactating Swine and Passive Protection of Neonates. Pathogens 2020, 9, 130. [Google Scholar] [CrossRef]
- Sun, D.; Wang, X.; Wei, S.; Chen, J.; Feng, L. Epidemiology and vaccine of porcine epidemic diarrhea virus in China: A mini-review. J. Vet. Med. Sci. 2016, 78, 355–363. [Google Scholar] [CrossRef]
- Liu, J.; Gao, R.; Shi, H.; Cong, G.; Chen, J.; Zhang, X.; Shi, D.; Cao, L.; Wang, X.; Zhang, J.; et al. Development of a rapid immunochromatographic strip test for the detection of porcine epidemic diarrhea virus specific SIgA in colostrum. J. Virol. Methods 2020, 279, 113855. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Liu, Q.; Luo, H.; Liang, K.; Han, Y.; Roland, K.L.; Curtiss, R., 3rd; Kong, Q. Bi-valent polysaccharides of Vi capsular and O9 O-antigen in attenuated Salmonella Typhimurium induce strong immune responses against these two antigens. NPJ Vaccines 2018, 3, 1. [Google Scholar] [CrossRef]
- Tam, S.K.; de Haan, B.J.; Faas, M.M.; Halle, J.P.; Yahia, L.; de Vos, P. Adsorption of human immunoglobulin to implantable alginate-poly-L-lysine microcapsules: Effect of microcapsule composition. J. Biomed. Mater. Res. A 2009, 89, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Khorshidian, N.; Mahboubi, A.; Kalantari, N.; Hosseini, H.; Yousefi, M.; Arab, M.; da Cruz, A.G.; Mortazavian, A.M.; Mahdavi, F.S. Chitosan-Coated Alginate Microcapsules Loaded with Herbal galactagogue Extract: Formulation Optimization and Characterization. Iran. J. Pharm. Res. 2019, 18, 1180–1195. [Google Scholar] [CrossRef]
- Vinner, G.K.; Vladisavljevic, G.T.; Clokie, M.R.J.; Malik, D.J. Microencapsulation of Clostridium difficile specific bacteriophages using microfluidic glass capillary devices for colon delivery using pH triggered release. PLoS ONE 2017, 12, e0186239. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Pacan, J.C.; Wang, Q.; Xu, Y.; Huang, X.; Korenevsky, A.; Sabour, P.M. Microencapsulation of bacteriophage felix O1 into chitosan-alginate microspheres for oral delivery. Appl. Environ. Microbiol. 2008, 74, 4799–4805. [Google Scholar] [CrossRef] [PubMed]
- Campos, D.A.; Madureira, A.R.; Sarmento, B.; Gomes, A.M.; Pintado, M.M. Stability of bioactive solid lipid nanoparticles loaded with herbal extracts when exposed to simulated gastrointestinal tract conditions. Food Res. Int. 2015, 78, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Tan, P.Y.; Tan, T.B.; Chang, H.W.; Tey, B.T.; Chan, E.S.; Lai, O.M.; Baharin, B.S.; Nehdi, I.A.; Tan, C.P. Effects of storage and yogurt matrix on the stability of tocotrienols encapsulated in chitosan-alginate microcapsules. Food Chem. 2018, 241, 79–85. [Google Scholar] [CrossRef]
- Jiang, T.; Singh, B.; Maharjan, S.; Li, H.S.; Kang, S.K.; Bok, J.D.; Cho, C.S.; Choi, Y.J. Oral delivery of probiotic expressing M cell homing peptide conjugated BmpB vaccine encapsulated into alginate/chitosan/alginate microcapsules. Eur. J. Pharm. Biopharm. 2014, 88, 768–777. [Google Scholar] [CrossRef] [PubMed]
- Cebron, N.; Maman, S.; Walachowski, S.; Gausseres, B.; Cunha, P.; Rainard, P.; Foucras, G. Th17-related mammary immunity, but not a high systemic Th1 immune response is associated with protection against E. coli mastitis. NPJ Vaccines 2020, 5, 108. [Google Scholar] [CrossRef]
- Hou, X.; Jiang, X.; Jiang, Y.; Tang, L.; Xu, Y.; Qiao, X.; Min, L.; Wen, C.; Ma, G.; Li, Y. Oral Immunization against PEDV with Recombinant Lactobacillus casei Expressing Dendritic Cell-Targeting Peptide Fusing COE Protein of PEDV in Piglets. Viruses 2018, 10, 106. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.; Choi, I.S.; Han, J.H.; Yoo, H.S. Chitosan and D-glucosamine induce expression of Th1 cytokine genes in porcine spleen cells. J. Vet. Med. Sci. 2002, 64, 645–648. [Google Scholar] [CrossRef]
- Wen, Z.S.; Xu, Y.L.; Zou, X.T.; Xu, Z.R. Chitosan nanoparticles act as an adjuvant to promote both Th1 and Th2 immune responses induced by ovalbumin in mice. Mar. Drugs 2011, 9, 1038–1055. [Google Scholar] [CrossRef]
- Li, G.P.; Liu, Z.G.; Liao, B.; Zhong, N.S. Induction of Th1-type immune response by chitosan nanoparticles containing plasmid DNA encoding house dust mite allergen Der p 2 for oral vaccination in mice. Cell. Mol. Immunol. 2009, 6, 45–50. [Google Scholar] [CrossRef]
- Raghuwanshi, D.; Mishra, V.; Das, D.; Kaur, K.; Suresh, M.R. Dendritic cell targeted chitosan nanoparticles for nasal DNA immunization against SARS CoV nucleocapsid protein. Mol. Pharm. 2012, 9, 946–956. [Google Scholar] [CrossRef] [PubMed]
- White, A.L.; Tutt, A.L.; James, S.; Wilkinson, K.A.; Castro, F.V.; Dixon, S.V.; Hitchcock, J.; Khan, M.; Al-Shamkhani, A.; Cunningham, A.F.; et al. Ligation of CD11c during vaccination promotes germinal centre induction and robust humoral responses without adjuvant. Immunology 2010, 131, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Hori, Y.; Winans, A.M.; Huang, C.C.; Horrigan, E.M.; Irvine, D.J. Injectable dendritic cell-carrying alginate gels for immunization and immunotherapy. Biomaterials 2008, 29, 3671–3682. [Google Scholar] [CrossRef] [PubMed]
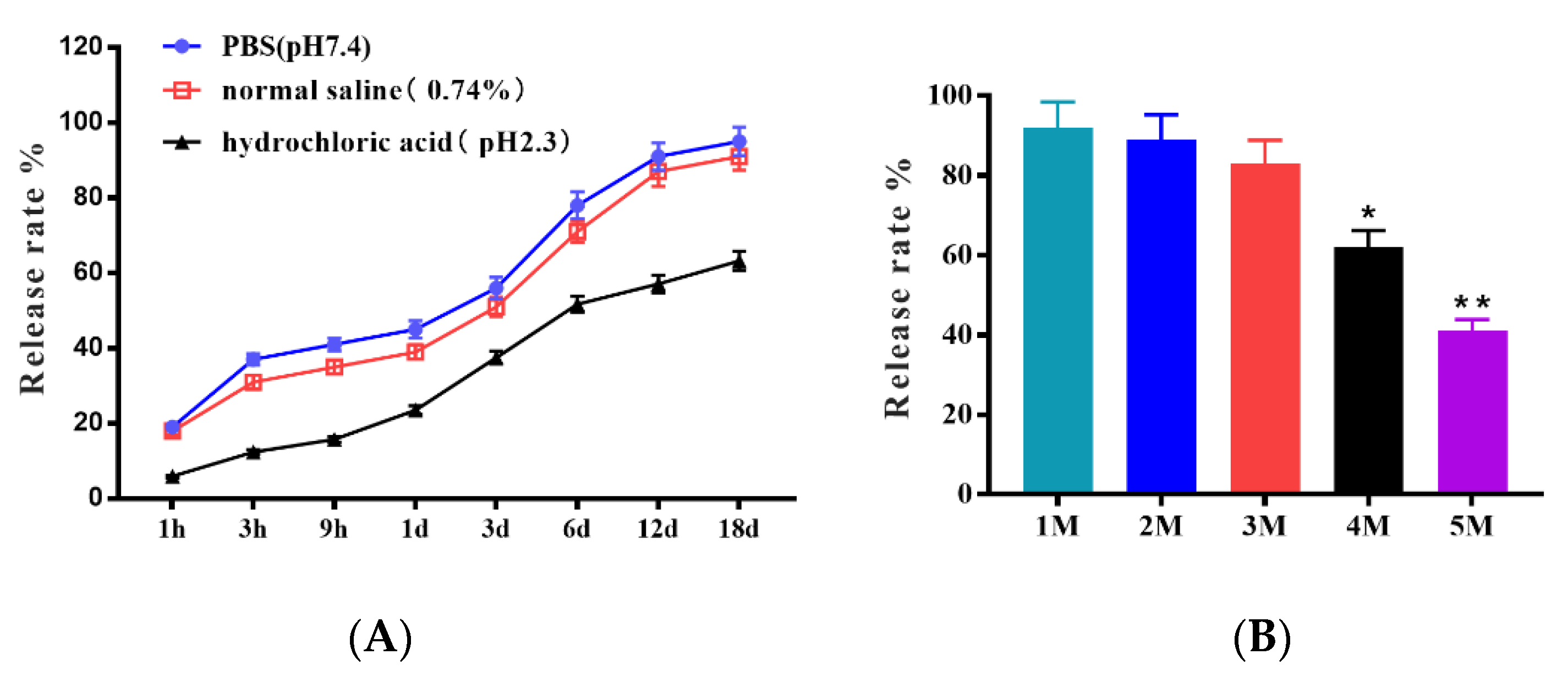
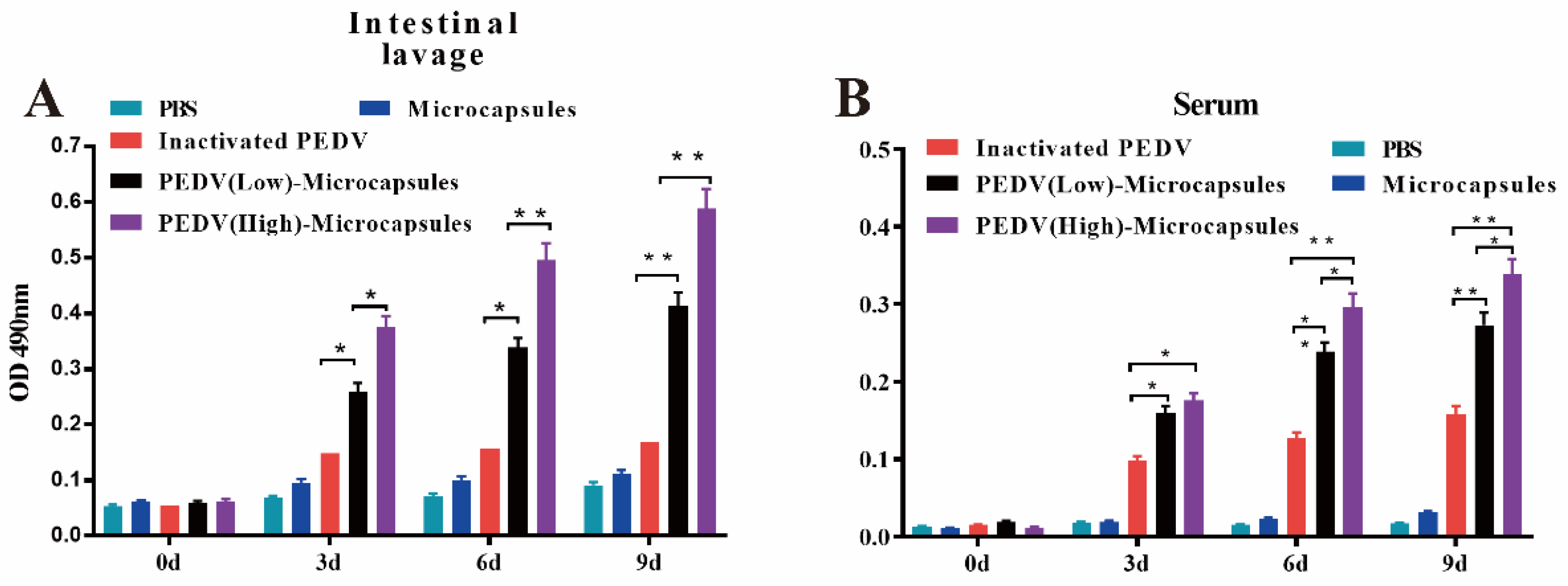


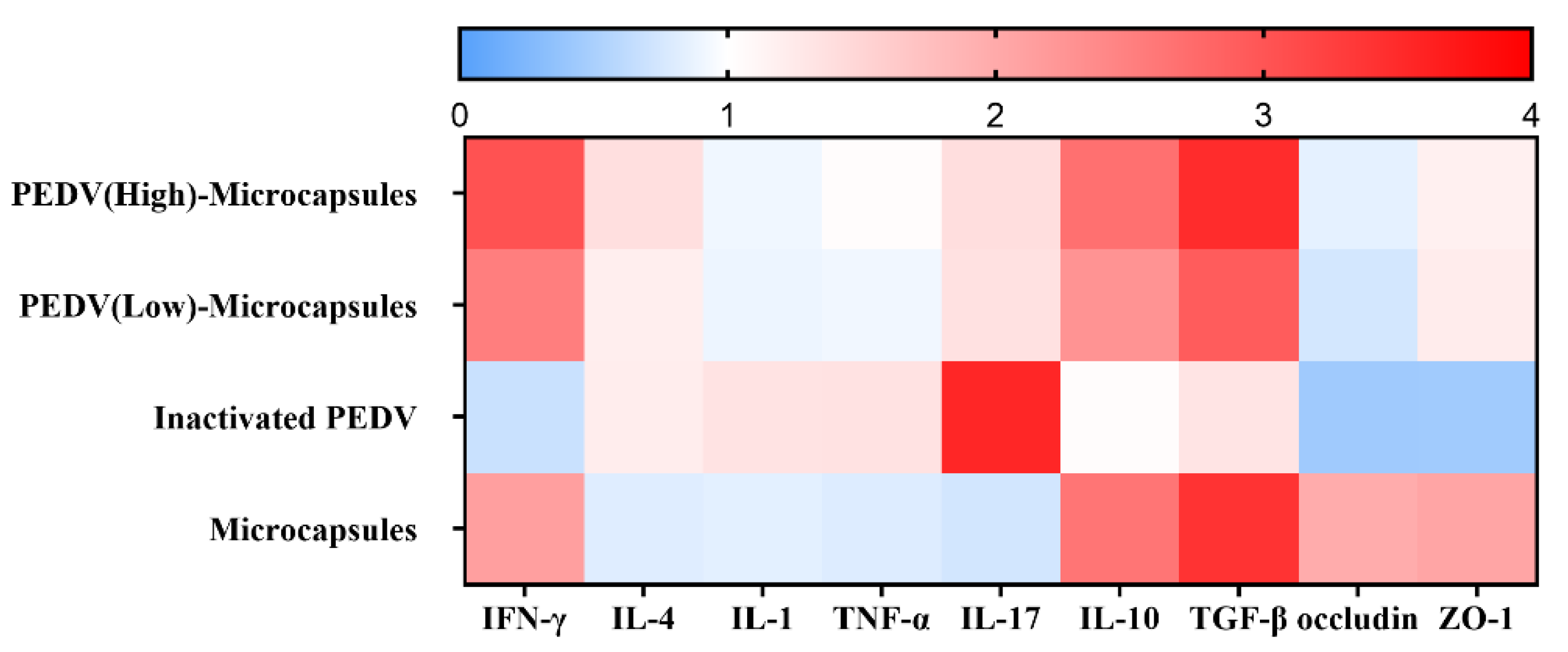
| Groups (n) | Type of Vaccine | 1st Vaccination | 2nd Vaccination | ||
|---|---|---|---|---|---|
| Route | Dose | Route | Dose | ||
| PBS (10) | Negative control of PBS buffer | O/A | 0.2 mL | O/A | 0.2 mL |
| Microcapsules (10) | PBS encapsulated in alginate and chitosan | O/A | 0.2 mL | O/A | 0.2 mL |
| Inactivated PEDV(10) | Inactivated PEDV vaccine | O/A | 0.2 mL | O/A | 0.2 mL |
| PEDV (low) microcapsule (10) | PEDV vaccine encapsulated in alginate and chitosan with low titer virus (6 × 106 PFU) | O/A | 0.2 mL | O/A | 0.2 mL |
| PEDV (high) microcapsule (10) | PEDV vaccine encapsulated in alginate and chitosan with high titer virus (6 × 107 PFU) | O/A | 0.2 mL | O/A | 0.2 mL |
| Groups | Stimulation Index Value | |
|---|---|---|
| 0.5 μg/mL PEDV | 1 μg/mL PEDV | |
| PBS(Control) | 1.054 ± 0. 13 | 1.025 ± 0.11 |
| Microcapsules | 1.272 ± 0. 149 | 1.231 ± 0.156 |
| Inactivated PEDV | 1.349 ± 0.151 | 1.358 ± 0.166 |
| PEDV (low) microcapsule | 1.721 ± 0.171 * | 2.529 ± 0.183 ** |
| PEDV (high) microcapsule | 1.987 ± 0.177 ** | 3.161 ± 0.191 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, Z.; Nai, Z.; Li, G.; He, X.; Wang, W.; Xia, J.; Chao, W.; Li, L.; Jiang, X.; Liu, D. The Oral Inactivated Porcine Epidemic Diarrhea Virus Presenting in the Intestine Induces Mucosal Immunity in Mice with Alginate–Chitosan Microcapsules. Animals 2023, 13, 889. https://doi.org/10.3390/ani13050889
Qin Z, Nai Z, Li G, He X, Wang W, Xia J, Chao W, Li L, Jiang X, Liu D. The Oral Inactivated Porcine Epidemic Diarrhea Virus Presenting in the Intestine Induces Mucosal Immunity in Mice with Alginate–Chitosan Microcapsules. Animals. 2023; 13(5):889. https://doi.org/10.3390/ani13050889
Chicago/Turabian StyleQin, Ziliang, Zida Nai, Gang Li, Xinmiao He, Wentao Wang, Jiqiao Xia, Wang Chao, Lu Li, Xinpeng Jiang, and Di Liu. 2023. "The Oral Inactivated Porcine Epidemic Diarrhea Virus Presenting in the Intestine Induces Mucosal Immunity in Mice with Alginate–Chitosan Microcapsules" Animals 13, no. 5: 889. https://doi.org/10.3390/ani13050889
APA StyleQin, Z., Nai, Z., Li, G., He, X., Wang, W., Xia, J., Chao, W., Li, L., Jiang, X., & Liu, D. (2023). The Oral Inactivated Porcine Epidemic Diarrhea Virus Presenting in the Intestine Induces Mucosal Immunity in Mice with Alginate–Chitosan Microcapsules. Animals, 13(5), 889. https://doi.org/10.3390/ani13050889






