IEg67 kDa Bovine Hydatid Cyst Antigen: A Candidate for Developing Sero-Diagnostic Assays for Cystic Echinococcosis, a Disease of One Health Importance
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site and Selection of Animals for Sera and Cyst Collection
2.2. Hydatid Cyst Collection
2.3. Microscopy of Hydatid Cysts
2.4. Molecular Identification of Echinococcus Granulosus
2.5. Preparation of Bovine Hydatid Cyst Fluid (BHCF) Antigen
2.6. SDS-PAGE and Western Blotting
2.7. Quantification of Native Antigen
2.8. ELISA
2.9. ELISA Sensitivity, Specificity, Positive Predictive Value (PPV), Negative Predictive Value (NPV), Likelihood Ratio Positive (LR+), Likelihood Ratio Negative (LR−) and Diagnostic Odds Ratio (DOR)
2.10. Statistical Analyses
3. Results
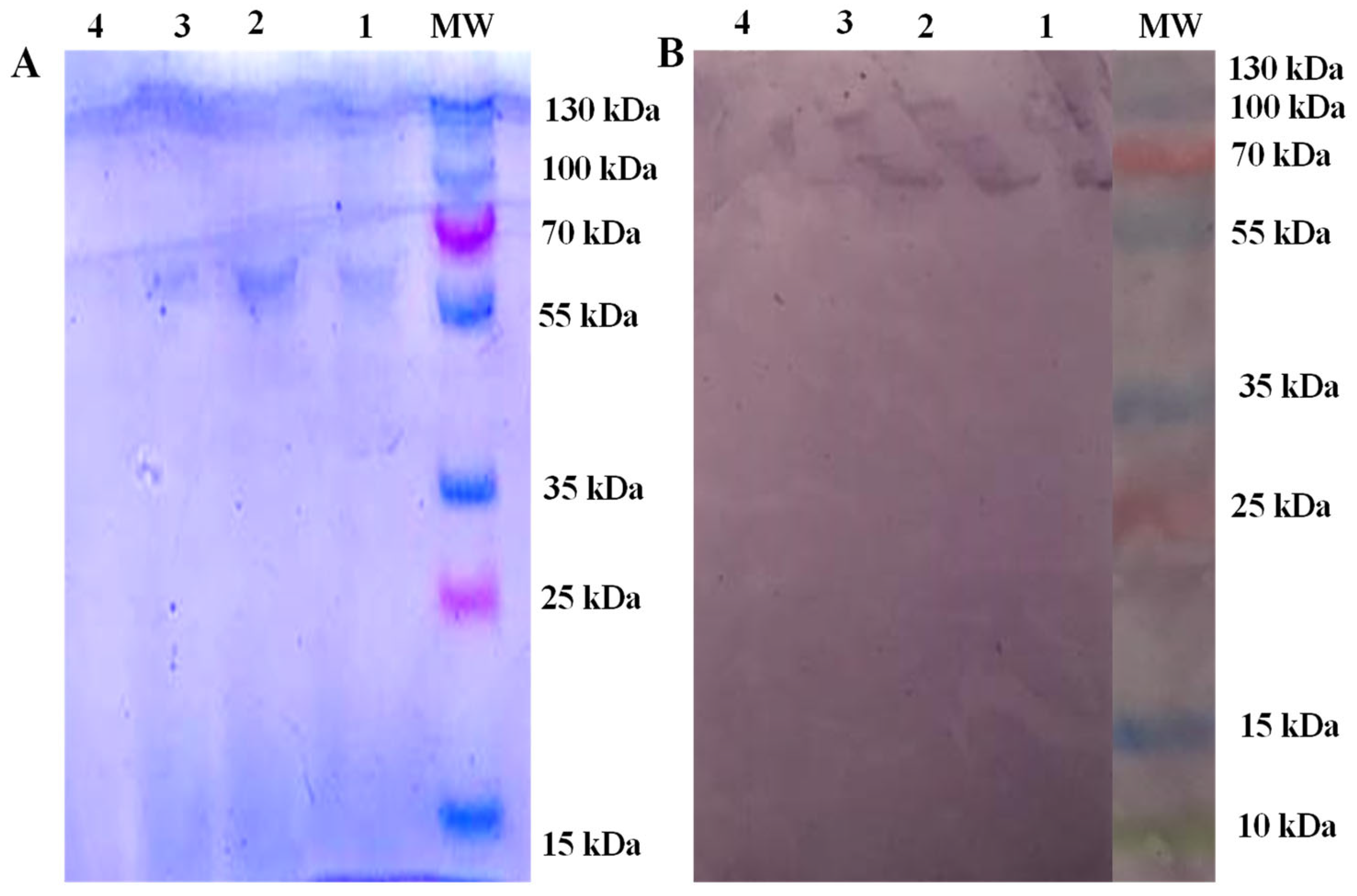
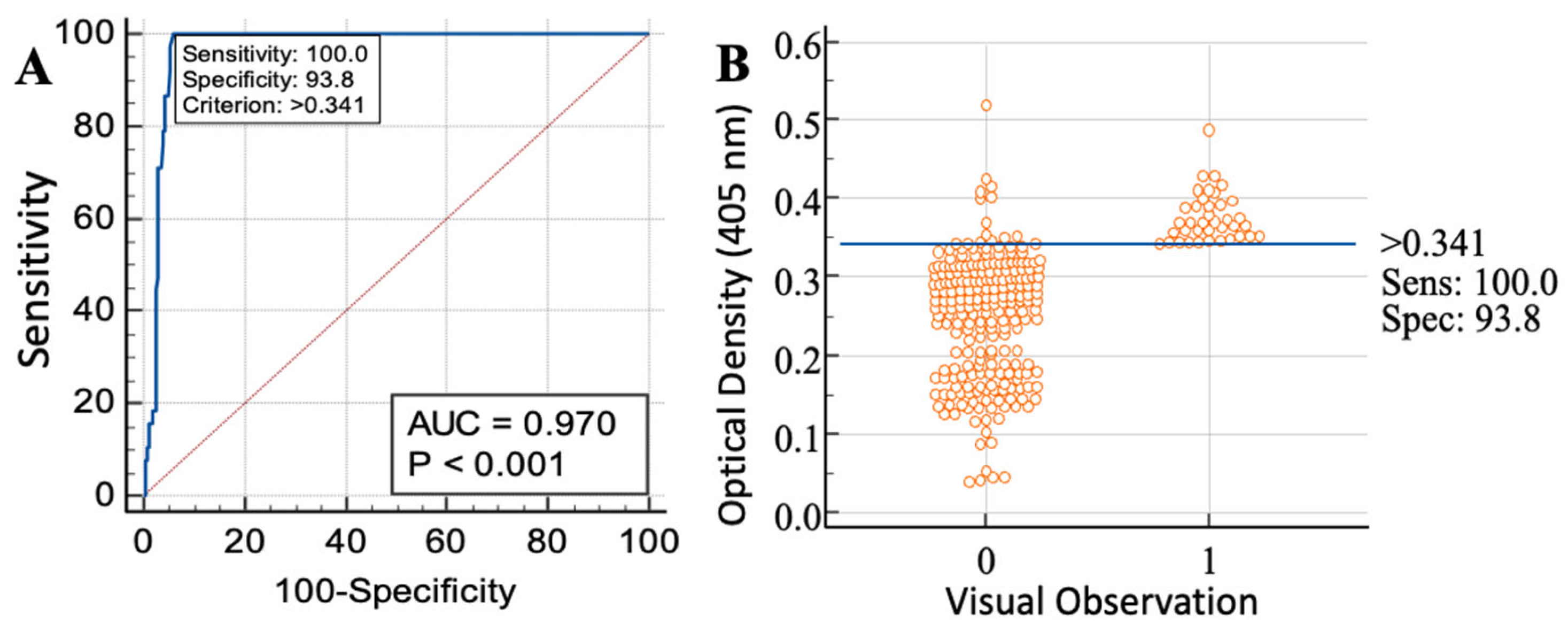
3.1. Molecular Confirmation, Identification of Echinococcus-Specific Immunogen and BHCF ELISA
3.2. Echinococcus Occurrence
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ali, I.; Panni, M.K.; Iqbal, A.; Munir, I.; Ahmad, S.; Ali, A. Molecular characterization of Echinococcus species in Khyber Pakhtunkhwa, Pakistan. Acta Sci. Vet. 2015, 43, 1–7. [Google Scholar]
- Rokni, M. Echinococcosis/hydatidosis in Iran. Iran. J. Parasitol. 2009, 4, 1–16. [Google Scholar]
- Khan, S.N.; Ali, R.; Khan, S.; Norin, S.; Rooman, M.; Akbar, N.; Khan, T.A.; Haleem, S.; Khan, M.A.; Ali, I. Cystic echinococcosis: An emerging zoonosis in southern regions of Khyber Pakhtunkhwa, Pakistan. BMC Vet. Res. 2021, 17, 139. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Naz, K.; Ahmed, H.; Simsek, S.; Afzal, M.S.; Haider, W.; Ahmad, S.S.; Farrakh, S.; Weiping, W.; Yayi, G. Knowledge, attitudes and practices related to cystic echinococcosis endemicity in Pakistan. Infect. Dis. Poverty 2018, 7, 4. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.; Ali, K.A.; Afzal, M.S.; Khan, A.A.; Raza, H.; Shah, Z.H.; Simsek, S. Why more research needs to be done on echinococcosis in Pakistan. Infect. Dis. Poverty 2017, 6, 90. [Google Scholar] [CrossRef] [PubMed]
- Lawson, J.R.; Gemmell, M. Hydatidosis and Cysticercosis: The Dynamics of Transmission. Adv. Parasitol. 1983, 22, 261–308. [Google Scholar] [CrossRef]
- Bourée, P. Hydatidosis: Dynamics of transmission. World J. Surg. 2001, 25, 4. [Google Scholar] [CrossRef] [PubMed]
- Nakao, M.; Yanagida, T.; Okamoto, M.; Knapp, J.; Nkouawa, A.; Sako, Y.; Ito, A. State-of-the-art Echinococcus and Taenia: Phylogenetic taxonomy of human-pathogenic tapeworms and its application to molecular diagnosis. Infect. Genet. Evol. 2010, 10, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Battelli, G. Echinococcosis: Costs, losses and social consequences of a neglected zoonosis. Veter Res. Commun. 2009, 33, 47–52. [Google Scholar] [CrossRef]
- Sarkar, M.; Pathania, M.; Jhobta, A.; Thakur, B.R.; Chopra, R. Cystic pulmonary hydatidosis. Lung India Off. Organ Indian Chest Soc. 2016, 33, 179. [Google Scholar] [CrossRef]
- Ali, R.; Khan, S.; Khan, M.; Adnan, M.; Ali, I.; Khan, T.A.; Haleem, S.; Rooman, M.; Norin, S.; Khan, S.N. A systematic review of medicinal plants used against Echinococcus granulosus. PLoS ONE 2020, 15, e0240456. [Google Scholar] [CrossRef] [PubMed]
- Daali, M.; Fakir, Y.; Hssaida, R.; Hajji, A.; Had, A. Hydatid cysts of the liver opening in the biliary tract. Report of 64 cases. Ann. Chir. 2001, 126, 242–245. [Google Scholar] [CrossRef] [PubMed]
- Gessese, A.T. Review on Epidemiology and Public Health Significance of Hydatidosis. Veter Med. Int. 2020, 2020, 8859116. [Google Scholar] [CrossRef] [PubMed]
- Bekele, J.; Butako, B. Occurrence and financial loss assessment of cystic echinococcosis (hydatidosis) in cattle slaughtered at Wolayita Sodo municipal abattoir, Southern Ethiopia. Trop. Anim. Health Prod. 2011, 43, 221–228. [Google Scholar] [CrossRef]
- Fan, S.; Dong, H.; Ma, H.; Wang, B.; Iqbal, M.; Zou, M.; Qi, M.; Cao, Z. Meta-analysis on the prevalence of bovine hydatid disease in China from 2000 to 2021. Microb. Pathog. 2022, 168, 105586. [Google Scholar] [CrossRef]
- Shumuye, N.A.; Ohiolei, J.A.; Gebremedhin, M.B.; Yan, H.-B.; Li, L.; Li, W.-H.; Zhang, N.-Z.; Fu, B.-Q.; Jia, W.-Z. A systematic review and meta-analysis on prevalence and distribution of Taenia and Echinococcus infections in Ethiopia. Parasites Vectors 2021, 14, 447. [Google Scholar] [CrossRef]
- Vaisi-Raygani, A.; Mohammadi, M.; Jalali, R.; Salari, N.; Hosseinian-Far, M. Prevalence of cystic echinococcosis in slaughtered livestock in Iran: A systematic review and meta-analysis. BMC Infect. Dis. 2021, 21, 429. [Google Scholar] [CrossRef]
- Grakh, K.; Prakash, A.; Mittal, D.; Kumar, P.; Kumar, R. Epidemiology, Risk Factors and Economics of Echinococcosis in India: A Review. Int. J. Livest. Res. 2020, 10. [Google Scholar] [CrossRef]
- Sarıözkan, S.; Yalçın, C. Estimating the production losses due to cystic echinococcosis in ruminants in Turkey. Vet. Parasitol. 2009, 163, 330–334. [Google Scholar] [CrossRef]
- Tasawar, Z.; Naz, F.; Lashari, M. The prevalence of hydatidosis in sheep and buffaloes at Multan, Punjab, Pakistan. Glob. Vet. 2014, 12, 332–335. [Google Scholar]
- Shafiq, M.T.; Athar, M. Epidemiology and Economical Aspects of Hydatidosis in Different Animals, Man and Its Control in Sheep with Indigenous Plants. Ph.D. Thesis, University of the Punjab, Punjab, Pakistan, 2004; pp. 1–653. [Google Scholar]
- Burgu, A.; Doğanay, A.; Gönenç, B.; Sarimehmetoğlu, H.O.; Kalinbacak, F. Analysis of fluids of hydatid cysts from sheep by SDS-PAGE, and determination of specific antigens in protein structure by western blotting. Turk. J. Vet. Anim. Sci. 2000, 24, 493–500. [Google Scholar]
- Pagnozzi, D.; Biosa, G.; Addis, M.F.; Mastrandrea, S.; Masala, G.; Uzzau, S. An Easy and Efficient Method for Native and Immunoreactive Echinococcus granulosus Antigen 5 Enrichment from Hydatid Cyst Fluid. PLoS ONE 2014, 9, e104962. [Google Scholar] [CrossRef] [PubMed]
- Golassa, L.; Abebe, T.; Hailu, A. Evaluation of crude hydatid cyst fluid antigens for the serological diagnosis of hydatidosis in cattle. J. Helminthol. 2010, 85, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.S.; Batabyal, S.; Chattopadhyay, U.K.; Raj, A.; Vinod, U.; Banik, A.; Bhattacharya, S.; Yogendra, M. Isolation, purification and immunobiochemical characterization of goat hydatid cyst fluid antigen. J. Entomol. Zool. Stud. 2020, 8, 1056–1060. [Google Scholar]
- Latif, A.; Tanveer, A.; Anjum, A.A.; Ali, M.A.; Rana, M.S.; Khan, M.R.; Ahmad, M.S. Characterization of hydatid cyst fluid from ruminants and humans by SDS-PAGE in Punjab, Pakistan. J. Anim. Plant Sci. 2013, 23, 405–410. [Google Scholar]
- Singh, B.B.; Sharma, J.K.; Tuli, A.; Sharma, R.; Bal, M.S.; Aulakh, R.S.; Gill, J.P.S. Prevalence and morphological characterisation of Echinococcus granulosus from north India. J. Parasit. Dis. 2012, 38, 36–40. [Google Scholar] [CrossRef]
- Guduro, G.G.; Desta, A.H. Cyst Viability and Economic Significance of Hydatidosis in Southern Ethiopia. J. Parasitol. Res. 2019, 2019, 2038628. [Google Scholar] [CrossRef]
- Boufana, B.; Boué, F.; Qiu, J.; Jenkins, D.; Chen, X.; Craig, P.; Umhang, G.; Lahmar, S. Development of Three PCR Assays for the Differentiation between Echinococcus shiquicus, E. granulosus (G1 genotype), and E. multilocularis DNA in the Co-Endemic Region of Qinghai-Tibet plateau, China. Am. J. Trop. Med. Hyg. 2013, 88, 795–802. [Google Scholar] [CrossRef]
- Bowles, J.; Blair, D.; McManus, D. Genetic variants within the genus Echinococcus identified by mitochondrial DNA sequencing. Mol. Biochem. Parasitol. 1992, 54, 165–173. [Google Scholar] [CrossRef]
- Mohammadzadeh, T.; Sako, Y.; Sadjjadi, S.M.; Sarkari, B.; Ito, A. Comparison of the usefulness of hydatid cyst fluid, native antigen B and recombinant antigen B8/1 for serological diagnosis of cystic echinococcosis. Trans. R. Soc. Trop. Med. Hyg. 2012, 106, 371–375. [Google Scholar] [CrossRef]
- Asghari, M.; Mohebali, M.; Kia, E.B.; Farahnak, A.; Aryaeipour, M.; Asadian, S.; Rokni, M.B. Seroepidemiology of human hydatidosis using AgB-ELISA test in Arak, central Iran. Iran. J. Public Health 2013, 42, 391. [Google Scholar]
- Nabi, H.; Rashid, I.; Ahmad, N.; Durrani, A.; Akbar, H.; Islam, S.; Bajwa, A.A.; Shehzad, W.; Ashraf, K.; Imran, N. Induction of specific humoral immune response in mice immunized with ROP18 nanospheres from Toxoplasma gondii. Parasitol. Res. 2017, 116, 359–370. [Google Scholar] [CrossRef]
- Rahman, S.U.; Akbar, H.; Shabbir, M.Z.; Ullah, U.; Rashid, M.I. Development of Human Toxo IgG ELISA Kit, and False-Positivity of Latex Agglutination Test for the Diagnosis of Toxoplasmosis. Pathogens 2021, 10, 1111. [Google Scholar] [CrossRef] [PubMed]
- Glas, A.S.; Lijmer, J.G.; Prins, M.H.; Bonsel, G.J.; Bossuyt, P.M.M. The diagnostic odds ratio: A single indicator of test performance. J. Clin. Epidemiol. 2003, 56, 1129–1135. [Google Scholar] [CrossRef] [PubMed]
- Bel-Ochi, N.C.; Bouratbine, A.; Mousli, M. Enzyme-Linked Immunosorbent Assay Using Recombinant SAG1 Antigen to Detect Toxoplasma gondii-Specific Immunoglobulin G Antibodies in Human Sera and Saliva. Clin. Vaccine Immunol. 2013, 20, 468–473. [Google Scholar] [CrossRef] [PubMed]
- Gatti, A.; Alvarez, A.R.; Araya, D.; Mancini, S.; Herrero, E.; Santillan, G.; Larrieu, E. Ovine echinococcosis: I. Immunological diagnosis by enzyme immunoassay. Vet. Parasitol. 2007, 143, 112–121. [Google Scholar] [CrossRef]
- Akbar, H.; Germon, S.; Berthon, P.; Dimier-Poisson, I.; Moiré, N. Depletion of CD25+ cells during acute toxoplasmosis does not significantly increase mortality in Swiss OF1 mice. Memórias Inst. Oswaldo Cruz 2012, 107, 155–162. [Google Scholar] [CrossRef]
- Ismael, A.B.; Sekkai, D.; Collin, C.; Bout, D.; Mévélec, M.-N. The MIC3 Gene of Toxoplasma gondii Is a Novel Potent Vaccine Candidate against Toxoplasmosis. Infect. Immun. 2003, 71, 6222–6228. [Google Scholar] [CrossRef]
- Mathewos, M.; Dawa, D.; Yirgalem, M.; Denano, T.; Fesseha, H. Cystic echinococcosis in cattle slaughtered at a slaughterhouse in Gessa, southern Ethiopia. Parasite Epidemiol. Control. 2022, 18, e00262. [Google Scholar] [CrossRef]
- El-Kattan, A.M.; Abdel-Ra’Ouf, A.M.; Yousef, R.R.; AbouElnga, T.R.; Mousa, W. Sensitivity and specificity of Indirect Enzyme Linked Immuno sorbent Assay (ELISA) for diagnosis of hydatidosis in dromedary camels using hydatid cyst fluid antigens. J. Vet. Med. Res. 2020, 27, 66–75. [Google Scholar] [CrossRef]
- Rahimi, H.; Sadjjadi, S.; Sarkari, B. Performance of Antigen B Isolated from Different Hosts and Cyst Locations in Diagnosis of Cystic Echinococcosis. Iran. J. Parasitol. 2011, 6, 12–19. [Google Scholar] [PubMed]
- Yakubu, R.A.; Nock, I.H.; Ndams, I.S.; Luka, S.A.; Yaro, C.A.; Alkazmi, L.; Batiha, G.E.-S. Detection of Echinococcus granulosus sensu lato cysts and seroprevalence of cystic echinococcosis in cattle and camels in Maiduguri Abattoir. J. Parasit. Dis. 2022, 46, 876–888. [Google Scholar] [CrossRef] [PubMed]
- Osman, F. Comparative study between serological analysis and visual meat inspection for hydatid cyst in camels. Assiut Vet. Med. J. 2014, 60, 1–6. [Google Scholar] [CrossRef]
- Kittelberger, R.; Reichel, M.P.; Jenner, J.; Heath, D.D.; Lightowlers, M.W.; Moro, P.; Ibrahem, M.M.; Craig, P.S.; O’Keefe, J.S. Evaluation of three enzyme-linked immunosorbent assays (ELISAs) for the detection of serum antibodies in sheep infected with Echinococcus granulosus. Vet. Parasitol. 2002, 110, 57–76. [Google Scholar] [CrossRef]
- Shepherd, J.C. Antigens for the Immunodiagnosis of Hydatid Disease. Ph.D. Thesis, University of London, Imperial College of Science and Technology, London, UK, 1988. [Google Scholar]
- Latif, A.A.; Tanveer, A.; Maqbool, A.; Siddiqi, N.; Kyaw-Tanner, M.; Traub, R.J. Morphological and molecular characterisation of Echinococcus granulosus in livestock and humans in Punjab, Pakistan. Vet. Parasitol. 2010, 170, 44–49. [Google Scholar] [CrossRef]
- Omrani, V.F.; Rouhani, S.; Kazemi, B.; Seyyedtabaei, S.J.; Kheirandish, F.; Rezapour, M. Seroprevalence of IgG Antibodies against Echinococcus granulosus by ELISA Method Using Recombinant Agb in Lorestan Province, Western Iran. Iran. J. Public Health 2017, 46, 1132–1138. [Google Scholar]
- Ibrahem, M.; Craig, P.; McVie, A.; Ersfeld, K.; Rogan, M. Echinococcus granulosus antigen B and seroreactivity in natural ovine hydatidosis. Res. Vet. Sci. 1996, 61, 102–106. [Google Scholar] [CrossRef]
- Kandil, O.; Hassan, N.; Sedky, D.; Ata, E.B. Studies on the specific immunodiagnosis of cystic echinococcosis in camels using enzyme-linked immunosorbent assay. Bulg. J. Vet. Med. 2019, 22, 305–313. [Google Scholar] [CrossRef]
- Singh, H.; Aulakh, R.S.; Sharma, R.; Singh, B.B. Prevalence and molecular characterisation of Echinococcus granulosus in disposed of bovine carcasses in Punjab, India. J. Parasit. Dis. 2020, 44, 521–527. [Google Scholar] [CrossRef]
- Craig, P.S. Detection of specific circulating antigen, immune complexes and antibodies in human hydatidosis from Turkana (Kenya) and Great Britain, by enzyme-immunoassay. Parasite Immunol. 1986, 8, 171–188. [Google Scholar] [CrossRef]
- Bellanger, A.; Wang, J.; Gbaguidi-Haore, H.; Barrera, C.; Bresson-Hadni, S.; Zlobec, I.; Lachenmayer, A.; Richou, C.; Turco, C.; Gottstein, B.; et al. Investigating new serological and tissue markers for the follow-up of patients operated for alveolar echinococcosis. Parasite Immunol. 2021, 43, e12827. [Google Scholar] [CrossRef] [PubMed]
- Haleem, S.; Niaz, S.; Qureshi, N.A.; Ullah, R.; Alsaid, M.S.; Alqahtani, A.S.; Shahat, A.A. Incidence, Risk Factors, and Epidemiology of Cystic Echinococcosis: A Complex Socioecological Emerging Infectious Disease in Khyber Pakhtunkhwa, Province of Pakistan. BioMed Res. Int. 2018, 2018, 5042430. [Google Scholar] [CrossRef] [PubMed]
- Asrat, M. Prevalence and economic significance of cystic hydatidosis: Bovine at Kombolcha Elfora Industrial Abattoir, North Wollo, Ethiopia. J. Anim. Res. 2015, 5, 707–711. [Google Scholar] [CrossRef]
- Azlaf, R.; Dakkak, A. Epidemiological study of the cystic echinococcosis in Morocco. Vet. Parasitol. 2006, 137, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Khan, J.; Basharat, N.; Khan, S.; Jamal, S.M.; Rahman, S.U.; Shah, A.A.; Khan, S.; Ali, R.; Khan, S.N.; Ali, I. Prevalence and Molecular Characterization of Cystic Echinococcosis in Livestock Population of the Malakand Division, Khyber Pakhtunkhwa, Pakistan. Front. Vet. Sci. 2021, 8, 757800. [Google Scholar] [CrossRef]
- Anwar, A.; Shamim, H.; Rana, H.; Khan, M.; Qudoos, A. Hydatidosis: Prevalence and biometrical studies in cattle (Bos indicus). Pak. J. Agric. Sci. 2000, 37, 29–32. [Google Scholar]
- Melaku, A.; Lukas, B.; Bogale, B. Cyst Viability, Organ Distribution and Financial Losses due to Hydatidosis in Cattle Slaughtered at Dessie Municipal Abattoir, North-eastern Ethiopia. Vet. World 2012, 5, 213–218. [Google Scholar] [CrossRef]
- Getaw, A.; Beyene, D.; Ayana, D.; Megersa, B.; Abunna, F. Hydatidosis: Prevalence and its economic importance in ruminants slaughtered at Adama municipal abattoir, Central Oromia, Ethiopia. Acta Trop. 2010, 113, 221–225. [Google Scholar] [CrossRef]
- Ahmadi, N.; Meshkehkar, M. An abattoir-based study on the prevalence and economic losses due to cystic echinococcosis in slaughtered herbivores in Ahwaz, south-western Iran. J. Helminthol. 2011, 85, 33–39. [Google Scholar] [CrossRef]
| Name | Definition | Formula |
|---|---|---|
| Sensitivity | “The capability of a test to correctly identify the patients with disease” | a/(a + c) × 100 |
| Specificity | “The potential of a screening test to identify the patients without the disease” | d/(b + d) × 100 |
| Positive predictive value (PPV) | “Prediction of positive patients before test (identifying true positive)” | a/a + b |
| Negative predictive value (NPV) | “Prediction of negative samples before test (identifying true negative)” | d/c + d |
| Likelihood ratio positive (LR+) | “Ratio of probability that a positive test outcome would be expected in a patient with a disease to the probability that a positive test outcome would be expected in a patient without a disease” | (a/a + c)/(b/b + d) |
| Likelihood ratio negative (LR−) | “The ratio of probability of a patient testing negative who has a disease to the probability of a patient testing negative who does not have a disease” | (c/a + c)/(d/b + d) |
| Diagnostic odd ratio (DOR) | “Odds of a positive test in those with disease relative to the odds of a positive test in those without disease” | LR+/LR− |
| Sensitivity | Specificity | PPV | NPV | LR+ | LR− | DOR |
|---|---|---|---|---|---|---|
| 100% | 93.8% | 73.07 | 99.96 | 16.33 | 0.012 | 1360.83 |
| Age Class | Buffalo | Cattle | ||
|---|---|---|---|---|
| Female | Male | Female | Male | |
| 2–3 years | 2/21 (9.5%) | 0/23 (0%) | 0/11 (0%) | 4/28 (14.2%) |
| 4–5 years | 2/31 (6.5%) | 3/19 (15.7%) | 12/31 (38.7%) | 4/22 (18.1%) |
| 6–7 years | 9/28 (32.1%) | 1/4 (25%) | 11/22 (50%) | 4/14 (28.5%) |
| Host Species | Sample Size | Post-Mortem (%) | ELISA (%) | Antigen Used in ELISA | References |
|---|---|---|---|---|---|
| Cattle | 128 | 19.5 | 27.3 | Ammonium sulphate ((NH4)2SO4) precipitated HCF | Current study |
| Buffalo | 136 | 9.5 | 12.5 | Ammonium sulphate ((NH4)2SO4) precipitated HCF | Current study |
| Cattle | 256 | 1.6 | 35.5 | Freeze-dried precipitated HCF | [43] |
| Camel | 304 | 14.14 | 52.6 | Freeze-dried precipitated HCF | [43] |
| Camel | 200 | 6 | 8 | Phosphotungstic acid and 2M magnesium chloride used for HCF precipitation | [44] |
| Sensitivity (%) | Specificity (%) | Host Origin | Antigen Size (kDa) | Type of Antigen | References |
|---|---|---|---|---|---|
| 100 | 93.8 | Buffalo | 67 | Crude antigen | Current study |
| 82.8 ↓ | 62.5 ↓ | Camel | Not mentioned | Crude antigen | [41] |
| 79.3 ↓ | 75 ↓ | Camel | Not mentioned | Partially purified crude antigen | [41] |
| 36 ↓ | 93 ↓ | Sheep | 8–24 | Crude antigen | [49] |
| 25 ↓ | 99 ↑ | Sheep | 6 | Recombinant antigen | [49] |
| 96% ↓ | 71% ↓ | Camel | Not mentioned | Crude antigen | [49] |
| 99% ↓ | 90% ↓ | Camel | Not mentioned | Camel antigen B | [49] |
| 89.2 ↓ | 89.5 ↓ | Sheep | 8–12 | Total hydatid liquid (LHT) | [37] |
| 80.0 ↓ | 93.9 ↑ | Sheep | 16 | Purified portion of total hydatid liquid (S2B) | [37] |
| 86.4 ↓ | 92.8 ↓ | Sheep | 20–24 | Purified antigen (B) | [37] |
| 83 ↓ | 70.3 ↓ | Camel | Not mentioned | Protoscolex antigen | [50] |
| 46.5 ↓ | 41.7 ↓ | Camel | Not mentioned | Germinal layer antigen | [50] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, S.; Cable, J.; Younus, M.; Rashid, M.I.; Hailer, F.; Akbar, H. IEg67 kDa Bovine Hydatid Cyst Antigen: A Candidate for Developing Sero-Diagnostic Assays for Cystic Echinococcosis, a Disease of One Health Importance. Animals 2023, 13, 866. https://doi.org/10.3390/ani13050866
Khan S, Cable J, Younus M, Rashid MI, Hailer F, Akbar H. IEg67 kDa Bovine Hydatid Cyst Antigen: A Candidate for Developing Sero-Diagnostic Assays for Cystic Echinococcosis, a Disease of One Health Importance. Animals. 2023; 13(5):866. https://doi.org/10.3390/ani13050866
Chicago/Turabian StyleKhan, Sakandar, Jo Cable, Muhammad Younus, Muhammad Imran Rashid, Frank Hailer, and Haroon Akbar. 2023. "IEg67 kDa Bovine Hydatid Cyst Antigen: A Candidate for Developing Sero-Diagnostic Assays for Cystic Echinococcosis, a Disease of One Health Importance" Animals 13, no. 5: 866. https://doi.org/10.3390/ani13050866
APA StyleKhan, S., Cable, J., Younus, M., Rashid, M. I., Hailer, F., & Akbar, H. (2023). IEg67 kDa Bovine Hydatid Cyst Antigen: A Candidate for Developing Sero-Diagnostic Assays for Cystic Echinococcosis, a Disease of One Health Importance. Animals, 13(5), 866. https://doi.org/10.3390/ani13050866





