Effects of Tea Tree Oil on Production Performance, Serum Parameter Indices, and Immunity in Postpartum Dairy Cows
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Tea Tree Oil
2.2. Animals
2.3. Sample Collection and Index Determination
2.3.1. Feed Intake Sampling and Analysis
2.3.2. Milk Sampling and Analysis
2.3.3. Blood Collection and Sampling Analysis
2.3.4. Elisa Analysis
2.4. Statistical Analysis
3. Results
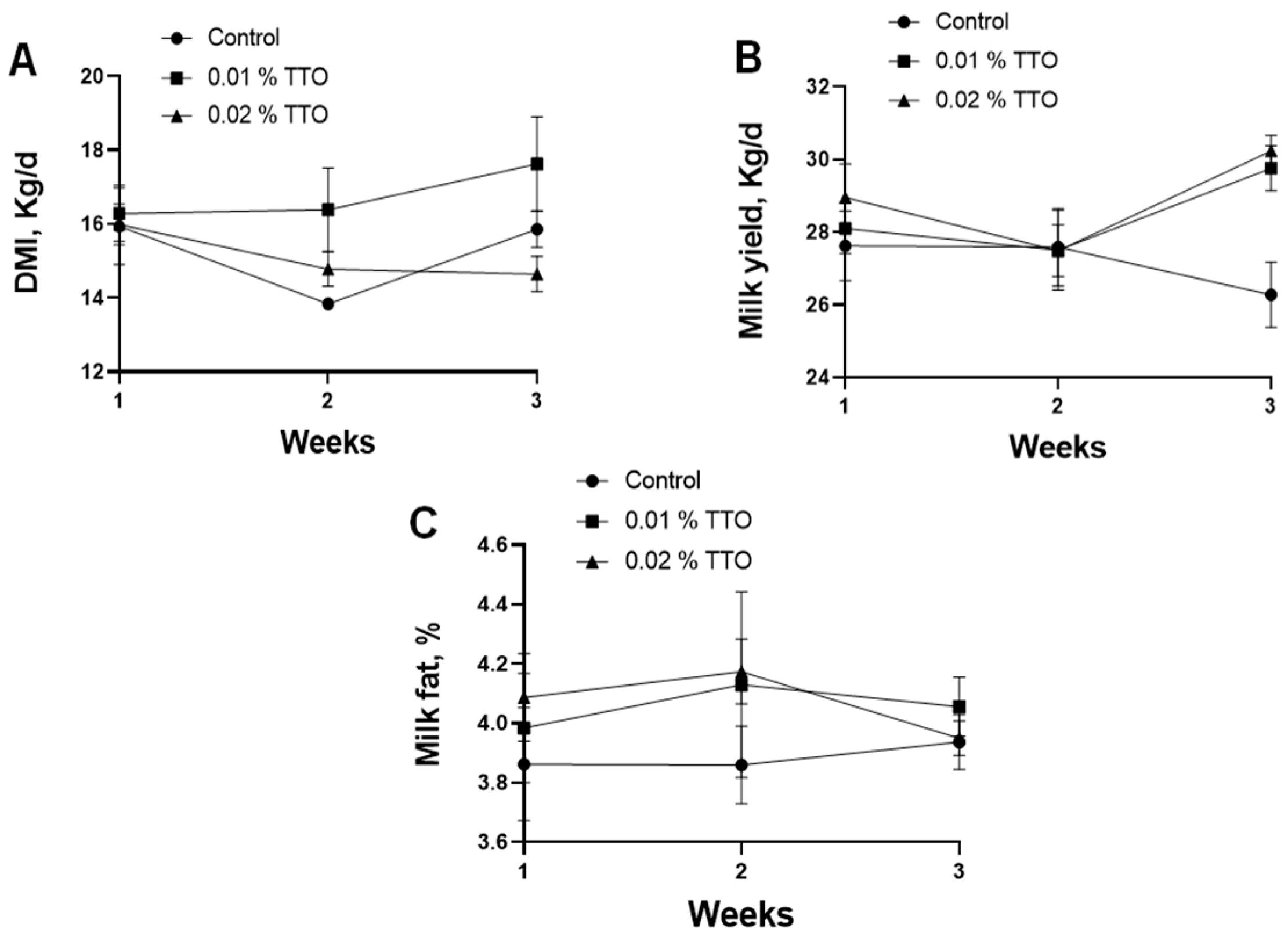
3.1. Effects of TTO on Production Performance in Postpartum Dairy Cows
3.2. Effects of TTO on Serum Biochemical Indices in Postpartum Dairy Cows
3.3. Effects of TTO on Serum Nitrogen Content in Postpartum Dairy Cows
3.4. Effects of TTO on Serum Glucolipid Metabolism in Postpartum Dairy Cows
3.5. Effects of TTO on Serum Immune Indices in Postpartum Dairy Cows
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hippen, A.R.; DeFrain, J.M.; Linke, P.L. Glycerol and other energy sources for metabolism and production of transition dairy bovine. Fla. Rumin. Nutr. Symp. 2008, 605, 1–16. [Google Scholar]
- Mezzetti, M.; Bionaz, M.; Trevisi, E. Interaction between inflammation and metabolism in periparturient dairy cows. J. Anim. Sci. 2020, 98, s155–s174. [Google Scholar] [CrossRef] [PubMed]
- Turner, H.S.; Uzi, M.; Hadar, K.; Gitit, K.; Yishai, L.; Laman, K.M.; Barry, J.B.; Maya, Z. Characterization of the liver proteome in dairy cows experiencing negative energy balance at early lactation. J. Proteom. 2021, 246, 104308. [Google Scholar]
- Miqueo, E.; Chiarle, A.; Giuliodori, M.; Relling, A. Association between prepartum metabolic status and resumption of postpartum ovulation in dairy cows. Domest. Anim. Endocrinol. 2019, 69, 62–67. [Google Scholar] [CrossRef]
- Federman, C.; Joo, J.; Almario, J.A.; Salaheen, S.; Biswas, D. Citrus-derived oil inhibits Staphylococcus aureus growth and alters its interactions with bovine mammary cells. Dairy Sci. 2016, 99, 3667–3674. [Google Scholar] [CrossRef]
- Kilani, S.; Ben Sghaier, M.; Limem, I.; Bouhlel, I.; Boubaker, J.; Bhouri, W.; Skandrani, I.; Neffatti, A.; Ben Ammar, R.; Dijoux-Franca, M.G.; et al. In vitro evaluation of antibacterial, antioxidant, cytotoxic and apoptotic activities of the tubers infusion and extracts of Cyperus rotundus. Bioresour. Technol. 2008, 99, 9004–9008. [Google Scholar] [CrossRef]
- Hu, Z.X.; Lin, M.; Ma, X.Y.; Zhao, G.Q.; Zhan, K. Effect of Tea Tree Oil on the Expression of Genes Involved in the Innate Immune System in Goat Rumen Epithelial Cells. Animals 2021, 11, 2460. [Google Scholar] [CrossRef]
- Yang, T.Y.; Jiang, M.C.; Cheng, Z.Q.; Datsomor, O.; Zhao, G.Q.; Zhan, K. The Role of Tea Tree Oil in Alleviating Palmitic Acid-Induced Lipid Accumulation in Bovine Hepatocytes. Front. Vet. Sci. 2022, 8, 814840. [Google Scholar] [CrossRef]
- Zhan, K.; Yang, T.Y.; Feng, B.B.; Zhu, X.Y.; Chen, Y.Y.; Huo, H.J.; Zhao, G.Q. The protective roles of tea tree oil extracts in bovine mammary epithelial cells and polymorphonuclear leukocytes. J. Anim. Sci. Biotechnol. 2020, 11, 62. [Google Scholar] [CrossRef]
- Urrutia, N.; Harvatine, K.J. Effect of conjugated linoleic acid and acetate on milk fat synthesis and adipose lipogenesis in lactating dairy cows. J. Dairy Sci. 2017, 100, 5792–5804. [Google Scholar] [CrossRef]
- Zhang, F.; Nan, X.; Wang, H.; Zhao, Y.; Guo, Y.; Xiong, B. Effects of Propylene Glycol on Negative Energy Balance of Postpartum Dairy Cows. Animals 2020, 10, 1526. [Google Scholar] [CrossRef] [PubMed]
- NRC. Nutrient Requirements of Dairy Cattle, 7th ed.; National Academy Press: Washington, DC, USA, 2001. [Google Scholar]
- Yang, T.Y.; Feng, F.F.; Zhan, K.; Ma, X.Y.; Jiang, M.C.; Datsomor, O.; Zhu, X.Y.; Huo, Y.J.; Zhao, G.Q. Effect of the Tea Tree Oil on Growth Performance, Meat Quality, Serum Biochemical Indices, and Antioxidant Capacity in Finishing Pigs. Front. Vet. Sci. 2022, 9, 916625. [Google Scholar] [CrossRef] [PubMed]
- Ran, M.; Cha, C.; Xu, Y.; Zhang, H.; Yang, Z.; Li, Z.; Wang, S. Traditional Chinese herbal medicine complex supplementation improves reproductive performance, serum biochemical parameters, and anti-oxidative capacity in periparturient dairy bovine. Anim. Biotechnol. 2022, 3, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.O.; Badry, K.A.; Zalzala, S.J.; Zakri, A.M. Activity of transaminase enzyme and testosterone hormone in blood of Awassi rams during different season. Asian Pac. J. Reprod. 2017, 6, 217. [Google Scholar]
- Zheng, J.; Liang, S.; Zhang, Y.; Sun, X.; Li, Y.; Diao, J.; Dong, L.; Ni, H.; Yin, Y.; Ren, J.; et al. Effects of Compound Chinese Herbal Medicine Additive on Growth Performance and Gut Microbiota Diversity of Zi Goose. Animals 2022, 12, 2942. [Google Scholar] [CrossRef]
- Wang, L.X.; Zhang, Y.; Liu, L.; Huang, F.; Dong, B. Effects of Three-Layer Encapsulated Tea Tree Oil on Growth Performance, Antioxidant Capacity, and Intestinal Microbiota of Weaned Pigs. Front. Vet. Sci. 2021, 8, 789225. [Google Scholar] [CrossRef]
- Koriem, K.M.M. Lead toxicity and the protective role of Cupressus sempervirens seeds growing in Egypt. Rev. Latinoam. Quim. 2009, 37, 230–242. [Google Scholar]
- Hashem, M.A.; Abd El Hamied, S.S.; Ahmed, E.M.A.; Amer, S.A.; El-Sharnouby, M.E. Mitigating the Growth, Biochemical Changes, Genotoxic and Pathological Effects of Copper Toxicity in Broiler Chickens by Supplementing Vitamins C and E. Animals 2021, 11, 1811. [Google Scholar] [CrossRef]
- Mohanta, K.N.; Subramanian, S.; Korikanthimath, V.S. Effect of Different Animal Protein Sources on Growth and Nutrient Utilization of Guppy, Poecilia reticulata Fingerlings. Proc. Zool. Soc. 2016, 69, 96–103. [Google Scholar] [CrossRef]
- Stanley, C.C.; Williams, C.C.; Jenny, B.F.; Fernandez, J.M.; Bateman Ii, H.G.; Nipper, W.A.; Lovejoy, J.C.; Gantt, D.T.; Goodier, G.E. Effects of Feeding Milk Replacer Once Versus Twice Daily on Glucose Metabolism in Holstein and Jersey Calves. J. Dairy Sci. 2002, 85, 2335–2343. [Google Scholar] [CrossRef]
- Michiels, J.; Maertens, L.; Buyse, J.; Lemme, A.; Rademacher, M.; Dierick, N.A.; De Smet, S. Supplementation of guanidinoacetic acid to broiler diets: Effects on performance, carcass characteristics, meat quality, and energy metabolism. Poult. Sci. 2012, 91, 402–412. [Google Scholar] [CrossRef] [PubMed]
- Yakubu, M.T.; Bilbis, L.S.; Lawal, M.; Akanji, M.A. Evaluation of selected parameters of rat liver and kidney function following repeated administration of yohimbine. Biokemistri 2003, 15, 50–56. [Google Scholar]
- Tang, X.; Xiong, K. Dietary Epidermal Growth Factor Supplementation Alleviates Intestinal Injury in Piglets with Intrauterine Growth Retardation via Reducing Oxidative Stress and Enhancing Intestinal Glucose Transport and Barrier Function. Animals 2022, 12, 2245. [Google Scholar] [CrossRef] [PubMed]
- Noya, A.; Casasús, I.; Ferrer, J.; Sanz, A. Long-Term Effects of Maternal Subnutrition in Early Pregnancy on Cow-Calf Performance, Immunological and Physiological Profiles during the Next Lactation. Animals 2019, 9, 936. [Google Scholar] [CrossRef]
- Lykos, T.; Varga, G.A. Varying Degradation Rates of Total Nonstructural Carbohydrates: Effects on Nutrient Uptake and Utilization by the Mammary Gland in High Producing Holstein Cows. J. Dairy Sci. 1997, 80, 3356–3367. [Google Scholar] [CrossRef]
- Pullen, D.L.; Liesman, J.S.; Emery, R.S. A species comparison of liver slice synthesis and secretion of triacylglycerol from nonesterified fatty acids in media. J. Anim. Sci. 1990, 68, 1395–1399. [Google Scholar] [CrossRef]
- Wheeler, T.L.; Davis, G.W.; Stoecker, B.J.; Harmon, C.J. Cholesterol concentration of longissimus muscle, subcutaneous fat and serum of two beef cattle breed types. J. Anim. Sci. 1987, 65, 1531–1537. [Google Scholar] [CrossRef]
- Gong, S.Q.; Ruprecht, R.M. Immunoglobulin M: An Ancient Antiviral Weapon—Rediscovered. Front. Immunol. 2020, 11, 1943. [Google Scholar] [CrossRef]
- Veerle, S.; Iain, R.P.; Eric, C. The IgA system: A comparison of structure and function in different species. Vet. Res. 2006, 37, 455–467. [Google Scholar]
- Viola, I.; Tizzani, P.; Perona, G.; Lussiana, C.; Mimosi, A.; Ponzio, P.; Cornale, P. Hazelnut Skin in Ewes’ Diet: Effects on Colostrum Immunoglobulin G and Passive Transfer of Immunity to the Lambs. Animals 2022, 12, 3220. [Google Scholar] [CrossRef]
- Ryu, C.H.; Kim, B.H.; Lee, S.; Bang, H.T.; Baek, Y.C. Effects of Supplemented Resveratrol on in Vitro Ruminal Fermentation and Growth Performance of Hanwoo Calves. Animals 2022, 12, 3420. [Google Scholar] [CrossRef] [PubMed]
| Item | % |
|---|---|
| Powder mix 1 | 22.73 |
| Expanded soybean meal | 2.19 |
| Soybean hulls | 2.19 |
| Whole cottonseed | 4.39 |
| Alfalfa | 3.95 |
| Oats | 3.29 |
| Alfalfa silage | 6.58 |
| Alfalfa corn | 48.25 |
| Molasses | 2.19 |
| Yeast | 0.07 |
| Fat powder | 0.66 |
| Lysine | 0.02 |
| Sodium bicarbonate | 0.64 |
| Sodium chloride | 1.21 |
| Methionine | 0.07 |
| Urea | 0.18 |
| Vitamin and mineral mix 2 | 1.39 |
| Nutrient composition | |
| NEL 3 (MJ/kg) | 6.78 |
| CP | 16.15 |
| NDF | 33.51 |
| ADF | 21.72 |
| Ash | 7.12 |
| Ca | 0.42 |
| P | 0.25 |
| EE | 3.75 |
| Item | Treatment 1 | SEM | p-Value | ||
|---|---|---|---|---|---|
| Control | 0.01% TTO | 0.02% TTO | |||
| DMI (kg/d) | 15.21 | 16.76 | 15.14 | 0.33 | 0.07 |
| Milk yield (kg/d) | 27.16 | 28.45 | 28.89 | 0.37 | 0.13 |
| Milk fat (%) | 3.88 | 4.05 | 4.07 | 0.39 | 0.58 |
| 4% FCM (kg/d) 2 | 26.68 | 28.67 | 29.41 | 0.53 | 0.09 |
| Feed efficiency 3 | 1.80 | 1.77 | 1.95 | 0.05 | 0.29 |
| Item | Treatment 1 | SEM | p-Value | ||
|---|---|---|---|---|---|
| Control | 0.01% TTO | 0.02% TTO | |||
| ALT | 24.20 | 20.54 | 18.30 | 1.33 | 0.19 |
| AST | 92.29 | 85.16 | 81.54 | 1.93 | 0.063 |
| ALP | 60.38 | 55.25 | 57.63 | 1.37 | 0.32 |
| Item | Treatment 1 | SEM | p-Value | ||
|---|---|---|---|---|---|
| Control | 0.01% TTO | 0.02% TTO | |||
| TP (g/L) | 71.55 | 72.54 | 73.21 | 0.56 | 0.50 |
| ALB (g/L) | 26.00 | 27.71 | 26.11 | 0.35 | 0.08 |
| GLO (g/L) | 45.55 b | 44.83 b | 47.10 a | 0.25 | <0.05 |
| CREA (μmol/L) | 60.01 | 61.41 | 60.53 | 0.80 | 0.79 |
| BUN (μmol/L) | 5.20 | 5.07 | 4.91 | 0.13 | 0.67 |
| Item | Treatment 1 | SEM | p-Value | ||
|---|---|---|---|---|---|
| Control | 0.01% TTO | 0.02% TTO | |||
| Glucose (mmol/L) | 2.79 b | 3.02 ab | 3.21 a | 0.06 | <0.05 |
| CHO (mmol/L) | 2.84 | 2.77 | 2.80 | 0.06 | 0.93 |
| TG (mmol/L) | 0.152 | 0.139 | 0.133 | 0.005 | 0.233 |
| HDL (mmol/L) | 2.99 | 2.87 | 3.11 | 0.51 | 0.15 |
| LDL (mmol/L) | 1.67 | 1.65 | 1.39 | 0.06 | 0.14 |
| Item (g/L) | Treatment 1 | SEM | p-Value | ||
|---|---|---|---|---|---|
| Control | 0.01% TTO | 0.02% TTO | |||
| IgG | 5.20 b | 5.36 ab | 5.59 a | 0.07 | 0.045 |
| IgM | 2.38 | 2.43 | 2.89 | 0.12 | 0.18 |
| IgA | 0.41 | 0.42 | 0.43 | 0.007 | 0.40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, C.; Ma, X.; Jiang, M.; Yang, T.; Lin, M.; Zhao, G.; Zhan, K. Effects of Tea Tree Oil on Production Performance, Serum Parameter Indices, and Immunity in Postpartum Dairy Cows. Animals 2023, 13, 682. https://doi.org/10.3390/ani13040682
Yuan C, Ma X, Jiang M, Yang T, Lin M, Zhao G, Zhan K. Effects of Tea Tree Oil on Production Performance, Serum Parameter Indices, and Immunity in Postpartum Dairy Cows. Animals. 2023; 13(4):682. https://doi.org/10.3390/ani13040682
Chicago/Turabian StyleYuan, Cong, Xiaoyu Ma, Maocheng Jiang, Tianyu Yang, Miao Lin, Guoqi Zhao, and Kang Zhan. 2023. "Effects of Tea Tree Oil on Production Performance, Serum Parameter Indices, and Immunity in Postpartum Dairy Cows" Animals 13, no. 4: 682. https://doi.org/10.3390/ani13040682
APA StyleYuan, C., Ma, X., Jiang, M., Yang, T., Lin, M., Zhao, G., & Zhan, K. (2023). Effects of Tea Tree Oil on Production Performance, Serum Parameter Indices, and Immunity in Postpartum Dairy Cows. Animals, 13(4), 682. https://doi.org/10.3390/ani13040682






