Simple Summary
A complete pelleted diet is one of the most popular and effective approaches for feeding livestock because of its contribution to improved flock productivity, low cost, and profitability. However, there are a number of negative effects associated with feeding such diets to livestock which can impact the livestock producer and consumer preferences; these include wool biting, wool eating, odor emission, and the dark color of ruminal tissue. The addition of yucca plays a beneficial role in the nutrition and welfare of livestock, particularly sheep, by reducing the negative effects of feeding complete pelleted diets. Animal diets supplemented with yucca target growth, productivity, and physiological responses. The aim of this study was to improve the quality of complete pelleted diets in order to avoid or mitigate their effects on fecal and urinary odor emission. The results found that supplementing Yucca schidgera extract at a level of 600 mg YS/kg dry matter (DM) in feed improved the fecal and urinary odors of lambs fed on pelleted diets.
Abstract
Sixty male Awassi lambs were used to investigate the effects of dietary Yucca schidgera extract (YS) on the production, fecal and urinary odor emissions, and carcass traits of growing lambs fed complete pellets. Lambs were fed either a complete pelleted diet without yucca (control) or supplemented with 300 or 600 mg YS/kg dry matter (DM) during the 84-day experiment. The weights and feed consumption of the lambs were measured weekly. Blood samples were taken on days 1, 28, 58, and 84, and ruminal fluid samples were collected on day 70. On day 90, the odor emissions from feces and urine were measured. On day 84, 12 lambs were slaughtered for the evaluation of carcass and meat quality. The final values for bodyweight, bodyweight gain, and feed efficiency of lambs fed the YS300 diet were 3.40%, 6.64%, and 6.17%, respectively, higher (p < 0.05) than those fed the YS600 diet. Additionally, the percentage of dressing, myofibril fragmentation index, and ruminal isovalerate percentage of lambs treated with YS600 were higher than those treated with YS300. Compared with the control, the addition of yucca reduced odor emissions from feces and urine. In conclusion, dietary YS300 had no additional benefits on growth rate, feed efficiency, and carcass traits, while dietary YS600 improved fecal and urinary odors.
1. Introduction
Extensive grazing systems do not always meet the nutrient requirements of the majority of livestock raised on them. Previous studies have successfully incorporated a variety of low-quality raw feed materials, such as cotton seed hulls, taramira oil cake, and sunflower hulls, into complete pelleted diets to fill this nutritional gap, resulting in economic gains for animal producers through improved animal growth rates, ruminal fermentation, and feed efficiency [1,2,3,4,5,6,7].
The use of natural feed additives in animal rations and the modification of feed formulations to improve the physical and microbiological processes of ruminal fermentation, thereby increasing the supply of volatile fatty acids (VFAs) and essential microbial proteins for ruminant growth [8,9], are gaining in popularity. These feed additives also play crucial roles in the regulation of toxic fermentation byproducts in the rumen [10,11]. There is a negative relationship between methane production and energy utilization in ruminants; therefore, the use of feed additives to reduce methane production and increase energy utilization is an attractive method of manipulating rumen fermentation [12,13].
Yucca is a medicinal plant that was grown originally in Mexico and the southwestern United States. Approximately 10% of the plant mass is dry matter [14,15]. Generally recognized as safe (GRAS), the US Food and Drug Administration has allowed the use of numerous YS products, including yucca extract and yucca powder, as additives in the food and drink industry, as well as in livestock and poultry feeds [16,17,18]. The biologically active surfactants in YS have been identified as steroid saponins and glycocomponents [18,19]. YS contains a number of polyphenolics which have antioxidant, anti-inflammatory, urease-inhibiting, and antiprotozoal characteristics, as well as properties related to the regulation of lipid metabolism [18,20,21,22]. Commercially, YS, as a feed additive, is used primarily in controlling environmental ammonia in livestock and poultry facilities in order to reduce odor emissions [23,24]. Several studies have indicated that the addition of YS powder to livestock feeds improves productivity and feed efficiency and increases ruminal VFA concentrations, as well as contributing to environmental control, microbial-activity modification, and blood biochemical parameters [23,24,25,26,27,28,29,30].
The effects of including dietary YS extract in completed pelleted diets on the productive performance of ruminant animals, particularly growing lambs, are largely unknown, and previous livestock nutrition studies have yielded contradictory results. Therefore, this study sought to determine whether feeding growing lambs on pelleted diets supplemented with varying levels of YS has beneficial effects on productivity, blood metabolic variables, ruminal fermentation, fecal and urine gas emissions, and carcass traits.
2. Materials and Methods
2.1. Experimental Design and Diets
The experiment was undertaken at the Animal Experimental Station, Department of Animal Production, College of Food and Agricultural Sciences, King Saud University, Riyadh, Saudi Arabia, and all procedures in this experiment followed the Animal Welfare Act of Practice for the Care and Use of Animals for Scientific Purposes, with approval from the King Saud University Research Ethics Committee (REC-KSU; Ethics Reference No.: KSU-SE-21-01). Sixty male Awassia lambs initially weighing 26.5, SD ± 2.0 kg, and aged between 3 and 4 months were purchased from the Riyadh livestock market and then transported to the Experimental Station. On the day of arrival, the animals’ weights were recorded and they were ear-tagged and treated against internal and external parasites and vaccinated against the commonest diseases. Animals were given a 14-day adaptation period, and, during this period, they were housed in shaded pens (4.0 m long, 3.0 m wide; 5 lambs in each pen). On the first day of the experiment, the lambs were randomly assigned to one of three treatments (four replicates for each treatment): (1) a complete pelleted diet (without YS supplementation; the basal diet; CON); (2) the basal diet with 300 mg YS/kg DM (YS300); and (3) the basal diet with 600 mg YS/kg DM (YS600). The Yucca schidgera extract BIOPOWDER®® was provided by Agroin (Ensenada, BC, Mexico). All experimental feeds were formulated according to NRC recommendations [31] for growing lambs (Table 1).

Table 1.
Ingredients and chemical composition of the basal diet used in the experiment 1.
2.2. Growth Preformance and Feed Intake
The lambs were weighed on day 1 and then every two weeks in order to determine growth performance (including bodyweight gain and average daily gain). Feed intake was recorded weekly for each pen by calculating the difference between feed offered and feed refused. The feed conversion ratio (FCR) for each lamb was determined by subtracting dry-matter intake consumed from the average daily gain, and then represented as kg of DMI to kg of BW.
2.3. Blood Sample Processing and Analysis
Blood samples from all lambs were taken in the morning before feeding on days 1, 28, 58, and 84. Serum was obtained after the centrifugation of blood samples at 2400× g for 15 min at 4 °C and was then frozen at −20 °C until analysis. Commercial biochemical regents from Randox Laboratories (Antrim, UK) and a microplate reader (Multiskan EX, Thermo Fisher Scientific, Inc., Waltham, MA, USA) were used to determine the concentrations of glucose, total protein, albumin, urea, and creatinine in serum, following the manufacturers’ instructions.
2.4. Rumen Fermentation Profiles
Before the morning feeding, samples of rumen fluid (50 mL) from 15 lambs in each treatment were collected using an oral stomach tube on day 70 for evaluation of the ruminal fermentation profiles. The samples were analyzed for pH values, strained through four layers of cheesecloth, transferred into plastic tubes, acidified with 2 mL of concentrated sulfuric acid, and then frozen at −20 °C for further analysis. The ruminal fluid samples were then thawed and analyzed for ammonia (using a TECO Diagnostics kit) and volatile fatty acid (VFA) proportions and totals, including acetic, propionic, butyric, isobutyric, valeric, and isovaleric acids, as described by [32], using 2-ethylbutyric acid as an internal standard and a gas chromatograph–mass spectrometer (7010C Triple Quadrupole GC/MS, Agilent Technologies, Palo Alto, CA, USA). The total volatile fatty acid concentrations were expressed as concentrations mol/l (mM), while individual VFA proportions were expressed as molar percentages of total molar VFAs (% mol).
2.5. Fecal and Urinary Odor Evaluation
On day 90, seven lambs from each treatment were removed into metabolism cages (7 days) for the digestibility trial. During the collection period (3 days), urine and feces from each animal were measured daily. Representative samples of the collected urine and feces were used for the evaluation of fecal and urinary odor emissions. Fecal (150 g) and urine (150 mL) samples were mixed in 3 L plastic boxes, and then the mixtures of feces and urine were kept at room temperature for 0, 12, and 24 h to ferment and produce odor gases. After each fermentation period, the total ammonia, hydrogen sulfide, and acetic acid contents in the mixtures were quantified using colorimetric gas detector tubes (model: Gv100s Gas Detection System, Gas Tec, Wangara, WS, Australia) and a gas chromatograph–mass spectrometer (7010C Triple Quadrupole GC/MS, Agilent Technologies, Palo Alto, CA, USA), following a methodology described by [33].
2.6. Carcass Traits and Meat Quality
At the end of the experiment (day 84), 12 lambs from each treatment were slaughtered after 16 h of feed deprivation. With the live bodyweights obtained, hot carcasses and different organs were weighed immediately after slaughtering and then stored at 4 for 24 h. The carcasses were weighed again the next day for evaluation of the cold carcasses, and the percentages of dressing and chilling losses for each carcass were recorded. The right side of each carcass was separated to measure the pH value, back-fat thickness, the area of the longissimus thoracis muscle, and meat color value. Samples of muscles (about 300 g) were taken for the determination of water-holding capacity (WHC) [34], cooking loss (CL) [35], and myofibril fragmentation index (MFI) [36], while meat hardness, cohesiveness, chewiness, and springiness were measured using a texture analyzer (TA; HD, Stable Micro Systems, Surrey, UK) with a compression-plate attachment.
2.7. Statistical Analyses
All data were analyzed using a complete randomized design with general linear model procedures of statistical analysis software (SAS Institute Inc., Cary, NC, USA). The statistical model included the level of yucca, the collection day, and the interaction of treatment and day as fixed effects, with animal within treatment (with the exception of using pens for feed intake data) as a random effect. Carcass and meat quality characteristics, ruminal fluid fermentation, and fecal–urinary odor emissions data were entered into the GLM model in SAS. Data are reported with the least-square means (±SEs), and differences between them were considered significant at p ≤ 0.05.
3. Results
3.1. Dry-Matter Intake (DMI), Average Daily Gain (ADG), and Feed Efficiency
The effects of the dietary treatments on the productive performance of the growing lambs are presented in Table 2. There were no differences (p > 0.05) in dry-matter intake (DMI) across the treatment groups. The mean DMI was similar for all the treatments (1.48 kg/day). In contrast, the final bodyweights, bodyweight changes, average daily gains, and feed efficiencies were lowest (p < 0.05) in the lambs in the YS300 group compared to the other groups.

Table 2.
Mean bodyweight (BW), bodyweight gain (BWG), average daily gain (ADG), dry-matter intake (DMI), and feed efficiency (G:F ratio) of growing lambs fed complete pelleted diets supplemented with different levels of yucca.
3.2. Serum Biochemical Variables
Differences in the serum concentrations of the biochemical variables of growing lambs according to treatment are presented in Table 3.

Table 3.
Effects of different levels of yucca supplementation on serum concentrations of biochemical variables of growing lambs.
The serum concentrations of total protein, albumin, and urea did not differ (p > 0.05) between the dietary treatments. When compared to lambs fed the CON diet, the YS300 and YS600 diets resulted in a decrease (p = 0.01) in serum glucose concentration and an increase (p = 0.01) in serum urea concentration. When compared with the YS300 and CON groups, the YS600 group had intermediate glucose concentrations.
3.3. Rumen Fermentation Profile
The means of pH values and the concentrations of total VFAs and ammonia in the ruminal fluid of growing lambs fed with different levels of yucca are presented in Table 4. The overall pH value across the treatments was 6.05, ranging from 5.98 to 6.12. Total VFA concentrations and proportions of the majority of individual VFAs in ruminal fluid were not affected (p > 0.05) by different levels of yucca supplementation. When compared with lambs fed the CON diet, yucca supplementation at 300 mg YS/kg DM increased the isobutyrate molar percentage in the ruminal fluid of growing lambs, whereas yucca supplementation at 600 mg YS/kg DM increased isovalerate molar percentage in comparison with CON. In addition, lambs in the YS600 treatment group had (p < 0.05) the lowest ruminal ammonia concentrations compared with the other treatments (Table 4).

Table 4.
Effects of different levels of yucca supplementation on the ruminal fermentation profiles of growing lambs.
3.4. Fecal and Urinary Odor Emissions
The effects of different levels of yucca dietary supplementation on the concentrations of fecal and urinary odor gases emitted by growing lambs are presented in Figure 1. The concentrations of all gases measured (acetic acid, hydrogen sulfide (H2S), ammonia (NH3), and carbon dioxide (CO2)) were affected (p < 0.05) by the yucca supplementation. The concentrations of NH3 and CO2 in lambs fed on YS diets (YS300 or YS600) were lower (p < 0.05) than those in lambs fed the CON diet. The addition of yucca at a level of 600 mg YS/kg DM to the lambs’ diets reduced (p = 0.03) the H2S concentration in the mixture (6.5 mg YS/kg DM) and increased (p = 0.01) the concentration of acetic acid in fecal and urinary odor emissions (11.3 mg YS/kg DM) compared to the lambs fed the complete pelleted diet without YS supplementation (CON group).
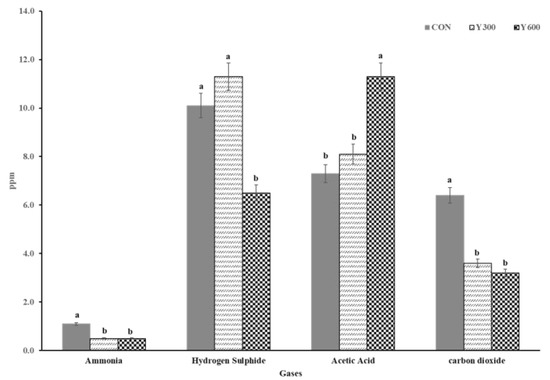
Figure 1.
Effect of dietary supplementation with different levels of yucca on fecal and urinary odor emissions of growing lambs. Values are means of growing lambs (n = 21) for 84 days. CON = a complete pelleted diet (the basal diet without YS supplementation); YS300 = the complete pelleted diet supplemented with YS at a level of 300 mg YS/kg DM; and YS600 = the complete pelleted diet supplemented with YS at a level of 600 mg YS/kg DM, Means without a common letter (a, b) differ (p < 0.05).
3.5. Carcass Characteristics and Meat Quality
The differences between the dietary treatments in terms of carcass characteristics and meat quality are presented in Table 5 and Table 6, respectively. In comparison with lambs in the YS600 group, lambs in the YS300 group had (p < 0.05) increased slaughter weights and shoulder percentages, which were similar to the values for lambs in the CON group. The YS600 group had higher chilling losses and lower shoulder percentages than the CON group. The dressing percentage was higher for the CON and YS600 groups compared with the YS300 group (p < 0.05). In addition, chilling losses were higher for the YS600 group compared with the CON group (p < 0.05), whereas these were intermediate for the YS300 group. There was no significant difference in organ weights between the treatments. The rack ribs and color components were not affected by the treatments (p > 0.05). Texture-profile analysis showed some significant differences according to the treatment. The myofibril fragmentation index (MFI) was significantly higher for the CON and YS600 groups compared with the YS300 group (p < 0.05). Shear force increased in the YS300 treatment group, but the difference was insignificant when compared with the YS600 group (p < 0.05). Generally, the meat samples from the YS300 and YS600 groups were harder, springier, and chewier (p < 0.05). Other parameters, such as cooking loss, WHC, and cohesiveness, were not affected by the treatments (p > 0.05).

Table 5.
Effects of different levels of dietary yucca supplementation on the carcass characteristics of growing lambs.

Table 6.
Effects of different levels of dietary yucca supplementation on meat quality of growing lambs.
4. Discussion
In Saudi Arabia, sheep production is a primary livestock industry in the agricultural sector. It plays a critical role in social and economic sustainability. According to [37], the sheep population in Saudi Arabia was estimated to be approximately 17.5 million head, representing approximately 70% of the total livestock population [37]. Under extensive grazing systems, sheep often do not receive their required nutrients because of shortages in natural resources, and this can cause reductions in the productive efficiency of flocks. Providing a range of feedstuffs, in particular forage, required by feeding ruminants is currently a major challenge facing the livestock industry in Saudi Arabia because of high feed prices.
According to [38], Awassi lambs fed complete pelleted diets had 10 and 21% higher ADGs and feed efficiencies, respectively, when compared to those fed on grain barley and alfalfa hay. Previous research found that feeding lambs a complete pelleted diet resulted in an increase in the color of ruminal tissue (L* value from 50.66 to 29.11) and a reduction in the pH values of ruminal fluid from 6.05 to 5.27 [39,40].
Saponins, a group of secondary compounds in plants, are able to alter rumen fermentation characteristics and encourage animal productivity [41]. Several studies have indicated that yucca saponins increased productive indicators, such as growth rate, meat, and milk production, in sheep when they were fed roughage diets [42,43]. A study reported in [25] observed that growing lambs fed a diet supplemented with YS at levels between 40 and 60 mg YS/kg DM had higher growth rates [25]. This is in agreement with the results obtained in our study: YS improved bodyweight, ADG, and feed efficiency ratio, in particular at 0 and 300 mg YS/kg DM. YS0 and YS300 generally performed better than YS600. Overall, lambs treated with YS300 had a higher final bodyweight (3.4%), ADG (6.6%), and feed efficiency (6.17%) in comparison with YS600 lambs.
Serum concentrations of total protein, albumin, and creatine were not affected by yucca supplementation in this study, and were all within the reference ranges reported in [44]. These results are consistent with those of previous studies [45,46,47] showing that neither the levels nor saponins of yucca supplementation affected total protein, albumin, and creatine levels in ruminants. A reduction in serum urea concentration was associated with the highest level of yucca supplementation (YS600) in our study, and the mean urea concentration was within the normal range for sheep (3.67–9.28 mM) [48]. Previous studies have indicated that yucca extract components might modify kidney function by increasing the rate of urea clearance and lowering blood urea concentrations [26].
In the current study, there was no response to the addition of yucca in terms of total VFA concentrations or in the proportions of the majority of individual VFAs in ruminal fluids. This is consistent with the results of previous studies, which indicated that dietary yucca supplementation had no beneficial effects on the VFA profiles of ruminant animals [49,50]. The authors of [51] suggested that saponins supplemented at higher concentrations might adversely affect rumen fermentation. Conversely, a report by [52] observed increases in gas and VFA production in dairy cattle fed a concentrate diet supplemented with yucca.
Reductions of 5.0 and 18.5% in ruminal ammonia concentrations were observed in lambs fed diets supplemented with 300 and 600 mg YS/kg DM, respectively, compared to those fed the CON diet (without yucca) in the current study. The complete pelleted diets of 300 and 600 mg YS/kg DM produced less ammonia compared with the control diet. The mechanism by which ammonia concentration is affected by the addition of YS could be attributed to an increase in ruminal nitrogen, as metabolic pathways (protein digestibility) and the involvement of saponins appeared to assist in reducing ammonia production in the rumen when yucca was added to the diets, while rumen ammonia concentration increases as a result of proteolysis in protein bacteria due to increases in rumen bacterial digestion by protozoa [50,53]. Thus, a reduction in ammonia production in the rumen could be attributed to the antiprotozoal activity utilized by saponins [53,54]. Similar findings have been reported in several previous studies [26,27,55].
Yucca has important applications in ruminant nutrition and plays an important role in the regulation of fecal and urinary odor emissions, this being one of the main reasons for adding it to ruminant diets. This role is related to its involvement in various physiological functions, including the improvement of ruminal fermentation characteristics and microbiome responses and alterations to digestion and metabolic pathways, and the high-value compounds present in yucca (e.g., steroid saponins, glycocomponents, phenolics, and several enzymes). For example, yucca plays beneficial roles in enhancing nitrogen utilization through digestion, absorption, and metabolic and excretion processes in ruminants and poultry [56,57,58]. These roles could be attributed to the reductions in the concentration of ammonia in fecal and urinary odor emissions that were observed in the current study when lambs were fed complete pelleted diets supplemented with yucca at a level of 600 mg/kg. Meanwhile, the release of fecal and urinary odors with the YS addition in the current study would have been due to the increase in acetic acid, which is involved in the regulation of a variety of enzymes; these include mono-oxygenases and the enzymatic family of aminotransferases. These enzymes are mainly responsible for the conversion of tryptophan in the diet into acetic acid [58].
Differences in carcass characteristics and meat quality between the dietary treatments showed that feeding lambs the 300 mg YS/kg DM yucca diet increased slaughter weight, hot-carcass yield percentage, and shoulder wholesale cut weight. The dressing percentage increased with the 600 mg YS/kg DM yucca diet, and chilling loss was reduced with the CON diet. In general, the meat samples from the yucca-supplemented groups were harder, springier, and chewier. The results obtained here are in disagreement with those obtained by the authors of [59], the latter reporting insignificant differences in the weights of parts of the carcass, meat, fat, and bones of lambs fed YS extracts at incremental levels from 0 to 400 mg YS/kg DM. In broilers fed with YS (100 mg YS/kg DM) supplements, the authors of [60] reported positive improvements in live weight and carcass characteristics, such as carcass yield, dressing percentage, and breast. Similarly, these results were confirmed by previous research, which found that YS supplementation of broilers improved evisceration weight and yield of breast and thigh meat [61,62]. The mechanism by which the carcass and meat quality characteristics are affected positively by the addition of YS into livestock diets could be attributed to the improvements in gut histomorphology and nutrient absorption and utilization caused by steroid saponins [60].
5. Conclusions
Under the conditions of the current study, the results indicated that the inclusion of dietary YS at a level of 300 mg YS/kg DM in the diet of growing lambs had no additional consistent effect on growth rate, feed efficiency, carcass yield, or meat quality. Although, there were reductions in growth rate and carcass traits associated with YS supplementation with 600 mg YS/kg DM, the high level of YS supplementation resulted in reductions in ammonia concentrations in ruminal fluids and fecal odor emissions. More research is required to clearly define the effects of YS supplementation and the mechanisms responsible for these effects on productivity and gas emissions. These studies should be considered to determine the most efficient sources and levels of YS supplementation for feeding sheep.
Author Contributions
Conceptualization, I.A.A.; methodology, I.S.A. and I.A.A.; formal analysis, I.S.A.; investigation, I.A.A.; data curation, I.A.A.; writing—original draft preparation, I.S.A.; writing—review and editing, I.A.A. and A.A.A.-H.; project administration, I.A.A. and A.A.A.-H.; supervision, A.A.A.-H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Research Supporting Project (RSPD2023R569), King Saud University, Saudi Arabia.
Institutional Review Board Statement
The current study and the use of all animals were approved by the Research Ethics Committee, King Saud University (REC-KSU; Ethics Reference No.: KSU-SE-21-01).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data and analyses presented in this paper are freely available from the corresponding author on reasonable request.
Acknowledgments
The authors would like to extend their sincere appreciation to the Research Supporting Project, King Saud University, for its funding of this research through project number RSPD2023R569.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chharang, D. Non Conventional Animal Feed Resources; Blue Rose Publishers: Delhi, India, 2022. [Google Scholar]
- Trabi, E.B.; Seddik, H.-E.; Xie, F.; Lin, L.; Mao, S. Comparison of the rumen bacterial community, rumen fermentation and growth performance of fattening lambs fed low-grain, pelleted or non-pelleted high grain total mixed ration. Anim. Feed. Sci. Technol. 2019, 253, 1–12. [Google Scholar] [CrossRef]
- Konka, R.; Kumar, D.S.; Ramana, J.; Ravi, A.; Rao, E.R. Fermentation pattern in Murrah buffalo bulls fed crop residue based complete rations vis-a-vis conventional feeding system. Anim. Nutr. Feed. Technol. 2016, 16, 171–179. [Google Scholar] [CrossRef]
- Rodríguez, A.B.; Bodas, R.; Fernández, B.; López-Campos, O.; Mantecon, A.; Giráldez, F.J. Feed intake and performance of growing lambs raised on concentrate-based diets under cafeteria feeding systems. Animal 2007, 1, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Lailer, P.; Dahiya, S.; Lal, D.; Chauhan, T. Complete feed for livestock concept, present status and future trend: A review. Indian J. Anim. Sci. 2005, 75, 84–91. [Google Scholar]
- Waje, S.; Singh, S.; Mudgal, V. Effect of using forest grass based complete rations on growth and nutrient utilization in growing crossbred calves. Anim. Nutr. Feed. Technol. 2010, 10, 229–234. [Google Scholar]
- Sharma, V.; Purohit, G.; Arya, R.; Harsh, M. Evaluation of some complete rations in sheep incorporating unconventional feed resources of arid zone of India. Anim. Nutr. Feed. Technol. 2006, 6, 135–141. [Google Scholar]
- Owens, F.N.; Basalan, M. Ruminal fermentation. In Rumenology; Springer: Berlin/Heidelberg, Germany, 2016; pp. 63–102. [Google Scholar]
- Gunun, N.; Ouppamong, T.; Khejornsart, P.; Cherdthong, A.; Wanapat, M.; Polyorach, S.; Kaewpila, C.; Kang, S.; Gunun, P. Effects of rubber seed kernel fermented with yeast on feed utilization, rumen fermentation and microbial protein synthesis in dairy heifers. Fermentation 2022, 8, 288. [Google Scholar] [CrossRef]
- Sari, N.F.; Ray, P.; Rymer, C.; Kliem, K.E.; Stergiadis, S. Garlic and its bioactive compounds: Implications for methane emissions and ruminant nutrition. Animals 2022, 12, 2998. [Google Scholar] [CrossRef]
- Pimentel, P.R.S.; dos Santos Brant, L.M.; de Oliveira Lima, A.G.V.; Cotrim, D.C.; Nascimento, T.; Oliveira, R.L. How can nutritional additives modify ruminant nutrition? Rev. Fac. Cienc. Agrar. UNCuyo 2022, 54, 175–189. [Google Scholar] [CrossRef]
- Sahebi Ala, M.; Pirmohammadi, R.; Khalilvandi-Behroozyar, H.; Anassori, E. Changes in vitro rumen fermentation, methane production and microbial populations in response to green tea extract. Ital. J. Anim. Sci. 2021, 20, 1114–1125. [Google Scholar] [CrossRef]
- Goel, G.; Makkar, H.; Becker, K. Changes in microbial community structure, methanogenesis and rumen fermentation in response to saponin-rich fractions from different plant materials. J. Appl. Microbiol. 2008, 105, 770–777. [Google Scholar] [CrossRef]
- Louderback, L.A.; Pavlik, B.M.; Spurling, A.M. Ethnographic and archaeological evidence corroborating Yucca as a food source, Mojave Desert, USA. J. Ethnobiol. 2013, 33, 281–297. [Google Scholar] [CrossRef]
- Sajad, M.; Thakur, S.C. Traditional Uses and Anti-Inflammatory Activities of Different Medicinal Plants: A Systematic Review. Int. J. Ayurvedic Herb. Med. 2018, 9, 3410–3432. [Google Scholar]
- Zúñiga-Serrano, A.; Barrios-García, H.B.; Anderson, R.C.; Hume, M.E.; Ruiz-Albarrán, M.; Bautista-Martínez, Y.; Sánchez-Guerra, N.A.; Vázquez-Villanueva, J.; Infante-Rodríguez, F.; Salinas-Chavira, J. Antimicrobial and Digestive Effects of Yucca schidigera Extracts Related to Production and Environment Implications of Ruminant and Non-Ruminant Animals: A Review. Agriculture 2022, 12, 1198. [Google Scholar] [CrossRef]
- Oleszek, M.; Oleszek, W. Saponins in Food; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Jiménez, G.G.; Durán, A.G.; Macías, F.A.; Simonet, A.M. Structure, Bioactivity and Analytical Methods for the Determination of Yucca Saponins. Molecules 2021, 26, 5251. [Google Scholar] [CrossRef]
- Kaya, S.; Keskin, M.; Gül, S. Effects of Yucca schidigera extract (Dk 35 Powder) on Awassi lambs performance. J. Anim. Vet. Adv. 2006, 5, 57–59. [Google Scholar]
- Pecio, Ł.; Kozachok, S.; Brinza, I.; Boiangiu, R.S.; Hritcu, L.; Mircea, C.; Burlec, A.F.; Cioanca, O.; Hancianu, M.; Wronikowska-Denysiuk, O. Neuroprotective Effect of Yucca schidigera Roezl ex Ortgies Bark Phenolic Fractions, Yuccaol B and Gloriosaol A on Scopolamine-Induced Memory Deficits in Zebrafish. Molecules 2022, 27, 3692. [Google Scholar] [CrossRef]
- Cheeke, P.; Piacente, S.; Oleszek, W. Anti-inflammatory and anti-arthritic effects of Yucca schidigera: A review. J. Inflamm. 2006, 3, 6. [Google Scholar] [CrossRef]
- Windisch, W.; Schedle, K.; Plitzner, C.; Kroismayr, A. Use of phytogenic products as feed additives for swine and poultry. J. Anim. Sci. 2008, 86, E140–E148. [Google Scholar] [CrossRef]
- Liu, W.; La, A.L.T.Z.; Evans, A.; Gao, S.; Yu, Z.; Ma, L.; Bu, D. Supplementation with Yucca schidigera improves antioxidant capability and immune function and decreases fecal score of dairy calves before weaning. J. Dairy Sci. 2021, 104, 4317–4325. [Google Scholar] [CrossRef]
- Abdel-Raheem, S.M.; Farghaly, M.M.; Hassan, E.H. Effect of dietary supplementation with Yucca schidigera powder on nutrient digestibility, rumen fermentation, ruminal enzyme activities and growth performance of buffalo calves. Biol. Rhythm. Res. 2022, 53, 854–866. [Google Scholar] [CrossRef]
- Eryavuz, A.; Dehority, B. Effect of Yucca schidigera extract on the concentration of rumen microorganisms in sheep. Anim. Feed. Sci. Technol. 2004, 117, 215–222. [Google Scholar] [CrossRef]
- Hristov, A.N.; McAllister, T.A.; Van Herk, F.H.; Cheng, K.-J.; Newbold, C.J.; Cheeke, P.R. Effect of Yucca schidigera on ruminal fermentation and nutrient digestion in heifers. J. Anim. Sci. 1999, 77, 2554–2563. [Google Scholar] [CrossRef] [PubMed]
- Lila, Z.; Mohammed, N.; Kanda, S.; Kamada, T.; Itabashi, H. Effect of sarsaponin on ruminal fermentation with particular reference to methane production in vitro. J. Dairy Sci. 2003, 86, 3330–3336. [Google Scholar] [CrossRef] [PubMed]
- Piacente, S.; Montoro, P.; Oleszek, W.; Pizza, C. Yucca s chidigera Bark: Phenolic Constituents and Antioxidant Activity. J. Nat. Prod. 2004, 67, 882–885. [Google Scholar] [CrossRef] [PubMed]
- Duffy, C.; Brooks, P.; Lyons, T.; Jacques, K. Using Yucca schidigera in pig diets: Effects on nitrogen metabolism. In Biotechnology in the Feed Industry; Alltach: Nicholasville, KY, USA, 1998; p. 61. [Google Scholar]
- Santoso, B.; Mwenya, B.; Sar, C.; Gamo, Y.; Kobayashi, T.; Morikawa, R.; Kimura, K.; Mizukoshi, H.; Takahashi, J. Effects of supplementing galacto-oligosaccharides, Yucca schidigera or nisin on rumen methanogenesis, nitrogen and energy metabolism in sheep. Livest. Prod. Sci. 2004, 91, 209–217. [Google Scholar] [CrossRef]
- NRC. Nutrient Requirements of Small Ruminants; National Academy Press: Washington, DC, USA, 2007. [Google Scholar]
- Jenkins, T. Effect of fats and fatty acid combinations on ruminal fermentation in semi-continuous in vitro cultures. J. Anim. Sci. 1987, 64, 1526–1532. [Google Scholar] [CrossRef] [PubMed]
- Hales, K.E.; Parker, D.B.; Cole, N.A. Potential odorous volatile organic compound emissions from feces and urine from cattle fed corn-based diets with wet distillers grains and solubles. Atmos. Environ. 2012, 60, 292–297. [Google Scholar] [CrossRef]
- Culler, R.; Smith, G.; Cross, H. Relationship of myofibril fragmentation index to certain chemical, physical and sensory characteristics of bovine longissimus muscle. J. Food Sci. 1978, 43, 1177–1180. [Google Scholar] [CrossRef]
- Wilhelm, A.E.; Maganhini, M.B.; Hernández-Blazquez, F.J.; Ida, E.I.; Shimokomaki, M. Protease activity and the ultrastructure of broiler chicken PSE (pale, soft, exudative) meat. Food Chem. 2010, 119, 1201–1204. [Google Scholar] [CrossRef]
- Al-Owaimer, A.; Suliman, G.; Sami, A.; Picard, B.; Hocquette, J.-F. Chemical composition and structural characteristics of Arabian camel (Camelus dromedarius) m. longissimus thoracis. Meat Sci. 2014, 96, 1233–1241. [Google Scholar] [CrossRef] [PubMed]
- GASTAT (General Authority for Statistics). Detailed Results of the Agriculture Census. 2015. Available online: https://www.stats.gov.sa/ (accessed on 23 October 2022).
- Abdelrahman, M.M.; Alhidary, I.A.; Suliman, G.M.; Alyemni, A.H.; Al-Saiady, M.Y.; Alshamiry, F.A.; Alobre, M.M.; Aljumaah, R.S. Impact of Feeding Different Levels of Neutral Detergent Fiber as Total Mixed Rations on Sensory Attributes, Carcass Characteristics and Meat Quality of Growing Lambs. Pak. J. Zool. 2018, 50, 2129–2134. [Google Scholar] [CrossRef]
- Abdelrahman, M.M.; Alhidary, I.; Alyemni, A.H.; Khan, R.U.; Bello, A.R.S.; Al-Saiady, M.Y.; Amran, R.A. Effect of alfalfa hay on rumen fermentation patterns and serum biochemical profile of growing Naemi lambs with ad libitum access to total mixed rations. Pak. J. Zool. 2017, 49, 1519–1522. [Google Scholar] [CrossRef]
- Alhidary, I.; Abdelrahman, M.M.; Alyemni, A.H.; Khan, R.U.; Al-Mubarak, A.H.; Albaadani, H.H. Characteristics of rumen in Naemi lamb: Morphological changes in response to altered feeding regimen. Acta Histochem. 2016, 118, 331–337. [Google Scholar] [CrossRef]
- Patra, A.; Saxena, J. The effect and mode of action of saponins on the microbial populations and fermentation in the rumen and ruminant production. Nutr. Res. Rev. 2009, 22, 204–219. [Google Scholar] [CrossRef]
- Jaques, K. Yucca sarsaponin mode of action: Effects independent of air quality problems. In Animal Feed, Biological Additives: Proceeding; Veterinary Science University of Sydney: Sydney, Australia, 1989; pp. 39–46. [Google Scholar]
- Wina, E.; Muetzel, S.; Becker, K. The impact of saponins or saponin-containing plant materials on ruminant production A Review. J. Agric. Food Chem. 2005, 53, 8093–8105. [Google Scholar] [CrossRef]
- Lepherd, M.; Canfield, P.; Hunt, G.B.; Bosward, K. Haematological, biochemical and selected acute phase protein reference intervals for weaned female Merino lambs. Aust. Vet. J. 2009, 87, 5–11. [Google Scholar] [CrossRef]
- Cheeke, P. Actual and potential applications of Yucca schidigera and Quillaja saponaria saponins in human and animal nutrition. In Saponins in Food, Feedstuffs and Medicinal Plants; Springer: Berlin/Heidelberg, Germany, 2000; pp. 241–254. [Google Scholar]
- Cline, J. Effect of feeding MICRO-AID on stillbirths, preweaning mortality, blood oxygen values of piglets and blood urea nitrogen in sows. J. Anim. Sci. 1996, 74, 189. [Google Scholar]
- Kaya, S.; Erdogan, Z.; Erdogan, S. Effect of different dietary levels of Yucca schidigera powder on the performance, blood parameters and egg yolk cholesterol of laying quails. J. Vet. Med. Ser. A 2003, 50, 14–17. [Google Scholar] [CrossRef]
- Aiello, S.E. The Merck Veterinary Manual, 8th ed.; Merck & Co., Inc.: Whitehouse Station, NJ, USA, 1998. [Google Scholar]
- Pen, B.; Takaura, K.; Yamaguchi, S.; Asa, R.; Takahashi, J. Effects of Yucca schidigera and Quillaja saponaria with or without β 1–4 galacto-oligosaccharides on ruminal fermentation, methane production and nitrogen utilization in sheep. Anim. Feed. Sci. Technol. 2007, 138, 75–88. [Google Scholar] [CrossRef]
- Lu, C.D.; Jorgensen, N.A. Alfalfa saponins affect site and extent of nutrient digestion in ruminants. J. Nutr. 1987, 117, 919–927. [Google Scholar] [CrossRef] [PubMed]
- Lovett, D.; Stack, L.; Lovell, S.; Callan, J.; Flynn, B.; Hawkins, M.; O’Mara, F. Effect of feeding Yucca schidigera extract on performance of lactating dairy cows and ruminal fermentation parameters in steers. Livest. Sci. 2006, 102, 23–32. [Google Scholar] [CrossRef]
- Wang, Y.; McAllister, T.A.; Yanke, L.J.; Xu, Z.J.; Cheeke, P.R.; Cheng, K.J. In vitro effects of steroidal saponins from Yucca schidigera extract on rumen microbial protein synthesis and ruminal fermentation. J. Sci. Food Agric. 2000, 80, 2114–2122. [Google Scholar] [CrossRef]
- Wallace, R.; Arthaud, L.; Newbold, C. Influence of Yucca shidigera extract on ruminal ammonia concentrations and ruminal microorganisms. Appl. Environ. Microbiol. 1994, 60, 1762–1767. [Google Scholar] [CrossRef]
- Makkar, H.P.; Sen, S.; Blümmel, M.; Becker, K. Effects of fractions containing saponins from Yucca schidigera, Quillaja saponaria, and Acacia auriculoformis on rumen fermentation. J. Agric. Food Chem. 1998, 46, 4324–4328. [Google Scholar] [CrossRef]
- Holtshausen, L.; Chaves, A.; Beauchemin, K.; McGinn, S.; McAllister, T.; Odongo, N.; Cheeke, P.; Benchaar, C. Feeding saponin-containing Yucca schidigera and Quillaja saponaria to decrease enteric methane production in dairy cows. J. Dairy Sci. 2009, 92, 2809–2821. [Google Scholar] [CrossRef]
- Hu, W.; Liu, J.; Wu, Y.; Guo, Y.; Ye, J. Effects of tea saponins on in vitro ruminal fermentation and growth performance in growing Boer goat. Arch. Anim. Nutr. 2006, 60, 89–97. [Google Scholar] [CrossRef]
- Giesy, R.; Harris, B., Jr.; Giesy, J.; Van Horn, H., Jr. Effectiveness of De-odorase in reducing ammonia levels in dairy barns during summer months. In Biotechnology in the Feed Industry; Alltech, Inc.: Nicholasville, KY, USA, 1992; pp. 16–18. [Google Scholar]
- Girard, I.; Newman, K.; Chandler, V. Fermentations in rumen-simulating cultures receiving yucca extract supple-mentation. In Biotechnology in the Feed Industry; Alltech, Inc.: Nicholasville, KY, USA, 1991; pp. 361–364. [Google Scholar]
- Selcuk, Z.; Tuncer, S.D. The effects of different levels of Yucca schidigera added to the lamb’s diets containing urea on growth performance, carcass characteristics, some rumen and blood parameters. J. Anim. Vet. Adv. 2010, 9, 654–660. [Google Scholar] [CrossRef]
- Alghirani, M.M.; Chung, E.L.T.; Sabri, D.S.M.; Tahir, M.N.J.M.; Kassim, N.A.; Kamalludin, M.H.; Nayan, N.; Jesse, F.F.A.; Sazili, A.Q.; Loh, T.C. Can Yucca schidigera Be Used to Enhance the Growth Performance, Nutrient Digestibility, Gut Histomorphology, Cecal Microflora, Carcass Characteristic, and Meat Quality of Commercial Broilers Raised under Tropical Conditions? Animals 2021, 11, 2276. [Google Scholar] [CrossRef]
- Ashour, E.; Alagawany, M.; Reda, F.; Abd El-Hack, M. Effect of Supplementation of Yucca schidigera Extract to Grovving Rabbit Diets on Grovvth Performance, Carcass Characteristics, Serum Biochemistry and Liver Oxidative Status. Asian J. Anim. Vet. Adv. 2014, 9, 732–742. [Google Scholar] [CrossRef]
- Miah, M.; Rahman, M.; Islam, M.; Monir, M. Effects of saponin and L-carnitine on the performance and reproductive fitness of male broiler. Int. J. Poult. Sci. 2004, 3, 530–533. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).