Dietary Protein Requirement of Juvenile Dotted Gizzard Shad Konosirus punctatus Based on the Variation of Fish Meal
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Diets
2.2. Experimental Fish and Feeding Trial
2.3. Sample Collection and Analysis
2.4. Calculation and Statistical Analysis
3. Results
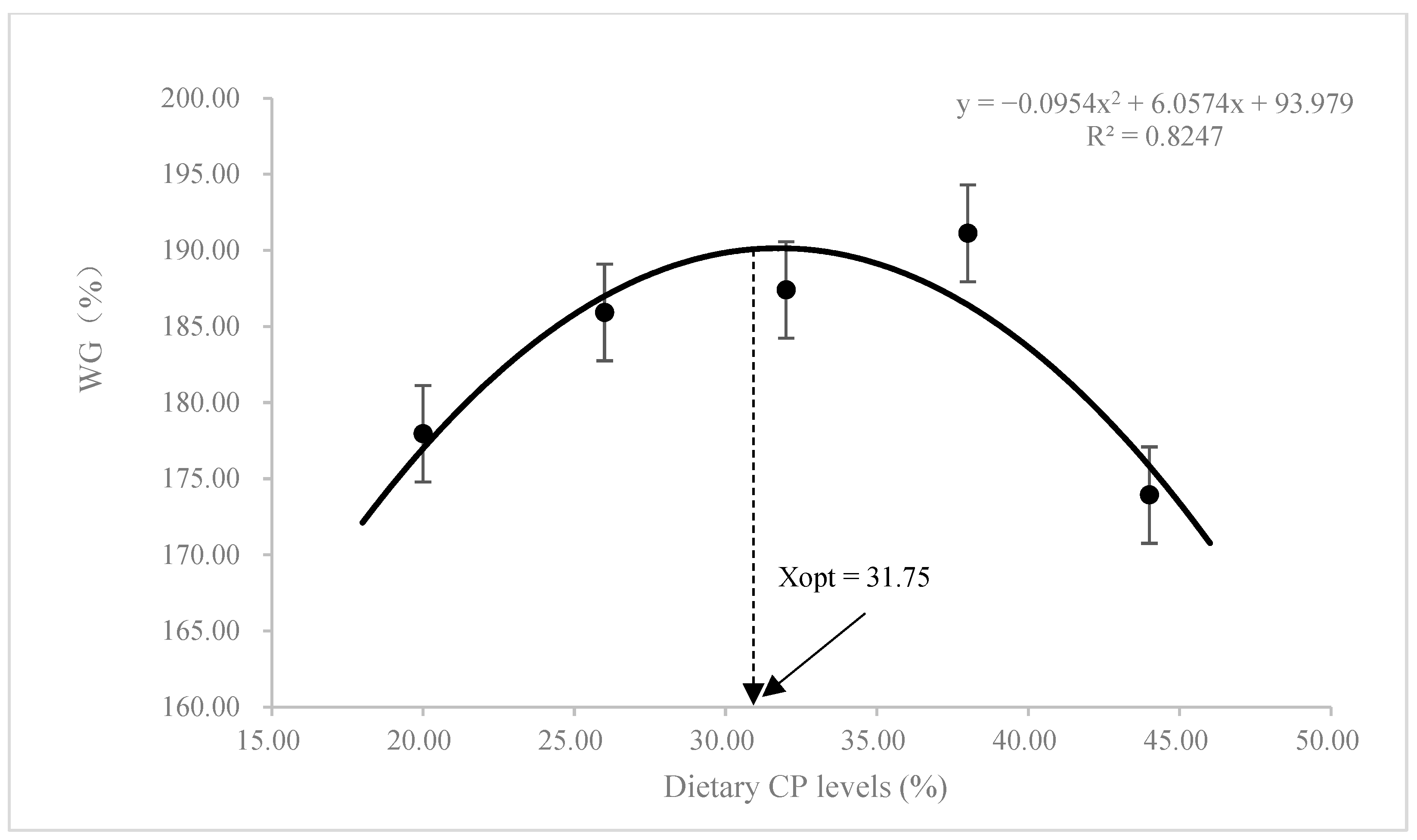
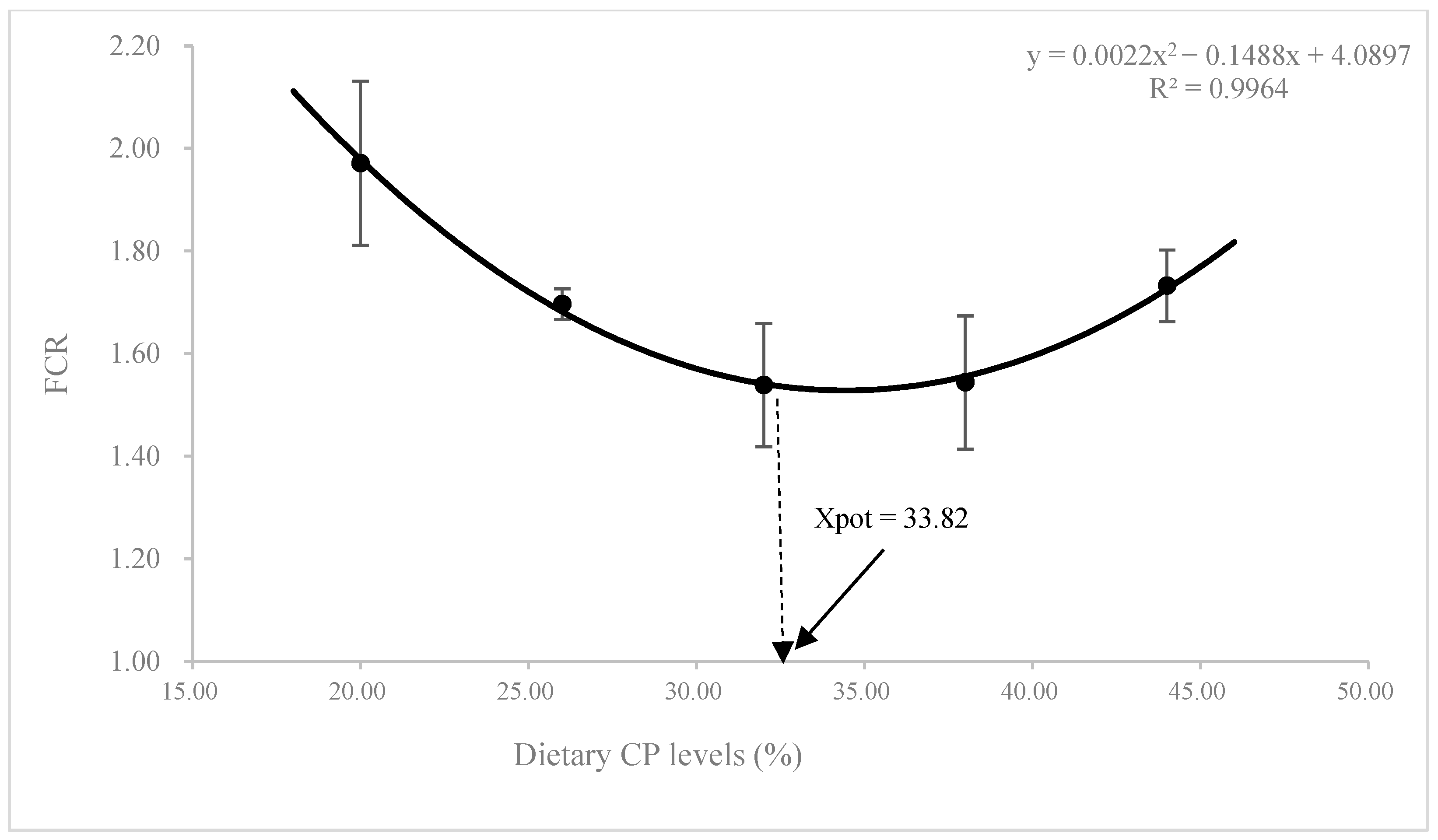
3.1. Growth Performance and Feed Utilization
3.2. Retention and Deposition of Energy, Nitrogen, and Lipid
3.3. The Whole-Body Composition
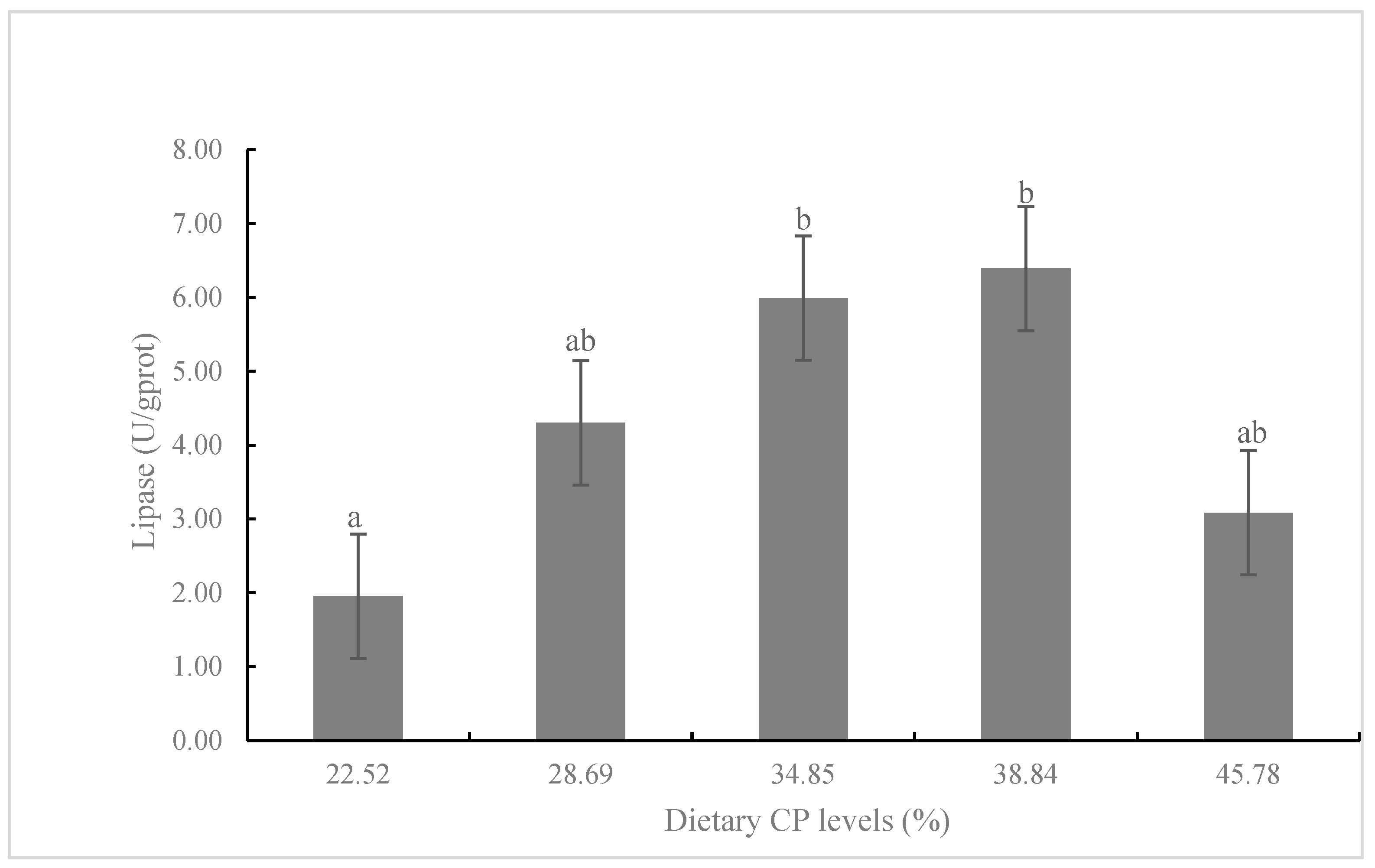
3.4. Liver Enzyme Activities
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wilson, R.P. Amino Acids and Proteins, 3rd ed.; Halver, J.E., Hardy, R.W., Eds.; Fish Nutrition, Academic Press: San Diego, CA, USA, 2002; pp. 143–179. [Google Scholar]
- Yang, S.D.; Liou, C.H.; Liu, F.G. Effects of dietary protein level on growth performance, carcass composition and ammonia excretion in juvenile silver perch (Bidyanus bidyanus). Aquaculture 2002, 213, 363–372. [Google Scholar] [CrossRef]
- Chen, J.M.; Ye, J.Y.; Pan, Q.; Shen, B.Q.; Wang, Y.H. Effect of dietary protein levels on growth performance and whole-body composition of summerling and winterling spotted barbel (Hemibarbus maculates, bleeker). Aquac. Nutrition. 2009, 16, 412–418. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, F.; Wang, L.; Shao, Q.; Xu, Z.; Xu, J. Dietary protein requirement of juvenile black sea bream, sparus macrocephalus. J. World Aquac. Soc. 2010, 41, 151–164. [Google Scholar] [CrossRef]
- Yang, C.G.; Jiang, M.; Lu, X.; Wen, H. Effects of Dietary Protein Level on the Gut Microbiome and Nutrient Metabolism in Tilapia (Oreochromis niloticus). Animals 2021, 11, 1024. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Wang, J.T.; Han, T.; Yang, Y.X.; Li, X.Y.; Jiang, Y.D. Dietary protein requirement of juvenile bluegill sunfish (Lepomis macrochirus). Aquaculture 2016, 459, 191–197. [Google Scholar] [CrossRef]
- Wang, J.T.; Jiang, Y.D.; Li, X.Y.; Han, T.; Yang, Y.X.; Hu, S.X.; Yang, M. Dietary protein requirement of juvenile red spotted grouper (Epinephelus kaara). Aquaculture 2016, 450, 289–294. [Google Scholar] [CrossRef]
- Hussain, M.; Hassan, H.U.; Siddique, M.A.M.; Mahmood, K.; Laghari, M.Y.; Abro, N.A.; Gabol, K.; Nisar; Rizwan, S. Effect of varying dietary protein levels on growth performance and survival of milkfish Chanos chanos fingerlings reared in brackish water pond ecosystem. Egypt. J. Aquat. Res. 2021, 2, 1687–4285. [Google Scholar] [CrossRef]
- Ye, C.X.; Wu, Y.L.; Sun, Z.Z.; Wang, A. Dietary protein requirement of juvenile obscure puffer, Takifugu obscurus. Aquac. Res. 2017, 48, 2064–2073. [Google Scholar] [CrossRef]
- Ye, J.D.; Liu, X.H.; Wang, Z.J.; Wang, K. Effect of partial fish meal replacement by soybean meal on the growth performance and biochemical indices of juvenile Japanese flounder Paralichthys olivaceus. Aquac. Int. 2011, 19, 143–153. [Google Scholar] [CrossRef]
- Zhang, C.X.; Rahimnejad, S.; Wang, Y.R.; Lu, K.L.; Song, K.; Wang, L.; Mai, K.S. Substituting fish meal with soybean meal in diets for Japanese seabass (Lateolabrax japonicus): Effects on growth, digestive enzymes activity, gut histology, and expression of gut inflammatory and transporter genes. Aquaculture 2018, 483, 173–182. [Google Scholar] [CrossRef]
- Liu, T.; Han, T.; Wang, J.T.; Han, T.; Bian, P.; Wang, Y.B.; Cai, X.J. Effects of replacing fish meal with soybean meal on growth performance, feed utilization and physiological status of juvenile redlip mullet Liza haematocheila. Aquac. Rep. 2021, 20, 100756. [Google Scholar] [CrossRef]
- Whitehead, P.J.P. FAO species catalogue. Vol. 7. Clupeoid fishes of the world (suborder Clupeoidei). Part 1: Chirocentridae, Clupeidae and Pristigasteridae. FAO Fish. Synop. 1985, 125, 305–379. [Google Scholar]
- Zhang, S.Y. Fauna Sinica-Ostichthyes: Acipenseriformes Elopiformes Clupeiformes Gonorhynchiformes; Science Press: Beijing, China, 2001. [Google Scholar]
- Song, N.; Gao, T.; Ying, Y.; Yanagimoto, T.; Han, Z. Is the Kuroshio Current a strong barrier for the dispersal of the gizzard shad (Konosirus punctatus) in the East China Sea. Mar. Fresh. Res. 2017, 68, 810–820. [Google Scholar] [CrossRef]
- Myoung, H.; Kim, J. Genetic diversity and population structure of the gizzard shad, Konosirus punctatus (Clupeidae, Pisces), in Korean waters based on mitochondrial DNA control region sequences. Genes Genom. 2014, 36, 591–598. [Google Scholar] [CrossRef]
- Park, S.Y.; Ha, S. Reduction of Escherichia coli and Vibrio parahaemolyticus Counts on Freshly Sliced Shad (Konosirus punctatus) by Combined Treatment of Slightly Acidic Electrolyzed Water and Ultrasound Using Response Surface Methodology. Food Bioprocess Tech. 2015, 8, 1762–1770. [Google Scholar] [CrossRef]
- Zhang, C.N.; Wang, S.D.; Sun, D.; Pan, Z.K.; Zhou, A.G.; Xie, S.L.; Wang, J.; Zou, J.X. Microplastic pollution in surface water from east coastal areas of Guangdong, South China and preliminary study on microplastics biomonitoring using two marine fish. Chemosphere 2020, 256, 127202. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, K.; Zhu, K.; Shafi, M.; Lü, Z. Population Genetics of Konosirus punctatus in Chinese Coastal Waters Inferred from Two mtDNA Genes (COI and Cytb). Front. Mar. Sci. 2020, 7, 534. [Google Scholar] [CrossRef]
- Li, M.; Shi, S.F.; Brown, C.L.; Yang, T.B. Phylogeographical pattern of Mazocraeoides gonialosae (Monogenea, Mazocraeidae) on the dotted gizzard shad, Konosirus punctatus, along the coast of China. Int. J. Parasitol. 2012, 41, 1263–1272. [Google Scholar] [CrossRef]
- Lou, F.; Qiu, S.; Tang, Y.; Wang, Z.; Wang, L. Comprehensive phylogeny of Konosirus punctatus (Clupeiformes: Clupeidae) based on transcriptomic data. Biosci. Rep. 2021, 41. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International, 16th ed.; Cunniff, P., Ed.; Official analytical chemists: Arlington, VA, USA, 1995; pp. 1141–1154. [Google Scholar]
- Li, J.S.; Li, J.L.; Wu, T.T. Distribution and properties of amylase and lipase in alimentary tract of tilapia Oreochromis niloticus × O.aureus. J. Fish. Sci. China 2004, 5, 473–477. [Google Scholar] [CrossRef]
- Reitman, S.; Frankel, S.A. A colourimetric method of serum glutamic oxalo acetic and glutamic pyruvic transaminases. Am. J. Clin. Pathol. 1957, 28, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Mohanta, K.N.; Mohanty, S.N.; Jena, J.K.; Sahu, N.P. Protein requirement of silver barb, Puntius gonionotus fingerlings. Aquac. Nutr. 2008, 14, 14–152. [Google Scholar] [CrossRef]
- Ozório, R.O.A.; Valente, L.M.P.; Correia, S.; PousÃo-ferreira, P.; Damasceno-oliveira, A.; Escórcio, C.; Oliva-teles, A. Protein requirement for maintenance and maximum growth of two-banded seabream (Diplodus vulgaris) juveniles. Aquac. Nutr. 2009, 15, 85–93. [Google Scholar] [CrossRef]
- Kim, S.S.; Lee, K.J. Dietary protein requirement of juvenile tiger puffer (Takifugu rubripes). Aquaculture 2009, 287, 219–222. [Google Scholar] [CrossRef]
- Jin, Y.; Tian, L.X.; Xie, S.W.; Guo, D.Q.; Yang, H.J.; Liang, G.Y.; Liu, Y.J. Interactions between dietary protein levels, growth performance, feed utilization, gene expression and metabolic products in juvenile grass carp (Ctenopharyngodon idella). Aquaculture 2015, 437, 75–83. [Google Scholar] [CrossRef]
- Lee, S.M.; Kim, K.D.; Park, H.G.; Kim, C.H.; Hong, K.E. Protein requirement of juvenile Manchurian trout Brachymystax lenok. Fish. Sci. 2001, 67, 46–51. [Google Scholar] [CrossRef]
- Chuapoehuk. Protein requirements of walking catfish, Calrias batrachus (Linnaeus), fry. Aquaculture 1987, 63, 215–219. [Google Scholar] [CrossRef]
- Shyong, W.J.; Huang, C.H.; Chen, H.C. Effects of dietary protein concentration on growth and muscle composition of juvenile Zacco barbata. Aquaculture 1998, 167, 35–42. [Google Scholar] [CrossRef]
- Rocha, C.B.; Fernandes, J.M.; Tavares, R.A.; Piedras, S.; Pouey, J.L.F. Dietary protein requirement for armoured catfish juveniles (Hypostomus commersoni Loricariidae). Braz. J. Aquat. Sci. Technol. 2016, 19, 33–37. [Google Scholar] [CrossRef]
- Zuanon, J.; Morais, J.A.; Carneiro, A.P.S.; Salaro, A. Dietary crude protein levels for juvenile betta splendens. Bol. Do Inst. De Pesca 2016, 42, 590–597. [Google Scholar] [CrossRef]
- Coutinho, F.; Peres, H.; Guerreiro, I.; Pousão-Ferreira, P.; Oliva-Teles, A. Dietary protein requirement of sharpsnout sea bream (Diplodus puntazzo, cetti 1777) juveniles. Aquaculture 2012, 356–357, 391–397. [Google Scholar] [CrossRef]
- Fournier, V.; Gouillou Coustans, M.F.; Metailler, R.; Vachot, C.; Guedes, M.J.; Tulli, F.; Kaushik, S.J. Protein and arginine requirements for maintenance and nitrogen gain in four teleosts. Br. J. Nutr. 2002, 87, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.D.; Lall, S.P. Effects of dietary protein level on growth and utilization of protein and energy by juvenile haddock (Melanogrammus aeglefinus). Aquaculture 2001, 195, 311–319. [Google Scholar] [CrossRef]
- Sá, R.; Gavilán, M.; Rioseco, M.J.; Llancabure, A.; Vargas-Chacodd, L.; Augsburger, A.; Bas, F. Dietary protein requirement of Patagonian blennie (Eleginops maclovinus, Cuvier 1830) juveniles. Aquaculture 2014, 428–429, 125–134. [Google Scholar] [CrossRef]
- Yang, M.; Wang, J.T.; Han, T.; Yang, Y.X.; Li, X.Y.; Tian, H.L.; Zheng, P.Q. Dietary protein requirement of juvenile triangular bream Megalobrama terminalis (richardson,1846). J. Appl. Ichthyol. 2017, 33, 971–977. [Google Scholar] [CrossRef]
- Lee, H.Y.M.; Cho, K.; Lee, J.; Yang, S. Dietary protein requirement of juvenile giant croaker, Nibea japonica Temminck & Schlegel. Aquac. Res. 2001, 32, 112–118. [Google Scholar] [CrossRef]
- National Research Council. Nutrient Requirements of Fish and Shrimp; National Academies Press: Washington, WA, USA, 2011. [Google Scholar]
- Wu, X.Y.; Gatlin, D.M., III. Effects of altering dietary protein content in morning and evening feedings on growth and ammonia excretion of red drum (Sciaenops ocellatus). Aquaculture 2014, 434, 33–37. [Google Scholar] [CrossRef]
- Martínez-Palacios, C.A.; Ríos-Durán, M.G.; Ambriz-Cervantes, L.; Jauncey, K.J.; Ross, L.G. Dietary protein requirement of juvenile Mexican silverside (Menidia estor Jordan 1879), a stomachless zooplanktophagous fish. Aquac. Nutr. 2007, 13, 304–310. [Google Scholar] [CrossRef]
- Mohammadinafchi, F.; Mohammadiazarm, H.; Yavari, V. Evaluation effect of soybean meal and baker’syeast on resistance to anoxia stress and blood biochemical parameters of fingerlings (Mesopotamichthys sharpeyi Günther, 1874). Int. J. Biosci. 2014, 5, 215–222. [Google Scholar] [CrossRef]
- Wang, L.G.; Hu, S.U.; Lou, B.; Chen, D.X.; Zhan, W.; Chen, R.Y.; Liu, F.; Xu, D.D. Effect of different dietary protein and lipid levels on the growth, body composition, and intestinal digestive enzyme activities of juvenile yellow drum Nibea albiflora (Richardson). J. Ocean. Univ. China 2018, 17, 1261–1267. [Google Scholar] [CrossRef]
- Ping, L.Y.; Ming, J.C.; Yun, Y.J.; Xiong, X.Y.; Ying, S.L.; Qian, B.S.; Liang, Z.Y.; Jian, G.; Ping, L.Y. The effects of diet protein levels on the growth, body composition and digestive enzyme activities of the Barbodes caldwelli juvenile. Chin. J. Agric. Biotechnol. 2009, 6, 199–205. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, X.M.; Le, K.X.; Han, Q.X.; Peng, R.B.; Liang, J.J.; Wang, P.S. Effects of dietary protein level on growth, muscle composition, and enzyme activity of Sepia lycidas during early growth period. Mar. Sci. 2016, 3, 8. (In Chinese) [Google Scholar] [CrossRef]
- Wang, X.X.; Zeng, B.H.; Liu, L.L.; Yang, R.B.; Liu, H.B. Effects of feed protein levels of growth, digestive enzyme activities, non-specific immunity and protein metabolism of Schizotho oconnori. Acta Hydrobiol. Sin. 2020, 44, 14. (In Chinese) [Google Scholar] [CrossRef]
- Li, X.Y.; Zheng, S.X.; Jia, S.C.; Song, F.; Zhou, C.P.; Wu, G.Y. Effects of dietary starch and lipid levels on the protein retention and growth of largemouth bass (Micropterus salmoides). Amino Acids. 2020, 52, 999–1016. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.F.; Sun, Z.Z.; Wang, A.N.; Ye, C.X.; Zhu, X. Effects of dietary protein and lipid levels on growth, body and plasma biochemical composition and selective gene expression in liver of hybrid snakehead (Channa maculata ♀× C. argus ♂) fingerlings. Aquaculture 2017, 468, 1–9. [Google Scholar] [CrossRef]


| Ingredients (g 100 g−1) | Dietary Crude Protein (CP) Levels (%) | ||||
|---|---|---|---|---|---|
| 22.52 (CP1) | 28.69 (CP2) | 34.85 (CP3) | 38.84 (CP4) | 45.78 (CP5) | |
| Fish meal 1 | 29.43 | 38.25 | 47.08 | 55.91 | 64.74 |
| Corn starch 2 | 34.02 | 28.02 | 22.02 | 16.02 | 10.02 |
| Fish oil 3 | 3.85 | 3.24 | 2.63 | 2.02 | 1.41 |
| Soybean oil 4 | 2.94 | 2.94 | 2.94 | 2.94 | 2.94 |
| Vitamin mix 5 | 2.50 | 2.50 | 2.50 | 2.50 | 2.50 |
| Mineral mix 6 | 1.50 | 1.50 | 1.50 | 1.50 | 1.50 |
| Vitamin C | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
| Choline chloride | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 |
| Sodium alginate | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 |
| Cellulose | 20.96 | 18.74 | 16.53 | 14.31 | 12.09 |
| Total | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 |
| Proximate composition (g 100 g−1 dry matter) | |||||
| Moisture | 4.10 | 4.18 | 3.99 | 5.04 | 4.67 |
| Crude protein | 22.52 | 28.69 | 34.85 | 38.84 | 45.78 |
| Crude lipid | 9.03 | 8.38 | 8.37 | 8.55 | 8.88 |
| Gross energy (kJ g−1) | 19.26 | 18.77 | 18.91 | 18.91 | 18.96 |
| Protein to energy ratio (mg kJ−1) | 11.69 | 15.29 | 18.43 | 20.54 | 24.15 |
| Items | Dietary CP Levels (%) | ||||
|---|---|---|---|---|---|
| 22.52 (CP1) | 28.69 (CP2) | 34.85 (CP3) | 38.84 (CP4) | 45.78 (CP5) | |
| IBW (g fish−1) 1 | 3.51 ± 0.06 | 3.46 ± 0.13 | 3.48 ± 0.11 | 3.68 ± 0.16 | 3.91 ± 0.15 |
| FBW (g fish−1) 2 | 9.77 ± 0.57 | 9.96 ± 0.64 | 9.94 ± 0.66 | 10.70 ± 0.82 | 10.70 ± 0.39 |
| Survival (%) | 96.97 ± 5.25 | 95.45 ± 4.55 | 93.94 ± 6.94 | 92.42 ± 6.94 | 92.42 ± 13.12 |
| WG (%) 3 | 177.96 ± 11.84 | 185.41 ± 7.94 | 187.92 ± 25.74 | 191.12 ± 21.95 | 173.93 ± 10.64 |
| SGR (% day−1) 4 | 1.82 ± 0.08 | 1.88 ± 0.05 | 1.87 ± 0.16 | 1.90 ± 0.14 | 1.80 ± 0.07 |
| FCR 5 | 1.97 ± 0.27 | 1.70 ± 0.04 | 1.54 ± 0.21 | 1.54 ± 0.20 | 1.73 ± 0.12 |
| DFI (g 100 g fish−1 day−1) 6 | 4.92 ± 0.71 b | 4.42 ± 0.96 ab | 3.92 ± 0.14 a | 3.83 ± 0.19 a | 3.85 ± 0.15 a |
| PER 7 | 2.18 ± 0.19 c | 1.97 ± 0.44 bc | 1.81 ± 0.24 b | 1.60 ± 0.20 b | 1.20 ± 0.81 a |
| Items | Dietary CP Levels (%) | ||||
|---|---|---|---|---|---|
| 22.52 (CP1) | 28.69 (CP2) | 34.85 (CP3) | 38.84 (CP4) | 45.78 (CP5) | |
| Nitrogen | |||||
| DNI (g kg−1 ABW−1 day−1) 1 | 1.51 ± 0.19 a | 1.73 ± 0.01 b | 1.89 ± 0.02 bc | 2.03 ± 0.05 c | 2.43 ± 0.07 d |
| DNG (g kg−1 ABW−1 day−1) 2 | 0.39 ± 0.05 | 0.44 ± 0.01 | 0.44 ± 0.03 | 0.40 ± 0.09 | 0.41 ± 0.04 |
| NR (%) 3 | 26.60 ± 6.24 b | 25.50 ± 0.40 b | 23.08 ± 1.71 ab | 19.93 ± 4.92 ab | 17.09 ± 1.92 a |
| Energy | |||||
| DEI (kJ kg−1 ABW−1 day−1) 4 | 8.03 ± 1.041 b | 7.08 ± 0.02 a | 6.41 ± 0.08 a | 6.16 ± 0.15 a | 6.29 ± 0.19 a |
| DEG (kJ kg−1 ABW−1 day−1) 5 | 3.89 ± 0.24 | 4.03 ± 0.18 | 3.80 ± 0.69 | 4.11 ± 0.17 | 3.91 ± 0.10 |
| ER (%) 6 | 48.82 ± 5.87 a | 56.86 ± 2.80 ab | 59.36 ± 10.88 ab | 66.80 ± 4.31 b | 62.15 ± 0.30 b |
| Lipid | |||||
| DLI (g kg−1 ABW−1 day−1) 7 | 3.77 ± 0.49 b | 3.16 ± 0.01 a | 2.84 ± 0.03 a | 2.79 ± 0.07 a | 2.95 ± 0.09 a |
| DLG (g kg−1 ABW−1 day−1) 8 | 1.75 ± 0.38 | 2.03 ± 0.12 | 1.71 ± 0.21 | 1.96 ± 0.44 | 1.83 ± 0.07 |
| LR (%) 9 | 47.82 ± 5.06 a | 64.08 ± 4.03 ab | 60.15 ± 7.15 ab | 70.52 ± 7.46 b | 62.03 ± 1.64 ab |
| Items (%) | Dietary CP Levels (%) | ||||
|---|---|---|---|---|---|
| 22.52 (CP1) | 28.69 (CP2) | 34.85 (CP3) | 38.84 (CP4) | 45.78 (CP5) | |
| Moisture | 71.93 ± 0.85 | 70.54 ± 0.56 | 71.25 ± 1.44 | 70.86 ± 1.07 | 71.04 ± 0.97 |
| Crude protein | 14.10 ± 1.21 | 15.03 ± 0.39 | 14.97 ± 0.12 | 14.01 ± 1.70 | 14.75 ± 0.78 |
| Crude lipid | 9.76 ± 1.49 | 10.63 ± 0.36 | 9.46 ± 0.37 | 10.30 ± 1.30 | 10.11 ± 0.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, T.; Weng, X.; Wang, J.; Han, T.; Wang, Y.; Chai, X. Dietary Protein Requirement of Juvenile Dotted Gizzard Shad Konosirus punctatus Based on the Variation of Fish Meal. Animals 2023, 13, 788. https://doi.org/10.3390/ani13050788
Liu T, Weng X, Wang J, Han T, Wang Y, Chai X. Dietary Protein Requirement of Juvenile Dotted Gizzard Shad Konosirus punctatus Based on the Variation of Fish Meal. Animals. 2023; 13(5):788. https://doi.org/10.3390/ani13050788
Chicago/Turabian StyleLiu, Tao, Xinzhi Weng, Jiteng Wang, Tao Han, Yuebin Wang, and Xuejun Chai. 2023. "Dietary Protein Requirement of Juvenile Dotted Gizzard Shad Konosirus punctatus Based on the Variation of Fish Meal" Animals 13, no. 5: 788. https://doi.org/10.3390/ani13050788
APA StyleLiu, T., Weng, X., Wang, J., Han, T., Wang, Y., & Chai, X. (2023). Dietary Protein Requirement of Juvenile Dotted Gizzard Shad Konosirus punctatus Based on the Variation of Fish Meal. Animals, 13(5), 788. https://doi.org/10.3390/ani13050788






