Simple Summary
Each year, approximately 167 billion Penaeus vannamei (white-leg shrimp) are farmed worldwide from an estimated total of more than 400 billion marines and freshwater shrimp farmed. In this context, the welfare of decapod crustaceans, the group with the most farmed animals on the planet, is becoming an increasingly important issue for researchers and society, and this debate will soon reach shrimp labs and farms. This article presents protocols specifically designed to measure the welfare of P. vannamei at all stages of their production cycle, from reproduction through larval rearing and postlarval transport to juvenile rearing in earthen ponds. These protocols were developed using four domains of welfare: nutrition, environment, health, and behaviour. Together, they help assess the fifth domain: psychology. The assessment protocols also include reference values for each indicator and three possible values for animal welfare on a continuum from positive (score 1) to very negative (score 3). Our assessment protocols can identify the critical points in the shrimp aquaculture process and are an essential step towards improving the welfare of farmed shrimp worldwide.
Abstract
Gradually, concern for the welfare of aquatic invertebrates produced on a commercial/industrial scale is crossing the boundaries of science and becoming a demand of other societal actors. The objective of this paper is to propose protocols for assessing the Penaeus vannamei welfare during the stages of reproduction, larval rearing, transport, and growing-out in earthen ponds and to discuss, based on a literature review, the processes and perspectives associated with the development and application of on-farm shrimp welfare protocols. Protocols were developed based on four of the five domains of animal welfare: nutrition, environment, health, and behaviour. The indicators related to the psychology domain were not considered a separate category, and the other proposed indicators indirectly assessed this domain. For each indicator, the corresponding reference values were defined based on literature and field experience, apart from the three possible scores related to animal experience on a continuum from positive (score 1) to very negative (score 3). It is very likely that non-invasive methods for measuring the farmed shrimp welfare, such as those proposed here, will become a standard tool for farms and laboratories and that it will become increasingly challenging to produce shrimp without considering their welfare throughout the production cycle.
1. Introduction
1.1. Animal Welfare and Progress in Shrimp Farming
Agricultural, aquacultural, and industrial production chains only develop (and grow) because they change. The shrimp farming production chain is considered one of the world’s most controversial agri-food systems for animal protein production [1,2,3,4,5]. The evolution of shrimp farming practices is currently taking place on several fronts. For example, there is a trend towards intensification of production systems for better utilisation of resources [6,7,8,9,10]; the pursuit of certifications and regulations to adapt shrimp production to the new demands of the market and society in general [11,12,13]; genetic improvement of animals [12,14,15]; improvement of shrimp feeding and nutrition [16,17]; and increased efforts towards hygienic-sanitary controls and biosecurity in shrimp farms [18,19], to name a few. This scenario of change, based on the scientific and technological development of the sector, in turn, helps to understand why the global production of a single species, the white-leg shrimp Penaeus vannamei, has increased by almost 53% in 5 years, from 3803.6 thousand tonnes to 5812.2 thousand tonnes between 2015 and 2020, so that this species already accounts for 51.7% of total shrimp production [20]. It is estimated that more than 167 billion shrimps of this species are produced annually [21] and that the total number of shrimps and prawns farmed annually reaches about 440 billion [22].
However, it is human nature that despite all this progress, there is often a temptation to cling to traditional production methods and concepts that seemed to work satisfactorily in the past, even though they certainly no longer work in the same way or are no longer acceptable under present and future conditions. Perhaps this is a challenge to overcome regarding welfare in shrimp farming.
The term “welfare” describes a potentially measurable quality of an animal’s life at a particular point in time and is, therefore, a scientific concept [23]. However, this concept is fundamentally dynamic and related to the adaptive abilities of the individual [24]. In other words, the welfare of a particular animal is always a temporary state related to the animal’s experiences and moves on a continuum from negative to positive [25]. Thus, to improve animal welfare, it is necessary to ensure that the animal’s experience is as positive as possible. Although society has recognised the importance of animal welfare in the commercial production of homeothermic vertebrates [26,27,28,29] and even ectotherms [30,31,32], there is still a glaring lack of recognition of the welfare of higher invertebrates, as in the case of shrimps [33,34,35].
The discussion on the need to apply the principles of animal welfare to shrimp farming includes two main aspects: those related to the current scientific evidence for sentience in these animals [36,37,38] and the ethical/legal aspects associated with the issue [39,40,41].
1.2. Shrimp Sentience
From the first perspective, the welfare of an organism is inseparable from the degree of suffering and the positive states that this individual experiences at a certain point in time [42]. Sentience, thus, refers to the ability of an animal to consciously perceive what is happening to it and what surrounds it, consciously perceive through the senses, and consciously feel or subjectively experience [43]. Crump et al. [44] explained that sentience needs to be understood and addressed in a broad sense, including sensory experiences (visual, auditory, tactile, and olfactory) as well as feelings of warmth or cold, fatigue, hunger, thirst, boredom, excitement, fear, pain, pleasure, and joy. These authors also pointed out that this ability to feel must be distinguished from other related abilities, since a sentient being, for example, is not necessarily able to think about or understand the feelings of other animals. Finally, they argue that in answering questions about the ability of invertebrate animals to feel, we must rely (at least partly) on behavioural and cognitive markers associated with knowledge of their nervous systems.
According to Birch et al. [45] and Feinberg and Mallatt [46], invertebrates’ brains differ even more radically from those of mammals and fish, the vertebrates most distant from humans. These authors explain that, although there are more than 500 million years of evolution between invertebrates and humans, it cannot be assumed that invertebrates lack sentience simply because their cerebral ganglia are organised differently from the brain of vertebrates [45,46]. In the higher invertebrates (chordates, echinoderms, arthropods, and mollusks) there are a variety of complex intra- and interspecific neuronal structures that determine the degree of their sentience [47]. According to Conte et al. [48], decapod crustaceans have sensory cells that enable the perception of noxious stimuli and show behavioural responses to these stimuli. However, the authors added that these responses could be nociceptive reflexes, that it cannot be ruled out that they occur without the involvement of sufficiently complex central processing thought to be necessary for the animals to feel pain, and that it has not yet been conclusively demonstrated that decapods possess sensory structures of sufficient complexity to feel pain.
On the other hand, Langworthy et al. [49] reported that some decapod crustaceans have cerebral ganglia as large and well-articulated as the brains of some fish. However, this does not seem to be the case with penaeid crustaceans. Crump et al. [44] established eight criteria, four neural and four cognitive-behavioural, focusing on pain to assess the sentience of crustaceans. They found evidence that true crustaceans (Infraorder Brachyura) met five criteria, leading the authors to classify the result as solid evidence for sentience. The anomural crustaceans (suborder Anomura) and lobsters (suborder Astacidea) would meet three criteria, leading the authors to classify them as substantial evidence for sentience.
In contrast, for other suborders, such as farmed shrimps (suborder Penaeidea), the evidence for sentience is less obvious, although the authors point to the glaring gaps in knowledge that still exist for these organisms. The lack of studies specifically aimed at assessing the sentience of decapod crustaceans should, therefore, not be confused with the absence of sentience in these organisms [50]. As Birch [51] suggested, “Where there are threats of serious, negative animal welfare outcomes, lack of full scientific certainty as to the sentience of the animals in question shall not be used as a reason for postponing cost-effective measures to prevent those outcomes”.
1.3. Shrimp Welfare and Legislation
Even if the question of the sentience of the Penaeidea has not yet been settled among researchers, it is a fact that it is no longer possible to treat shrimps (as well as any other animal) as simple “production machines”—they are not. Whether due to scientific findings or ethical reasons, several countries have already started to enact regulations for the species-appropriate and humane slaughter of crustaceans. In Europe, although the European Union only sets rules for the slaughter of vertebrates (and excludes reptiles and amphibians) [51], the EFSA (European Food Safety Authority) concluded that decapod crustaceans could feel pain and suffering [52]. Countries such as Norway and Switzerland already include crustaceans in animal welfare legislation [53]. France enacted a “service directive” more than 10 years ago to ensure the proper handling of crustaceans before sale and consumption [54]. The Swiss government has established as a rule that only electrical stunning or “mechanical destruction” of the brain are recognised stunning methods, including for crustaceans, and requires lobsters and crayfish to be stunned before slaughter [55,56,57]. The UK government amended its Animal Welfare (Sentience) Act 2022 to include decapod crustaceans [58], which are also protected by animal welfare legislation in New Zealand [53] and Australia [58], a country that adopted the Australian Animal Welfare Strategy (AAWS) in 2004, but where the protection of crustaceans is limited to a few states.
In Asia, where some of the world’s largest shrimp producers are located, codes of conduct already include animal welfare as one of the pillars, along with food safety, animal health, and environmental integrity [33,59,60]. There is, thus, a clear tendency for the world’s largest shrimp import markets, particularly the North American and European markets, to require their suppliers to comply with norms and animal welfare standards during the production process of these organisms [20,61].
Thus far, the most important economic power in the world, the United States, has taken an opposite position to that of the European Union in conflicts between trade and animal welfare, prioritising economic concerns [62]. Nevertheless, due to scientific advances in recognising the sentience of decapod crustaceans or consumer demand—probably both—it will become increasingly challenging to produce shrimp without regard to animal welfare. This is due to consumer pressure, which affects all actors in the value chain, as well as issues such as sustainability certification, organic farming, and animal welfare, which have already been included in the social responsibility programmes of large companies [63,64].
The challenge, then, is to define indicators that are more measurable and less subjective that encompass different aquaculture production systems [3,65] and that focus on the welfare of farmed animals and not just the quality of the product because although these two factors are linked, they are not the same thing [4]. However, since it is impossible to ask a shrimp directly how it is doing, it is assumed that the animal’s welfare is directly related to satisfying its needs [66]. That is how the animal relates biologically, behaviourally, and emotionally to its environment [67]. Albalat et al. [21] suggested using the five domains of animal welfare (i.e., nutrition, physical environment, health, behaviour, and psychological needs) as indicators of penaeid shrimp welfare, which in turn correspond to the five freedoms established by the Farm Animal Welfare Council [68]. In this way, any measurements or observations made in a shrimp laboratory or on a shrimp farm that provide information about the extent to which the needs of the shrimp are being met can be considered potential indicators of their welfare. This paper aims to propose protocols consisting of the indicators, respective reference values and scores for assessing the welfare of P. vannamei in the phases of reproduction, larval rearing, transport, and growth in earthen ponds, and to discuss, based on a literature review, the processes and perspectives related to the development and application of shrimp welfare protocols.
2. Materials and Methods
2.1. The Welfare Domains and Indicators
The indicators selected to assess the welfare of P. vannamei at the different stages of the production process (reproduction, larval rearing, transport, and grow-out) in earthen ponds (Figure 1) were established following the same logic already used for farmed fish species such as Atlantic salmon [69], Nile tilapia [70], and grass carp [32]. These indicators were grouped according to four of the five domains: (1) environmental, (2) sanitary, (3) nutritional, and (4) behavioural. The indicators related to psychological freedom were not considered a separate category, as the other proposed indicators assessed this freedom indirectly.
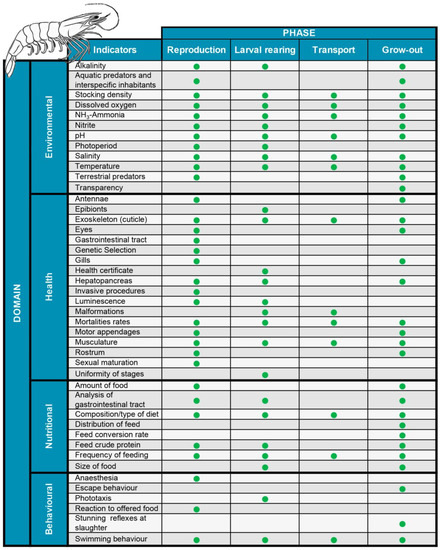
Figure 1.
Domains and welfare indicators during the production process of white-leg shrimp, Penaeus vannamei.
2.2. Identification and Selection of Documents for Bibliographic Review and Reference Levels
The environmental, health, nutritional, and behavioural domains associated with P. vannamei and the indicators and their respective reference values during the reproductive, larval rearing, transport, and grow-out phases were identified based on a literature search using Google Scholar as the research platform.
Books, technical and scientific articles, case studies, manuals, and handouts developed by international institutions, theses, and dissertations were sought. The search period covered 1976 to 2023. To determine the indicators and their respective reference values, texts and documents were evaluated with the following combination of previously defined terms in their title, abstract or keywords: vannamei AND shrimp AND “pond” -biofloc AND; the specific indicators are shown in Table 1.

Table 1.
Specific indicators used to obtain bibliographic data to define the reference values for the four domains of marine shrimp welfare studied.
A database of preselected texts and documents was then set up. Subsequently, this material was analysed to evaluate the results and the methodology used by the authors to obtain them. The results were selected in situations likely to reflect biological and/or operational conditions in shrimp production.
Based on the information available in the literature, three scores were assigned (1, 2, and 3). Score 1 can be interpreted as covering the ideal variation limits for the target species. Score 2 refers to variations within the limits that animals usually tolerate. Even if such variations cause or adversely affect the animals, these are sublethal. An exception to this criterion is the indicator of mortality, which is evaluated at an intensity that can be lethal as long as it is at a lower level than in nature and, at the same time, at an intermediate level when the rearing environment is taken into account. Score 3 refers to reference levels that affect the animals’ physiological, health, and behavioural status to an unacceptable degree, so their welfare and survival are at risk.
2.3. Field Evaluation of the Preselected Indicators
The applicability and operational feasibility, as well as the measurement technique of each of the preselected indicators, were assessed in the field. To this end, technical visits were conducted to monitor procedures and routine management in laboratories and farms producing marine shrimp in northeastern Brazil. More specifically, we visited one farm, and one larval rearing laboratory in the state of Rio Grande do Norte (municipality of Canguaretama), three farms (in the cities of Aracati and Jaguaruana), and two larval rearing laboratories (in Aracati and Parajuru), in the state of Ceará. The companies were selected after prior contacts and expressions of interest from people responsible for improving the welfare of farmed shrimp.
The indicators that could not be collected on-site for technical or operational reasons were discarded. After this final check, the protocols were restructured in the format in which they are presented in Tables 2–14.
3. Results
3.1. Reproduction Stage
The reproductive stage ranges from the selection of animals for the formation of breeding banks to their care (in tanks or earthen ponds), to mating and spawning. According to Ceballos-Vázques et al. [71] the optimal reproductive potential of females of P. vannamei, based on the frequency of spawning and the quality of larvae, is reached at 12 months. As these authors point out, body weight appears to be of secondary importance at this age, although optimal breeding conditions are required for adequate growth and nutrition. This exact age is also recommended for males since the sperm quality of the animals is high from this period [72].
Eleven environmental indicators were selected to assess the welfare of shrimp-farmed breeders. In addition to the parameters commonly used to determine water quality in a farm (temperature, pH, alkalinity, ammonia, nitrite, and salinity), photoperiod (when this variable is controlled), absence of predators (terrestrial or aquatic), and stocking density were considered (Table 2). The controlled presence of terrestrial predators means that the predators are physically present in the environment but do not have direct access to the shrimp. This is the case, for example, when protective fencing prevents birds from accessing ponds. In these cases, however, even indirect contact with the predator could be detrimental to the welfare of the shrimp, e.g., as a carrier of infectious diseases. For the aquatic predators indicator, we considered controlled presence of inter-specific inhabitants in case that the producer obtained knowledge of the existence of other species in the pond, for instance, in case of polyculture.

Table 2.
Environmental indicators for Penaeus vannamei breeders.
Health indicators of P. vannamei breeding animals can be primarily measured by direct visual observation of the anatomical features of the animals. Luminescence, on the other hand, when observed in animals in dark environments, either in rearing facilities or in the laboratory, is indicative of the presence of bacteria of the genus Vibrio. Sexual maturity refers to the characteristics of animals that have already been selected for reproduction and spawning in the laboratory. Invasive procedures (especially removal of the eyestalk in females) are considered the most critical point in shrimp welfare at this stage of the production process and should be avoided. Mortality rates must be assessed cumulatively (from the beginning of this stage to the time of analysis or at the end of the reproductive process). Genetic selection means the application or non-application of properly standardised protocols used by the laboratory in the selection and husbandry of farmed animals (Table 3).

Table 3.
Health indicators for Penaeus vannamei breeders.
Nutritional indicators of P. vannamei breeders include, in addition to a direct indicator (the filling of the digestive tract with food), some essential aspects of the feeding routine, such as the composition and type of feed offered, the proportion of crude protein in the breeders’ artificial diet, the amount fed (as a percentage of shrimp biomass) and the feeding frequency (Table 4).

Table 4.
Nutritional indicators for Penaeus vannamei breeders.
Behavioural indicators refer to shrimp swimming, feeding behaviour during management, and outcomes related to the use of stunning methods when invasive procedures are performed on the animals (Table 5).

Table 5.
Behavioural indicators for Penaeus vannamei breeders.
3.2. Larvae Rearing Phase
According to Kitani [149], the eggs of P. vannamei are 0.26–0.29 mm in diameter. About 13 h after spawning, they hatch into a naupliar larva that feeds on its yolk and passes through six sub-stages (N1 to N6) before growing to about 0.46 mm in length, transforming into a zoea. At this stage, the larva feeds on phytoplankton and passes through three sub-stages (Z1 to Z3). When it reaches a size of about 2.1 mm in length, it transforms into a mysis larva. When shrimp in captivity reach this larval stage, they prefer to feed on rotifers, Artemia, or artificial food. The mysis measures up to 3.80 mm after passing through three sub-stages (M1 to M3) and swims upside down or holds its body in a vertical position with its head down. In the next stage, postlarva (PL1), the shrimp, about 4.2 mm long, holds its body horizontally and becomes a benthic organism. This stage continues until the animal completes its morphological development and becomes a juvenile. In a laboratory, larval rearing extends from nauplii to postlarvae when the animals are ready for marketing.
The environmental Indicators adopted for the larval rearing stage here are essentially those already used for livestock rearing, except for the presence of predators, which are unlikely to be present in the ponds used for larval rearing. The reference values are adjusted accordingly for this life stage of the shrimp (Table 6).

Table 6.
Environmental indicators for larvae and postlarvae of Penaeus vannamei.
The health indicators for the larvae and postlarvae of P. vannamei are pretty specific and concern issues related to the laboratory itself (whether or not health certificates are issued for the animals sold), the conditions in the larval rearing tanks (presence of bioluminescence in the larvae or even in the water); questions directly related to the health status of the larvae at the time of assessment (uniformity of larval stages present in the tank, presence of poorly formed larvae, presence of epibionts, muscle necrosis or melanisation of the exoskeleton, presence of lipid droplets and staining of the hepatopancreas of the postlarvae); and finally, the observed cumulative mortality rate concerning the batch analysed (Table 7).

Table 7.
Health indicators for larvae and postlarvae of Penaeus vannamei.
Nutritional indicators of larval and postlarval welfare are analysed directly about shrimp feed intake (analysis of gastrointestinal tract filling) and also to diet (size of feed at each larval stage, composition of feed, and percentage of crude protein in artificial feed), as well as to the diet chosen and handling (analysis of gastrointestinal tract filling, frequency of artificial feed intake, and composition of feed), as shown in Table 8.

Table 8.
Nutritional indicators for larvae and postlarvae of Penaeus vannamei.
The behavioural indicators of larvae and postlarvae were limited to the positive phototaxic behaviour of nauplii and zoeae and the swimming activity of the animals (Table 9).

Table 9.
Behavioural indicators for larvae and postlarvae of Penaeus vannamei.
3.3. Postlarvae Transport
Although it is known that in some cases, transport of nauplii takes place (sold between laboratories doing reproduction and larval culture and others doing larval culture only), only transport of postlarvae (which takes place between laboratories and farms) was considered here.
We have proposed 12 indicators for assessing the welfare of P. vannamei postlarvae, to be evaluated when the postlarvae arrive at the farm, including six environmental, two health, two nutritional, and two behavioural indicators (Table 10).

Table 10.
Indicator for the transport of postlarvae of Penaeus vannamei.
3.4. Grow-Out Stage
The growth phase begins with the transfer of postlarvae to the nurseries (in the case of biphasic rearing) or the grow-out facilities (in the case of monophasic rearing) and ends with the selection of animals for breeding banks or slaughter for marketing and consumption. The indicators proposed here and their respective reference values have been established based on rearing in earthen ponds and do not necessarily apply to other rearing systems such as bioflocs or raceways.
The environmental indicators are practically the same as those already described for the breeders, except for the photoperiod, because since the cultures are carried out in earthen ponds, the photoperiod is always the natural one. However, water transparency was included here, an indirect indicator of the number of planktonic organisms in the water (Table 11). It can be observed that the reference values for parameters such as temperature, pH, dissolved oxygen, salinity, and stocking density also differ between juveniles and breeders.

Table 11.
Environmental indicators for juvenile and adult Penaeus vannamei. It is recommended that postlarvae are previously acclimatised in ponds for 10 to 15 min for every degree of temperature difference and salinity unit (81; 149) and at least 60 min per different 0.5 pH unit (81; 88).
The welfare degree of the young can be assessed by anatomical indicators that can be analysed without invasive methods (in Table 12, it is suggested that producers/caretakers can identify such parameters during the biometric survey or harvest). Mortality rates can be assessed through daily monitoring, removal, and counting of dead shrimp from the pond, assessment of feed intake, and accurate quantitative surveys after harvest.

Table 12.
Health indicators for Penaeus vannamei juveniles.
The nutritional indicators used to assess the welfare of the juvenile are essentially indirect, except for the visual assessment of the filling of the digestive tract (Table 13). The aim is to ensure adequate feeding conditions and, thus, good nutrition for the animals. Therefore, the reference values and corresponding scores for some of the indicators (size of feed, amount of initial feed, frequency of feeding in the ponds, percentage of crude protein in the feed, and apparent feed conversion ratio) were established based on four size classes in the production process: for shrimp below 0.9 g, for juveniles from 1 to 3.9 g, and in two size classes where they can already be marketed for consumption, from 4 to 8.9 g and from 9 to 15 g. The other three indicators are independent of the size of the animals. Two scenarios were considered for the distribution of the feed: the distribution of the feed over the pond surface and the use of feeding trays.

Table 13.
Nutritional indicators for Penaeus vannamei juveniles and adults.
The behavioural indicators (Table 14) are specific to the different stages of the production process in a shrimp farm. During routine management, it is often possible to observe the swimming behaviour of shrimp, especially at the surface and edges of the ponds or near the water inlet and outlet structures. During harvesting, it is possible to assess the behaviour of the animals depending on the harvesting method used (fixed nets, at the monk’s exit, or trawls). The more restless the animals become, the more they jump (escape behaviour) and become stressed. The clinical reflexes detected during stunning are technical analyses that must be performed in detail because they are essential to assess the effectiveness of the slaughter, which is one of the most critical points for the welfare of the animals in the final grow-out stage.

Table 14.
Behavioural indicators for Penaeus vannamei juveniles and adults.
4. Discussion
4.1. Indicators for Shrimp Welfare
Sustainability of production, the environment, and animal welfare are critical issues in global food production and part of meaningful international sustainability discussions and policies, such as the United Nations Sustainable Development Goals [222]. In this context, the welfare of farmed shrimp cannot be neglected, considering how representative they are of aquaculture and how many billions of animals are produced and marketed worldwide each year [22].
The (few) studies on this subject still focus mainly on the reproduction and rearing/growth stages. As far as breeders are concerned, the principal reported risks are related to the removal of the eyestalk of females [21,223,224,225,226], which has several undesirable consequences, including physiological and biochemical imbalance, reproductive exhaustion, physical trauma, stress, increased energy requirements, weight loss, and increased animal mortality [21]. The main risks to shrimp welfare during the grow-out phase are related to the occurrence of diseases, especially those of viral and bacterial origin [21,227] and to the processes that eventually lead to the outbreak of these diseases, such as the problematic environmental factors related to loss of water quality or other stressful conditions to which shrimp are exposed during rearing [228,229]. Another risk to shrimp welfare that has merited the attention of researchers relates to inadequate slaughter conditions, mainly due to the absence or inadequacy of prior stunning, since immersion of animals in ice, a widely used method in shrimp slaughter worldwide, is not a stunning procedure, unlike electric shock [56]. In most publications, however, the term “shrimp welfare” now appears only sporadically and not infrequently in overly general contexts, generally referring to the most diverse aspects of the production process.
However, for shrimp welfare to be put into practise by aquaculture producers and entrepreneurs or even included in international certification standards for companies and product marketing, concepts, and indicators that can effectively measure welfare need to be defined. Indicators of animal welfare can describe the extent to which nutritional, environmental, health, behavioural, and psychological needs are met or not met, and thus, provide information on the state of the quality of life of farm animals [227,230]. Preferably, these indicators should be relevant, valid, standardised, easy to use, reliable, comparable, and appropriate for specific systems or routines, and it is also desirable that they are simple and inexpensive [231].
We have attempted to incorporate such requirements into the indicators proposed here, following a method already proposed by our group in defining indicators of grass carp welfare [32]. In both cases, we used protocols that were always scored with a maximum of three scores (1, 2, 3) for each indicator, and we tried to define the most significant possible number of non-invasive indicators of welfare. The scores range from a positive state (score 1) to a negative one (score 3). This makes the assessment less subjective and variable compared to welfare protocols that use a larger number of scores. We also advocate for indicators of P. vannamei welfare that can be effectively and accurately measured and scored in the field. Indeed, some of these indicators are already integrated into routine data collection and monitoring of the production process by laboratories and shrimp farms, mainly those historically applicable through good aquaculture practises (GAP) (see examples in [232,233,234,235,236]).
We have tested and confirmed the practical feasibility of the proposed indicators in Brazilian shrimp farms and commercial laboratories and concluded that the protocols presented here do not affect the operations of these enterprises, although they may require operational adjustments. This does not mean animal welfare protocols must be mandated in aquaculture. However, if they are difficult to apply or require high investment, they are unlikely to be adopted voluntarily by the production sector, which, as Barreto et al. [237] warned, would limit their relevance to a purely experimental context, which is not our aim. In this way, we would like to reinforce the concept that it is not incompatible in principle to adopt farming practises that promote the welfare of farmed shrimp while bringing technical, economic, and commercial benefits to aquaculture producers and entrepreneurs.
On the other hand, we do not include physiological biomarkers that require invasive interventions among our indicators. Since there are no uniform definitions for the terms ‘invasive’ and ‘non-invasive’ when applied to animal-assisted methods [234], we assume here that “invasive” involves those procedures in which the integument of an animal is injured [37,238]. That is, we do not propose here methods in which haemolymph [239] is collected, as in the analysis of haematological [240,241], physiological [241,242,243], or biochemical [196] biomarkers of shrimp. However, this does not mean that the animals cannot be manipulated at some point to obtain relevant information. Direct observations and measurements are always essential and provide information about the welfare of the animals, as they are not themselves the cause of excessive impairment of the welfare of these animals. In the case of shrimp, invasive interventions are likely to affect their welfare seriously, so we tried to minimise them in our protocols. We believe there is always a “maximum acceptable price” to be paid for scientific knowledge. As Valente [239] questioned, is it ethically and morally correct to subject potentially sentient animals to painful or distressing tests to obtain information about their welfare? In our opinion, no.
4.2. The Establishment of Farmed Shrimp Welfare Protocols
There have been several challenges in establishing indicators that reflect the welfare of P. vannamei. The first is related to the considerable gap that exists due to the lack of information on many aspects of the biology of this species, as pointed out by Saraiva et al. [223]. Such a gap does not mean that assumptions about animal welfare are invalid, but without it, the debate about stress or shrimp welfare runs the risk of not getting beyond the emotional or subjective level [244]. In other words, robust animal welfare protocols cannot be established without consistent scientific data. We have identified and used the most up-to-date and reliable information available in the technical and scientific literature to meet this challenge. However, we hope, recommend, and wish that the indicators proposed here, as well as the respective reference values for each of the three associated scores, will be regularly analysed and revised in the light of the knowledge that can be gained on the individual topics.
The second challenge is related to the life stages of animals. We are trying to establish protocols that apply to all stages of the production cycle of farmed P. vannamei and, thus, to all stages of its life cycle, including the larval stages. However, if the understanding of the sentience of juvenile and adult shrimps is still full of doubts and gaps, what about their sentience during the larval stages? This question is not as evident as it may seem. It is already known that the shrimp’s nervous system begins to structure itself immediately after hatching. Already at the beginning of the naupliar stage (N1), the neuropil is formed; the anterior part of the nervous system of P. vannamei begins to take structural shape in N3 when the ganglia in the anterior part of the head coalesce; and the complete structure of the central nervous system, including proto-, deutero-, and tritocerebrum, can be seen in N6 [245]. Without going into the core of the debate as to whether the larval stages of P. vannamei are sentient or not—which is not our aim here—we have, therefore, decided to follow the precautionary principle proposed by Birc [246]: “Where there is a threat of serious negative consequences for animal welfare, the absence of complete scientific certainty about the sentience of the animals concerned should not be used as a reason for postponing cost-effective measures to avoid these consequences”. Furthermore, we believe that if we can propose methods and breeding procedures that better meet the biological needs of shrimp even at this life stage, and if we can help to ensure that producers benefit after the larval stage by including them in the GAP, why not?
The third challenge is to recognize that the degree of tolerance or the desired level of a particular factor/indicator depends not only on the animal’s life stage but also on the production system used and the environmental conditions in which they are kept [21]. In this article, due to space constraints, it is neither possible nor necessary to discuss individually all the indicators proposed here for the welfare of P. vannamei at the different breeding stages, as both the indicators and the reference values are intended to be self-explanatory. However, it should be emphasised that the welfare indicators almost always interact directly and indirectly. In the following, we present two classic cases that illustrate this: the indicators “salinity” and “stocking density”.
Penaeus vannamei is known as one of the euryhaline shrimps [247]. Their reproduction takes place in oceanic waters (with high salinity), while postlarvae are settled in coastal areas (estuaries and lagoons with variable salinity) [248], where they arrive after developing their physiological abilities to overcome the fluctuating nature of these environments [249]. In these coastal regions, animals grow throughout the juvenile stage before returning to marine areas, where they reach sexual maturity and reproduce [248]. It is known that the mechanisms of euryhalinity and osmoregulation change during the ontogenetic development of P. vannamei. The hyper-hypo osmoregulatory pattern exhibited by juveniles and adults appears to be established at the beginning of the first postlarval stage (PL1), with greater tolerance to salinity fluctuations observed in PL2, PL4, and PL22, suggesting that P. vannamei exhibits a progressive increase in the efficiency of the osmoregulatory mechanism after the last larval stage [247]. However, it is known that salinity is not only an indicator that directly affects growth, survival [250], and, thus, the welfare of the species, but that in a husbandry system, it also directly affects changes in other parameters that determine water quality and thus shrimp welfare, such as solubility of oxygen [251]. At the same time, salinity can also influence the oxygen consumption of the shrimp themselves [252] and even their tolerance to low dissolved oxygen concentrations [253].
The stocking density in the rearing phase is admittedly a critical decision in farmed shrimp production [204,205]. The density, and thus, the sustainable biomass to be achieved, is closely related to the carrying capacity of the chosen system and varies considerably between different farming systems [254]. In extreme cases, shrimp may arrive at a farm and initially be kept in nursery tanks with densities exceeding 1500 postlarvae/m2 [255] and then exceed 400 shrimp/m2 during the final stages if the chosen husbandry system is Biofloc [256], or exceed 150 shrimp/m2 in earthen ponds [257]. From an animal welfare perspective, it is recommended that the maximum stocking density in ponds during the rearing phase does not exceed 50 shrimp/m2 [205]. Higher stocking densities tend to increase the stress to which shrimp are subjected, which from a technical point of view can compromise the results to be achieved by the operator (e.g., by reducing and compromising growth and survival rates, feed consumption, and final biomass achieved) and from the animals’ point of view can severely damage their immune system [258] affecting their welfare [21]. The expression of a particular disease—another critical issue for the welfare of farmed shrimp—is almost always the result of a complex interplay of factors related not only to stocking density but also to intrinsic aspects of the cultivated species, the pathogen in particular, and the environment [259]. This requires that disease control programmes take a multifactorial approach and that any innovation introduced should be seen as just one more element in this complex process [19].
The ecological and biological variables are so dynamic and interactive that we believe it is impossible to think of standardised management techniques or control environmental variables in ponds and hatcheries for shrimp farming. On the other hand, it is perfectly possible, for example, to establish acceptable ranges of variation in environmental parameters; to ensure the supply of natural food to the animals; to offer pre- and probiotics as part of the shrimp diet; to stimulate the growth of beneficial microbial communities; to carry out regular tests on the health status of the farmed animals; to promote their stunning before slaughter. Our protocols aim to help measure the impact of all these practises on the welfare of farmed P. vannamei.
5. Conclusions
The welfare of farmed shrimp has only recently attracted the interest of researchers, producers, policy makers, and other stakeholders in this valuable production chain [260]. As we noted in our literature review, this paper is the first attempt to propose indicators that encompass all stages of the production process of a shrimp species and, in this case, an essential shrimp species for global aquaculture. However, it is foreseeable that technologies for monitoring the welfare of farmed shrimp and those specifically targeting GAP will increasingly converge from now on. This development will typically take place through so-called “precision aquaculture” [256], which will include technologies such as the use of biosensors, data loggers, and early warning systems [21]; computer vision for animal monitoring, sensor networks (wireless and long range), robotics, and decision support tools, such as algorithms, the Internet of Things, and decision support systems [261,262,263]; as well as the use of high-quality and sensitive cameras for automatic detection and analysis of shrimp behaviour even in turbid waters [145,264]. Such technologies will, in turn, provide shrimp farmers with important information to manage feed supply with minimum waste and maximum feed efficiency; assess organic waste accumulation in ponds to optimise the water quality available to shrimp; improve management decisions related to animal health; and increasingly use animal behavioural signals as indicators of their welfare and the efficiency of management practices applied.
Likely, these non-invasive methods of measuring shrimp welfare will soon become routine in farms and laboratories, and it will become increasingly challenging to produce shrimp without taking into account the welfare of the organisms that are farmed, slaughtered, and offered to consumers, whether due to scientific advances in decapod sensitivity, consumer market demand, or changes in international regulations governing shrimp production and marketing—or all of these factors combined. The best evidence that this is entirely plausible is that remote water quality monitoring technologies are already a reality in several places worldwide [265,266,267,268]. Further evidence is that large companies are already incorporating issues such as sustainability certification, organic farming, and animal welfare into their social responsibility programmes [63,64]. Although the possible technological revolution has the potential to facilitate the assessment of welfare indicators, it will not render the use of these indicators superfluous. On the contrary, as is already the case with remote water quality monitoring, there is a tendency to integrate it with this and other technological tools. However, until this happens, any shrimp farmer can already measure the welfare of P. vannamei during the different stages and breeding processes.
Author Contributions
Conceptualization, A.S.P. and A.O.; methodology, A.S.P., C.P.d.S.T. and A.O.; validation, N.C. and U.d.A.T.d.S.; investigation, A.S.P., C.P.d.S.T., N.C. and A.O.; resources, A.S.P., C.P.d.S.T. and M.H.Q.; data curation, N.C. and A.O.; writing—original draft, A.S.P., N.C. and A.O.; writing—review and editing, A.S.P. and A.O.; supervision, M.H.Q. and A.O.; project administration, M.H.Q.; formal analysis A.S.P. and A.O. All authors have read and agreed to the published version of the manuscript.
Funding
FAI Farms Limited funded this work.
Institutional Review Board Statement
The study was developed in accordance with the Directives of the Ethics Committee on Animal Use of the Agrarian Sciences Sector of the Federal University of Paraná (CEUA-UFPR), Brazil.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We thank all the aquaculturists involved for their trust and permission to carry out this work and the CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) for awarding a productivity and research grant to Antonio Ostrensky (Process 304451/2021-5).
Conflicts of Interest
M.H.Q. was employed by FAI Farms. The remaining authors declare that the research was conducted without any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Stonich, S.C. The Environmental Quality and Social Justice Implications of Shrimp Mariculture Development in Honduras. Hum. Ecol. 1995, 23, 143–168. [Google Scholar] [CrossRef]
- Swapan, M.S.H. Michael Gavin A Desert in the Delta: Participatory Assessment of Changing Livelihoods Induced by Commercial Shrimp Farming in Southwest Bangladesh. Ocean Coast. Manag. 2011, 54, 45–54. [Google Scholar] [CrossRef]
- Queiroz, L.; Rossi, S.; Meireles, J.; Coelho, C. Shrimp Aquaculture in the Federal State of Ceará, 1970–2012: Trends after Mangrove Forest Privatization in Brazil. Ocean Coast. Manag. 2013, 73, 54–62. [Google Scholar] [CrossRef]
- Jayanthi, M.; Thirumurthy, S.; Muralidhar, M.; Ravichandran, P. Impact of Shrimp Aquaculture Development on Important Ecosystems in India. Glob. Environ. Chang. 2018, 52, 10–21. [Google Scholar] [CrossRef]
- Treviño, M.; Murillo-Sandoval, P.J. Uneven Consequences: Gendered Impacts of Shrimp Aquaculture Development on Mangrove Dependent Communities. Ocean Coast. Manag. 2021, 210, 105688. [Google Scholar] [CrossRef]
- Nguyen, T.A.T.; Nguyen, K.A.T.; Jolly, C. Is Super-Intensification the Solution to Shrimp Production and Export Sustainability? Sustainability 2019, 11, 5277. [Google Scholar] [CrossRef]
- Joffre, O.M.; Klerkx, L.; Khoa, T.N.D. Aquaculture Innovation System Analysis of Transition to Sustainable Intensification in Shrimp Farming. Agron. Sustain. Dev. 2018, 38, 1–11. [Google Scholar] [CrossRef]
- Boyd, C.E.; Davis, R.P.; McNevin, A.A. Perspectives on the Mangrove Conundrum, Land Use, and Benefits of Yield Intensification in Farmed Shrimp Production: A Review. J. World Aquac. Soc. 2022, 53, 8–46. [Google Scholar] [CrossRef]
- Cozer, N.; Horodesky, A.; Rossi, V.G.; Pont, G.D.; Ostrensky, A. Challenges and Potentialities of the Integrated Production Regime Implementation in the Brazilian Marine Shrimp Farming: A Systematic Review. Aquac. Int. 2019, 27, 539–553. [Google Scholar] [CrossRef]
- Cozer, N.; Pont, G.D.; Horodesky, A.; Ostrensky, A. Infrastructure, Management and Energy Efficiency in a Hypothetical Semi-Intensive Shrimp Model Farm in Brazil: A Systematic Review and Meta-Analysis. Rev. Aquac. 2020, 12, 1072–1089. [Google Scholar] [CrossRef]
- Bush, S.R. Understanding the Potential of Eco-Certification in Salmon and Shrimp Aquaculture Value Chains. Aquaculture 2018, 493, 376–383. [Google Scholar] [CrossRef]
- Tlusty, M.F.; Thompson, M.; Tausig, H. Statistical Tools to Assess the Breadth and Depth of Shrimp Aquaculture Certification Schemes. Fish. Res. 2016, 182, 172–176. [Google Scholar] [CrossRef]
- Samerwong, P.; Bush, S.R.; Oosterveer, P. Implications of Multiple National Certification Standards for Thai Shrimp Aquaculture. Aquaculture 2018, 493, 319–327. [Google Scholar] [CrossRef]
- Vijayan, K.K. Domestication and Genetic Improvement of Indian White Shrimp, Penaeus Indicus: A Complimentary Native Option to Exotic Pacific White Shrimp, Penaeus Vannamei. J. Coast. Res. 2019, 86, 270–276. [Google Scholar] [CrossRef]
- Alday-Sanz, V.; Brock, J.; Flegel, T.W.; McIntosh, R.; Bondad-Reantaso, M.G.; Salazar, M.; Subasinghe, R. Facts, Truths and Myths about SPF Shrimp in Aquaculture. Rev. Aquac. 2020, 12, 76–84. [Google Scholar] [CrossRef]
- Sookying, D.; Davis, D.A.; Soller Dias da Silva, F. A Review of the Development and Application of Soybean-Based Diets for Pacific White Shrimp Litopenaeus Vannamei. Aquac. Nutr. 2013, 19, 441–448. [Google Scholar] [CrossRef]
- Butt, U.D.; Lin, N.; Akhter, N.; Siddiqui, T.; Li, S.; Wu, B. Overview of the Latest Developments in the Role of Probiotics, Prebiotics and Symbiotics in Shrimp Aquaculture. Fish Shellfish Immunol. 2021, 114, 263–281. [Google Scholar] [CrossRef] [PubMed]
- Hoseinifar, S.H.; Sun, Y.Z.; Wang, A.; Zhou, Z. Probiotics as Means of Diseases Control in Aquaculture, a Review of Current Knowledge and Future Perspectives. Front. Microbiol. 2018, 9, 2429. [Google Scholar] [CrossRef] [PubMed]
- Flegel, T.W. A Future Vision for Disease Control in Shrimp Aquaculture. J. World Aquac. Soc. 2019, 50, 249–266. [Google Scholar] [CrossRef]
- FAO. The State of the World Fisheries and Aquaculture 2020: Towards Blue Transformation; FAO (Food and Agriculture Organization of the United Nations): Rome, Italy, 2020. [Google Scholar] [CrossRef]
- Albalat, A.; Zacarias, S.; Coates, C.J.; Neil, D.M.; Planellas, S.R. Welfare in Farmed Decapod Crustaceans, With Particular Reference to Penaeus Vannamei. Front. Mar. Sci. 2022, 9, 677. [Google Scholar] [CrossRef]
- Waldhorn, D.R.; Autric, E. Shrimp Production: Understanding the Scope of the Problem; Center for Open Science: San Francisco, CA, USA, 2022; pp. 1–32. [Google Scholar] [CrossRef]
- Broom, D.M. A History of Animal Welfare Science. Acta Biotheor. 2011, 59, 121–137. [Google Scholar] [CrossRef] [PubMed]
- Ohl, F.; van der Staay, F.J. Animal Welfare: At the Interface between Science and Society. Vet. J. 2012, 192, 13–19. [Google Scholar] [CrossRef]
- Fernandes, J.N.; Hemsworth, P.H.; Coleman, G.J.; Tilbrook, A.J. Costs and Benefits of Improving Farm Animal Welfare. Agriculture 2021, 11, 104. [Google Scholar] [CrossRef]
- Kaygisiz, F.; Bolat, B.A.; Bulut, D. Determining Factors Affecting Consumer’s Decision to Purchase Organic Chicken Meat. Braz. J. Poult. Sci. 2019, 21. [Google Scholar] [CrossRef]
- Beaver, A.; Proudfoot, K.L.; von Keyserlingk, M.A.G. Symposium Review: Considerations for the Future of Dairy Cattle Housing: An Animal Welfare Perspective. J. Dairy Sci. 2020, 103, 5746–5758. [Google Scholar] [CrossRef] [PubMed]
- Vaärikkala, S.; Artukka, S.M.; Hänninen, L.; Nevas, M. Finnish Cattle and Pig Farmers’ Perceptions of Animal Welfare Inspections. Anim. Welf. 2018, 27, 369–377. [Google Scholar] [CrossRef]
- Yunes, M.C.; Osório-Santos, Z.; von Keyserlingk, M.A.G.; Hötzel, M.J. Gene Editing for Improved Animal Welfare and Production Traits in Cattle: Will This Technology Be Embraced or Rejected by the Public? Sustainability 2021, 13, 4966. [Google Scholar] [CrossRef]
- Ankamah-Yeboah, I.; Jacobsen, J.B.; Olsen, S.B.; Nielsen, M.; Nielsen, R. The Impact of Animal Welfare and Environmental Information on the Choice of Organic Fish: An Empirical Investigation of German Trout Consumers. Mar. Resour. Econ. 2019, 34, 248–266. [Google Scholar] [CrossRef]
- Maesano, G.; di Vita, G.; Chinnici, G.; Pappalardo, G.; D’amico, M. The Role of Credence Attributes in Consumer Choices of Sustainable Fish Products: A Review. Sustainability 2020, 12, 10008. [Google Scholar] [CrossRef]
- Pedrazzani, A.S.; Tavares, C.P.d.S.; Quintiliano, M.; Cozer, N.; Ostrensky, A. New Indices for the Diagnosis of Fish Welfare and Their Application to the Grass Carp (Ctenopharyngodon Idella) Reared in Earthen Ponds. Aquac. Res. 2022, 53, 5825–5845. [Google Scholar] [CrossRef]
- David, P.N.F.; Manipol, N.E.P.; Madamba, J.A.B.; Mariano, R.A. Good Aquaculture Practices Adoption and Certification of Shrimp Aquaculture Farms in Bulacan, Philippines: Status, Issues and Prospects. J Glob Bus Trade 2020, 15, 11–26. [Google Scholar] [CrossRef]
- Xuan, B.B.; Sandorf, E.D.; Ngoc, Q.T.K. Stakeholder Perceptions towards Sustainable Shrimp Aquaculture in Vietnam. J. Environ. Manag. 2021, 290, 112585. [Google Scholar] [CrossRef]
- Booncharoen, C.; Anal, A.K. Attitudes, Perceptions, and On-Farm Self-Reported Practices of Shrimp Farmers’ towards Adoption of Good Aquaculture Practices (GAP) in Thailand. Sustainability 2021, 13, 5194. [Google Scholar] [CrossRef]
- Cooper, J.J.; Tinarwo, A.; Ventura, B.A. Decapods as Food, Companions and Research Animals: Legal Impact of Ascribing Sentience. Anim. Sentience 2022, 7, 27. [Google Scholar] [CrossRef]
- Souza Valente, C.D. Decapod Sentience: Broadening the Framework. Anim. Sentience 2022, 7, 8. [Google Scholar] [CrossRef]
- Gorman, R. What Might Decapod Sentience Mean for Policy, Practice, and Public? Anim. Sentience 2022, 32, 1–4. [Google Scholar] [CrossRef]
- Passantino, A.; Elwood, R.W.; Coluccio, P. Why Protect Decapod Crustaceans Used as Models in Biomedical Research and in Ecotoxicology? Ethical and Legislative Considerations. Animals 2021, 11, 73. [Google Scholar] [CrossRef]
- Mather, J.A. Philosophical Background of Attitudes toward and Treatment of Invertebrates. ILAR J. 2011, 52, 205–212. [Google Scholar] [CrossRef]
- Mikhalevich, I.; Powell, R. Minds without Spines: Evolutionarily Inclusive Animal Ethics. Anim. Sentience 2020, 29. [Google Scholar] [CrossRef]
- Beausoleil, N.J.; Mellor, D.J.; Beausoleil, N.J. Extending the “Five Domains” Model for Animal Welfare Assessment to Incorporate Positive Welfare States The Veterinarian’s Role in End-of-Life Management of Older and Chronically Ill Cats in New Zealand View Project Breathless Birds: Does Air Hunger Impact the Welfare of Poultry at Slaughter? View Project Extending the “Five Domains” Model for Animal Welfare Assessment to Incorporate Positive Welfare States. Anim. Welf. 2015, 24, 241–253. [Google Scholar] [CrossRef]
- Mellor, D.J. Welfare-Aligned Sentience: Enhanced Capacities to Experience, Interact, Anticipate, Choose and Survive. Animals 2019, 9, 440. [Google Scholar] [CrossRef]
- Crump, A.; Browning, H.; Schnell, A.; Burn, C.; Birch, J. Sentience in Decapod Crustaceans: A General Framework and Review of the Evidence. Anim. Sentience 2022, 7, 1. [Google Scholar] [CrossRef]
- Birch, J.; Burn, C.; Schnell, A.; Browning, H.; Crump, A. Review of the Evidence of Sentience in Cephalopod Molluscs and Decapod Crustaceans I; LSE Consulting. LSE Enterprise Ltd.; The London School of Economics and Political Science: London, UK, 2021. [Google Scholar]
- Feinberg, T.E.; Mallatt, J.M. The Ancient Origins of Consciousness: How the Brain Created Experience; MIT Press: Cambridge, MA, USA, 2016. [Google Scholar]
- Birch, J.; Broom, D.M.; Browning, H.; Crump, A.; Ginsburg, S.; Halina, M.; Harrison, D.; Jablonka, E.; Lee, A.Y.; Kammerer, F.; et al. How Should We Study Animal Consciousness Scientifically? J. Conscious Stud. 2022, 29, 8–28. [Google Scholar] [CrossRef]
- Conte, F.; Voslarova, E.; Vecerek, V.; Elwood, R.W.; Coluccio, P.; Pugliese, M.; Passantino, A. Humane Slaughter of Edible Decapod Crustaceans. Animals 2021, 11, 1089. [Google Scholar] [CrossRef]
- Langworthy, K.; Helluy, S.; Benton, J.; Beltz, B. Amines and Peptides in the Brain of the American Lobster: Immunocytochemical Localization Patterns and Implications for Brain Function. Cell Tissue Res. 1997, 288, 191–206. [Google Scholar] [CrossRef]
- Briffa, M. Sentience in Decapods: An Open Question. Anim. Sentience 2022, 7, 1–6. [Google Scholar] [CrossRef]
- European Union Council Regulation (EC) No 1099/2009 of 24 September 2009 on the Protection of Animals at the Time of Killing. Off. J. Eur. Union 2009, 7, 1–30. Available online: http://data.europa.eu/eli/reg/2009/1099/oj (accessed on 29 January 2023).
- EFSA Scientific Panel on Animal Health and Welfare. Aspects of the Biology and Welfare of Animals Used for Experimental and Other Scientific Purposes. EFSA J. 2005, 292, 1–46. [Google Scholar]
- Smith, J.A.; Andrews, P.L.R.; Hawkins, P.; Louhimies, S.; Ponte, G.; Dickel, L. Cephalopod Research and EU Directive 2010/63/EU: Requirements, Impacts and Ethical Review. J. Exp. Mar. Biol. Ecol. 2013, 447, 31–45. [Google Scholar] [CrossRef]
- French Ministry of Agriculture, Agrifood and Forestry. Service Note DGAL/SDSSA/N2012-8219, Dated 20 November 2012. In Authorisation and Health Inspection of Storage Tanks for Crustaceans and Seawater and Freshwater Fish; French Ministry of Agriculture, Agrifood and Forestry: Paris, France, 2012. [Google Scholar]
- Conte, F.; Voslarova, E.; Passantino, A. Destinozí Korýši: Otázka Welfare v Souvislosti s Jejich Využitím Jako Potraviny [Decapod Crustaceans: Some Issues Related to Welfare and Their Use as Food]. Maso 2018, 7, 51–54. [Google Scholar]
- Weineck, K.; Ray, A.J.; Fleckenstein, L.J.; Medley, M.; Dzubuk, N.; Piana, E.; Cooper, R.L. Physiological Changes as a Measure of Crustacean Welfare under Different Standardized Stunning Techniques: Cooling and Electroshock. Animals 2018, 8, 158. [Google Scholar] [CrossRef]
- Crump, A.; Birch, J. Animal Consciousness: The Interplay of Neural and Behavioural Evidence. J. Conscious Stud. 2022, 29, 104–128. [Google Scholar] [CrossRef]
- Johnston, C.; Jungalwalla, P. Aquatic Animal Welfare Guidelines: Guidelines on Welfare of Fish and Crustaceans in Aquaculture and/or in Live Holding Systems for Human Consumption; National Aquaculture Council of Australia: Deakin, ACT, Australia, 2005. [Google Scholar]
- PNS/BAFS 197:2017; BAFS Philippine National Standards Code of Good Aquaculture Practices for Shrimp and Crab. Bureau of Agriculture and Fisheries Standards: Quezon City, Philippines, 2017.
- Nguyen, T.B.T. Good Aquaculture Practices (VietGAP) and Sustainable Aquaculture Development in Viet Nam; Romana-Eguia, M.R.R., ParadoEstepa, F., Salayo, N., Lebata-Ramos, M.J.H., Eds.; Resource Enhancement and Sustainable Aquaculture Practices in Southeast Asia: Challenges in Responsible Production of Aquatic Species: Proceedings of the International Workshop on Resource Enhancement and Sustainable Aquaculture Practices in Southeast Asia 2014 (RESA); Aquaculture Dept., Southeast Asian Fisheries Development Center: Tigbauan, Philippines, 2015; pp. 85–92. Available online: http://hdl.handle.net/10862/2766 (accessed on 29 January 2023).
- Paschke, M. Applying U.S. Animal Law Extraterritoriality to Improve Animal Welfare Standards Abroad and Avoid a Race to the Bottom. Denver J. Int. Law Policy 2021, 49, 13. [Google Scholar]
- Vesilind, P.A. Continental Drift: Agricultural Trade and the Widening Gap Between European Union and United States Animal Welfare Laws. Int. Trade eJ 2010, 12, 223. [Google Scholar] [CrossRef]
- Alfnes, F.; Chen, X.; Rickertsen, K. Labeling Farmed Seafood: A Review. Aquac. Econ. Manag. 2018, 22, 1–26. [Google Scholar] [CrossRef]
- Alfnes, F. Selling Only Sustainable Seafood: Attitudes toward Public Regulation and Retailer Policies. Mar. Policy 2017, 78, 74–79. [Google Scholar] [CrossRef]
- Coates, C.J.; Söderhäll, K. The Stress–Immunity Axis in Shellfish. J. Invertebr. Pathol. 2021, 186, 107492. [Google Scholar] [CrossRef]
- Scherer, L.; Tomasik, B.; Rueda, O.; Pfister, S. Framework for Integrating Animal Welfare into Life Cycle Sustainability Assessment. Int. J. Life Cycle Assess. 2018, 23, 1476–1490. [Google Scholar] [CrossRef]
- Franks, B.; Ewell, C.; Jacquet, J. Animal Welfare Risks of Global Aquaculture. Sci. Adv. 2021, 7, eabg0677. [Google Scholar] [CrossRef]
- FAWC Prevention of Cruelty to Animals Act 1979. Available online: https://legislation.nsw.gov.au/view/html/inforce/current/act-1979-200 (accessed on 29 December 2022).
- Stien, L.H.; Bracke, M.B.M.; Folkedal, O.; Nilsson, J.; Oppedal, F.; Torgersen, T.; Kittilsen, S.; Midtlyng, P.J.; Vindas, M.A.; Øverli, Ø.; et al. Salmon Welfare Index Model (SWIM 1.0): A Semantic Model for Overall Welfare Assessment of Caged Atlantic Salmon: Review of the Selected Welfare Indicators and Model Presentation. Rev. Aquac. 2013, 5, 33–57. [Google Scholar] [CrossRef]
- Pedrazzani, A.S.; Quintiliano, M.H.; Bolfe, F.; Sans, E.C.d.O.; Molento, C.F.M. Tilapia On-Farm Welfare Assessment Protocol for Semi-Intensive Production Systems. Front. Vet. Sci. 2020, 7, 991. [Google Scholar] [CrossRef]
- Ceballos-Vázquez, B.P.; Palacios, E.; Aguilar-Villavicencio, J.; Racotta, I.S. Gonadal Development in Male and Female Domesticated Whiteleg Shrimp, Litopenaeus Vannamei, in Relation to Age and Weight. Aquaculture 2010, 308, 116–123. [Google Scholar] [CrossRef]
- Ceballos-Vázquez, B.P.; Rosas, C.; Racotta, I.S. Sperm Quality in Relation to Age and Weight of White Shrimp Litopenaeus Vannamei. Aquaculture 2003, 228, 141–151. [Google Scholar] [CrossRef]
- González, R.A.; Díaz, F.; Licea, A.; Denisse Re, A.; Noemí Sánchez, L.; García-Esquivel, Z. Thermal Preference, Tolerance and Oxygen Consumption of Adult White Shrimp Litopenaeus Vannamei (Boone) Exposed to Different Acclimation Temperatures. J. Therm. Biol. 2010, 35, 218–224. [Google Scholar] [CrossRef]
- Perez-Velazquez, M.; Bray, W.A.; Lawrence, A.L.; Gatlin, D.M.; Gonzalez-Felix, M.L. Effect of Temperature on Sperm Quality of Captive Litopenaeus Vannamei Broodstock. Aquaculture 2001, 198, 209–218. [Google Scholar] [CrossRef]
- Barbieri, C.; Ostrensky, A. Camarões Marinhos–Reprodução, Maturação e Larvicultura; Aprenda Fácil: Viçosa, Brazil, 2001; p. 370. [Google Scholar]
- Balasubramanian, C.P.; Anand, S.; Kannappan, S.; Biju, I.F. Training Manual on Recent Advances in Farming of Pacific White Shrimp, Penaeus Vannamei. Train. Man. Ser. 2018, 14, 126. [Google Scholar]
- Simpson, J.J.; Zirino, A. Biological Control of PH in the Peruvian Coastal Upwelling Area. Deep Sea Res. Part A Oceanogr. Res. Pap. 1980, 27, 733–743. [Google Scholar] [CrossRef]
- Zuta, S.; Guillén, O. Oceanografía de Las Aguas Costeras Del Perú. Bol. Inst. Mar. Perú. 1970, 2, 157–324. [Google Scholar]
- FAO Health Management and Biosecurity Maintenance in White Shrimp (Penaeus Vannamei) Hatcheries in Latin America. Fish TechPap 2003, 450, 62.
- Treece, G.D. Shrimp Maturation and Spawning; UJNR Technical Report No. 28; Texas A&M University, Sea Grant College Program: Bryan, TX, USA, 2000; pp. 121–134. [Google Scholar]
- Ostrensky, A.; Stevanato, D.J.; Pont, G.D.; Castilho-Westphal, G.G.; Girotto, M.V.F.; Cozer, N.; García-Madrigal, R.F.d.A.; Silva, U. A Produção Integrada Na Carcinicultura Brasileira (Volume 1); Ostrensky, A., Cozer, N., Eds.; Instituto GIA: Curitiba, PR, Brasil, 2017. [Google Scholar]
- Peixoto, S.; Wasielesky, W.; Louzada, L. Comparative Analysis of Pink Shrimp, Farfantepenaeus Paulensis, and Pacific White Shrimp, Litopenaeus Vannamei, Culture in Extreme Southern Brazil. J. Appl. Aquac. 2003, 14, 101–111. [Google Scholar] [CrossRef]
- Venkateswarlu, V.; Seshaiah, P.; Arun, P.; Behra, P. A Study on Water Quality Parameters in Shrimp L. Vannamei Semi-Intensive Grow out Culture Farms in Coastal Districts of Andhra Pradesh, India. Int. J. Fish, Aquat. Stud. 2019, 7, 394–397. [Google Scholar]
- Fast, A.W.; Lester, L.J. Marine Shrimp Culture: Principles and Practices, 1st ed.; Elsevier: Amsterdam, The Netherlands, 1992; p. 862. ISBN 978-0-444-88606-4. [Google Scholar]
- Nunes, A.J.P. O cultivo de camarões marinhos no Nordeste do Brasil. Rev. Panor. Aqüic. 2001, 11, 65. [Google Scholar]
- Nogueira, D.B.; Souza, L.S.B.; Santos, W.R.; Júnior, G.d.N.A.; Souza, C.A.A.; Jardim, A.M.R.F.; Silva, T.G.F. Understanding the Influence of Photoperiod on the Cultivation of Litopenaeus Vannamei: Revisiting Studies Carried out for the 2005–2020 Cropping Period. Res. Soc. Dev. 2021, 10. [Google Scholar] [CrossRef]
- ABCC. MCR Apostila Técnica de Boas Práticas de Manejo Para a Capacitação de Pequenos Produtores, Natal-RN; Associação Brasileira de Criadores de Camarão: Candelária, Natal, RN, Brasil, 2010. [Google Scholar]
- Fonseca, C.S. Manual de Boas Práticas de Manejo e de Biossegurança Para a Carcinicultura Brasileira; Associação Brasileira de Criadores de Camarão: Candelária, RN, Brasil, 2021. [Google Scholar]
- Nunes AJ, P.; Gesteira TC, V.; Oliveira, G.G.; Lima, R.C.; Miranda PT, C.; Madrid, R.M. Princípios Para Boas Práticas de Manejo Na Engorda de Camarão Marinho No Estado Do Ceará; Universidade Federal do Ceará–LABOMAR: Fortaleza, CE, Brasil, 2005. [Google Scholar]
- Rojas, A.A.; Haws, M.C.; y Cabanillas, J.A. Buenas Prácticas de Manejo Para el Cultivo de Camarón, 1st ed.; The David and Lucile Packard Foundation; United States Agency for International Development: Washington, DC, USA, 2005; pp. 4–51. [Google Scholar]
- Zhang, P.; Zhang, X.; Li, J.; Huang, G. The Effects of Body Weight, Temperature, Salinity, PH, Light Intensity and Feeding Condition on Lethal DO Levels of Whiteleg Shrimp, Litopenaeus Vannamei (Boone, 1931). Aquaculture 2006, 256, 579–587. [Google Scholar] [CrossRef]
- Han, S.; Wang, B.; Wang, M.; Liu, Q.; Zhao, W.; Wang, L. Effects of Ammonia and Nitrite Accumulation on the Survival and Growth Performance of White Shrimp Litopenaeus Vannamei. Invert. Surv. J. 2017, 14, 221–232. [Google Scholar] [CrossRef]
- Chen, J. Acute Toxicity of Ammonia on Litopenaeus Vannamei Boone Juveniles at Different Salinity Levels. J. Exp. Mar. Biol. Ecol. 2001, 259, 109–119. [Google Scholar]
- Gross, A.; Abutbul, S.; Zilberg, D. Acute and Chronic Effects of Nitrite on White Shrimp, Litopenaeus Vannamei, Cultured in Low-Salinity Brackish Water. J. World Aquac. Soc. 2004, 35, 315–321. [Google Scholar] [CrossRef]
- Lin, Y.C.; Chen, J.C. Acute Toxicity of Nitrite on Litopenaeus Vannamei (Boone) Juveniles at Different Salinity Levels. Aquaculture 2003, 224, 193–201. [Google Scholar] [CrossRef]
- Tan, J.; Luan, S.; Cao, B.; Luo, K.; Meng, X.; Kong, J. Comparison of Growth and Reproduction Performance of Broodstock Pacific White Shrimp Litopenaeus Vannamei Reared in Oceanic and Brackish Water. Aquac. Res. 2019, 50, 1893–1902. [Google Scholar] [CrossRef]
- Flores, R.; Espino, M.; Luque, G.; Quispe, J. Patrones de Variabilidad Ambiental En El Mar Peruano. Rev. Peru Biol. 2013, 20, 021–028. [Google Scholar] [CrossRef]
- Aquacop. Penaeid Reared Brood Stock: Closing the Cycle. Meeting of the World Mariculture Society. J. World Maricult. Soc. 1979, 10, 445–452. [Google Scholar] [CrossRef]
- Alday-Sanz, V. The Shrimp Book, 1st ed.; Nottingham University Press: Nottingham, UK, 2012; ISBN 9781904761594. [Google Scholar]
- Chen, F.; Reid, B.; Arnold, C.R. Maturing, Spawning and Egg Collecting of the White Shrimp Penaeus Vannamei Boone in a Recirculating System. J. World Aquac. Soc. 1991, 22, 167–172. [Google Scholar] [CrossRef]
- Basso, E.; Drever, M.C.; Fonseca, J.; Navedo, J.G. Semi-intensive Shrimp Farms as Experimental Arenas for the Study of Predation Risk from Falcons to Shorebirds. Ecol. Evol. 2021, 11, 13379. [Google Scholar] [CrossRef]
- Kungvankij, P.; Chua, T.E.; Pudadera, B.J., Jr.; Corre, K.G. Shrimp Culture: Pond Design, Operation and Management. 1988. Available online: https://www.fao.org/3/ac210e/AC210E00.htm#TOC (accessed on 29 January 2023).
- Terazaki, M.; Tharnbuppa, P.; Nakayama, Y. Eradication of Predatory Fishes in Shrimp Farms by Utilization of Thai Tea Seed. Aquaculture 1980, 19, 235–242. [Google Scholar] [CrossRef]
- Palanikumar, P.; Velmurugan, S.; Citarasu, T. Factors Influencing in Success of Penaeus Vannamei Culture. Aquac. Asia Mag. 2011, 15, 10–15. [Google Scholar]
- Arulmoorthy, M.P.; Anandajothi, E.; Vasudevan, S.; Suresh, E. Major Viral Diseases in Culturable Penaeid Shrimps: A Review. Aquac. Int. 2020, 28, 1939–1967. [Google Scholar] [CrossRef]
- Furtado, P.S.; Campos, B.R.; Serra, F.P.; Klosterhoff, M.; Romano, L.A.; Wasielesky, W. Effects of Nitrate Toxicity in the Pacific White Shrimp, Litopenaeus Vannamei, Reared with Biofloc Technology (BFT). Aquac. Int. 2015, 23, 315–327. [Google Scholar] [CrossRef]
- Lee, D.; Yu, Y.B.; Choi, J.H.; Jo, A.H.; Hong, S.M.; Kang, J.C.; Kim, J.H. Viral Shrimp Diseases Listed by the OIE: A Review. Viruses 2022, 14, 585. [Google Scholar] [CrossRef]
- Johnson, S.K. Handbook of Shrimp Diseases; Texas A&M Univer sity Sea Grant College Program, the Texas A&M Department of Wildlife and Fisheries Sciences and the Texas Agricultural Extension Service: Bryan, TX, USA, 1995. [Google Scholar]
- Gunalan, B.; Soundarapandian, P.; Anand, T.; Kotiya, A.S.; Simon, N.T. Disease Occurrence in Litopenaeus Vannamei Shrimp Culture Systems in Different Geographical Regions of India. Int. J. Aquac. 2014. [Google Scholar] [CrossRef]
- Lightner, D.V. A Handbook of Shrimp Pathology and Diagnostic Procedures for Disease of Cultured Penaeid Shrimp; World Aqua.: Baton Rouge, LA, USA, 1996.111. Smith, P.T. Diseases of the Eye of Farmed Shrimp Penaeus Monodon. Dis. Aquat. Organ. 2000, 43, 159–173. [Google Scholar] [CrossRef]
- Bondad-Reantaso, M.G.; McGladdery, S.E.; East, I.; Subasinghe, R.P. Asia Diagnostic Guide to Aquatic Animal Diseases; FAO Fisheries Technical Paper 402, Supplement 2; FAO: Rome, Italy, 2001; 240p. [Google Scholar]
- Dewangan, N.K.; Gopalakrishnan, A.; Kannan, D.; Shettu, N.; Singh, R.R. Black Gill Disease of Pacific White Leg Shrimp (Litopenaeus Vannamei) by Aspergillus Flavus. J. Coast. Life Med. 2015, 3, 761–765. [Google Scholar] [CrossRef]
- Sivakamavalli, J.; Park, K.; Kwak, I.; Baskaralingam, V. Bacterial Disease Control Methods in Shrimp ( Penaeus, 1798) Farming Sector in Asian Countries. In Arthropods-Are They Beneficial for Mankind? IntechOpen: London, UK, 2021; ISBN 978-1-78984-166-4. [Google Scholar]
- Dey, B.K.; Dugassa, G.H.; Hinzano, S.M.; Bossier, P. Causative Agent, Diagnosis and Management of White Spot Disease in Shrimp: A Review. Rev. Aquac. 2020, 12, 822–865. [Google Scholar] [CrossRef]
- Zhang, Q.; Xu, T.; Wan, X.; Liu, S.; Wang, X.; Li, X.; Dong, X.; Yang, B.; Huang, J. Prevalence and Distribution of Covert Mortality Nodavirus (CMNV) in Cultured Crustacean. Virus Res. 2017, 233, 113–119. [Google Scholar] [CrossRef]
- Prasad, K.P.; Shyam, K.U.; Banu, H.; Jeena, K.; Krishnan, R. Infectious Myonecrosis Virus (IMNV)—An Alarming Viral Pathogen to Penaeid Shrimps. Aquaculture 2017, 477, 99–105. [Google Scholar] [CrossRef]
- Prachumwat, A.; Munkongwongsiri, N.; Eamsaard, W.; Lertsiri, K.; Flegel, T.W.; Stentiford, G.D.; Sritunyalucksana, K. A Potential Prokaryotic and Microsporidian Pathobiome That May Cause Shrimp White Feces Syndrome (WFS). bioRxiv 2021, 12120. [Google Scholar] [CrossRef]
- Kannan, D.; Thirunavukkarasu, P.; Jagadeesan, K.; Shettu, N.; Kumar, A. Procedure for Maturation and Spawning of Imported Shrimp Litopenaeus Vannamei in Commercial Hatchery, South East Coast of India. Fish. Aquac. J. 2015, 6. [Google Scholar] [CrossRef]
- Taylor, J.; Vinatea, L.; Ozorio, R.; Schuweitzer, R.; Andreatta, E.R. Minimizing the Effects of Stress during Eyestalk Ablation of Litopenaeus Vannamei Females with Topical Anesthetic and a Coagulating Agent. Aquaculture 2004, 233, 173–179. [Google Scholar] [CrossRef]
- Menezes, T.B.B. de Efeito Da Não Ablação No Processo de Reprodução de Fêmeas de Penaeus Vannamei. 54 f. Master’s Thesis, (Mestrado em Engenharia de Pesca)–Universidade Federal do Ceará, Fortaleza, Brazil, 2019. [Google Scholar]
- Ren, S.; Prentis, P.; Mather, P.B.; Li, Y.; Tang, B.; Hurwood, D.A. Genetic Parameters for Growth and Survival Traits in a Base Population of Pacific White Shrimp (Litopenaeus Vannamei) Developed from Domesticated Strains in China. Aquaculture 2020, 523, 735148. [Google Scholar] [CrossRef]
- Ogle, J.T.; Beaugez, K.; Lotz, J.M. Effects of Salinity on Survival and Growth of Postlarval Penaeus Vannamei. Gulf. Caribb Res. 1992, 8, 415–421. [Google Scholar] [CrossRef]
- Doyle, R.W. Inbreeding and Disease in Tropical Shrimp Aquaculture: A Reappraisal and Caution. Aquac. Res. 2016, 47, 21–35. [Google Scholar] [CrossRef]
- Pontes, C.S.; de F. Arruda, M. Acesso Ao Alimento Artificial e Enchimento Do Trato Digestivo de Juvenis Do Camarão Marinho Litopenaeus Vannamei (Boone) (Crustacea, Decapoda, Penaeidae) Durante as Fases Clara e Escura Do Período de 24 Horas. Rev. Bras. Zool. 2005, 22, 1039–1043. [Google Scholar] [CrossRef]
- Wyk, P. Van Nutrition and Feeding of Litopenaeus Vannamei in Intensive Culture System. Farming Mar. Shrimp Recirc. Freshw. Syst. 1999, 2, 125–139. [Google Scholar]
- Alencar, C.A. Relatório Sobre o Acompanhamento de Um Cultivo de Camarão Branco Do Pacífico, Litopenaeus Vannamei (Boone,1931), Em Viveiros Escavados, Realizado Na Fazenda Santa Isabel, No Município de Aracati–CE. Relatório de Estágio Supervisionado. Relatório supervisionado; Departamento de Engenharia de Pesca do Centro de Ciências Agrárias, Universidade Federal do Ceará: Fortaleza, CE, Brasil, 2003. [Google Scholar]
- de Sousa, R.R. Descrição Do Cultivo de Camarões Marinho Em Duas Fazendas Da CPH Aquacultura LTDA; Trabalho de Conclusão de Curso: Fortaleza, CE, Brasil, 2009. [Google Scholar]
- Felix, S. Advances in Shrimp Aquaculture Management, 1st ed.; Daya Publishing House: New Delhi, India, 2009; ISBN 9788170355472. [Google Scholar]
- Carvalho, E.A. Efeito Da Frequencia Alimentar Sovbre o Desempenho Zootécnico Do Camarão Branco Do Pacífico Litopenaeus Vannamei Em Condições de Cultivo. Diploma Thesis, Universidade de São Paulo–USP, São Paulo, SP, Brasil, 2005. [Google Scholar]
- Brito, M.D.L.S. Arraçoamento Do Litopenaeus Vannamei: Desempenho, Sobrevivência e Qualidade Do Efluente. Diploma Thesis, Universidade de São Paulo–USP, São Paulo, SP, Brasil, 2017. [Google Scholar]
- De Lima, P.P.; Pontes, C.S.; Arruda, M.D.F. Activity Pattern of the Marine Shrimp Litopenaeus Vannamei (Boone 1931) in Laboratory as a Function of Different Feeding Frequencies. Aquac. Res. 2009, 41, 53–60. [Google Scholar] [CrossRef]
- Wouters, R.; Lavens, P.; Nieto, J.; Sorgeloos, P. Penaeid Shrimp Broodstock Nutrition: An Updated Review on Research and Development. Aquaculture 2001, 202, 1–21. [Google Scholar] [CrossRef]
- Hoa, N.D.; Wouters, R.; Wille, M.; Thanh, V.; Dong, T.K.; Van Hao, N.; Sorgeloos, P. A Fresh-Food Maturation Diet with an Adequate HUFA Composition for Broodstock Nutrition Studies in Black Tiger Shrimp Penaeus Monodon (Fabricius, 1798). Aquaculture 2009, 297, 116–121. [Google Scholar] [CrossRef]
- Racotta, I.S.; Palacios, E.; Ibarra, A.M. Shrimp Larval Quality in Relation to Broodstock Condition. Aquaculture 2003, 227, 107–130. [Google Scholar] [CrossRef]
- Harrison, K.E. The Role of Nutrition in Maturation Reproduction and Embryonic Development of Decapod Crustaceans a Review. J. Shellfish. Res. 1990, 9, 1–28. [Google Scholar] [CrossRef]
- Vaghei, G.R.; Abolhasani, M.H.; Matinfar, A.; Dadgar, S.; Ghorbani, R. Production of Artificial Diets for Female Broodstock of Western White Shrimp (Litopenaeus Vannamei) and Study on Their Singular Effect. Iran J. Fish. Sci. 2017, 16, 1204–1213. [Google Scholar]
- Rocha, N.S. Maturação Do Camarão Marinho Litopenaeus Vannamel: O Modelo de Produção Da Aquacrusta Ltda (Acarau-Ce). Diploma Thesis, Universidade Federal do Ceará, Aracau, CE, Brasil, 2007. [Google Scholar]
- de Oliveira, L.L. Larvicultura Do Camarão Marinho Litopenaeus Vannamei; Graduation thesis in Fishing Engineering; Universidade Federal do Ceará: Aracaú, CE, Brasil, 2006. [Google Scholar]
- Cuzon, G.; Lawrence, A.; Gaxiola, G.; Rosas, C.; Guillaume, J. Nutrition of Litopenaeus Vannamei Reared in Tanks or in Ponds. Aquaculture 2004, 235, 513–551. [Google Scholar] [CrossRef]
- Bardera, G.; Owen, M.A.G.; Façanha, F.N.; Sloman, K.A.; Alexander, M.E. The Influence of Sex on Feeding Behaviour in Pacific White Shrimp (Litopenaeus Vannamei). Appl. Anim. Behav. Sci. 2020, 224, 104946. [Google Scholar] [CrossRef]
- Venero, J.A.; Davis, D.A.; Rouse, D.B. Variable Feed Allowance with Constant Protein Input for the Pacific White Shrimp Litopenaeus Vannamei Reared under Semi-Intensive Conditions in Tanks and Ponds. Aquaculture 2007, 269, 490–503. [Google Scholar] [CrossRef]
- Severino, D.V. Formação de Reprodutores de Camarões Marinhos (Litopenaeus Vannamei) Em Sistema Biosseguro de Bioflocos. Diploma Thesis, Universidade Federal de Santa Catarina, Florianópolis, SC, Brasil, 2014. [Google Scholar]
- Zheng, C.; Zhao, Q.; Li, E.; Zhao, D.; Sun, S. Role of Hypoxia in the Behaviour, Physiology, Immunity and Response Mechanisms of Crustaceans: A Review. Rev. Aquac. 2022, 14, 676–687. [Google Scholar] [CrossRef]
- Darodes de Tailly, J.B.; Keitel, J.; Owen, M.A.G.; Alcaraz-Calero, J.M.; Alexander, M.E.; Sloman, K.A. Monitoring Methods of Feeding Behaviour to Answer Key Questions in Penaeid Shrimp Feeding. Rev. Aquac. 2021, 13, 1828–1843. [Google Scholar] [CrossRef]
- Parodi, T.V.; Cunha, M.A.; Heldwein, C.G.; De Souza, D.M.; Martins, Á.C.; Garcia, L.D.O.; Junior, W.W.; Monserrat, J.M.; Schmidt, D.; Caron, B.O.; et al. The Anesthetic Efficacy of Eugenol and the Essential Oils of Lippia Alba and Aloysia Triphylla in Post-Larvae and Sub-Adults of Litopenaeus Vannamei (Crustacea, Penaeidae). Comp. Biochem. Physiol. C Toxicol. Pharmac. 2012, 155, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Becker, A.J.; Vaz, L.J.; De Garcia, L.O.; Wasielesky, W.; Heinzmann, B.M.; Baldisserotto, B. Anesthetic Potential of Different Essential Oils for Two Shrimp Species, Farfantepenaeus Paulensis and Litopenaeus Vannamei (Decapoda, Crustacea). Cienc. Rural. 2021, 51. [Google Scholar] [CrossRef]
- de Souza Valente, C. Anaesthesia of Decapod Crustaceans. Vet. Anim. Sci. 2022, 16, 100252. [Google Scholar] [CrossRef]
- Serviço Nacional de Aprendizagem Rural. Larvicultura de Camarão Marinho: Do Náuplio a Pós-Larva, 1st ed.; Serviço Nacional de Aprendizagem Rural: Brasília, DF, Brasil, 2016; Volume 1. [Google Scholar]
- Bermudes-Lizárraga, J.; Nieves-Soto, M.; Medina-Jasso, A.; Piña-Valdez, P.; Bermudes-Lizárraga, J.; Nieves-Soto, M.; Medina-Jasso, A.; Piña-Valdez, P. Effect of Temperature and Salinity on Larval Survival and Development of Litopenaeus Vannamei. Rev. MVZ Cordoba 2017, 22, 5844–5853. [Google Scholar] [CrossRef]
- Ravichandran, P.; Devarajan, K.; Pandian, S.K. Unit-7 Seed Production and Larval Rearing of Shrimps. In Block-2 Hatchery Technology for Prawns and Fishes; Indira Gandhi National Open University New Delhi: New Delhi, India, 2004; p. 11. [Google Scholar]
- Nunes, H.R.; Andreatta, E.R. Efeito Da Salinidade e Temperatura Sobre a Taxa de Metamorfose de Náuplios Para Protozoea e Sobre a Qualidade Das Larvas de Litopenaeus Vannamei. Atlântica 2011, 33, 87–96. [Google Scholar] [CrossRef]
- Ponce-Palafox, J.; Martinez-Palacios, C.A.; Ross, L.G. The Effects of Salinity and Temperature on the Growth and Survival Rates of Juvenile White Shrimp, Penaeus Vannamei, Boone, 1931. Aquaculture 1997, 157, 107–115. [Google Scholar] [CrossRef]
- Viet, L.Q.; Hai, T.N.; Phu, T.M.; Ngan, T.V. Effects of Photoperiods on Growth and Quality of White Leg Shrimp (Litopenaeus van-Namei) in Biofloc System. Can Tho Univ. J. Sci. 2017, 6, 83. [Google Scholar] [CrossRef]
- Sanudin, N.; Daning Tuzan, A.; Seok, A.; Yong, K. Feeding Activity and Growth Performance of Shrimp Post Larvae Litopenaeus Vannamei Under Light and Dark Condition. J. Agric. Sci. 2014, 6, p103. [Google Scholar] [CrossRef]
- Cobo, M.D.L.; Sonnenholzner, S.; Wille, M.; Sorgeloos, P. Ammonia Tolerance of Litopenaeus Vannamei (Boone) Larvae. Aquac. Res. 2014, 45, 470–475. [Google Scholar] [CrossRef]
- Valencia-Castañeda, G.; Frías-Espericueta, M.G.; Vanegas-Pérez, R.C.; Pérez-Ramírez, J.A.; Chávez-Sánchez, M.C.; Páez-Osuna, F. Acute Toxicity of Ammonia, Nitrite and Nitrate to Shrimp Litopenaeus Vannamei Postlarvae in Low-Salinity Water. Bull. Environ. Contam. Toxicol. 2018, 101, 229–234. [Google Scholar] [CrossRef]
- Frías-Espericueta, M.G.; Harfush-Melendez, M.; Páez-Osuna, F. Effects of Ammonia on Mortality and Feeding of Postlarvae Shrimp Litopenaeus Vannamei. Bull. Environ. Contam. Toxicol. 2000, 65, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, B.L.F. de Adição Do Nitrito de Sódio Em Sistema de Biofloco e Toxicidade Do Nitrito Para o Camarão Litopenaeus Vannamei. Diploma Thesis, Universidade Federal Rural de Pernambuco, Recife, PE, Brasil, 2013. [Google Scholar]
- Branco, L.F.C. Avaliação Da Taxa de Sobrevivência Final Na Larvicultura Do Camarão Marinho Litopenaeus Vannamei Através de Diferentes Densidades de Estocagem. Diploma Thesis, Universidade Federal Rural do Semi-Árido, Mossoró, RN, Brasil, 2021. [Google Scholar]
- López Peraza, D.J.; Bermudes Lizárraga, J.F.; Flores Alarcón, J.M.; Nieves Soto, M. Effect of the Larval Density and Food Ratio on Litopenaeus Vannamei (Boone, 1931) Zoea. Agro Prod. 2022. [Google Scholar] [CrossRef]
- Knoll, R.C. Sistema de Avaliação Da Qualidade de Pós-Larvas Do Camarão Marinho Litopenaeus Vannamei (Boone, 1931). Diploma Thesis, Universidade Federal de Santa Catarina, Florianópolis, SC, Brasil, 2012. [Google Scholar]
- Magalhães, M.E. Cultivo do Camarão Marinho Litopenaeus Vannamei (BOONE,1931) em Sistema Multifásico–Pesquisa Google. Diploma Thesis, Universidade Federal Rural de Pernambuco, Recife, Brazil, 2004. [Google Scholar]
- Peregrino, L.H.S.; Correia, E.S.; Oliveira, A. Revista da ABCC; Associação Brasileira de Criadores de Camarão: Recife, PE, Brazil, 2002; pp. 1–70. [Google Scholar]
- Alencar, C.A. Acompanhamento de um Cultivo Intensivo de Camarão Branco do Pacífico, Litopenaeus Vannamei (BOONE,1931), em Viveiros Escavados, Realizado na Fazenda Santa Isabel, No Municipio de Aracati-CE. Diploma Thesis, Universidade Federal do Ceará, Fortaleza, CE, Brazil, 2003. [Google Scholar]
- Gelabert, R.; Pacheco, A. Selectivity of Particle Size by the Shrimp Litopenaeus Vannamei (Boone, 1931) Larvae. Aquac. Nutr. 2011, 17, 244–247. [Google Scholar] [CrossRef]
- Barbieri, R.C.; Ostrensky, A. Camarões Marinhos–Engorda Ostrensky; Viçosa: Aprenda Fácil, Brazil, 2002; 326p. [Google Scholar]
- Van Wyk, P.; Davis-Hodgkins, M.; Laramore, R.; Main, K.L.; Mountain, J.; Scarpa, J. Farming Marine Shrimp in Recirculating Freshwater Systems Harbor Branch Oceanographic Institution. In Farming Marine Shrimp in Recirculating Freshwater Systems; van Wyk, P., Davis-Hodgkins, M., Laramore, R., Main, K.L., Mountain, J., Scarpa, J., Eds.; Harbor Branch Oceanographic Institution: Fort Pierce, FL, USA, 1999; Volume 220, pp. 125–140. [Google Scholar]
- Jiménez-Yan, L.; Brito, A.; Cuzon, G.; Gaxiola, G.; García, T.; Taboada, G.; Soto, L.A.; Brito, R. Energy Balance of Litopenaeus Vannamei Postlarvae Fed on Animal or Vegetable Protein Based Compounded Feeds. Aquaculture 2006, 260, 337–345. [Google Scholar] [CrossRef]
- Colvin, L.B.; Brand, C.W. The protein requirement of penaeid shrimp at various life-cycle stages in controlled environment systems. Proc. Annu. Meet. World Maric. Soc. 1977, 8, 821–840. [Google Scholar] [CrossRef]
- Robertson, L.; Wrence, A.L.L.; Castille, F.L. Effect of Feeding Frequency and Feeding Time on Growth of Penaeus Vannamei (Boone). Aquac. Res. 1993, 24, 1–6. [Google Scholar] [CrossRef]
- Aalimahmoudi, M.; Reyshahri, A.; Salehipour Bavarsad, S.; Maniat, M.; Mazdak Aalimahmoudi, C. Effects of Feeding Frequency on Growth, Feed Conversion Ratio, Survival Rate and Water Quality of White Leg Shrimp (Litopenaeus Vannamei, Boone, 1931). Int. J. Fish. Aquat. Stud. 2016, 4, 293–297. [Google Scholar]
- Kuban, F.D.; Lawrence, A.L.; Wilkenfeld, J.S. Survival, Metamorphosis and Growth of Larvae from Four Penaeid Species Fed Six Food Combinations. Aquaculture 1985, 47, 151–162. [Google Scholar] [CrossRef]
- Chu, K.H.; Shing, C.K. Feeding Behavior of the Shrimp, Metapenaeus Ensis, on Artemia Nauplii. Aquaculture 1986, 58, 175–184. [Google Scholar] [CrossRef]
- Piña, P.; Nieves, M.; Ramos-Brito, L.; Chavira-Ortega, C.O.; Voltolina, D. Survival, Growth and Feeding Efficiency of Litopenaeus Vannamei Protozoea Larvae Fed Different Rations of the Diatom Chaetoceros Muelleri. Aquaculture 2005, 249, 431–437. [Google Scholar] [CrossRef]
- Martín, L.; Arenal, A.; Fajardo, J.; Pimentel, E.; Hidalgo, L.; Pacheco, M.; García, C.; Santiesteban, D. Complete and Partial Replacement of Artemia Nauplii by Moina Micrura during Early Postlarval Culture of White Shrimp (Litopenaeus Schmitti). Aquac. Nutr. 2006, 12, 89–96. [Google Scholar] [CrossRef]
- Kolkovski, S. Digestive Enzymes in Fish Larvae and Juveniles—Implications and Applications to Formulated Diets. Aquaculture 2001, 200, 181–201. [Google Scholar] [CrossRef]
- Barajas, F.J.M.; Villegas, R.S.; Clark, G.P.; Moreno, B.L. Litopenaeus Vannamei (Boone) Post-Larval Survival Related to Age, Temperature, PH and Ammonium Concentration. Aquac. Res. 2006, 37, 492–499. [Google Scholar] [CrossRef]
- Soares, M.; Evangelista, D.K.R.; Pereira, A.M.L. Boas Práticas de Manejo e de Biossegurança Na Carcinicultura Para Convivência Com Enfermidades; Embrapa Pesca e Aquicultura: Brasília, DF, Brasil, 2021; ISSN 2318-1400. [Google Scholar]
- Moraes, R.d.C. Influência Da Densidade e Idade No Transporte de Pós-Larvas Do Camarão Marinho Litopenaeus Vannamei. Diploma Thesis, Universidade Federal de Santa Catarina, Florianópolis, SC, Brasil, 2004. [Google Scholar]
- Saputra, H.K.; Hamka, M.S.; Kurniaji, A.; Susanti, L.; Firman, S.W.; Dwiarto, A.; Alam, H.S. The Technology of Shrimp Larvae Transportation: Ecophysiology and Bioeconomy Effects on High Stocking Density Shrimp Litopenaeus Vannamei. E3S Web Conf. 2022, 348, 1–38. [Google Scholar] [CrossRef]
- ABCC. Projeto de Desenvolvimento Tecnológico Com Boas Práticas de Manejo e Biossegurança Para a Carcinicultura No Nordeste; Associação Brasileira de Criadores de Camarão: Candelária, RN, Brazil, 2017; p. 75. [Google Scholar]
- Vinza, A.; Emma, G.; Loaiza, L.; Nicole, P. Elaboración de Una Guía de Transporte Para Postlarvas de Penaeus Vannamei. 2020. Graduation Thesis in Aquaculture Engineering. Escuela Superior Politécnica del Litoral, Guayaquil, Ecuador. Available online: http://www.dspace.espol.edu.ec/xmlui/handle/123456789/50598 (accessed on 29 January 2023).
- Ostrensky, A.; Stevanato, D.J.; Pont, G.D.; Castilho-Westphal, G.G.; Girotto, M.V.F.; Cozer, N.; García-Madrigal, R.F.d.A.; Silva, U. A Produção Integrada Na Carcinicultura Brasileira (Volume 2), 1st ed.; Ostrensky, A., Cozer, N., Eds.; Instituto GIA: Curitiba, PR, Brazil, 2017. [Google Scholar]
- Yonghui, Y.; Guangbin, H.; Zhong, P.; Zulei, J. Analysis of Mortality Rate of Penaeus Vannamei Larvae Transferred from Large Culture Ponds to Small Culture Tanks. J. Fish. Livest Prod. 2021, 9, 1–4. [Google Scholar]
- Liu, H.L.; Yang, S.P.; Wang, C.G.; Chan, S.M.; Wang, W.X.; Feng, Z.H.; Sun, C.B. Effect of Air Exposure and Resubmersion on the Behavior and Oxidative Stress of Pacific White Shrimp Litopenaeus Vannamei. N. Am. J. Aquac. 2015, 77, 43–49. [Google Scholar] [CrossRef]
- Akbari, S.; Khoshnod, M.J.; Rajaian, H.; Afsharnasab, M. The Use of Eugenol as an Anesthetic in Transportation of With Indian Shrimp (Fenneropenaeus Indicus) Post Larvae. Turk. J. Fish. Aquat. Sci. 2010, 10, 423–429. [Google Scholar] [CrossRef]
- Kumlu, M.; Kumlu, M.; Turkmen, S. Combined Effects of Temperature and Salinity on Critical Thermal Minima of Pacific White Shrimp Litopenaeus Vannamei (Crustacea: Penaeidae). J. Therm. Biol. 2010, 35, 302–304. [Google Scholar] [CrossRef]
- Chamberlain, G.W.; Hutchins, D.L.; Lawrence, A.L.; Parker, J.C. Winter Culture of Penaeus Vannamei in Ponds Receiving Thermal Effluent at Different Rates. Proceed World Maricul. Soc. 2009, 11, 30–43. [Google Scholar] [CrossRef]
- Wyban, J.; Walsh, W.A.; Godin, D.M. Temperature Effects on Growth, Feeding Rate and Feed Conversion of the Pacific White Shrimp (Penaeus Vannamei). Aquaculture 1995, 138, 267–279. [Google Scholar] [CrossRef]
- Abdelrahman, H.A.; Abebe, A.; Boyd, C.E. Influence of Variation in Water Temperature on Survival, Growth and Yield of Pacific White Shrimp Litopenaeus Vannamei in Inland Ponds for Low-Salinity Culture. Aquac. Res. 2019, 50, 658–672. [Google Scholar] [CrossRef]
- Bückle, F.L.; Barón, B.; Hernández, M. Osmoregulatory Capacity of the Shrimp Litopenaeus Vannamei at Different Temperatures and Salinities, and Optimal Culture Environment. Available online: https://www.scielo.sa.cr/scielo.php?script=sci_arttext&pid=S0034-77442006000300005 (accessed on 26 June 2022).
- Hernández, R.M.; Bückle, R.L.F.; Palacios, E.; Barón, S.B. Preferential Behavior of White Shrimp Litopenaeus Vannamei (Boone 1931) by Progressive Temperature–Salinity Simultaneous Interaction. J. Therm. Biol. 2006, 31, 565–572. [Google Scholar] [CrossRef]
- Lima, P.P. de Influência Da Salinidade e Temperatura Da Água Nas Respostas Comportamental e Fisiológica de Camarões Marinhos Litopenaeus Vannamei (BOONE 1931); Universidade Federal do Rio Grande do Norte: Lagoa Nova, RN, Brazil, 2011. [Google Scholar]
- Boyd, C.E. Management Practices for Reducing the Environmental Impacts of Shrimp Farming in Methods for Improving Shrimp Farming in Central Americ UCA University Press Managua, Nicaragua. In Bottom Soils, Sediment, and Pond Aquaculture; Chapman and Hall: New York, NY, USA, 2001; p. 348. [Google Scholar]
- Furtado, P.S.; Fugimura, M.M.S.; Monserrat, J.M.; Souza, D.M.; Garcia, L.d.O.; Wasielesky, W. Acute Effects of Extreme PH and Its Influences on the Survival and Biochemical Biomarkers of Juvenile White Shrimp, Litopenaeus Vannamei. Mar. Fresh Behav. Physiol. 2015, 48, 417–429. [Google Scholar] [CrossRef]
- Girotto, M.V.F. Efeitos Da Amônia Sobre Juvenis de Litopenaeus Vannamei (BOONE, 1931) e Litopenaeus Schmitti ( BURKENROAD, 1936): Excreção e Toxicidade. Diploma Thesis, Universidade Federal do Paraná, Curitiba, PR, Brazil, 2010. [Google Scholar]
- Re, A.D.; Díaz, F.; Ponce-Rivas, E.; Giffard, I.; Muñoz-Marquez, M.E.; Sigala-Andrade, H.M. Combined effect of temperature and salinity on the thermotolerance and osmotic pressure of juvenile white shrimp Litopenaeus vannamei (Boone). J. Therm. Biol. 2012, 37, 413–418. [Google Scholar] [CrossRef]
- da Fonseca, S.B.; de Paula Mendes, P.; de Lyra Albertim, C.J.; Bittencourt, C.F.; da Silva, J.H.V. Cultivo Do Camarão Marinho Em Água Doce Em Diferentes Densidades de Estocagem. Pesq. Agropec. Bras. 2009, 44, 1352–1358. [Google Scholar] [CrossRef]
- Kotiya, A.S.; Vadher, K.H. Effect of Different Stocking Density on Growth, Survival on Litopenaeus Vannamei (Boone, 1931) in Summer and Monsoon Crop in Province of Gujarat States in India. J. Surv. Fish. Sci. 2021, 7, 71–99. [Google Scholar] [CrossRef]
- Mena-Herrera, A.; Gutierrez-Corona, C.; Linan-Cabello, M.; Sumano-Lopez, H. Effects of Stocking Densities on Growth of the Pacific White Shrimp (Litopenaeus Vannamei) in Earthen Ponds. Isr. J. Aquac. Bamidgeh 2006, 58, 205–213. [Google Scholar] [CrossRef]
- Sookying, D.; Silva, F.S.D.; Davis, D.A.; Hanson, T.R. Effects of Stocking Density on the Performance of Pacific White Shrimp Litopenaeus Vannamei Cultured under Pond and Outdoor Tank Conditions Using a High Soybean Meal Diet. Aquaculture 2011, 319, 232–239. [Google Scholar] [CrossRef]
- Wyban, J.A.; Lee, C.S.; Sato, V.T.; Sweeney, J.N.; Richards, W.K. Effect of Stocking Density on Shrimp Growth Rates in Manure-Fertilized Ponds. Aquaculture 1987, 61, 23–32. [Google Scholar] [CrossRef]
- Bardera, G.; Owen, M.A.G.; Façanha, F.N.; Alcaraz-Calero, J.M.; Alexander, M.E.; Sloman, K.A. The Influence of Density and Dominance on Pacific White Shrimp (Litopenaeus Vannamei) Feeding Behaviour. Aquaculture 2021, 531, 735949. [Google Scholar] [CrossRef]
- da Costa, F.P.; Gomes, B.S.F.; Pereira, S.D.d.N.A.; de Fátima Arruda, M. Influence of Stocking Density on the Behaviour of Juvenile Litopenaeus Vannamei (Boone, 1931). Aquac. Res. 2016, 47, 912–924. [Google Scholar] [CrossRef]
- Barbieri, R.C.; Ostrensky, A. Camarões Marinhos: Engorda II; Aprenda Fácil Editora: Viçosa, Brasil, 2020. [Google Scholar]
- Martinez-Cordova, L.R.; Porchas-Cornejo, M.A.; Villarreal-Colemnares, H.; Calderon-Perez, J.A.; Naranjo-Paramo, J. Evaluation of Three Feeding Strategies on the Culture of White Shrimp Penaeus Vannamei Boone 1931 in Low Water Exchange Ponds. Aquac. Eng. 1998, 17, 21–28. [Google Scholar] [CrossRef]
- Carvalho, E.A.; Nunes, A.J.P. Effects of Feeding Frequency on Feed Leaching Loss and Grow-out Patterns of the White Shrimp Litopenaeus Vannamei Fed under a Diurnal Feeding Regime in Pond Enclosures. Aquaculture 2006, 252, 494–502. [Google Scholar] [CrossRef]
- Sousa, P. Análise Da Eficiência Da Produção. 2003. [Google Scholar]
- Amaya, E.; Davis, D.A.; Rouse, D.B. Alternative Diets for the Pacific White Shrimp Litopenaeus Vannamei. Aquaculture 2007, 262, 419–425. [Google Scholar] [CrossRef]
- Albertim-Santos, C.J.; Santos, D.L.; Mendes, P. pAULA Uso de Modelos Matemáticos Para Avaliação Das Variáveis de Manejo Do Litopenaeus Vannamei (Boone, 1931)/Use of Mathematical Models for Evolution of the Management Variables Litopenaeus Vannamei (Boone, 1931). Acta Fish. Aquatic. Resour. 2014, 2, 28–39. [Google Scholar] [CrossRef]
- Maia, E.P. Produzindo Camarão Em Sistema Trifásico. Rev. Panor. Aquic. 2017, 1, 5–32. [Google Scholar]
- Boyd, C.E.; Davis, R.P.; Wilson, A.G.; Marcillo, F.; Brian, S.; McNevin, A.A. Resource Use in Whiteleg Shrimp Litopenaeus Vannamei Farming in Ecuador. J. World Aquac. Soc. 2021, 52, 772–788. [Google Scholar] [CrossRef]
- Nunes, A.J.P.; Sandoval, P.F.C. Dados de Produção e Qualidade de Água de Um Cultivo Comercial Semi-Intensivo Dos Camarões Penaeus Subtilis e P. Vannamei Com a Utilização de Bandejas de Alimentação. Rev. Panor. Aquic. 1997, 1, 12–15. [Google Scholar]
- Farias, G.R. Eficiência de Bandejas Na Alimentação de Juvenis Do Camarão Branco, Litopenaeus Vannamei (BOONE, 1931) Avaliado Por Meio de Imagens Subaquáticas. Diploma Thesis, Universidade Federal do Ceará, Fortaleza, CE, Brasil, 2013. [Google Scholar]
- Nunes, A.J.P. Alimentação Para Camarões Marinhos–Parte II. Panor. Aquic. 2001, 63, 17–21. [Google Scholar]
- Pontes, C.S.; Arruda, M.F. Comportamento de Litopenaeus vannamei (Boone)(Crustacea, Decapoda, Penaeidae) em função da oferta do alimento artificial nas fases clara e escura do período de 24 horas. Rev. Bras. Zool. 2005, 22, 648–652. [Google Scholar] [CrossRef]
- Robles-Romo, A.; Zenteno-Savín, T.; Racotta, I.S. Bioenergetic Status and Oxidative Stress during Escape Response until Exhaustion in Whiteleg Shrimp Litopenaeus Vannamei. J. Exp. Mar. Biol. Ecol. 2016, 478, 16–23. [Google Scholar] [CrossRef]
- Becker, A.J.; Ramos, P.B.; Monserrat, J.M.; Wasielesky, W.; Baldisserotto, B. Behavioural and Biochemical Responses in Adult Pacific White Shrimp, Litopenaeus Vannamei, Exposed to the Essential Oil of Cymbopogon Citratus. Aquac. Res. 2021, 52, 6205–6217. [Google Scholar] [CrossRef]
- Stoner, A.W. Evaluating Vitality and Predicting Mortality in Spot Prawn, Pandalus Platyceros, Using Reflex Behaviors. Fish. Res. 2012, 119–120, 108–114. [Google Scholar] [CrossRef]
- Tomlinson, M. Crustacean Compassion: Should decapod crustaceans be legally defined as animals? Fish. Vet. J. 2018, 16, 30–35. [Google Scholar]
- Keeling, L.; Tunón, H.; Olmos Antillón, G.; Berg, C.; Jones, M.; Stuardo, L.; Swanson, J.; Wallenbeck, A.; Winckler, C.; Blokhuis, H. Animal Welfare and the United Nations Sustainable Development Goals. Front. Vet. Sci. 2019, 6, 336. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, J.; Volstorf, J. Whiteleg Shrimp Litopenaeus Vannamei. Available online: https://fishethobase.net/db/21/farm/shortprofile/ (accessed on 6 January 2023).
- Wright, J. Seajoy’s Ablation-Free Shrimp Answers Emerging Welfare Concern-Responsible Seafood Advocate. Available online: https://www.globalseafood.org/advocate/seajoys-ablation-free-shrimp-answers-emerging-welfare-concern/ (accessed on 6 January 2023).
- Uawisetwathana, U.; Leelatanawit, R.; Klanchui, A.; Prommoon, J.; Klinbunga, S.; Karoonuthaisiri, N. Insights into Eyestalk Ablation Mechanism to Induce Ovarian Maturation in the Black Tiger Shrimp. PLoS ONE 2011, 6, e24427. [Google Scholar] [CrossRef]
- Okumura, T. Perspectives on Hormonal Manipulation of Shrimp Reproduction. Jpn. Agric. Res. Q. JARQ 2004, 38, 49–54. [Google Scholar] [CrossRef]
- Segner, H.; Sundh, H.; Buchmann, K.; Douxfils, J.; Sundell, K.S.; Mathieu, C.; Ruane, N.; Jutfelt, F.; Toften, H.; Vaughan, L. Health of Farmed Fish: Its Relation to Fish Welfare and Its Utility as Welfare Indicator. Fish. Physiol. Biochem. 2012, 38, 85–105. [Google Scholar] [CrossRef]
- Millard, R.S.; Ellis, R.P.; Bateman, K.S.; Bickley, L.K.; Tyler, C.R.; van Aerle, R.; Santos, E.M. How Do Abiotic Environmental Conditions Influence Shrimp Susceptibility to Disease? A Critical Analysis Focussed on White Spot Disease. J. Invertebr. Pathol. 2021, 186, 1–13. [Google Scholar] [CrossRef]
- ASC. ASC Shrimp Standard Version 1.1; Aquaculture Stewardship Council: Utrecht, The Netherlands, 2019. [Google Scholar]
- Stien, L.H.; Bracke, M.; Noble, C.; Kristiansen, T.S. Assessing Fish Welfare in Aquaculture. In The Welfare of Fish; Springer: Cham, Switzerland, 2020; pp. 303–321. [Google Scholar]
- Noble, C.; Gismervik, K.; Iversen, M.H.; Kolarevic, J.; Nilsson, J.; Stien, L.H.; Turnbull, J.F. Welfare Indicators for Farmed Atlantic Salmon–Part A. Knowledge and Theoretical Background. In Welfare Indicators for Farmed Atlantic Salmon: Tools for Assessing Fish Welfare; NOFIMA: Tromsø, Norway, 2018; pp. 10–145. ISBN 9788282965569. [Google Scholar]
- Sivaraman, I.; Krishnan, M.; Radhakrishnan, K. Better Management Practices for Sustainable Small-Scale Shrimp Farming. J. Clean. Prod. 2019, 214, 559–572. [Google Scholar] [CrossRef]
- Kim, N.T.Q.; van Hien, H.; Doan Khoi, L.N.; Yagi, N.; Lerøy Riple, A.K. Quality Management Practices of Intensive Whiteleg Shrimp (Litopenaeus Vannamei) Farming: A Study of the Mekong Delta, Vietnam. Sustainability 2020, 12, 4520. [Google Scholar] [CrossRef]
- Florina, P.; Hartoyo, S.; Kunci, K.; Vannamei, L.; Udang, T.; Management Practice, G.; Nilai, R.; Kasus Berganda, S. Adoption of Good Management Practice (GMP) in Small and Medium Scale Vannamei Shrimp Farms on the Northern Shore of East Java. J. Manaj. Agribis 2012, 9, 79–88. [Google Scholar]
- Madhu, K.; Madhu, R. School Course Manual on “Recent Advances in Breeding and Larviculture of Marine Finfish and Shellfish”; Central Marine Fisheries Research Institute (Indian Council of Agricultural Research): Cochin, Kerala, India, 2008; Volume 12, pp. 1–12. [Google Scholar]
- Otta, S.K.; Patil, P.K. Training Programme on Management of Emerging Diseases of Shrimp with Special Reference to Pacific White Shrimp, Litopenaeus Vannamei; Indian Council of Agricultural Research: Chennai, Tamil Nadu, India, 2012; Volume 111, pp. 42–88. [Google Scholar]
- Barreto, M.O.; Rey Planellas, S.; Yang, Y.; Phillips, C.; Descovich, K. Emerging Indicators of Fish Welfare in Aquaculture. Rev. Aquac. 2022, 14, 343–361. [Google Scholar] [CrossRef]
- Lefort, M.-C.; Cruickshank, R.H.; Descovich, K.; Adams, N.J.; Barun, A.; Emami-Khoyi, A.; Ridden, J.; Smith, V.R.; Sprague, R.; Waterhouse, B.; et al. Blood, Sweat and Tears: A Review of Non-Invasive DNA Sampling. Peer Community J. 2022, 2. [Google Scholar] [CrossRef]
- Garshelis, D.L. On the Allure of Noninvasive Genetic Sampling–Putting a Face to the Name-Web of Science Core Collection. URSUS 2006, 17, 109–123. [Google Scholar] [CrossRef]
- Tu, H.T.; Silvestre, F.; Wang, N.; Thome, J.P.; Phuong, N.T.; Kestemont, P. A Multi-Biomarker Approach to Assess the Impact of Farming Systems on Black Tiger Shrimp (Penaeus Monodon). Chemosphere 2010, 81, 1204–1211. [Google Scholar] [CrossRef]
- Battison, A.L.; Horney, B.; Ciaramella, M.A. Measurement of Tissue Lipid Reserves in the American Lobster (Homarus Americanus): Hemolymph Metabolites as Potential Biomarkers of Nutritional Status. J. Crustac. Biol. 2014, 34, 629–638. [Google Scholar] [CrossRef]
- Albalat, A.; Johnson, L.; Coates, C.J.; Dykes, G.C.; Hitte, F.; Morro, B.; Dick, J.; Todd, K.; Neil, D.M. The Effect of Temperature on the Physiological Condition and Immune-Capacity of European Lobsters (Homarus Gammarus) During Long-Term Starvation. Front. Mar. Sci. 2019, 6, 281. [Google Scholar] [CrossRef]
- Nguyen, T.; Alfaro, A.C.; Rodríguez, J.; Bayot, B.; Sonnenholzner, S. Changes in Metabolic Profiling of Whiteleg Shrimp (Penaeus Vannamei) under Hypoxic Stress. J. Invertebr. Pathol. 2022, 193, 107798. [Google Scholar] [CrossRef] [PubMed]
- Moberg, G.P. Biological Response to Stress: Key to Assessment of Animal Well-Being? In Animal Stress; Moberg, G.P., Ed.; Springer: New York, NY, USA, 1985; pp. 27–49. [Google Scholar]
- Muhammad, F.; Zhang, Z.F.; Shao, M.Y.; Dong, Y.P.; Muhammad, S.; Balouch, W.A. Early Development of Nervous System in Litopenaeus Vannamei (Boone, 1931) (Crustacea: Decapoda) Larval Nervous System, Genesis, Differentiation. Sindh. Univ. Res. J. (Sci. Ser.) 2012, 44, 29–34. [Google Scholar]
- Birch, J. Animal Sentience and the Precautionary Principle. Anim. Sentience 2017, 16. [Google Scholar] [CrossRef]
- Chong-Robles, J.; Charmantier, G.; Boulo, V.; Lizárraga-Valdéz, J.; Enríquez-Paredes, L.M.; Giffard-Mena, I. Osmoregulation Pattern and Salinity Tolerance of the White Shrimp Litopenaeus Vannamei (Boone, 1931) during Post-Embryonic Development. Aquaculture 2014, 422–423, 261–267. [Google Scholar] [CrossRef]
- Medina-Reyna, C.E. Growth and Emigration of White Shrimp, Litopenaeus Vannamei, in the Mar Muerto Lagoon, Southern Mexico. Naga Iclarm Q. 2001, 24, 30–34. [Google Scholar]
- McKenney, C.L.; Celestial, D.M. Interactions among Salinity, Temperature, and Age on Growth of the Estuarine Mysid Mysidopsis Bahia Reared in the Laboratory through a Complete Life Cycle. I.Body Mass and Age-Specific Growth Rate. J. Crustac. Biol. 1995, 15, 169–178. [Google Scholar] [CrossRef]
- O’brien, C.J. The effects of temperature and salinity on growth and survival of juvenile tiger prawns Penaeus esculentus (Haswell). J. Exp. Mar. Biol. Ecol. 1994, 183, 133–145. [Google Scholar] [CrossRef]
- Boyd, C.E.; Pillai, V.K. Water Quality Management in Aquaculture; CMFRI Special Publication, 22; Central Marine Fisheries Research Institute: Cochin, India, 1985; p. 71. [Google Scholar]
- Rosas, C.; López, N.; Mercado, P.; Martínez, E. Effect of Salinity Acclimation on Oxygen Consumption of Juveniles of the White Shrimp Litopenaeus Vannamei. J. Crustac. Biol. 2001, 21, 912–922. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, X.; Li, J.; Gao, T. Effect of salinity on survival, growth, oxygen consumption and ammonia-N excretion of juvenile whiteleg shrimp, Litopenaeus vannamei. Aquac. Res. 2009, 40, 1419–1427. [Google Scholar] [CrossRef]
- Kautsky, N.; Rönnbäck, P.; Tedengren, M.; Troell, M. Ecosystem Perspectives on Management of Disease in Shrimp Pond Farming. Aquaculture 2000, 191, 145–161. [Google Scholar] [CrossRef]
- Moss, K.R.K.; Moss, S.M. Effects of Artificial Substrate and Stocking Density on the Nursery Production of Pacific White Shrimp Litopenaeus Vannamei. J. World Aquac. Soc. 2004, 35, 536–542. [Google Scholar] [CrossRef]
- Krummenauer, D.; Peixoto, S.; Cavalli, R.O.; Poersch, L.H.; Wasielesky, W. Superintensive Culture of White Shrimp, Litopenaeus Vannamei, in a Biofloc Technology System in Southern Brazil at Different Stocking Densities. J. World Aquac. Soc. 2011, 42. [Google Scholar] [CrossRef]
- Samocha, T.M.; Hamper, L.; Emberson, C.R.; Davis, A.D.; McIntosh, D.; Lawrence, A.L.; van Wyk, P.M. Review of Some Recent Developments in Sustainable Shrimp Farming Practices in Texas, Arizona, and Florida. J. Appl. Aquac. 2002, 12, 1–42. [Google Scholar] [CrossRef]
- Liu, G.; Zhu, S.; Liu, D.; Guo, X.; Ye, Z. Effects of Stocking Density of the White Shrimp Litopenaeus Vannamei (Boone) on Immunities, Antioxidant Status, and Resistance against Vibrio Harveyi in a Biofloc System. Fish Shellfish. Immunol. 2017, 67, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Lightner, D.; Redman, R.M. Shrimp Diseases and Current Diagnostic Methods. Aquaculture 1998, 164, 201–220. [Google Scholar] [CrossRef]
- Corsin, F.; Mohan, C.V. Towards Responsible Shrimp Farming. In The Shrimp Book; Aldey-Sanz, V., Ed.; Nottingham University Press: Nottingham, UK, 2010; pp. 353–375. [Google Scholar]
- O’Donncha, F.; Stockwell, C.L.; Planellas, S.R.; Micallef, G.; Palmes, P.; Webb, C.; Filgueira, R.; Grant, J. Data Driven Insight Into Fish Behaviour and Their Use for Precision Aquaculture. Front. Anim. Sci. 2021, 2, 30. [Google Scholar] [CrossRef]
- Antonucci, F. Corrado Costa Precision Aquaculture: A Short Review on Engineering Innovations. Aquac. Int. 2020, 28, 41–57. [Google Scholar] [CrossRef]
- de Avila, L.R.; da Costa Botelho, S.S.; Pias, M.R.; de Vargas Guterres, B.; Junior, J.N.J. On Aquaculture Enhancements Through Robotic Behaviour Actuation. In Proceedings of the 2021 Latin American Robotics Symposium (LARS), 2021 Brazilian Symposium on Robotics (SBR), and 2021 Workshop on Robotics in Education (WRE), Natal, Brazil, 11–15 October 2021; pp. 294–299. [Google Scholar] [CrossRef]
- Hung, C.C.; Tsao, S.C.; Huang, K.H.; Jang, J.P.; Chang, H.K.; Dobbs, F.C. A Highly Sensitive Underwater Video System for Use in Turbid Aquaculture Ponds. Sci. Rep. 2016, 6, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Sreepada, R.A.; Kuzkamil, S.; Suryavanshi2, U.; Ingole, B.S.; Drensgstigl, A.; Braa, B. Water Quality Management in Shrimp Aquaculture Ponds Using Remote Water Quality Logging System; School of Industrial Fisheries, CUSAT: Kerala, India, 2006. [Google Scholar]
- Simbeye, D.S.; Zhao, J.; Yang, S. Design and Deployment of Wireless Sensor Networks for Aquaculture Monitoring and Control Based on Virtual Instruments. Comput. Electron. Agric. 2014, 102, 31–42. [Google Scholar] [CrossRef]
- Gusmawati, N.; Soulard, B.; Selmaoui-Folcher, N.; Proisy, C.; Mustafa, A.; le Gendre, R.; Laugier, T.; Lemonnier, H. Surveying Shrimp Aquaculture Pond Activity Using Multitemporal VHSR Satellite Images–Case Study from the Perancak Estuary, Bali, Indonesia. Mar. Pollut. Bull. 2018, 131, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Irawan, Y.; Fonda, H.; Sabna, E.; Febriani, A. Intelligent Quality Control of Shrimp Aquaculture Based On Real-Time System and IoT Using Mobile Device. Int. J. Eng. Trends Tech. 2021, 69, 49–56. [Google Scholar] [CrossRef]
- Capelo, J.; Ruiz, E.; Asanza, V.; Toscano-Quiroga, T.; Sánchez-Pozo, N.N.; Lorente-Leyva, L.L.; Peluffo-Ordóñez, D.H. Raspberry Pi-Based IoT for Shrimp Farms Real-Time Remote Monitoring with Automated System. In Proceedings of the International Conference on Applied Electronics, University of West Bohemia, Pilsen, Czech Republic, 7 September 2021; IEEE Computer Society: Pilsen, Czech Republic, 2021; pp. 1–4. ISBN 978-80-261-0973-0. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).