Ocular Ultrasonography in Healthy Calves with Different Transducers
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
- -
- Age < 30 days
- -
- Clinically healthy
- -
- No detectable ocular lesions.
- -
- Axial length of the globe (AL): distance from the mid-cornea to the posterior pole of the globe including the scleroretinal rim;
- -
- Depth of the anterior chamber (DAC): distance from the mid-cornea to the anterior lens capsule;
- -
- Vitreous depth (VD): distance from the lens to the scleroretinal rim;
- -
- Corneal thickness (CT): distance from the anterior to the posterior corneal epithelium;
- -
- Scleroretinal rim thickness (SRT): distance from the internal epithelium to the external scleroretinal rim;
- -
- Lens length (LL): distance from the anterior to the posterior chamber of the lens;
- -
- Lens thickness (LTK): distance between the equatorial poles of the lens;
- -
- Optic nerve diameter (OD).
3. Results
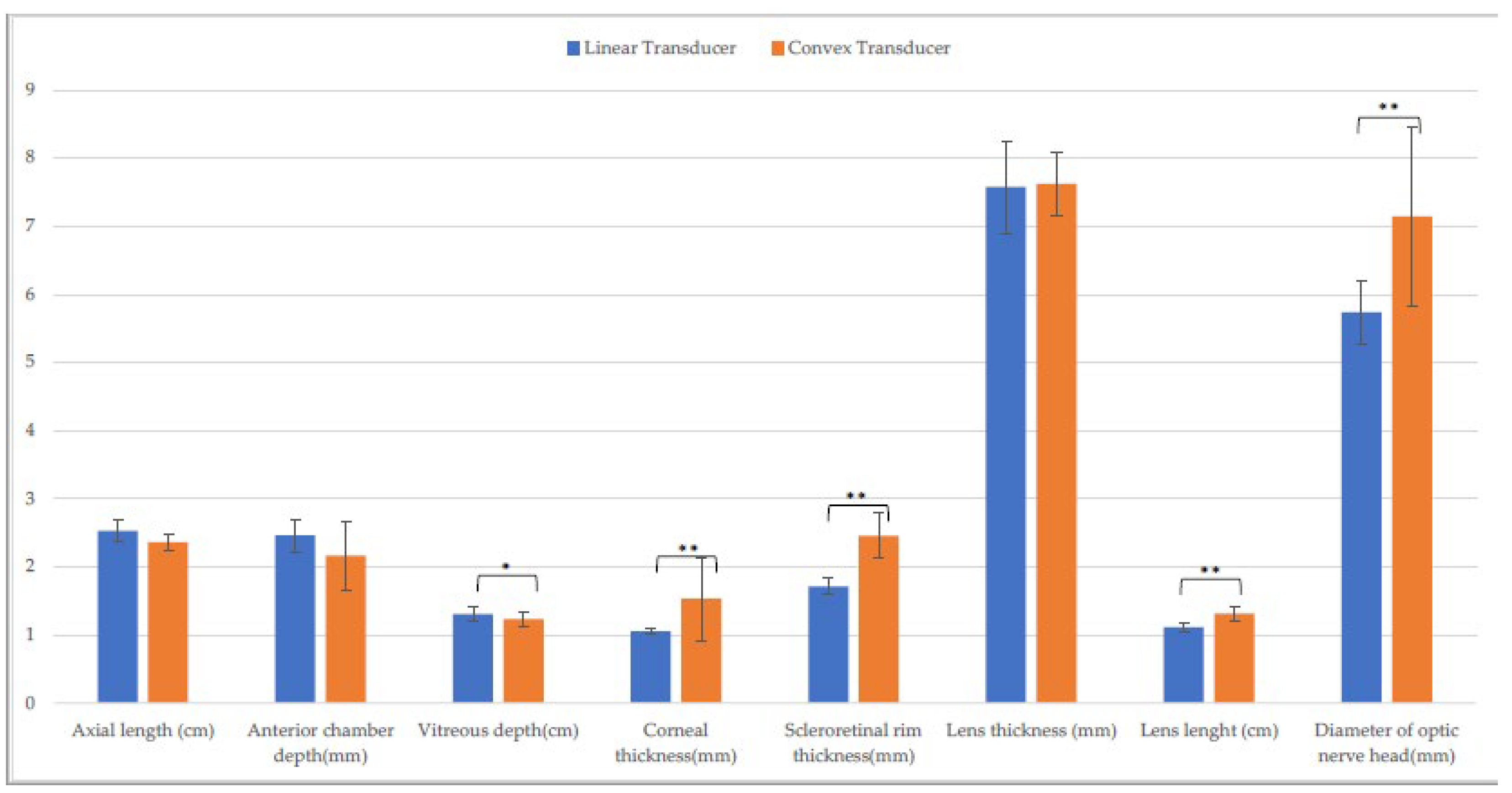
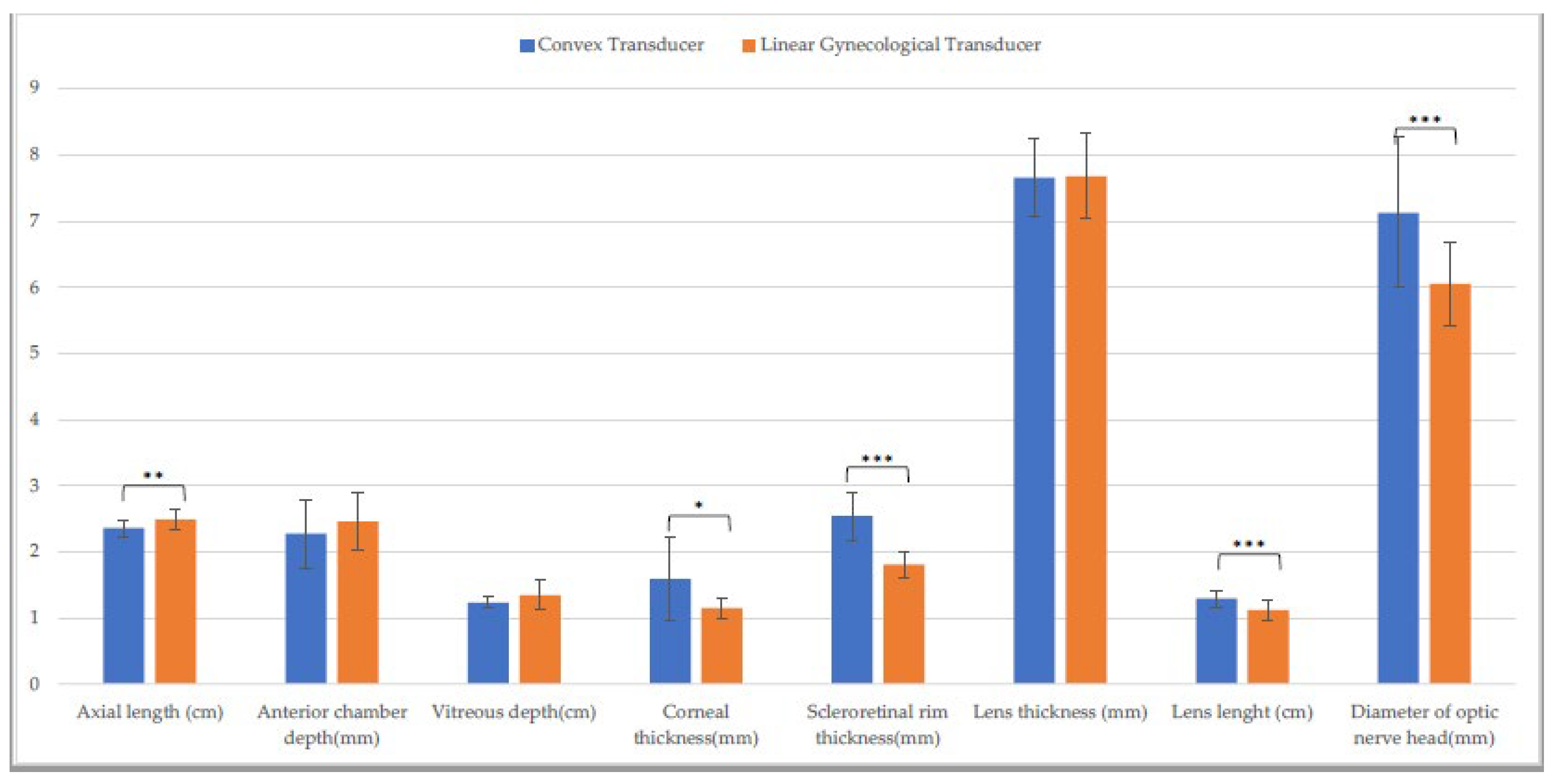
3.1. Entire Sample
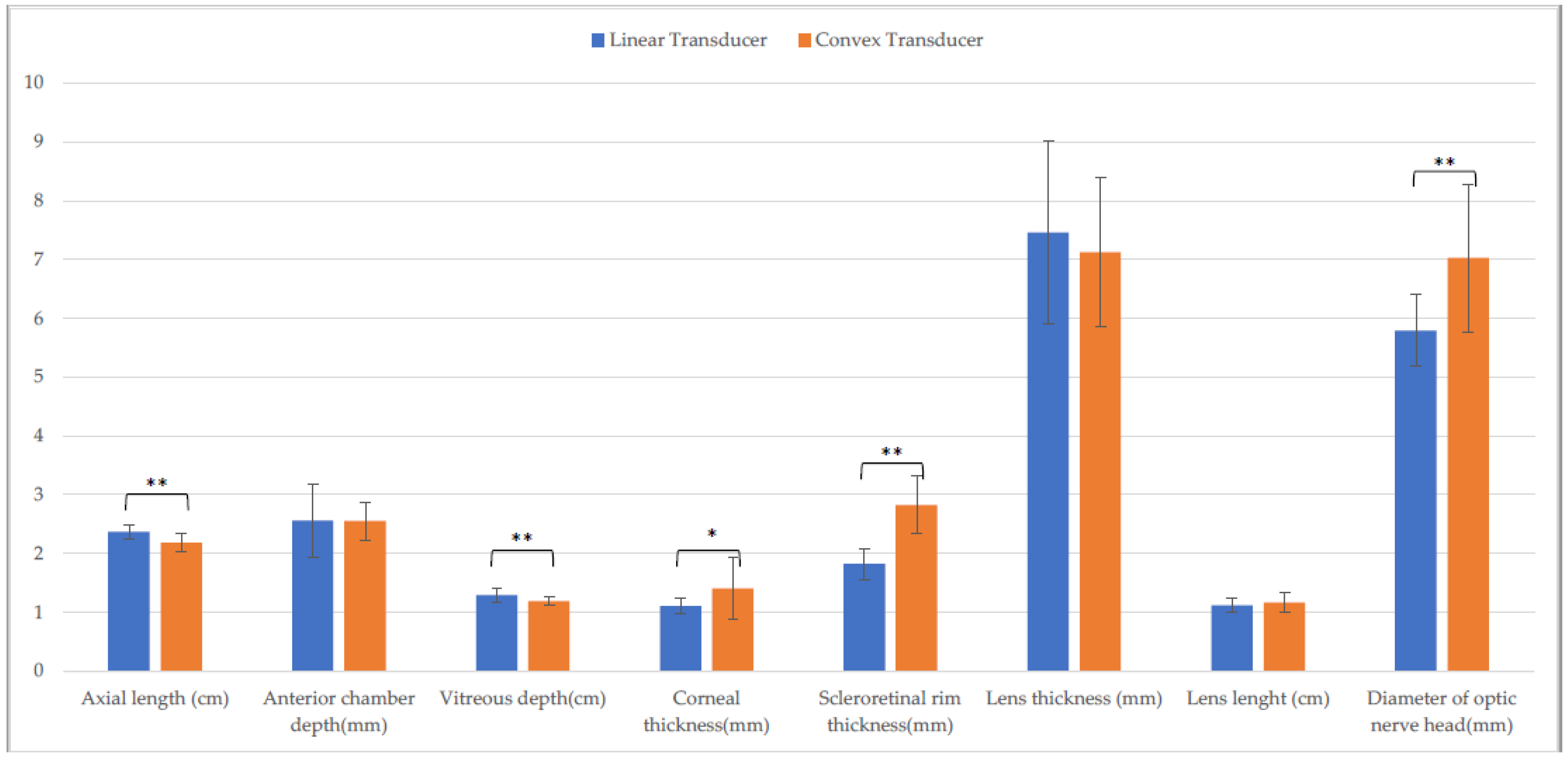
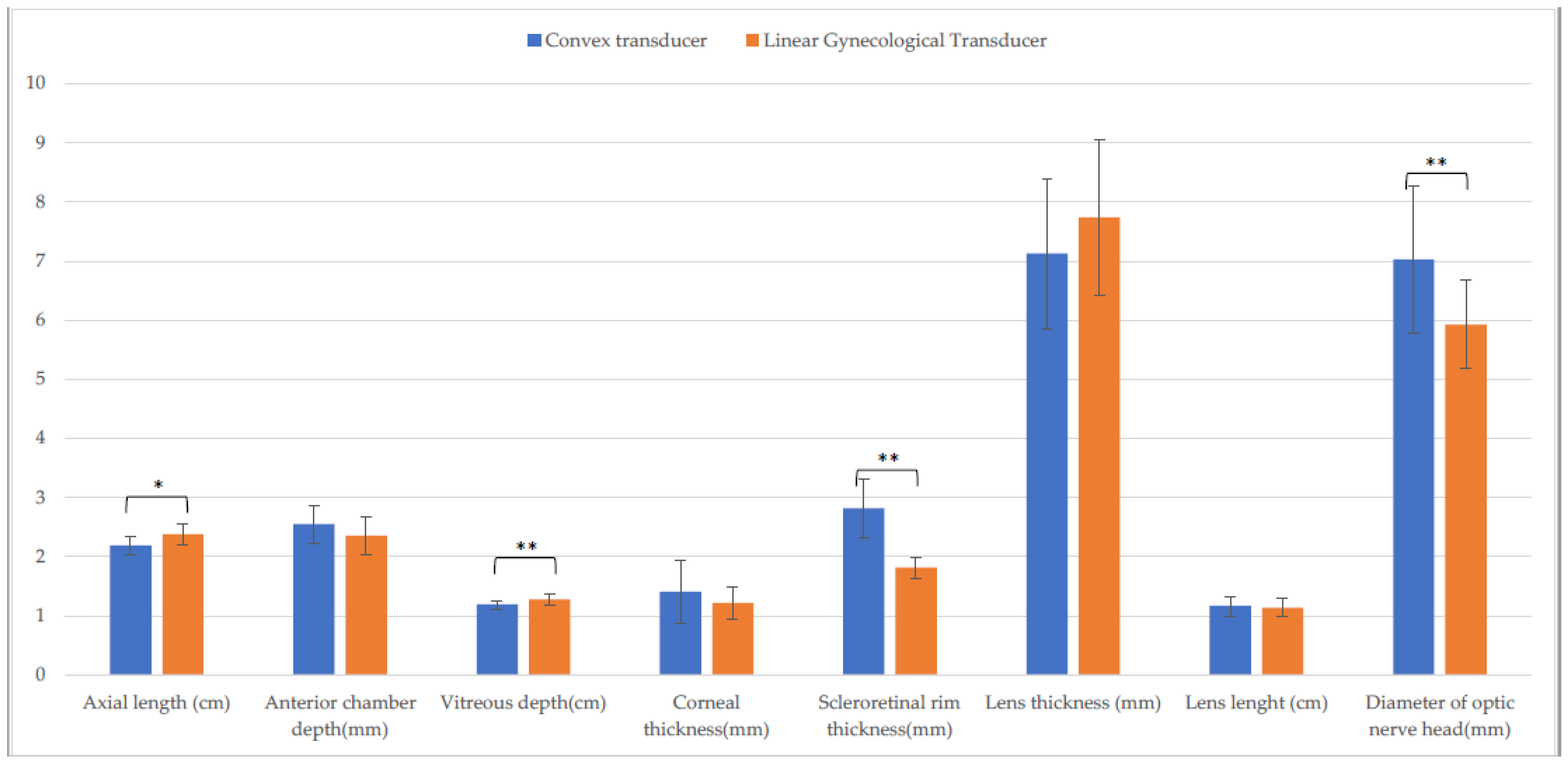
3.2. Holstein Calves
3.3. Piedmontese Calves
3.4. Comparison between Breeds
3.5. Non-Valuable Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Blohm, K.O.; Hittmair, K.M.; Tichy, A.; Nell, B. Quantitative, Noninvasive Assessment of Intra- and Extraocular Perfusion by Contrast-Enhanced Ultrasonography and Its Clinical Applicability in Healthy Dogs. Vet. Ophthalmol. 2019, 22, 767–777. [Google Scholar] [CrossRef] [PubMed]
- Ojaghihaghighi, S.; Lombardi, K.M.; Davis, S.; Vahdati, S.S.; Sorkhabi, R.; Pourmand, A. Diagnosis of Traumatic Eye Injuries With Point-of-Care Ocular Ultrasonography in the Emergency Department. Ann. Emerg. Med. 2019, 74, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Potter, T.J.; Hallowell, G.D.; Bowen, I.M. Ultrasonographic Anatomy of the Bovine Eye. Vet. Radiol. Ultrasound 2008, 49, 172–175. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, N.; Tsuka, T.; Sunden, Y.; Morita, T.; Islam, S.; Yamato, O.; Yoshimura, T. Ophthalmic Findings in a Septic Calf with the Concurrent Exhibition of Meningitis and Endophthalmitis Running Head: Detached retina in calf endophthalmitis. J. Vet. Med. Sci. 2021, 83, 1648–1652. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.L. Lens Morphometry Determined by B-Mode Ultrasonography of the Normal and Cataractous Canine Lens. Vet. Ophthalmol. 2004, 7, 91–95. [Google Scholar] [CrossRef] [PubMed]
- McMullen, R.J.; Gilger, B.G. Keratometry, Biometry and Prediction of Intraocular Lens Power in the Equine Eye. Vet. Ophthalmol. 2006, 9, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Townsend, W.M. Examination Techniques and Therapeutic Regimens for the Ruminant and Camelid Eye. Vet. Clin. N. Am. Food Anim. Pract. 2010, 26, 437–458. [Google Scholar] [CrossRef] [PubMed]
- Cellina, M.; Cè, M.; Marziali, S.; Irmici, G.; Gibelli, D.; Oliva, G.; Carrafiello, G. Computed Tomography in Traumatic Orbital Emergencies: A Pictorial Essay-Imaging Findings, Tips, and Report Flowchart. Insights Imaging 2022, 13, 4. [Google Scholar] [CrossRef] [PubMed]
- Beeston, T.; Israeliantz Gunz, N.; Leigh, H.; Lopez, C.; Lord, S.; Liuti, T. Anatomical Localisation of the Maxillary Nerve with the Use of Computed Tomography to Aid Nerve Block Placement in Dogs. Vet. Rec. 2022, 191, e1388. [Google Scholar] [CrossRef] [PubMed]
- El-Maghraby, H.M.; Nyland, T.G.; Bellhorn, R.W. Ultrasonographic and biometric evaluation of sheep and cattle eyes. Vet. Radiol. Ultrasound 1995, 36, 148–151. [Google Scholar] [CrossRef]
- Hatar, H.; Parrah, U.; Handoo, N.; Qayoom Mir, A.; Udin Dar, M.; Qureshi, B.; Vet Anim Sci, T.J.; Athar, H.; Udin, P.J.; Qayoom, M.A.; et al. Ocular Ultrasonography and Echobiometry in Sheep Ocular Ultrasonography and Echobiometry in Sheep Turkish Journal of Veterinary and Animal Sciences Ocular Ultrasonography and Echobiometry in Sheep. Turk. J. Vet. Anim. Sci. 2021, 45, 221–228. [Google Scholar] [CrossRef]
- Gayrard, P.; Carrière, P.D.; Des Côteaux, L. Principles and Recommendations, Essential Concepts, and Common Artifacts in Ultrasound Imaging. In Practical Atlas of Ruminant and Camelid Reproductive Ultrasonography; DesCôteaux, L., Gnemmi, G., Cotton, J.W.B., Eds.; Wiley-Blackwell Publishing Ltd.: Ames, IA, USA, 2010; pp. 3–20. [Google Scholar]
- Ivan, D.; Ohlerth, S.; Richter, H.; Verdino, D.; Rampazzo, A.; Pot, S. 3T High-Resolution Magnetic Resonance Imaging, Conventional Ultrasonography and Ultrasound Biomicroscopy of the Normal Canine Eye. BMC Vet. Res. 2022, 18, 67. [Google Scholar] [CrossRef] [PubMed]
- Philp, H.S.; Epstein, S.E.; Hopper, K. Clinical and Clinicopathological Characteristics, Treatment, and Outcome for Dogs and Cats with Confirmed Foxtail Foreign Body Lesions: 791 Cases (2009–2018). J. Vet. Emerg. Crit. Care 2022, 32, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Drolet, C.; Pinard, C.; Gaitero, L.; Monteith, G.; Bateman, S. Study of the Effect of Anaesthesia on the Canine Ultrasonographic Optic Nerve Sheath Diameter. J. Small Anim. Pract. 2021, 62, 1070–1078. [Google Scholar] [CrossRef] [PubMed]
- Shank, A.M.M.; Teixeira, L.B.C.; Dubielzig, R.R. Ocular Porcupine Quilling in Dogs: Gross, Clinical and Histopathologic Findings in 17 Cases (1986–2018). Vet. Ophthalmol. 2021, 24, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, I.Q.; Delgado, E. Congenital Ocular Malformations in Dogs and Cats: 123 Cases. Vet. Ophthalmol. 2020, 23, 964–978. [Google Scholar] [CrossRef] [PubMed]
- Cho, J. Ocular Ultrasound Abnormalities and Optic Nerve Sheath Diameter in Dogs and Cats. Vet. Clin. N. Am.-Small Anim. Pract. 2021, 51, 1295–1314. [Google Scholar] [CrossRef] [PubMed]
- Evangelisti, M.A.; Carta, G.; Burrai, G.P.; Pinna Parpaglia, M.L.; Cubeddu, F.; Ballocco, I.; Puggioni, A.; Manunta, M.L. Repeatability of Ultrasound Examination of the Optic Nerve Sheath Diameter in the Adult Cat: Comparison between Healthy Cats and Cats Suffering from Presumed Intracranial Hypertension. J. Feline Med. Surg. 2020, 22, 959–965. [Google Scholar] [CrossRef] [PubMed]
- Lochner, P.; Leone, M.A.; Fassbender, K.; Cantello, R.; Coppo, L.; Nardone, R.; Zorzi, G.; Lesmeister, M.; Comi, C.; Brigo, F. Transorbital Sonography and Visual Outcome for the Diagnosis and Monitoring of Optic Neuritis. J. Neuroimaging 2017, 27, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Şık, N.; Erbaş, İ.M.; Demir, K.; Yılmaz, D.; Duman, M. Bedside Sonographic Measurements of Optic Nerve Sheath Diameter in Children with Diabetic Ketoacidosis. Pediatr. Diabetes 2021, 22, 618–624. [Google Scholar] [CrossRef] [PubMed]
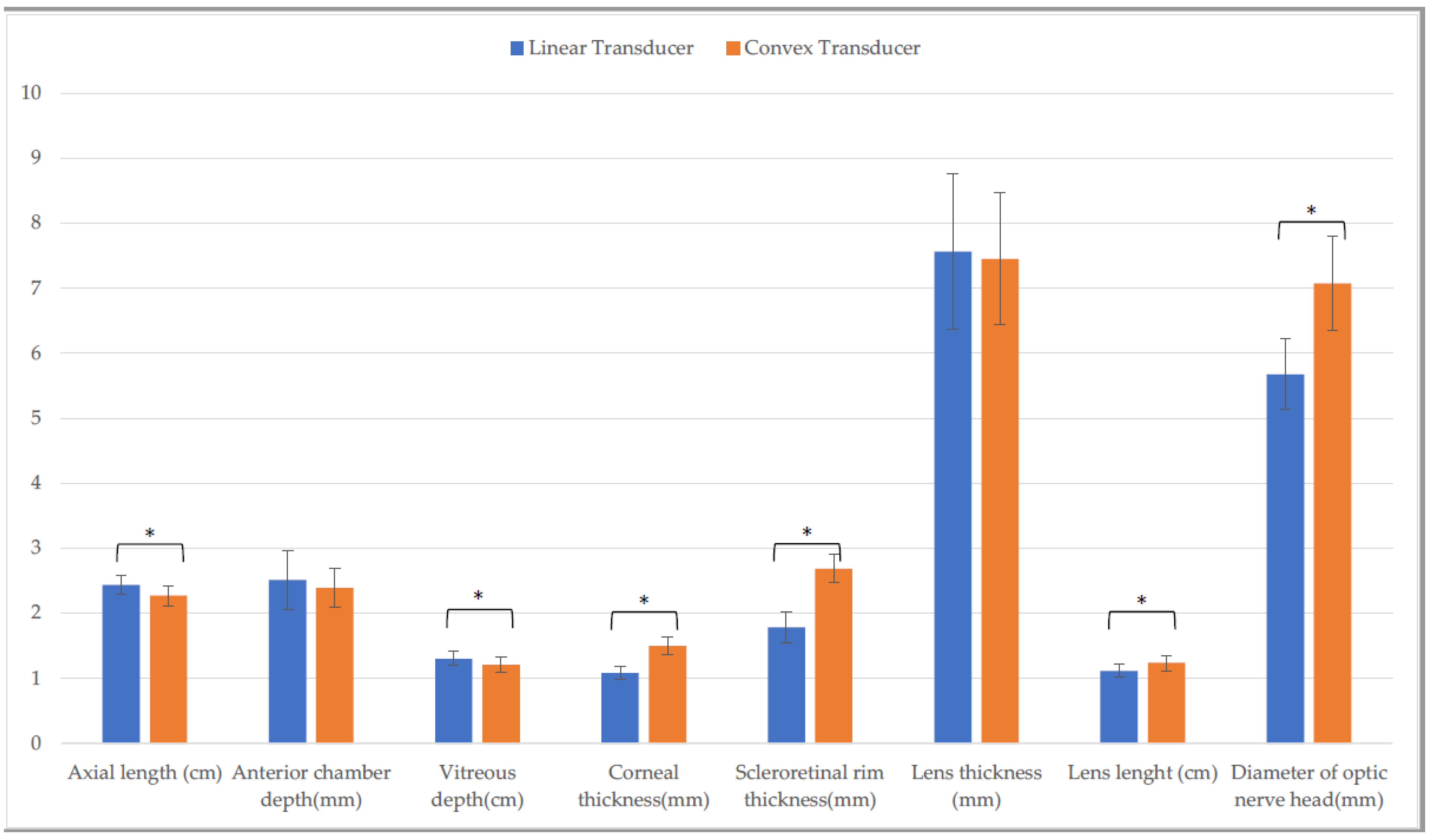
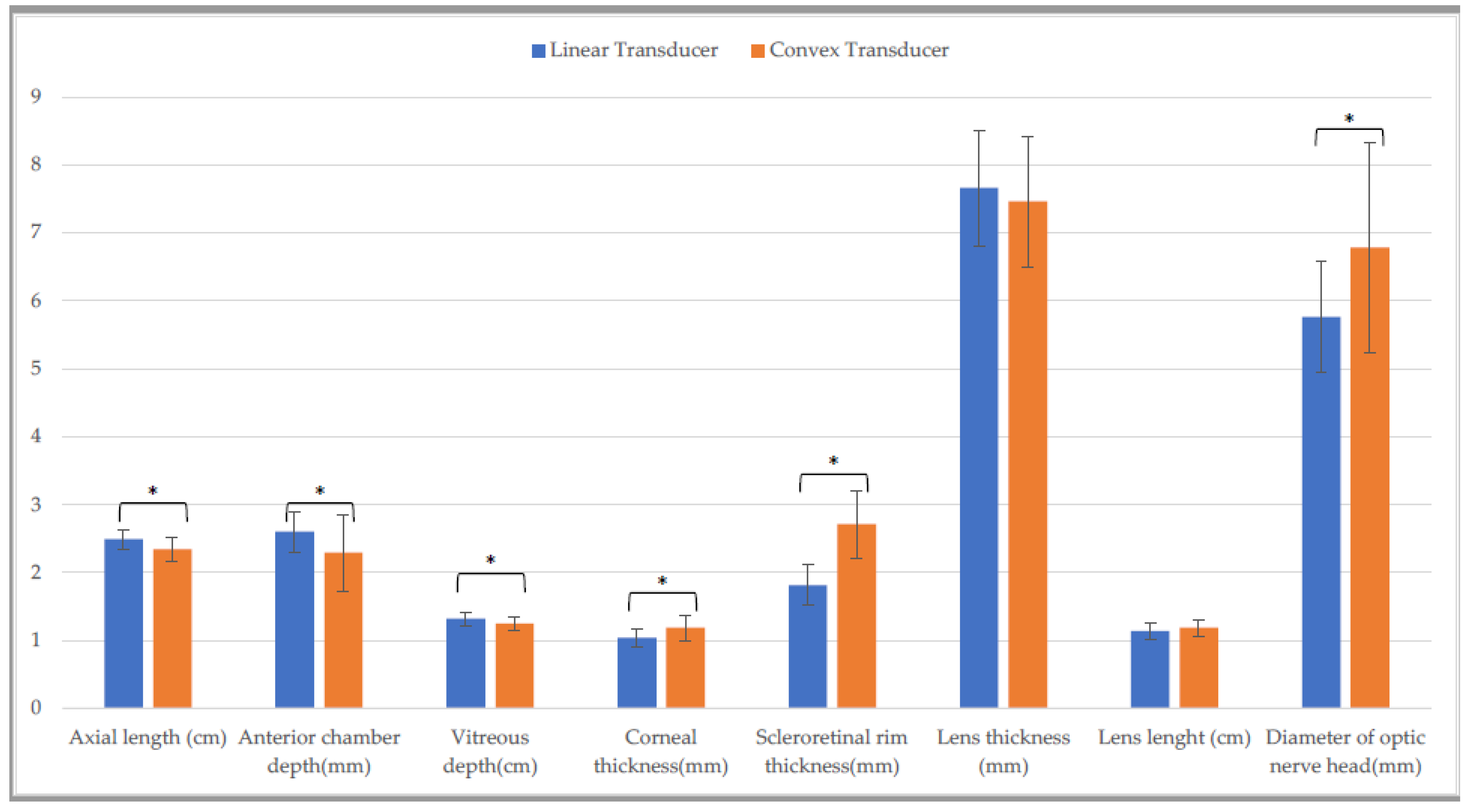
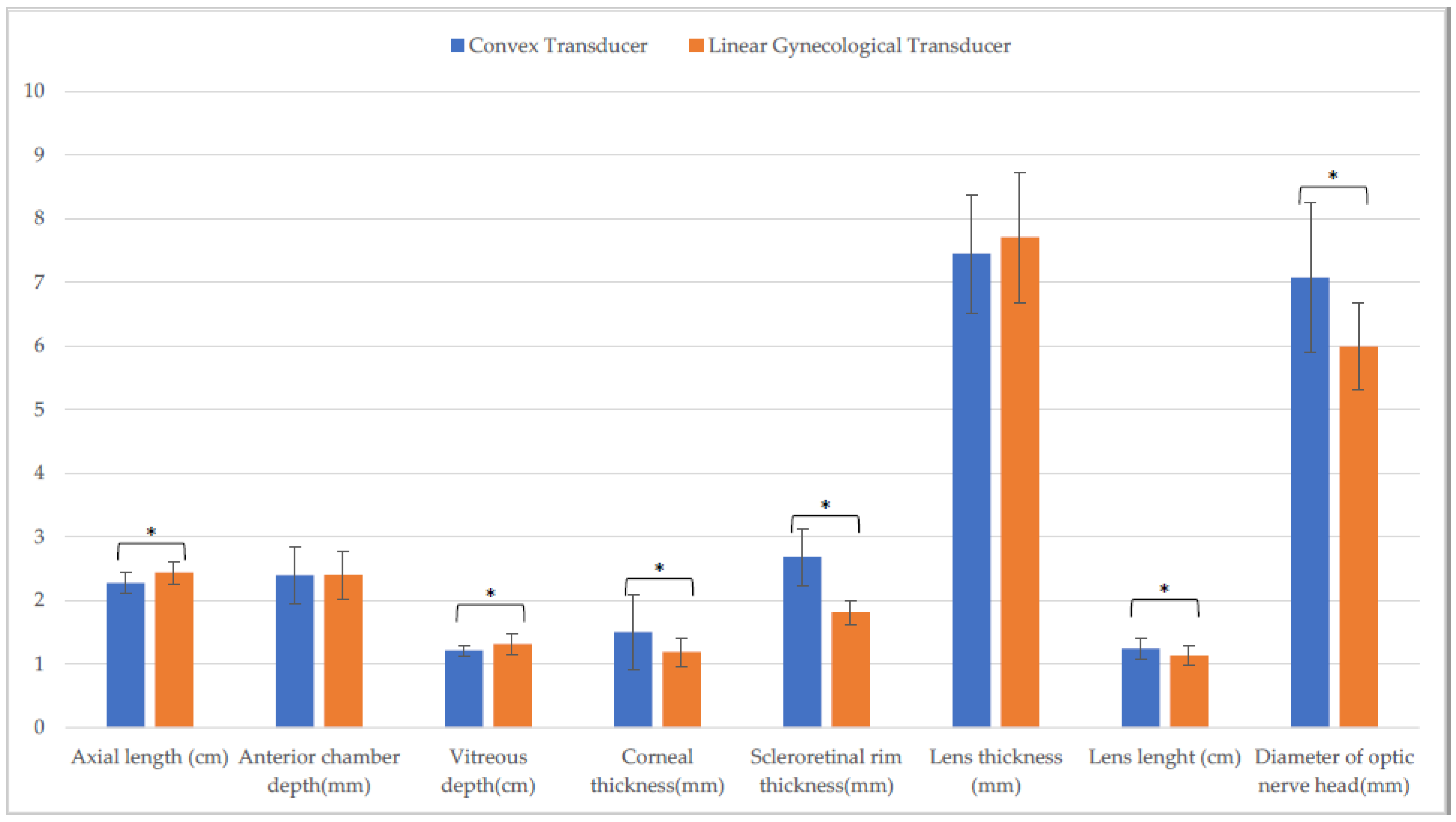
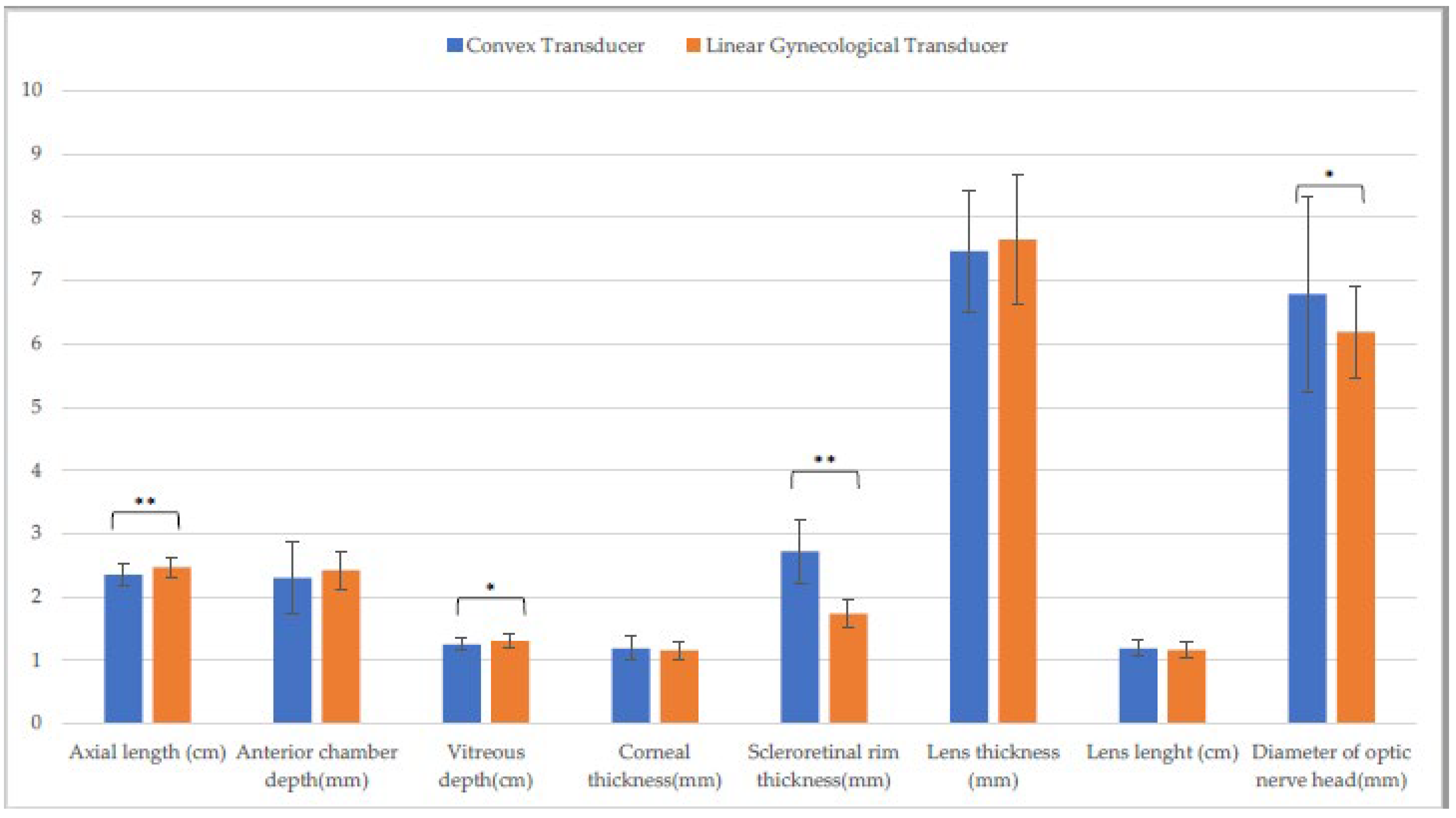

| Linear Transducer | Convex Transducer | Linear Gynecological Transducer | ||||
|---|---|---|---|---|---|---|
| Parameter | Horizontal | Vertical | Horizontal | Vertical | Horizontal | Vertical |
| Axial length (cm) | 2.5 ± (0.1) | 2.5 ± (0.1) C | 2.4 ± (0.1) L | 2.4 ± (0.1) C,Q | 2.5 ± (0.2) L | 2.5 ± (0.1) Q |
| Anterior chamber depth (mm) | 2.5 ± (0.2) | 2.6 ± (0.3) H | 2.3 ± (0.5) | 2.2 ± (0.6) | 2.5 ± (0.4) | 2.4 ± (0.3) H |
| Vitreous depth (cm) | 1.3 ± (0.1) A | 1.3 ± (0.1) | 1.2 ± (0.1) A | 1.3 ± (0.1) | 1.3 ± (0.2) | 1.3 ± (0.1) |
| Corneal thickness (mm) | 1.1 ± (0.1) A,F | 1.0 ± (0.1) D,I | 1.6 ± (0.6) a,B,M | 1.1 ± (0.2) a,D | 1.2 ± (0.2) C,F,M | 1.1 ± (0.1) I |
| Scleroretinal rim thickness (mm) | 1.8 ± (0.2) A | 1.7 ± (0.3) E | 2.5 ± (0.4) B,N | 2.5 ± (0.3) E,R | 1.8 ± (0.2) N | 1.7 ± (0.2) R |
| Lens thickness (mm) | 7.7 ± (0.7) | 7.7 ± (0.6) | 7.7 ± (0.2) | 7.6 ± (0.9) | 7.7 ± (0.7) | 8.0 ± (0.8) |
| Lens length (cm) | 1.1 ± (0.1) | 1.2 ± (0.1) | 1.3 ± (0.1) b,O | 1.2 ± (0.1) b | 1.1 ± (0.2) O | 1.2 ± (0.1) |
| Optic nerve head diameter (mm) | 5.6 ± (0.5) G | 5.7 ± (0.7) | 7.1 ± (1.1) P | 6.6 ± (2.0) | 6.1 ± (0.6) G.P | 6.1 ± (0.8) |
| Linear | Convex | Linear Gynecological | ||||
|---|---|---|---|---|---|---|
| Parameter | Horizontal | Vertical | Horizontal | Vertical | Horizontal | Vertical |
| Axial length (cm) | 2.4 ± (0.1) A | 2.4 ± (0.1) F | 2.2 ± (0.2) b,A,O | 2.3 ± (0.2) b,F,S | 2.4 ± (0.2) O | 2.4 ± (0.2) S |
| Anterior chamber depth (mm) | 2.6 ± (0.6) | 2.7 ± (0.2) | 2.6 ± (0.3) | 2.4 ± (0.5) | 2.4 ± (0.3) | 2.5 ± (0.2) |
| Vitreous depth (cm) | 1.3 ± (0.1) B | 1.3 ± (0.1) G | 1.2 ± (0.1) B,P | 1.2 ± (0.1) G,T | 1.3 ± (0.1) P | 1.1 ± (0.1) T |
| Corneal thickness (mm) | 1.1 ± (0.1) C,M | 1.1 ± (0.2) H,N | 1.4 ± (0.5) C | 1.2 ± (0.2) H | 1.2 ± (0.3) M | 1.2 ± (0.2) N |
| Scleroretinal rim thickness (mm) | 1.8 ± (0.3) a,D | 2.0 ± (0.2) a,I,O | 2.8 ± (0.5) D,Q | 3.0 ± (0.6) I,U | 1.8 ± (0.2) Q | 1.8 ± (0.2) O,U |
| Lens thickness (mm) | 7.5 ± (1.6) | 7.6 ± (1.1) | 7.1 ± (1.3) | 7.2 ± (1.0) | 7.7 ± (1.3) | 7.3 ± (1.2) |
| Lens length (cm) | 1.1 ± (0.1) | 1.1 ± (0.2) | 1.2 ± (0.2) | 1.2 ± (0.1) | 1.1 ± (0.2) | 1.2 ± (0.1) |
| Diameter of optic nerve head (mm) | 5.8 ± (0.6) E | 5.8 ± (0.9) L | 7.0 ± (1.3) E,R | 7.0 ± (1.0) L,V | 5.9 ± (0.8) R | 6.3 ± (0.7) V |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borriello, G.; Valentini, F.; Rampinelli, M.; Ferrini, S.; Cagnotti, G.; D’angelo, A.; Bellino, C. Ocular Ultrasonography in Healthy Calves with Different Transducers. Animals 2023, 13, 742. https://doi.org/10.3390/ani13040742
Borriello G, Valentini F, Rampinelli M, Ferrini S, Cagnotti G, D’angelo A, Bellino C. Ocular Ultrasonography in Healthy Calves with Different Transducers. Animals. 2023; 13(4):742. https://doi.org/10.3390/ani13040742
Chicago/Turabian StyleBorriello, Giuliano, Flaminia Valentini, Mauro Rampinelli, Sara Ferrini, Giulia Cagnotti, Antonio D’angelo, and Claudio Bellino. 2023. "Ocular Ultrasonography in Healthy Calves with Different Transducers" Animals 13, no. 4: 742. https://doi.org/10.3390/ani13040742
APA StyleBorriello, G., Valentini, F., Rampinelli, M., Ferrini, S., Cagnotti, G., D’angelo, A., & Bellino, C. (2023). Ocular Ultrasonography in Healthy Calves with Different Transducers. Animals, 13(4), 742. https://doi.org/10.3390/ani13040742






